Abstract
Expression of sphingosine kinase-1 (SphK-1) correlates with a poor survival rate of tumor patients. This effect is probably due to the ability of SphK-1 to be released into the extracellular medium where it catalyzes the biosynthesis of sphingosine-1-phosphate (S1P), a signaling molecule endowed with profound proangiogenic effects. SphK-1 is a leaderless protein which is secreted by an unconventional mechanism. In this paper, we will show that in human hepatocarcinoma Sk-Hep1 cells, extracellular signaling is followed by targeting the enzyme to the cell surface and parallels targeting of FGF-2 to the budding vesicles. We will also show that SphK-1 is present in a catalitycally active form in vesicles shed by SK-Hep1 and human breast carcinoma 8701-BC cells. The enzyme substrate sphingosine is present in shed vesicles where it is produced by neutral ceramidase. Shed vesicles are therefore a site for S1P production in the extracellular medium and conceivably also within host cell following vesicle endocytosis.
1. Introduction
Malignant tumors have the remarkable ability to adapt their stromal environment to their benefit. They alter the surrounding extracellular matrix and modify normal cell behavior to facilitate tumor cell growth, invasion, immune evasion, and angiogenesis [1].
Most of these effects are mediated by the release of small vesicles from the tumor cells into the extracellular medium. Shed vesicles are known to facilitate tumor invasion [2–4], mainly by proteolytic enzymes associated with their membrane [5–9]. Indeed, the vesicle membranes are selectively enriched in some components including MMP-9 [7] and other proteolytic enzymes [4, 6], together with β1 Integrin and class I HLA molecules [7]. Enrichment of ganglioside GD3 and caveolin-1 has also been reported [10]. Moreover, vesicles use several mechanisms to contribute to tumor escape from immune reactions [11–16].
Notably, vesicles carry many proangiogenic growth factors, expressed differently depending on the vesicle origin, and that act on endothelial cells to promote angiogenesis. Indeed, FGF-2 was detected in vesicles shed by human hepatocarcinoma Sk-Hep1 cells [17, 18]; VEGF was found to be present in vesicles shed by human ovarian carcinoma cells [19] and in vesicles shed by neurons and astrocytes [20, 21]; angiogenin, IL-6, IL-8, VEGF, and TIMPs were found in vesicles shed by glioblastoma tumor cells [22]. Additionally, the sphingolipid fraction of vesicles shed by HT1080 fibrosarcoma and DU-145 human prostate carcinoma cells also showed proangiogenic activity [23]. Sphingomyelin is a normal component of plasma membranes where it is largely clustered in the outer membrane leaflet. It is subjected to intense metabolism which is responsible for the formation of a number of bioactive metabolites including ceramide, ceramide-1-phosphate, sphingosine, and sphingosine-1-phosphate (S1P) [24]. Ceramide, generated by sphingomyelinase (SMase) action on spingomyelin, appears to be a critical regulator of cell growth arrest, differentiation, and apoptosis [25, 26]. Sphingosine is formed by ceramide deacylation catalyzed by at least three different isoforms of ceramidase, which differ in optimal pH, primary structure, and cellular localization [27]. The enzyme sphingosine kinase (SphK) catalyzes the formation of S1P from sphingosine and ATP [28]. Two distinct SphK isoforms, SphK-1 and SphK-2, have been cloned [29, 30]. SphK-1, the more intensely researched isoform, is primarily localized in the cytosol, but, following ERK dependent phosphorylation elicited by various stimuli, it becomes translocated to the plasma membrane [31]. SphK-1 has been shown to regulate a wide variety of cellular processes, including the promotion of cell proliferation, survival, and motility [32] and, just as importantly, it possesses oncogenic potential [33]. Previous studies have established that SphK-1, like FGF-2 and several other proteins, can be released in the extracellular environment although it lacks a conventional secretory signal sequence. The mechanism of SphK-1 secretion is unconventional and likely involves a nonstandard pathway independent of the endoplasmic reticulum/Golgi system; the SphK-1 secretion mechanism is only known to require functional actin dynamics [34]. Notably, the SphK product S1P, among multiple biological activities, exerts a strong proangiogenic effect which is known to act synergistically with growth factors such as FGF-2 [35, 36] and VEGF [35].
In this study we investigated whether vesicles shed by hepatocarcinoma and carcinoma cultured cells contain S1P-generating enzymes. The data from this research demonstrates that neutral ceramidase (nCDase) and SphK-1 are localized in vesicles, supporting the view that S1P participates in the proangiogenic activity exerted by these particles.
2. Materials and Methods
2.1. Cells and Culture Media
Human SK-Hep1 hepatocarcinoma cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (FCS; Euroclone, Celbio). Human breast carcinoma 8701-BC cells, kindly provided by Profcessor Minafra [37], were grown in RPMI 1640 supplemented with 10% fetal calf serum (FCS; Euroclone, Celbio). Bovine GM7373 fetal aortic endothelial cells were grown in Eagle's minimal essential medium (Euroclone, Celbio) supplemented with 10% FCS, vitamins, and essential and nonessential amino acids.
2.2. Cell Extraction
Cells were removed from plate by a scraper and centrifuged at 2000 g for 5 minutes; pelleted cells were then resuspended in 300 μL of Triton X100 (1%) on phosphate buffer saline (PBS). After 10- minute incubation at room temperature, the cell extract was centrifuged at 800 g for 10 minutes The amount of protein extracted from cells was determined using the Bradford microassay method (Bio-Rad, Segrate, Milan, Italy) employing bovine serum albumin (Sigma-Aldrich) as a standard.
2.3. Vesicle Purification from Conditioned Medium
Vesicles were purified from the conditioned medium as described above [38]. Briefly, the medium was conditioned by culturing subconfluent healthy cells for 3 or 24 hours and were centrifuged at 2000 g for 10 minutes and at 4000 g for 15 minutes The supernatant was ultracentrifuged at 105,000 g in a Ti-60 Rotor (Backman) for 90 minutes Pelleted vesicles were resuspended in PBS. The amount of isolated vesicles was determined by measuring the protein concentration using the Bradford microassay method (Bio-Rad, Segrate, Milan, Italy) using bovine serum albumin (Sigma-Aldrich) as a standard.
2.4. Western Blotting
After SDS-PAGE electrophoresis were cast in 7.5% gels, proteins was blotted onto a nitrocellulose membrane (Hybond; Amersham Biosciences) that was saturated with 3% nonfat dry milk in Tris Buffer Saline 50 mM pH 7.9/Tween 0.05% (TBS-T). After 5 washes in TBS-T for 5 minutes each, the nitrocellulose membranes were incubated overnight at 4°C, with mouse monoclonal anti-nCDase antibody 1 : 200 (kindly donated by Professor Ito, Fukuoka, Japan) [39]. The primary antibody was followed by peroxidase-conjugated anti-mouse antibodies (1 : 10000) (Amersham Biosciences) for 1 hour at room temperature. Immunocomplexes were visualized with the ECL Western blotting kit (Amersham Biosciences) using Hyperfilm.
2.5. Confocal Immunofluorescence
Cells, seeded at low density (2.000 cells/well) onto microscope cover slips in 12-well culture plates (Nunc), were grown overnight in the complete medium and, when needed, for 3 more days in a serum-free medium with three medium changes. SphK-1 and SphK-2 were detected by using as primary antibodies, rabbit polyclonal anti-SphK-1 antibody (kindly donated by Prof. Obeid, Charleston, SC, USA) [40] 1 : 100, and rabbit polyclonal anti-SphK-2 antibodies 1 : 100 (kindly provided by Dr. Nakamura, Kobe, Japan), respectively, [41]. Secondary antibodies used were antirabbit TRITC-conjugated antibodies (1 : 200 Sigma); β 1 Integrin was detected using C27 anti-β 1 Integrin rat primary monoclonal antibody 1 : 150 [42] and antirat TRITC conjugated secondary antibody (1 : 320, Sigma). FGF-2 was detected using mouse monoclonal anti-FGF-2 antibody (0.5 mg/mL 1 : 200, Upstate Biotechnology type II) and Texas Redconjugated antimouse antibody (1 : 200, Amersham Biosciences).
In order to stain nuclei, cells were fixed in 3.7% formaldehyde and then stained for 10 minutes with propidium iodide (Sigma).
Immunostained cells were analyzed by confocal microscopy (Olympus 1X70 with Melles Griot laser system).
2.6. Staining of Vesicle Lipids
Vesicle lipids were stained with the lipophilic styryl compound FM4-64 (Molecular Probes). Purified vesicles (180 μg) were resuspended in 1 ml PBS and stained with FM4-64 dissolved in PBS without calcium and magnesium. FM4-64 was added at a final concentration of 5 μg/ml; samples were incubated at room temperature for 15 minutes. Stained vesicles were collected by centrifugation at 50,000 g for 1 hour, resuspended in 50 μl PBS and added to GM7373 cells to monitor vesicle targeting.
2.7. Transient Cell Transfection
SK-Hep1 cells were plated in six-well culture plates at 3 × 105 cells/well and maintained overnight in high-glucose DMEM containing 10% fetal calf serum, 2 mM L-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin. The next day, cells were transfected with SKpeGFP plasmid encoding for SphK-GFP chimera (kindly donated by Professor Spiegel, Richmond, VA, USA) [43]. Transfection was carried out using Lipofectamine Reagent (GIBCO Life Technologies), according to the manufacturer's instructions.
2.8. Sphingosine Kinase Assay
SphK activity was assayed in isolated vesicles or serum-free conditioned medium as described by Olivera et al. [28]. Briefly, 50 μg vesicle proteins were resuspended in 100 μl of the reaction mixture which contained 20 mM Tris-HCl, pH 7.4, 20% (v/v) glycerol, 1 mM β-mercaptoethanol, 1 mM EDTA, 1 mM sodium orthovanadate, 15 mM sodium fluoride, protease inhibitors (10 mg/ml leupeptin,10 mg/ml aprotinin, and 1 mM PMSF), and 0.5 mM 4-deoxypyridoxine.
The serum-free conditioned medium was concentrated approximately 40-fold before being employed for enzymatic activity measurement.
The enzymatic reaction was initiated by adding 50 μM sphingosine and 1 mM [γ 32P] ATP. In some cases, assays were performed which omitted sphingosine to evaluate the availability of endogenous sphingosine. After 30- minutes incubation at 37°C, the reaction was terminated by adding 20 μl 1 N HCl and 900 μl of chloroform/methanol/HCl (100 : 200 : 1, v/v). Lipids were then extracted, separated by TLC, labeled SIP and quantified by liquid scintillation essentially as previously described [44]. Specific activity of SphK was expressed as pmol of S1P produced/min/mg of protein.
2.9. Neutral Ceramidase Activity Assay
NCDase activity was determined using C12-NBD-ceramide as a substrate as previously described [45]. Briefly, 100 pmol of C12-NBD-ceramide (NBD-C12:0, d18:1) was incubated for 2 h at 37°C with an appropriate amount of proteins in 20 μl of 25 mM Tris-HCl buffer pH 7.5 and 0.25% (w/v) Triton X-100. Samples were then applied to a TLC plate, which was developed with chloroform, methanol, and 25% ammonia (90 : 20: 0.5, v/v). Spots corresponding to NBD-dodecanoic acid and C12-NBD-ceramide were scraped, incubated with methanol at 37°C to extract the compounds from the silica, and their fluorescence at 470/525 nm excitation/emission wavelengths was measured using a Shimadzu 9000 spectrophotofluorimeter. The compounds were quantified using a standard curve of known amounts of C12-NBD-ceramide and NBD-dodecanoic acid.
3. Results
3.1. Immunolocalization of SphK-1 and Sphk-2 in 8701-BC and Sk-Hep-1 Cells
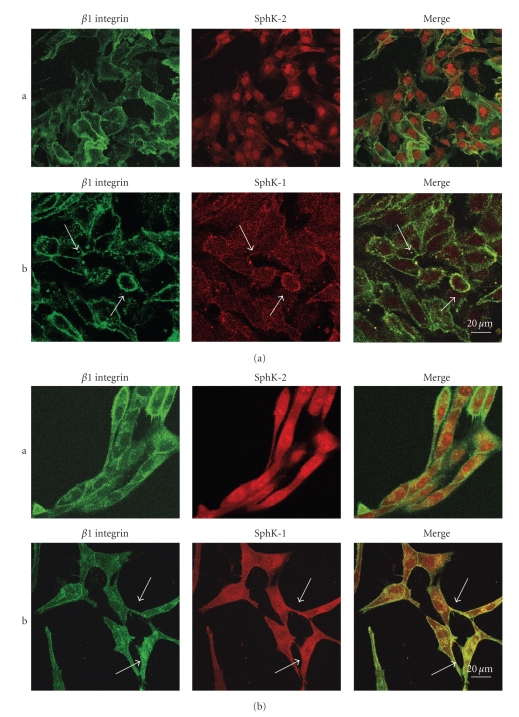
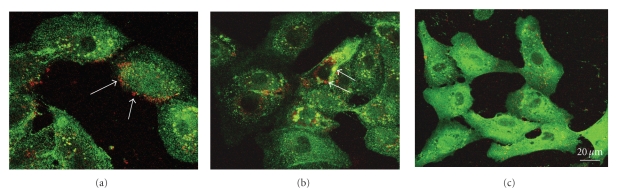
In a first group of experiments, expression and localization of SphK-1 and SphK-2 were analyzed by immunofluorescence in 8701 BC carcinoma cells and in Sk-Hep1 hepatocarcinoma cells (Figure 1).
Figure 1.
Comparative analysis of β1 Integrin, SphK-2, and SphK-1 immunolocalization. (a) Localization in 8701-BC cells. (b) Localization in Sk-Hep1 cells. Line a: Immunolocalization of β1 Integrin and SphK-2 showing a different distribution of the two molecules. Line b: Immunolocalization of β1 Integrin and SphK-1. Arrows indicate colocalization areas. β1 Integrin was detected using FITC-conjugated secondary antibodies and SphK-2 and SphK-1 using Texas red-conjugated secondary antibodies. Arrows indicate colocalization areas.
Moreover, since it had been previously demonstrated that β 1 integrin is clustered in shed vesicles [7, 17], distribution of the two proteins was compared with distribution of β 1 integrin.
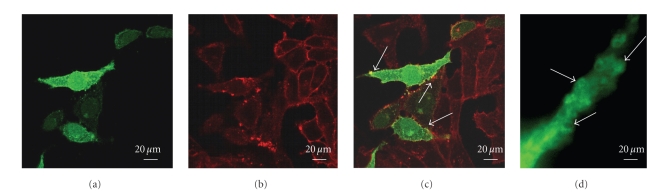
In both cell lines, the distribution of SphK-2 was quite different from the distribution of β 1 integrin (Figures 1(a) and 1(b) line a). SphK-2 was indeed clustered in the cell nucleus. However, it was absent in the cell membrane where instead β 1 integrin was located. In contrast, SphK-1 and β 1 integrin appear to colocalize at the plasma membrane (Figures 1(a) and 1(b) line b). Moreover, as can more clearly be seen in cells transiently transfected with GFP-SphK-1 (Figure 2), both SphK-1 and β 1 integrin seem to be more dense in specific areas of the plasma membrane, and clustering appears to occur in areas of the cell membrane from which vesicles are released (Figure 2(d)).
Figure 2.
Comparative analysis of SphK-1 and of β1 Integrin localization in transfected Sk-Hep1 cells. (a) GFP-bound SphK-1 localization in transfected cells. The protein is localized in cell membranes where it shows uneven clustering in small spots. (b) Immunolocalization of β1 Integrin detected using Texas red-conjugated secondary antibodies. β1 Integrin is seen in cell membranes of both transfected and non-transfected cells. Like SphK-1, β1 Integrin shows uneven clustering in small spots. (c) Double staining shows colocalization of the two proteins in some areas of the plasma membrane (indicated by arrows). (d) Enlargement of a cell protrusion showing budding areas (indicated by arrows) in which SphK-1 appears to have a preferential localization.
3.2. Effects of Serum Addition on SphK-1 Trafficking toward the Cell Periphery
In a previous study, we observed that vesicles shed by Sk-Hep1 cells mediate FGF-2 release and that vesicle shedding and release of FGF-2 were simultaneously induced by the addition of serum to starved cells [17]. By monitoring intracellular movements of the growth factor subsequent to serum addition, we showed that within one hour FGF-2 was targeted to the cell periphery and to the cell nucleus and nucleolus. FGF-2 movements toward the cell periphery required actin filament integrity [18].
Similarly to FGF-2, SphK-1 is a leaderless protein secreted by unconventional mechanisms [29, 34] whose movement toward the cell periphery is mediated by actin filaments [34]. We therefore theorized that the two proteins could share a similar export mechanism and we analyzed whether intracellular movements of SphK-1 were influenced by serum addition and whether the enzyme colocalized with FGF-2.
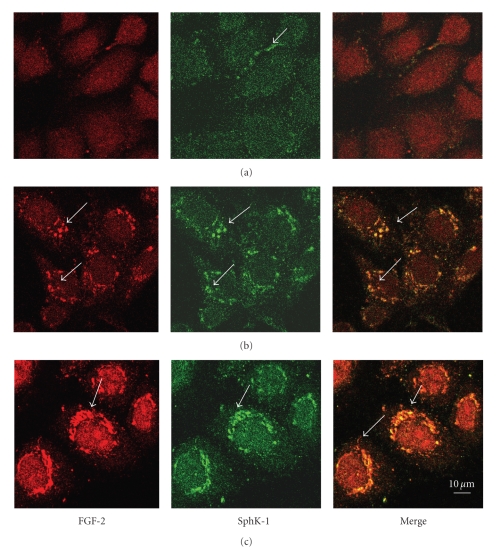
Intracellular distribution of SphK-1 was therefore analyzed by immunolocalization in starved cells as well as at time intervals after serum addition and compared with FGF-2 distribution. As shown in Figure 3, in starved cells the two proteins did not colocalize. In SphK-1 they were partially localized in small granules and in FGF-2 they were totally dispersed.
Figure 3.
Course of endogenous SphK-1 and endogenous FGF-2 targeting to the cell periphery over time, observed by immunolocalization experiments. FGF-2 and SphK-1 immunolocalization at 0, 30, and 60 minutes after serum addition (lines (a), (b), (c), resp.) Sections 3 μm from surface. FGF-2 was detected using Texas red-conjugated secondary antibodies; SphK-1 was detected using FITC-conjugated antibodies. Arrows indicate granules of protein localization.
Thirty minutes after the serum was added both proteins were clustered in granules and showed a clear colocalization. One hour after the serum was added large granules containing both proteins were present near the cell membrane. In contrast, cell nuclei were exclusively stained by anti-FGF-2 antibodies.
These results suggest that FGF-2 and SphK-1 share a similar transport mechanism toward the cell periphery and that the two proteins are both likely to be targeted to the budding vesicles.
3.3. Detection of an Active Form of SphK-1 in Shed Vesicles
In order to establish if an active form of SphK-1 is shed as a component of membrane vesicles, we ascertained SphK activity in vesicles or cell-conditioned media in some experiments. Results reported in Table 1 show that SphK-1 was clearly detectable in vesicles shed by Sk-Hep1 and 8701-BC cells, although the activity was found to be greater in vesicles shed by Sk-Hep1 cells.
Table 1.
Enzimatic assay of SphK-1 activity.
| Sample | Incubation mixture with exogenous sphingosine | Incubation mixture without exogenous sphingosine |
|---|---|---|
| SK-Hep1 vesicles | 43.93 pmol/min/mg of protein | 26.70 pmol/min/mg of protein |
| SK-Hep1 vesicles* | 43.59 pmol/min/mg of protein | |
| SK-Hep1 C.M. | 2.23 pmol/min/mg of protein | |
| 8701 BC vesicles | 14.44 pmol/min/mg of protein | 12.61 pmol/min/mg of protein |
| 8701 BC vesicles* | 16.70 pmol/min/mg of protein | |
| 8701 BC CM | 2.16 pmol/min/mg of protein |
*Vesicles recovered from medium in which 2 M NaCl had been added.
SphK-1 activity was also tested in vesicle-deprived conditioned media. No enzymatic activity could be detected in serum-containing media, even when it was concentrated. Instead, it was possible to detect a low SphK activity in 40-fold-concentrated serum-free medium that had been conditioned by maintaining cells in culture for 24 h. As shown in Table 1, when vesicles were collected from a medium to which 2M NaCl had been added in order to solubilize proteoglycans and other molecules unspecifically bound to the vesicle membrane, the enzymatic activity was not affected.
The addition of sphingosine increased labeled S1P production; however, the catalytic activity of the enzyme was also observed when this substrate was not added. This result indicates that sphingosine is already present in shed components of membrane vesicles.
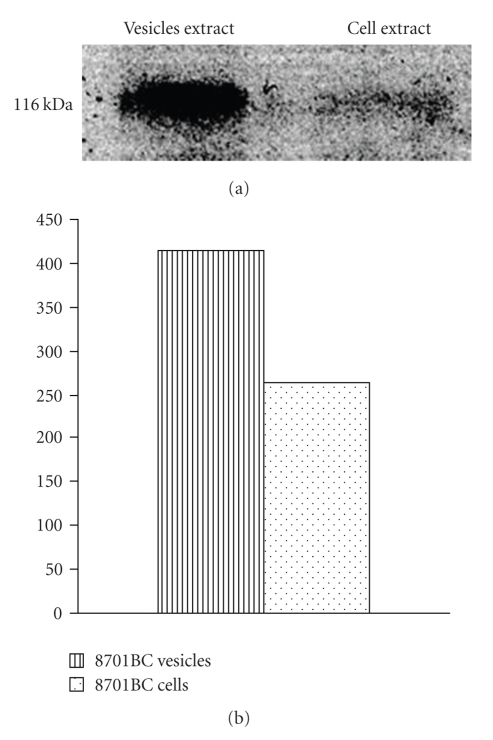
Sphingosine is likely produced by nCDase, which is known to be localized in plasma membranes and also found in the extracellular medium [46, 47]. An enzymatically active form of nCDase was actually found to be present in vesicles shed by 8701 BC (Figure 4 and Table 2). Moreover, in Sk-Hep1 cells, we observed colocalization of nCDase and β 1 integrin (data not shown), indicating that it is also likely that nCDase is present in vesicles shed by this cell line.
Figure 4.
Western Blot analysis for nCDase (a) and its densitometric analisys (b) on 8701BC vesicles and cell extracts.
Table 2.
| Sample | Enzimatic activity of nCDase |
|---|---|
| 8701 BC cells | 2,08 pmol/min/mg of protein |
| 8701 BC vesicles | 5,25 pmol/min/mg of protein |
3.4. Fate of Shed Vesicles
Since S1P can be produced in the membrane of shed vesicles, the molecule could remain, at least in part, within the vesicle. Vesicles could adhere to the plasma membrane of cells surrounding the tumor and S1P could exert its effects by interacting with receptors localized at the cell surface. On the other hand, vesicles could be internalized by the host cell and consequently release S1P inside cells where it could act as intracellular messenger. In order to verify these hypotheses, we analyzed the targets of shed vesicles after adding them to GM7373 cells, an immortalized line of embryonic bovine aortic endothelial cells.
For this purpose, vesicles released by Sk-Hep1 cells were labeled for 15 minutes with the lipid marker FM4-64. Labeled vesicles were then added to in vitro cultured GM7373 cells which in turn had been labeled with antibodies against β 1 integrin. As shown in Figure 5, at 10 minutes after incubation vesicles were observed to be bound to the cell membrane, while after 20-minutes incubation most vesicles were internalized and visible in the cytoplasm. At 30 minutes, the signal borne by lipid marker FM4-64 was no longer visible, indicating that the lipids of the vesicle membranes had degraded. In principle therefore, vesicle-associated S1P could act on both membrane receptors and intracellular targets.
Figure 5.
Interactions of shed vesicles with endothelial cells. Vesicles shed by SK-Hep1 cells, labelled with lipid styryl dye FM4-64 (red fluorescence), were added to GM7373 endothelial cells in which β1 Integrin was stained using FITC-conjugated secondary antibodies (green fluorescence). Cells were incubated with vesicles, respectively, for (a) 10 minutes, (b) 20 minutes, and (c) 30 minutes. The arrows indicate vesicle localization.
4. Conclusions
Membrane vesicles shed by tumor cells appear to exert a variety of effects on the surrounding cells. Vesicles are rich in enzymatic activities able to modify extracellular medium composition, thus facilitating tumor cell migration and angiogenesis. Depending on their origin, they also convey different signaling molecules which exert their effects on lymphocytes, mesenchymal cells, and endothelial cells. Shed vesicles have been shown to induce angiogenesis using a variety of mechanisms including the action of proteins such as FGF-2, VEGF, angiogenin, IL-6, IL-8, and TIMPs, and lipid molecules such as sphingomyelin.
Based on the present results, nCDase and SphK-1 can now be included among the signaling molecules transferred by shed membrane vesicles, suggesting that S1P formed at level of the vesicle membranes plays a role in the biological processes regulated by these particles.
Interestingly, nCDase, here identified as a component of shed vesicles, was previously identified in various subcellular compartments such as endosomes, mitochondria, and microdomains of the plasma membrane [39, 48] but was also found to be involved in extracellular sphingolipid metabolism [47]. In this regard it was demonstrated that although nCDase is localized at the plasma membrane as a type II integral membrane protein, the enzyme is released in the extracellular medium after the proteolytic action of secretases [49, 50]. Moreover, in agreement with the present results, nCDase, together with acid SMase, was identified as a component of a complex in the cell membrane domain which is subjected to budding as well as in conditioned medium associated with caveolin-1, a key structural protein of caveole [51] which was also detected in shed vesicles [52]. SphK-1 is a secreted leaderless protein, and the shedding of membrane vesicles appears to represent a mechanism which accounts for its secretion. The presence of the enzymatic protein in shed vesicles does not per se exclude the face that other mechanisms may also participate in the release of SphK-1. Indeed it was reported that, in FGF-1 overexpressing NIH 3T3 cells, SphK-1 is secreted together with FGF-1 as a component of a high molecular weight complex [53, 54]. However, SphK-1 is also secreted by cells which do not express FGF-1, and in the absence of stress signaling which induces FGF-1 secretion. Here we have demonstrated that at least in some instances SphK-1 is secreted as a component of shed vesicles. Since shed vesicles also contain nCDase, which provides the rate-limiting substrate for S1P production by catalyzing sphingosine generation, it is likely that these particles cause sustained S1P production.
SphK-1 and S1P produced by its enzymatic activity are able to mediate a network of paracrine signaling. It is well known that acting on the two membrane receptors SIP1 and SIP3, S1P induces morphogenesis in HUVEC cells [35]. Moreover, since vesicles carry several other molecules able to affect angiogenesis, the overall effects of vesicles on surrounding endothelial cells will be amplified and differently modulated depending on the specific composition of the vesicles.
The exact mechanism by which the S1P message borne by shed vesicles is delivered to the host cell remains to be explored. Indeed, an attractive hypothesis is that after interacting with the recipient cell plasma membrane, vesicles are internalized via endocytosis. SphK-1 and SIP would therefore be delivered into the cytoplasm of the receiving cell, where, as already known, SIP could exert its intracellular effects, regulating various processes among which cell survival is prominent [55, 56]. Indeed vesicles were shown to convey molecules, such as mRNA and iRNA, to the cytoplasm of surrounding cells, and it was recently reported that mRNA included in shed vesicles can be translated in recipient cells following endocytosis [22].
Alternatively, because SphK-1 association with cell membranes is strengthened by interaction with phosphatidylserine following its phosphorylation [57] and since exposure of phosphatidylserine on the outer leaflet is a hallmark of shed membrane vesicles [58, 59], it can also be speculated that SphK-1, localized in these particles, can generate S1P in the extracellular environment. If this is the case, the bioactive lipid generated outside the endothelial cells could determine key effects on development and proliferation of endothelial cells, acting as a ligand of S1P1 and/or S1P3 receptors [60, 61]. Independent of the action mechanism, the ability of shed vesicles to carry on key enzymes for S1P production which this study brought to light illuminates a novel aspect of their biochemical properties which is relevant to a complete understanding of their proangiogenic activity.
Acknowledgments
This work was supported by the Italian Ministry of University and Scientific and Technological Research (to M.L.V. and P.B.) and by grants from University of Florence and “Ente Cassa di Risparmio di Firenze” (to P.B.). they would like to thank Salvatore Agnello for his technical assistance with the confocal microscopy. They would also like to thank Professor Salvatore Minafra for providing them with 8701-BC cells.
References
- 1.Singer CF, Gschwantler-Kaulich D, Fink-Retter A, et al. Differential gene expression profile in breast cancer-derived stromal fibroblasts. Breast Cancer Research and Treatment. 2008;110(2):273–281. doi: 10.1007/s10549-007-9725-2. [DOI] [PubMed] [Google Scholar]
- 2.Poste G, Nicolson GL. Arrest and metastasis of blood-borne tumor cells are modified by fusion of plasma membrane vesicles from highly metastatic cells. Proceedings of the National Academy of Sciences of the United States of America. 1980;77(1):399–403. doi: 10.1073/pnas.77.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor DD, Taylor CG, Jiang CG, Black PH. Characterization of plasma membrane shedding from murine melanoma cells. International Journal of Cancer. 1988;41(4):629–635. doi: 10.1002/ijc.2910410425. [DOI] [PubMed] [Google Scholar]
- 4.Ginestra A, La Placa MD, Saladino F, Cassara D, Nagase H, Vittorelli ML. The amount and proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasiveness. Anticancer Research. 1998;18(5):3433–3437. [PubMed] [Google Scholar]
- 5.Zucker S, Wieman JM, Lysik RM, Wilkie DP, Ramamurthy N, Lane B. Metastatic mouse melanoma cells release collagen-gelatin degrading metalloproteinases as components of shed membrane vesicles. Biochimica et Biophysica Acta. 1987;924(1):225–237. doi: 10.1016/0304-4165(87)90091-2. [DOI] [PubMed] [Google Scholar]
- 6.Ginestra A, Monea S, Seghezzi G, et al. Urokinase plasminogen activator and gelatinases are associated with membrane vesicles shed by human HT1080 fibrosarcoma cells. Journal of Biological Chemistry. 1997;272(27):17216–17222. doi: 10.1074/jbc.272.27.17216. [DOI] [PubMed] [Google Scholar]
- 7.Dolo V, Ginestra A, Cassara D, et al. Selective localization of matrix metalloproteinase 9, β1 integrins, and human lymphocyte antigen class I molecules on membrane vesicles shed by 8701-BC breast carcinoma cells. Cancer Research. 1998;58(19):4468–4474. [PubMed] [Google Scholar]
- 8.Taraboletti G, D’Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. American Journal of Pathology. 2002;160(2):673–680. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graves LE, Ariztia EV, Navari JR, Matzel HJ, Stack MS, Fishman DA. Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Research. 2004;64(19):7045–7049. doi: 10.1158/0008-5472.CAN-04-1800. [DOI] [PubMed] [Google Scholar]
- 10.Dolo V, Li R, Dillinger M, et al. Enrichment and localization of ganglioside GD3 and caveolin-1 in shed tumor cell membrane vesicles. Biochimica et Biophysica Acta. 2000;1486(2-3):265–274. doi: 10.1016/s1388-1981(00)00063-9. [DOI] [PubMed] [Google Scholar]
- 11.Alexander P. Escape from immune destruction by the host through shedding of surface antigens: is this a characteristic shared by malignant and embryonic cells? Cancer Research. 1974;34(8):2077–2082. [PubMed] [Google Scholar]
- 12.Poutsiaka DD, Schroder EW, Taylor DD, Levy EM, Black PH. Membrane vesicles shed by murine melanoma cells selectively inhibit the expression of Ia antigen by macrophages. Journal of Immunology. 1985;134(1):138–144. [PubMed] [Google Scholar]
- 13.Dolo V, Adobati E, Canevari S, Picone MA, Vittorelli ML. Membrane vesicles shed into the extracellular medium by human breast carcinoma cells carry tumor-associated surface antigens. Clinical and Experimental Metastasis. 1995;13(4):277–286. doi: 10.1007/BF00133483. [DOI] [PubMed] [Google Scholar]
- 14.Zuccato E, Blott EJ, Holt O, et al. Sorting of Fas ligand to secretory lysosomes is regulated by mono-ubiquitylation and phosphorylation. Journal of Cell Science. 2007;120(1):191–199. doi: 10.1242/jcs.03315. [DOI] [PubMed] [Google Scholar]
- 15.Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Research. 2007;67(15):7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 16.Gabrilovich DI. Molecular mechanisms and therapeutic reversal of immune suppression in cancer. Current Cancer Drug Targets. 2007;7(1):p. 1. [PubMed] [Google Scholar]
- 17.Taverna S, Ghersi G, Ginestra A, et al. Shedding of membrane vesicles mediates fibroblast growth factor-2 release from cells. Journal of Biological Chemistry. 2003;278(51):51911–51919. doi: 10.1074/jbc.M304192200. [DOI] [PubMed] [Google Scholar]
- 18.Taverna S, Rigogliuso S, Salamone M, Vittorelli ML. Intracellular trafficking of endogenous fibroblast growth factor-2. FEBS Journal. 2008;275(7):1579–1592. doi: 10.1111/j.1742-4658.2008.06316.x. [DOI] [PubMed] [Google Scholar]
- 19.Taraboletti G, D’Ascenzo S, Giusti I, et al. Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia. 2006;8(2):96–103. doi: 10.1593/neo.05583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiera G, Proia P, Alberti C, Mineo M, Savettieri G, Di Liegro I. Neurons produce FGF2 and VEGF and secrete them at least in part by shedding extracellular vesicles. Journal of Cellular and Molecular Medicine. 2007;11(6):1384–1394. doi: 10.1111/j.1582-4934.2007.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Proia P, Schiera G, Mineo M, et al. Astrocytes shed extracellular vesicles that contain fibroblast growth factor-2 and vascular endothelial growth factor. International Journal of Molecular Medicine. 2008;21(1):63–67. [PubMed] [Google Scholar]
- 22.Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nature Cell Biology. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim CW, Lee HM, Lee TH, Kang C, Kleinman HK, Gho YS. Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Research. 2002;62(21):6312–6317. [PubMed] [Google Scholar]
- 24.Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochemical Journal. 2000;349(2):385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274(5294):1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 26.Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annual Review of Physiology. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- 27.Ito M, Okino N, Tani M, Mitsutake S, Mori K. Molecular evolution of neutral ceramidase: signalling molecule and virulence factor. Tanpakushitsu Kakusan Koso. 2002;47:455–462. [PubMed] [Google Scholar]
- 28.Olivera A, Kohama T, Tu Z, Milstien S, Spiegel S. Purification and characterization of rat kidney sphingosine kinase. Journal of Biological Chemistry. 1998;273(20):12576–12583. doi: 10.1074/jbc.273.20.12576. [DOI] [PubMed] [Google Scholar]
- 29.Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. Journal of Biological Chemistry. 1998;273(37):23722–23728. doi: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Sugiura M, Nava VE, et al. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. Journal of Biological Chemistry. 2000;275(26):19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 31.Pitson SM, Moretti PAB, Zebol JR, et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO Journal. 2003;22(20):5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiegel S, Milstien S. Exogenous and intracellularly generated sphingosine 1-phosphate can regulate cellular processes by divergent pathways. Biochemical Society Transactions. 2003;31(6):1216–1219. doi: 10.1042/bst0311216. [DOI] [PubMed] [Google Scholar]
- 33.Pitson SM, Xia P, Leclercq TM, et al. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. Journal of Experimental Medicine. 2005;201(1):49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ancellin N, Colmont C, Su J, et al. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. Journal of Biological Chemistry. 2002;277(8):6667–6675. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- 35.Lee M-J, Thangada S, Claffey KP, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99(3):301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 36.Harvey K, Siddiqui RA, Sliva D, Garcia JGN, English D. Serum factors involved in human microvascular endothelial cell morphogenesis. Journal of Laboratory and Clinical Medicine. 2002;140(3):188–198. doi: 10.1067/mlc.2002.126827. [DOI] [PubMed] [Google Scholar]
- 37.Minafra S, Morello V, Glorioso F, et al. A new cell line (8701-BC) from primary ductal infiltrating carcinoma of human breast. British Journal of Cancer. 1989;60(2):185–192. doi: 10.1038/bjc.1989.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dolo V, Ginestra A, Ghersi G, Nagase H, Vittorelli ML. Human breast carcinoma cells cultured in the presence of serum shed membrane vesicles rich in gelatinolytic activities. Journal of Submicroscopic Cytology and Pathology. 1994;26(2):173–180. [PubMed] [Google Scholar]
- 39.Mitsutake S, Tani M, Okino N, et al. Purification, characterization, molecular cloning, and subcellular distribution of neutral ceramidase of rat kidney. Journal of Biological Chemistry. 2001;276(28):26249–26259. doi: 10.1074/jbc.M102233200. [DOI] [PubMed] [Google Scholar]
- 40.Johnson KR, Becker KP, Facchinetti MM, Hannun YA, Obeid LM. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate (PMA) Journal of Biological Chemistry. 2002;277(38):35257–35262. doi: 10.1074/jbc.M203033200. [DOI] [PubMed] [Google Scholar]
- 41.Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S-I. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. Journal of Biological Chemistry. 2003;278(47):46832–46839. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- 42.Monsky WL, Lin C-Y, Aoyama A, et al. A potential marker protease of invasiveness, seprase, is localized on invadopodia of human malignant melanoma cells. Cancer Research. 1994;54(21):5702–5710. [PubMed] [Google Scholar]
- 43.Olivera A, Rosenfeldt HM, Bektas M, et al. Sphingosine kinase type 1 induces G12/13-mediated stress fiber formation, yet promotes growth and survival independent of G protein-coupled receptors. Journal of Biological Chemistry. 2003;278(47):46452–46460. doi: 10.1074/jbc.M308749200. [DOI] [PubMed] [Google Scholar]
- 44.Donati C, Cencetti F, De Palma C, et al. TGFβ protects mesoangioblasts from apoptosis via sphingosine kinase-1 regulation. Cellular Signalling. 2009;21(2):228–236. doi: 10.1016/j.cellsig.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Romiti E, Meacci E, Tani M, et al. Neutral/alkaline and acid ceramidase activities are actively released by murine endothelial cells. Biochemical and Biophysical Research Communications. 2000;275(3):746–751. doi: 10.1006/bbrc.2000.3370. [DOI] [PubMed] [Google Scholar]
- 46.Hwang Y-H, Tani M, Nakagawa T, Okino N, Ito M. Subcellular localization of human neutral ceramidase expressed in HEK293 cells. Biochemical and Biophysical Research Communications. 2005;331(1):37–42. doi: 10.1016/j.bbrc.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 47.Tani M, Ito M, Igarashi Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cellular Signalling. 2007;19(2):229–237. doi: 10.1016/j.cellsig.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Tani M, Okino N, Mori K, Tanigawa T, Izu H, Ito M. Molecular cloning of the full-length cDNA encoding mouse neutral ceramidase. A novel but highly conserved gene family of neutral/alkaline ceramidases. Journal of Biological Chemistry. 2000;275(15):11229–11234. doi: 10.1074/jbc.275.15.11229. [DOI] [PubMed] [Google Scholar]
- 49.Tani M, Iida H, Ito M. O-glycosylation of mucin-like domain retains the neutral ceramidase on the plasma membranes as a type II integral membrane protein. Journal of Biological Chemistry. 2003;278(12):10523–10530. doi: 10.1074/jbc.M207932200. [DOI] [PubMed] [Google Scholar]
- 50.Yoshimura Y, Tani M, Okino N, Iida H, Ito M. Molecular cloning and functional analysis of zebrafish neutral ceramidase. Journal of Biological Chemistry. 2004;279(42):44012–44022. doi: 10.1074/jbc.M405598200. [DOI] [PubMed] [Google Scholar]
- 51.Romiti E, Meacci E, Donati C, et al. Neutral ceramidase secreted by endothelial cells is released in part associated with caveolin-1. Archives of Biochemistry and Biophysics. 2003;417(1):27–33. doi: 10.1016/s0003-9861(03)00212-1. [DOI] [PubMed] [Google Scholar]
- 52.Dolo V, D’Ascenzo S, Sorice M, et al. New approaches to the study of sphingolipid enriched membrane domains: the use of electron microscopic autoradiography to reveal metabolically tritium labeled sphingolipids in cell cultures. Glycoconjugate Journal. 2000;17(3-4):261–268. doi: 10.1023/a:1026505710607. [DOI] [PubMed] [Google Scholar]
- 53.Prudovsky I, Mandinova A, Soldi R, et al. The non-classical export routes: FGF1 and IL-1α point the way. Journal of Cell Science. 2003;116(24):4871–4881. doi: 10.1242/jcs.00872. [DOI] [PubMed] [Google Scholar]
- 54.Soldi R, Mandinova A, Venkataraman K, et al. Sphingosine kinase 1 is a critical component of the copper-dependent FGF1 export pathway. Experimental Cell Research. 2007;313(15):3308–3318. doi: 10.1016/j.yexcr.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circulation Research. 2004;94(6):724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- 56.Bassi R, Anelli V, Giussani P, Tettamanti G, Viani P, Riboni L. Sphingosine-1-phosphate is released by cerebellar astrocytes in response to bFGF and induces astrocyte proliferation through Gi-protein-coupled receptors. Glia. 2006;53(6):621–630. doi: 10.1002/glia.20324. [DOI] [PubMed] [Google Scholar]
- 57.Stahelin RV, Hwang JH, Kim J-H, et al. The mechanism of membrane targeting of human sphingosine kinase 1. Journal of Biological Chemistry. 2005;280(52):43030–43038. doi: 10.1074/jbc.M507574200. [DOI] [PubMed] [Google Scholar]
- 58.Heijnen HFG, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and α-granules. Blood. 1999;94(11):3791–3799. [PubMed] [Google Scholar]
- 59.MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1β by microvesicle shedding. Immunity. 2001;15(5):825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 60.Pyne S, Pyne NJ. Sphingosine 1 phosphate signaling via the endothelial differentation gene family of G protein coupled receptors. Pharmacology and Therapeutics. 2000:115–131. doi: 10.1016/s0163-7258(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y, Wada R, Yamashita T, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. Journal of Clinical Investigation. 2000;106(8):951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]