Abstract
This study aims to develop an amperometric glucose biosensor, based on carbon nanotubes material for reverse iontophoresis, fabricated by immobilizing a mixture of glucose oxidase (GOD) and multiwalled carbon nanotubes (MWCNT) epoxy-composite, on a planar screen-printed carbon electrode. MWCNT was employed to ensure proper incorporation into the epoxy mixture and faster electron transfer between the GOD and the transducer. Results showed this biosensor possesses a low detection potential (+500 mV), good sensitivity (4 μA/mM) and an excellent linear response range (r2 = 0.999; 0–4 mM) of glucose detection at +500 mV (versus Ag/AgCl). The response time of the biosensor was about 25 s. In addition, the biosensor could be used in conjunction with reverse iontophoresis technique. In an actual evaluation model, an excellent linear relationship (r2 = 0.986) was found between the glucose concentration of the actual model and the biosensor’s current response. Thus, a glucose biosensor based on carbon nanotube composites and incorporated with reverse iontophoresis function was developed.
Keywords: amperometric, carbon nanotubes, glucose monitoring, biosensors, reverse iontophoresis
Introduction
The discovery of carbon nanotube (CNT) in 1991, led to many new technical developments and applications because of the characteristics of large surface area, unique electronic properties, and relatively high mechanical strength associated with it.1 Recent studies demonstrated high electrocatalytic effect and fast electron-transfer rate in CNT material2–6 and thereby provide a new material for fabricating biosensors.7,8 Moreover, CNT can reduce the surface fouling on electrochemical devices without a mediator. The ability of CNT in facilitating electron transfer of hydrogen peroxide (H2O2) shows great promise as oxidase-based amperometric biosensors.6,9 To the best of our knowledge there is no report on the use of CNT composites in glucose biosensor, incorporated with reverse iontophoresis function, for noninvasive glucose monitoring.
Reverse iontophoresis is a technique using a small electric charge to extract both charged and neutral molecules through the skin, recently used for patient monitoring.10–15 The authors only found reports of a glucose sensor integrated with reverse iontophoresis function.11,14–30 This glucose biosensor, approved by the US Food and Drug Administration, is GlucoWatch® biographer, which passes a small current between two skin-surface hydrogel electrodes to extract glucose-containing interstitial fluid into hydrogel pads, incorporating a glucose oxidase (GOD) biosensor.14,17,31 Unfortunately, GlucoWatch causes several problems, such as skin irritation or rash under the device in a number of patients. This problem may be due to the immobilization of GOD inside the hydrogel pads releasing peroxide. Even worse, GlucoWatch is unreliable in detecting hypoglycemia and hyperglycemia.16
The aim of this study is thus to find out the optimum combination of multiwalled carbon nanotubes and GOD for a glucose biosensor, in order to develop a new glucose biosensor incorporated with reverse iontophoresis function for noninvasive glucose monitoring. The glucose biosensor for reverse iontophoresis was eventually evaluated in this study.
Materials and methods
Reagents and solutions
All reagents used in this study, were commercially available and used without further purification: GOD (type VII), β–D(+) glucose, phosphate buffered saline, multiwalled carbon nanotubes (MWCNT, 0.5–50 μm, >95% purity) powders were purchased from Sigma Chemical Co. (St. Louis, MO, USA); Methylcellulose (MC, Methocel A4M Prem) from Dow Chemical Co. (Midland, MI, USA). Deionized water purified by a Millipore System (Milli-Q UFplus; Bedford, MA, USA) was used to prepare all solutions. Graphite paste, silver paste, silver-silver chloride (Ag/AgCl) paste, and insulating paste were purchased from Advanced Conductive Materials (Atascadero, CA, USA). Epoxy (EPO-TEK® 509FM-1) was purchased from Epoxy Technology (Billerica, MA, USA). Polyethylene terephthalate (PETE) sheet was purchased from 3M.
Construction of the glucose biosensor
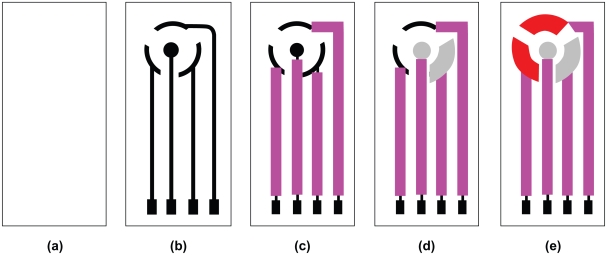
The construction steps of a planar three-electrode transducer were shown schematically in Figure 1, according to the procedure described earlier.32 The transducer was then used for glucose biosensor construction.
Figure 1.
Construction steps for the planar configuration of the screen-printed transducer. a) (PETE) support material; b) conducting silver basal track; c) insulation layer; d) Ag/AgCl pads, the iontophoresis electrode (the central circular one) for reverse iontophoresis and the reference electrode; e) graphite pads, the working and counter electrodes.
A biocomposite paste was prepared by mixing MWCNT (18.0%–19.5% w/w) with GOD (0.5%–2.0% w/w), followed by the incorporation of epoxy (80.0% w/w) and further 30 minutes mixing, in order to obtain a homogeneous biocomposite paste. This paste (10 μL) was then coated onto the surface of a graphite pad (working electrode) of the transducer and dried for 3 days at 30°C. Unused glucose biosensors were kept in the dark at 4°C.
Hydrodynamic voltammetry measurements of the glucose biosensor
Using an electrochemical interface (electrochemical interface SI1286, Schlumberger Technologies, England), measurements were taken at 25°C with the biosensor in a solution of 4 mM glucose in 0.1 M phosphate buffer (pH 7.0) subjected to constant stirring. Incremental potentials (0–1000 mV, 100 mV increments) versus Ag/AgCl pad were applied to the working electrode of the biosensor and the current responses of the biosensor to glucose were measured.
Measurements of the biosensor current response to glucose
Measurements were carried out at 25°C at the electrochemical interface. The biosensor was placed in a cell containing 0.1 M phosphate buffer solution pH 7.0, subjected to constant stirring, employed as supporting electrolyte. An applied potential of +500 mV versus Ag/AgCl pad was applied to the working electrode of the biosensor. After the background current stabilized, different glucose solution was added to the constantly-stirred buffer solution and the current response of the biosensor to glucose was then measured.
Evaluation of the ferrocene-mediated glucose biosensor for reverse iontophoresis
A 90 μL of a 4% MC gel (prepared by mixing 4 g of MC with 100 mL of 0.1 M phosphate buffer pH 7.0) was laced onto two biosensors made up of optimum combination of MWCNT (18.0% w/w) and GOD (2.0% w/w). The two biosensors were then fixed onto the nanoporous membrane (Spectra/Por® CE, MWCO: 500, Spectrum Laboratories, Inc., Canada) of our custom-developed diffusion cell,33 filled with different concentrations of glucose solutions, and the iontophoresis electrode center of the biosensors was 23 mm apart. Using our custom-developed reverse iontophoresis device34 at room temperature (22–24°C), a bipolar current (0.3 mA/cm2, period = 30 minutes, ie, 555.6 μHz) was passed between the iontophoresis electrodes for 90 minutes. At the end of the experiment, biosensors were connected to the electrochemical interface for current response measurement and an applied potential of +500 mV versus Ag/AgCl pad was applied to the working electrode.
Results and discussion
The amperometric transducer
Good inert behavior of the counter electrode is very important and hence elements such as gold and platinum are commonly used.35,36 On the other hand, there is no report of any meaningful relationship between current density and the materials used and their geometries.37 Therefore graphite, an inert material, was used as counter electrode material in this study based on their availability and affordability.
The ratio of counter electrode area to working electrode area (AC/AW), can exert influence on the supply of determined current to the working electrode without limiting its response.38 The optimal AC/AW ratio was reported to be in the range of 1 and 12.39–43 Composite material used in this study allows fabrication of an inert graphite counter electrode with an AC/AW ratio of at least 0.125. Furthermore, there is no great difference between the current density of transducer constructed with AC/AW ratio 0.11 and 9.20.37 Therefore the AC/AW ratio of 1 was employed on constructing the amperometric transducer in this study.
Hydrodynamic voltammogram of the glucose biosensor
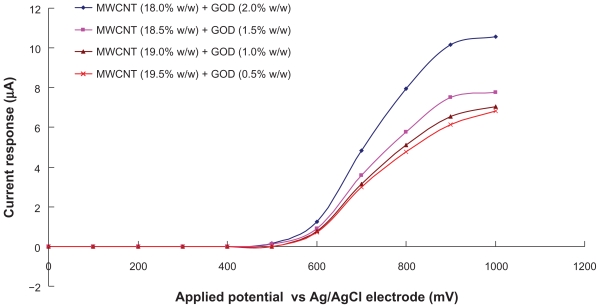
Several reports7,8 have suggested that carbon nanotube can significantly facilitates catalytic effect, both on the reduction and oxidation of hydrogen peroxide (H2O2), making it highly sensitive in detection of H2O2. Figure 2 illustrated the hydrodynamic voltammogram of the biosensors at a constantly-stirred solution of 4 mM glucose in 0.1 M phosphate buffer (pH 7.0). It was found that the oxidation currents generated by the biosensors begin at potential about +500 mV. Carbon nanotube therefore can significantly facilitate the electron transfer between H2O2 molecules.7,8 Moreover, carbon nanotube promotes the low oxidation potential amperometric determination of glucose. As shown in Figure 2, biosensor with the combination of MWCNT (18.0% w/w) and GOD (2.0% w/w) is optimal for detecting and generating glucose signals, than mixture with other ratios.
Figure 2.
Hydrodynamic voltammogram of the biosensors in constantly-stirred solution of 4 mM glucose with 0.1 M phosphate buffer (pH 7.0).
Abbreviations: GOD, glucose oxidase; MWCNT, multiwalled carbon nanotubes.
Calibration of the biosensor to glucose
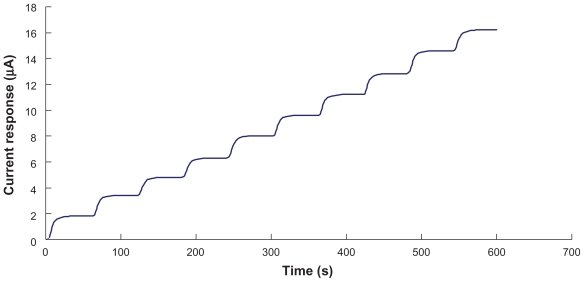
Figure 3 showed a current-time curve, where steps correspond to current response of the biosensor (MWCNT [18.0% w/w] and GOD [2.0% w/w] mixture) at the applied potential of +500 mV, for successive additions of 4 mM glucose in 0.1 M phosphate buffer solution (pH 7.0). Immediately after the addition of glucose solution, the reductive current increased and reached 95% of the steady state current at about 25 seconds. The response time of our biosensor is comparable to that reported by Antiochia and Gorton.44
Figure 3.
Current-time curve obtained by successive addition of 4 mM glucose solution to the biosensor. Data is obtained from biosensor with the combination of MWCNT (18.0% w/w) and GOD (2.0% w/w).
Abbreviations: GOD, glucose oxidase; MWCNT, multiwalled carbon nanotubes.
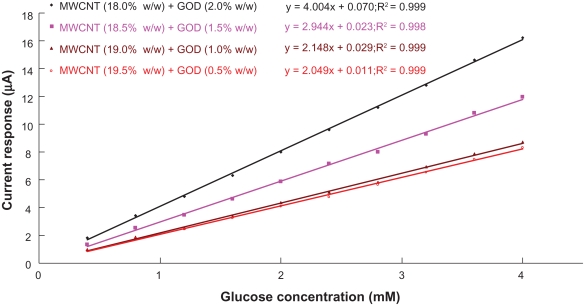
From the data of current-time curves, the magnitude of current response was plotted against the glucose concentration (see Figure 4) and the sensitivity of the biosensor was determined by the slope of linear regression of this current response versus glucose concentration. The current response is directly proportional to the glucose concentration over a wide range of concentration (0–4 mM), where correlation coefficient is greater than 0.997. Since the amount of transdermal glucose extraction by reverse iontophoresis is in the order of μM,28 the range of measurement of glucose biosensor designed in this study can detect the normal concentrations of transdermally extracted glucose with good accuracy.
Figure 4.
Calibration curves of the biosensors to glucose concentration. The lines are best fit found by linear regression. Sensitivity of the biosensor is indicated by the slope of the linear regression line.
Abbreviations: GOD, glucose oxidase; MWCNT, multiwalled carbon nanotubes.
Biosensor constructed with MWCNT (18.0% w/w) and GOD (2.0% w/w), as shown in Figure 4, has the greatest sensitivity of 4.0 μA/mM, more than 1.36 times the other biosensors. Therefore, the combination of 18.0% w/w of MWCNT and 2.0% w/w of GOD is considered as the optimum combination for constructing glucose biosensor in this study.
The reproducibility in the construction of the biosensor was evaluated from standard deviation of biosensor sensitivity. As shown in Table 1, the relative standard deviation of biosensor sensitivity indicated a batch reproducibility of biosensor construction of about 4% (n = 5).
Table 1.
The sensitivity (n = 5) of the biosensor at different combinations of glucose oxidase (GOD) loading and multiwalled carbon nanotubes (MWCNT) loading
| Biosensor | MWCNT (%) | GOD (%) | Sensitivity (μA/mM) | Relative SD |
|---|---|---|---|---|
| 1 | 18.0 | 2.0 | 4.13 ± 0.14 | 3.4% |
| 2 | 18.5 | 1.5 | 3.02 ± 0.11 | 3.6% |
| 3 | 19.0 | 1.0 | 2.05 ± 0.07 | 3.4% |
| 4 | 19.5 | 0.5 | 2.01 ± 0.08 | 4.0% |
Although the glucose biosensor of the GlucoWatch has a linear (r2 ~ 0.98) glucose response range of 0–28 mM which is larger than the one in this study (r2 ~ 1.00, 0–4 mM), the amount of transdermal glucose extraction by reverse iontophoresis are several orders of magnitude lower than those present in the blood (~5 μM vs ~ 5 mM).28 Therefore, our glucose biosensor can cover the concentrations of the transdermally extracted glucose and be useful in a clinical setting.
The use of the biosensor for noninvasive glucose measurement by reverse iontophoresis
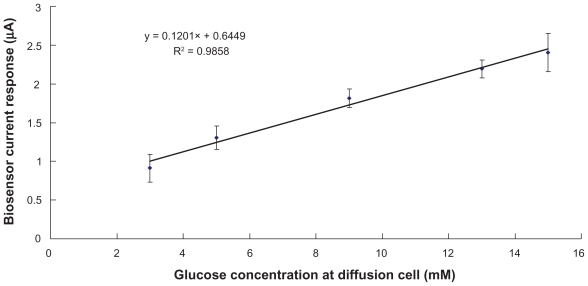
The biosensor, with the optimum combination of MWCNT (18.0% w/w) and GOD (2.0% w/w), coupled to our custom-developed reverse iontophoresis device34 has been tested in this study. Transmembrane (the membrane of the diffusion cell) glucose extraction, achieved by means of the reverse iontophoresis, was then quantified by the biosensor. The magnitude of the biosensor current response was plotted against the glucose concentration inside the diffusion cell (see Figure 5). In order to simulate hypoglycemia, normal and hyperglycemia situations, glucose concentrations of 3 mM, 5 mM and 15 mM was chosen respectively for testing inside the diffusion cell. An excellent linear relationship (r2 = 0.99) was found between the diffusion cell glucose concentration (3–15 mM) and biosensor current response. Based on the results, our glucose biosensor was accurate enough to be used to measure glucose levels in both condition of hypoglycemia and hyperglycemia, as opposed to GlucoWatch®’s unreliability in detecting hypoglycemia and hyperglycemia.45
Figure 5.
Evaluation of the biosensor for noninvasive glucose measurement by reverse iontophoresis. An excellent linear relationship (r2 = 0.986) between the biosensor current response and glucose concentration in diffusion cell was found. Data (n = 5) was obtained from biosensor with the optimum combination of MWCNT (18.0% w/w) and GOD (2.0% w/w). The diffusion cell was filled with an electrolyte solution comprising 0.1 M phosphate buffer (pH 7.0) and 3–15mM glucose.
Abbreviations: GOD, glucose oxidase; MWCNT, multiwalled carbon nanotubes.
Conclusion
A simple, low-cost carbon nanotube composite-based glucose biosensor with low oxidation potential and high sensitivity was developed in this study. The use of the biosensor incorporated with reverse iontophoresis for noninvasive glucose determination at glucose range for hypoglycemia, normal and hyperglycemia situations were also demonstrated to be successful and potentially useful in clinical setting, better than the presently commercialized product.
Acknowledgments
This work was supported by grants (NSC 98-2221-E-260- 002- and NSC 98-2221-E-260-024-MY3) from National Science Council, Taiwan, Republic of China. Also, this work was partially supported by grant (98A032) from the National Chi Nan University and Puli Christian Hospital, Taichung, Taiwan, Republic of China.
Footnotes
Disclosures
The authors report no conflicts of interest in this work.
References
- 1.Moore RR, Banks CE, Compton RG. Basal plane pyrolytic graphite modified electrodes: comparison of carbon nanotubes and graphite powder as electrocatalysts. Anal Chem. 2004;76:2677–2682. doi: 10.1021/ac040017q. [DOI] [PubMed] [Google Scholar]
- 2.Gong K, Zhang M, Yan Y, et al. Sol-gel-derived ceramic-carbon nanotube nanocomposite electrodes: tunable electrode dimension and potential electrochemical applications. Anal Chem. 2004;76:6500–6505. doi: 10.1021/ac0492867. [DOI] [PubMed] [Google Scholar]
- 3.Lin Y, Rao AM, Sadanadan B, et al. Functionalizing multi-walled carbon nanotubes with aminopolymers. J Phys Chem B. 2002;106:1294–1298. [Google Scholar]
- 4.Jiang K, Eitan A, Schadler LS, et al. Selective attachment of gold nanoparticles to nitrogen-doped carbon nanotubes. Nano Lett. 2003;3:275–277. [Google Scholar]
- 5.Eitan A, Jiang KY, Dukes D, et al. Surface modification of multiwalled carbon nanotubes: toward the tailoring of the interface in polymer composites. Chem Mater. 2003;15:3198–3201. [Google Scholar]
- 6.Zhang MG, Smith A, Gorski W. Carbon nanotube-chitosan system for electrochemical sensing based on dehydrogenase enzymes. Anal Chem. 2004;76:5045–5050. doi: 10.1021/ac049519u. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Q, Gan Z, Zhuang Q. Electrochemical sensors based on carbon nanotubes. Electroanal. 2002;14:1609–1613. [Google Scholar]
- 8.Li N, Wang J, Li M. Electrochemistry at carbon nanotube electrodes. Rev Anal Chem. 2003;22:19–34. [Google Scholar]
- 9.Jiang LY, Wang RX, Li XM, et al. Electrochemical oxidation behavior of nitrite on a chitosan-carboxylated multiwall carbon nanotube modified electrod electrochem. Electrochem Commun. 2005;597:597–601. [Google Scholar]
- 10.Degim IT, Ilbasmis S, Dundaroz R, Oguz Y. Reverse iontophoresis: a noninvasive technique for measuring blood urea level. Pediatr Nephrol. 2003;18:1032–1037. doi: 10.1007/s00467-003-1217-y. [DOI] [PubMed] [Google Scholar]
- 11.Pitzer KR, Desai S, Dunn T, et al. Detection of hypoglycaemia with the GlucoWatch biographer. Diabetes Care. 2001;24:881–885. doi: 10.2337/diacare.24.5.881. [DOI] [PubMed] [Google Scholar]
- 12.Potts RO, Tamada JA, Tierney MJ. Glucose monitoring by reverse iontophoresis. Diabetes Metab Res Rev. 2002;18:S49–53. doi: 10.1002/dmrr.210. [DOI] [PubMed] [Google Scholar]
- 13.Rao G, Guy RH, Glikfeld P, et al. Reverse iontophoresis: noninvasive glucose monitoring in vivo in human. Pharm Res. 1995;12:1869–1873. doi: 10.1023/a:1016271301814. [DOI] [PubMed] [Google Scholar]
- 14.Tamada JA, Garg S, Jovanovic L, et al. Noninvasive glucose monitoring: comprehensive clinical results. Cygnus Research Team. JAMA. 1999;282:1839–1844. doi: 10.1001/jama.282.19.1839. [DOI] [PubMed] [Google Scholar]
- 15.Tierney MJ, Tamada JA, Potts RO, et al. Clinical evaluation of the GlucoWatch® biographer: a continual, non-invasive glucose monitor for patients with diabetes. Biosens Bioelectron. 2001;16:621–629. doi: 10.1016/s0956-5663(01)00189-0. [DOI] [PubMed] [Google Scholar]
- 16.Diabetes Research in Children Network (DirecNet) Study Group. Accuracy of the GlucoWatch G2 Biographer and the continuous glucose monitoring system during hypoglycemia: experience of the Diabetes Research in Children Network. Diabetes Care. 2004;27:722–726. doi: 10.2337/diacare.27.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg SK, Potts RO, Ackerman NR, et al. Correlation of fingerstick blood glucose measurements with GlucoWatch biographer glucose results in young subjects with type 1 diabetes. Diabetes Care. 1999;22:1708–1714. doi: 10.2337/diacare.22.10.1708. [DOI] [PubMed] [Google Scholar]
- 18.Tierney MJ, Garg S, Ackerman NR, et al. Effect of acetaminophen on the accuracy of glucose measurements obtained with the GlucoWatch biographer. Diabetes Technol Ther. 2000;2:199–207. doi: 10.1089/15209150050025140. [DOI] [PubMed] [Google Scholar]
- 19.Eastman RC, Chase HP, Buckingham B, et al. Use of the GlucoWatch biographer in children and adolescents with diabetes. Pediatr Diab. 2002;3:127–134. doi: 10.1034/j.1399-5448.2002.30302.x. [DOI] [PubMed] [Google Scholar]
- 20.Gandrud LM, Paguntalan HU, Van Wyhe MM, et al. Use of the Cygnus GlucoWatch biographer at a diabetes camp. Pediatrics. 2004;113:108–111. doi: 10.1542/peds.113.1.108. [DOI] [PubMed] [Google Scholar]
- 21.Nunnold T, Colberg SR, Herriott MT, Somma CT. Use of the noninvasive GlucoWatch Biographer during exercise of varying intensity. Diabetes Technol Ther. 2004;6:454–462. doi: 10.1089/1520915041705848. [DOI] [PubMed] [Google Scholar]
- 22.Sieg A, Guy RH, Delgado-Charro MB. Noninvasive glucose monitoring by reverse iontophoresis in vivo: application of the internal standard concept. Clin Chem. 2004;50:1383–1390. doi: 10.1373/clinchem.2004.032862. [DOI] [PubMed] [Google Scholar]
- 23.Dunn TC, Eastman RC, Tamada JA. Rates of glucose change measured by blood glucose meter and the GlucoWatch Biographer during day, night, and around mealtimes. Diabetes Care. 2004;27:2161–2165. doi: 10.2337/diacare.27.9.2161. [DOI] [PubMed] [Google Scholar]
- 24.Tsalikian E, Kollman C, Mauras N, et al. GlucoWatch G2 Biographer alarm reliability during hypoglycemia in children. Diabetes Technol Ther. 2004;6:559–566. doi: 10.1089/dia.2004.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hathout E, Patel N, Southern C, et al. Home use of the GlucoWatch G2 biographer in children with diabetes. Pediatrics. 2005;115:662–666. doi: 10.1542/peds.2004-0820. [DOI] [PubMed] [Google Scholar]
- 26.Fiallo-Scharer R for Diabetes Research in Children Network Study Group. Eight-point glucose testing versus the continuous glucose monitoring system in evaluation of glycemic control in Type 1 diabetes. J Clin Endocrinol Metab. 2005;90:3387–3391. doi: 10.1210/jc.2004-2510. [DOI] [PubMed] [Google Scholar]
- 27.Buckingham B, Block J, Burdick J, et al. Diabetes Research in Children Network, response to nocturnal alarms using a real-time glucose sensor. Diabetes Technol Ther. 2005;7:440–447. doi: 10.1089/dia.2005.7.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tierney MJ, Jayalakshmi Y, Parris NA, et al. Design of a biosensor for continual, transdermal glucose monitoring. Clin Chem. 1999;45:1681–1683. [PubMed] [Google Scholar]
- 29.Kurnik RT, Berner B, Tamada J, Potts RO. Design and simulation of a reverse lontophoretic glucose monitoring device. J Electrochem Soc. 1998;145:4119–4125. [Google Scholar]
- 30.Kurnika RT, Oliver JJ, Waterhouse SR, et al. Application of the Mixtures of Experts algorithm for signal processing in a noninvasive glucose monitoring system. Sensor Actuat B-Chem. 1999;60:19–26. [Google Scholar]
- 31.Tamada JA, Bohannon NJ, Potts RO. Measurement of glucose in diabetic subjects using noninvasive glucose extraction. Nat Med. 1995;1:1198–1201. doi: 10.1038/nm1195-1198. [DOI] [PubMed] [Google Scholar]
- 32.Ching CTS, Sun TP, Huang SH, et al. A mediated glucose biosensor incorporated with reverse iontophoresis function for noninvasive glucose monitoring. Ann Biomed Eng. 2010 doi: 10.1007/s10439-010- 9918-4. [DOI] [PubMed] [Google Scholar]
- 33.Ching CTS, Connolly P. A novel diffusion cell ideal for the study of membrane extraction/permeation processes and for device/sensor development. Sensor Actuat B-Chem. 2008;129:30–34. [Google Scholar]
- 34.Ching CTS, Camilleri I, Connolly P. A low-cost, programmable device for versatile current delivery in iontophoresis applications. Sensor Actuat B-Chem. 2005;106:534–540. [Google Scholar]
- 35.De La Guardia M. Biochemical sensors: the state of art. Microchim Acta. 1995;120:243–255. [Google Scholar]
- 36.Khan GF, Wernet W. Design of enzyme electrodes for extended use and storage life. Anal Chem. 1997;69:2682–2687. [Google Scholar]
- 37.Galan-Vidal CA, Munoz J, Dominguez C, et al. Glucose biosensor strip in a three electrode configuration based on composite and biocomposite materials applied by planar thick film technology. Sensor Actuat B-Chem. 1998;52:257–263. [Google Scholar]
- 38.Angerstein-Kozlowska H. Comprehensive treatise of electrochemistry. New York: Plenum Press; 1984. [Google Scholar]
- 39.Jager A, Bilitewski U. Screen printed enzyme electrode for the determination of lactose. Analyst. 1994;119:1251–1255. [Google Scholar]
- 40.White SF, Tothill IE, Newman JD, Turner APF. Development of a mass-producible glucose biosensor and flow-injection analysis system suitable for on line monitoring during fermentations. Anal Chim Acta. 1996;321:165–172. [Google Scholar]
- 41.Nagata R, Yokoyama K, Clark SA, Karube I. A glucose biosensor fabricated by the screen printing technique. Biosensens Bioelectron. 1995;10:261–267. doi: 10.1016/0956-5663(95)96845-p. [DOI] [PubMed] [Google Scholar]
- 42.Lambrechts M, Sansen W. Biosensors: microchemical devices. Bristol: IOP publishing; 1992. [Google Scholar]
- 43.Gunther A, Bilitewski U. Characterization of inhibitors of acetylcholinesterase by an automated amperometric flow-injection system. Anal Chim Acta. 1995;300:117–125. [Google Scholar]
- 44.Antiochia R, Gorton L. Development of a carbon nanotube paste electrode osmium polymer-mediated biosensor for determination of glucose in alcoholic beverages. Biosens Bioelectron. 2007;22:2611–2617. doi: 10.1016/j.bios.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 45.Chase HP, Beck R, Tamborlane W, et al. A randomized multicenter trial comparing the GlucoWatch biographer with standard glucose monitoring in children with type 1 diabetes. Diabetes Care. 2005;28:1101–1106. doi: 10.2337/diacare.28.5.1101. [DOI] [PubMed] [Google Scholar]