Abstract
Background
Since 1996, six new drugs have been introduced for the treatment of metastatic colorectal cancer. While promising, these drugs are frequently given in the palliative, and are much more expensive than older treatments. The objective of this study is to measure the cost implications of treatment with sequential regimens that include chemotherapy and/or monoclonal antibodies.
Methods
A Markov model evaluated a hypothetical cohort of 1000 patients with newly diagnosed metastatic colorectal cancer. Patients are treated with up to three lines of treatment prior to supportive care and subsequent death. Data was obtained from published multicenter phase II and randomized phase III clinical trials. Sensitivity analyses were conducted on the efficacy, toxicity and cost.
Results
Using drug costs alone, treatment including new chemotherapeutic agents increases survival at an incremental cost effectiveness ratio (ICER) of $100,000/discounted life year (DLY). Addition of monoclonal antibodies improves survival at an ICER of over $170,000/DLY. Results are most sensitive to changes in the initial regimen. Even with significant improvements in clinical characteristics (efficacy and toxicity), treatment with the most effective regimens still have an very high ICERs
Conclusions
Treatment of metastatic colorectal cancer with the most effective regimens comes at very high incremental costs. Cost effectiveness analyses should be a routine component of the drug development process, so that physicians and patients are appropriately informed regarding the value of new innovation.
Introduction
The treatment of metastatic colorectal cancer has changed dramatically in the last decade. Introduced in the 1950's, 5 Fluorouracil (5FU) was the first drug available for treatment of metastatic colorectal cancer. Overall survival remained at approximately one year, despite biomodulation with leucovorin. Irinotecan was approved in 1996, followed by oxaliplatin in 2000. In 2004, the monoclonal antibodies cetuximab and bevacizumab were introduced for treatment of metastatic colorectal cancer. Panitumumab gained approval in late 2006 for patients who had failed chemotherapy. With these new agents, there has been a paradigm shift in the treatment of patients with metastatic colorectal cancer with a doubling of median survival over 5FU alone and a potential for long term survival in a significant minority of patients.1
Unfortunately, the vast majority are not cured and each new drug offers only modest survival benefits. Irinotecan increases median overall survival by 2.2 months when added to 5FU/LV.2 Oxaliplatin, when given in combination with infusional 5FU (FOLFOX) improves median overall survival by 4.4 months compared to irinotecan, bolus 5-FU and leucovorin (IFL).3 Bevacizumab received FDA approval based upon 4.7 month improvement in survival when added to IFL.4 Cetuximab, when given alone has a median time to progression (TTP) of 1.5 months; combining this antibody with irinotecan increases median TTP to 4.1 months.5 The progression free survival (PFS) of chemotherapy refractory patients treated with panitumumab is 8 weeks compared to 7.3 weeks in patients treated with best supportive care alone6.
The National Comprehensive Cancer Network (NCCN) Practice Guidelines for Colorectal Cancer recommend that patients with good tolerance for intensive chemotherapy be treated initially with 5FU-based combination chemotherapy and bevacizumab. The guidelines suggest additional chemotherapy and/or cetuximab or panitumumab in the second and third line settings, ultimately exposing patients to all available classes of agents.7
With the exception of 5-FU and irinotecan (which recently came off patent), these drugs are very expensive and the cost implications of these promising medications, are the subject of significant debate.8-10 There are limited data in the published literature regarding the cost effectiveness of treatments for patients with metastatic colorectal cancer. Hillner used data from the multicenter study NCCTG 9741 to compare treatment with IFL to FOLFOX and found that the 4.4 month median survival benefit of the FOLFOX arm was accompanied by an incremental cost of $29,953, resulting in an incremental cost effectiveness ratio (ICER) of $80,410 per life year gained.11 Starling examined the role of cetuximab and irinotecan compared to best supportive care from the perspective of the United Kingdom National Health Service and found an ICER of 42,975 pounds per life year gained. 12 To our knowledge, there has not been a comprehensive cost effectiveness analysis that includes multiple lines of therapy.
In an effort to characterize the cost of modern treatment for metastatic colorectal cancer, we developed a model that measures the added benefits and costs of sequential combination regimens. Rather than performing a direct comparison of specific agents or regimens, our model evaluates broad categories of therapies, such as antibody-containing regimens versus non-antibody containing regimens, and treatment strategies that include one, two or three lines of therapy to better understand the cost implications of the changing paradigm of metastatic colorectal cancer treatment.
Methods
Model
We used TreeAge Pro 2006 to develop a Markov model (Figure 1) to follow the natural history of patients treated for metastatic colorectal cancer. Patients can be treated with one, two or three lines of therapy prior to supportive care and death. Patients are evaluated at weekly intervals (Markov Cycles) and can either remain on therapy at stable doses, develop toxicity or die. Patients who develop toxicity will die, continue on therapy at a dose reduction, or change therapy.
Figure 1.
Stylized Markov Model: Patients can receive up to three lines of therapy. Patients can also die in any health state from all cause mortality. (not shown)
Perspective
Third party payor.
Comparators
For this model, we used nine possible treatment strategies (Table 1). Although there are many possible other treatment sequences, these were selected to reflect the sequential advances in colorectal cancer treatment. Sequence A includes 5FU/LV alone followed by supportive care. Regimens B, C, D, and E are increasingly complex regimens. F and G contain all available regimens but use irinotecan and oxaliplatin in different orders; H and I are identical to F and G but use combination irinotecan and cetuximab in the third line. Doses, schedules and costs are shown in Table 2.
Table 1.
Sequences Used in Model (fixed)
| Sequence | First Line | Second Line | Third Line | Fourth Line | Discounted life expectancy | Discounted total cost |
|---|---|---|---|---|---|---|
| A | 5FU/LV (Mayo) | Supportive Care | 47.4 | $23,164 | ||
| B | FOLFOX | Supportive Care | 65.2 | $78,815 | ||
| C | FOLFIRI | FOLFOX | Supportive Care | 83.6 | $94,563 | |
| D | FOLFOX Bevacizumab | Irinotecan | Supportive Care | 99.8 | $165,649 | |
| E | FOLFOX | Irinotecan | Cetuximab | Supportive Care | 87.9 | $106,833 |
| F | FOLFOX Bevacizumab | Irinotecan | Cetuximab | Supportive Care | 105.2 | $175,964 |
| G | FOLFIRI Bevacizumab | FOLFOX | Cetuximab | Supportive Care | 105.2 | $165,360 |
| H | FOLFIRI Bevacizumab | FOLFOX | Cetuximab Irinotecan | Supportive Care | 112.9 | $191,773 |
| I | FOLFOX Bevacizumab | Irinotecan | Cetuximab Irinotecan | Supportive Care | 113.7 | $205,384 |
Shaded sequences represent sequences used to calculate ICERs and sensitivity analyses.
Table 2.
Regimens, Doses and Costs
| Regimen | Estimated Cost over 6 months (24 weeks) | |
|---|---|---|
| 5FU/LV | 5FU 425mg/m2 and LV 20 m/gm2 daily for 5 days, every 28 days | $96 |
| Capecitabine* | 1000 mg/m2 twice daily for 14 days every 21 days | $11,304 |
| FOLFOX | 5FU 400 mg/m2m, LV 400 mg/mg, Oxaliplatin 85 mg/m2 then 5FU 2400m/mg2 every 14 days | $33,270 |
| FOLFIRI | 5FU 400 mg/m2m, LV 400 mg/mg, Irinotecan 180mg/m2 then 5FU 2400m/mg2 every 14 days | $23,529 |
| Irinotecan | Irinotecan 350m/gm2 every 21 days | $30,100 |
| Cetuximab | 400mg/m2 followed by 250mg/m2 weekly | $52,080 |
| Panitumumab* | 6 mg/kg every 14 days | $41,457 |
| Bevacizumab | Bevacizumab 5mg/kg every 14 days | $24,123 |
Not included in model
Costs
We based cost on average sales price (ASP) for a 75 kg patient who was 69 inches tall (BSA=1.9 m2) provided by the Centers for Medicare and Medicaid Services (http://www.cms.hhs.gov/McrPartBDrugAvgSalesPrice accessed April 20,2008). Geeneric irinotecan was approved in February 20, 2008; however impact of generic irinotecan was not reflected in the Medicare payment amount until October 1, 2008.13 We included an estimated pump rental cost for regimens containing infusional 5FU ($300 per week). We assumed no vial wastage, and did not include supportive care medications, costs related to management of toxicity, radiographic studies, physician visits or indirect costs
Life Expectancy
We measured overall survival, and assumed that living patients in all health states have equivalent values. In every health state and cycle, patients face competing all-cause mortality rates. Since the median age at diagnosis of colon cancer is approximately 70 years, we used US life table data for a 70 year-old man in our base case analysis.
Data Sources
Estimates of rates of progression and toxicity were obtained from published results of multicenter Phase II and randomized Phase III studies (Table 3). In addition, we included unpublished aggregate data from NCCTG 9741 (FOLFOX vs. IFL in first line therapy). We restricted data to peer reviewed publications and excluded abstract presentations.
Table 3.
Estimates for base case scenario
| Median PFS/TTP (months) | % of patients with grade 3 or 4 toxicity | Results of toxicity | Source | |||
|---|---|---|---|---|---|---|
| Dose reduction or delay | Treatment-related death | Discontinuation of therapy | ||||
| 1st Line | ||||||
| 5FU/LV (Mayo) | 4.7 | 43%* | 35% | 1% | 7% | 37 |
| FOLFOX | 8.7 | 81% | 63.2% | 2% | 16%* | Sargent, 2005, unpublished data |
| FOLFIRI | 8.5 | 53% | 43%* | 4%** | 6% | 18 |
| FOLFOX and Bevacizumab | 13.1 | 81% | 63.2 | 2% | 16%* | |
| FOLFIRI and Bevacizumab | 12.9 | 53% | 43%* | 4%** | 6% | |
| 2nd/3rd Line | ||||||
| Irinotecan | 4.2 | 69% | 59% | 0 | 10% | 38 |
| FOLFOX | 4.6 | 36% | 26% | 2% | 8% | 39 |
| Cetuximab | 1.5 | 43.5% | 42.3% | 0 | 1.2% | 5 |
| Cetuximab and Irinotecan | 4.1 | 61.5% | 61.5% | 0 | 1.2% | 5 |
calculated from available data.
Progression
We used time to progression (TTP) or progression free survival (PFS) data to estimate the length of time patients were on treatment. When more than one source was available, we chose the source with the larger sample size. To estimate the benefit of the addition of bevacizumab to FOLFIRI or FOLFOX, we added 4.6 months (the increased TTP of IFL plus bevacizumab compared to IFL alone) 4 to the TTP of these regimens.
Toxicity
We used reported rates of grade 3 (severe) or 4 (life-threatening) toxicity. We used toxicity rates of FOLFOX and FOLFIRI alone as the toxicity rates for the respective bevacizumab-containing regimens, which provide a conservative estimate of treatment-related toxicity. We assumed that patients could have up to 2 toxic events before discontinuing treatment. We assumed that the probability of having a second toxic event was 10%; we fit the model using this estimate to general clinical outcomes consistent with clinical practice and varied this figure in sensitivity analyses.
Calculations of Probability for Progression and Toxicity
We assumed that toxicity and progression were independent and mutually exclusive events over the course of a one-week cycle. Progression rates were abstracted from trial results and converted to weekly probabilities using the declining exponential approximation of life expectancy (DEALE).14, 15 Toxicities were converted to probabilities using the DEALE and fractionated into fatal and non-fatal outcomes. After running the Markov analyses, expected survivals expressed as means were transformed back to median PFS/TTP intervals.
Supportive Care
The model is designed so that patients spend approximately 24 weeks (undiscounted) on supportive care, based on the observation that patients who received supportive care rather than second line irinotecan had a median survival of six months.16 In addition, Starling estimated that patients who received best supportive instead of cetuximab and irinotecan lived 5.6 months.12 We used cost of $135/day, based on Medicare reimbursement17
Incremental Cost Effectiveness Ratios (ICER)
For clarity of presentation, we present regimens A, C, G and I. These regimens were selected because they reflect increasingly effective treatments:
Pair 1: Comparing C to A measures the survival advantage offered by modern chemotherapy agents (irinotecan and oxaliplatin) compared to 5FU/LV alone.
Pair 2: Comparing G to C measures the survival advantage offered by the addition of antibodies to modern chemotherapy.
Pair 3: Comparing I to G represents the survival advantage offered by the most aggressive sequence in this model.
Sensitivity Analyses
We performed one-way sensitivity analyses in which we measured the effects of changes in toxicity, progression, drug costs, probability of second toxicity, time on supportive care and cost of supportive care on the overall cost effectiveness of the model. We report the ICERs of Pairs 1, 2 and 3. We performed a separate sensitivity analysis to measure the impact of generic irinotecan. (estimated to be approximately one third the cost of brand irinotecan).13
Monte Carlo Simulation
To measure the impact of uncertainty of all variables, we performed two dimensional sensitivity analyses using a Monte Carlo simulation. We studied 1000 hypothetical patients as they transitioned through the model over their beta probability distributions of toxicity and progression. To measure the impact of lower drug prices on the cost effectiveness ratios, so we varied the rates between 25 and 106% of ASP on a triangular distribution.
Discounting
Life expectancy and costs were discounted 3% per year.
Results
Base Case
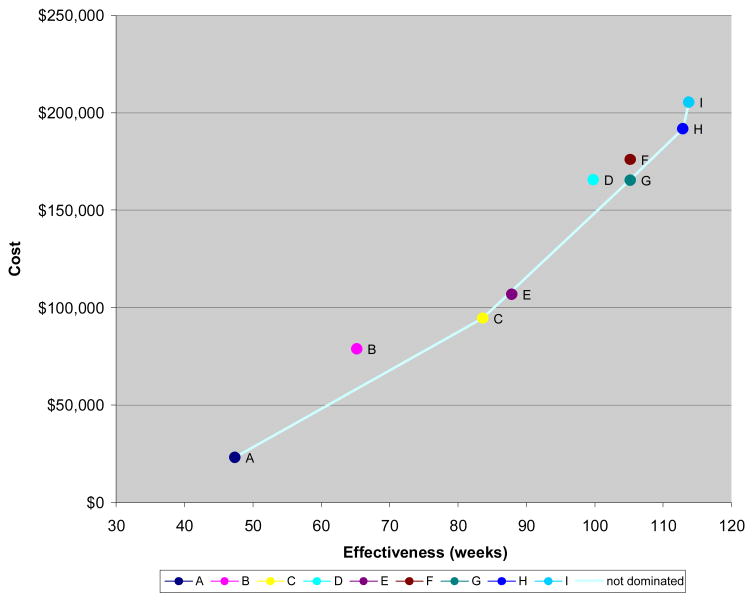
The cost and effectiveness for each sequence is presented in Table 1. The cost effectiveness frontier for the base case scenario is presented in Figure 2. Similar sequences that vary the order in which irinotecan and oxaliplatin are given (F and G; H and I) have similar life expectancies.
Figure 2.
Cost Effectiveness Frontier (Discounted Cost and Effectiveness) (fixed)
Incremental Cost Effectiveness Ratios (ICERS)
ICERS for selected sequences are shown in Table 4. The ICER per discounted life year (DLY) gained for adding the modern chemotherapy agents is approximately $100,000/DLY gained. The benefits of adding antibody come at higher costs ($170,000/DLY). The modest additional benefit of the most effective regimen in this model (using both cetuximab and irinotecan in the third line setting) generate even higher costs ($240,000/DLY)
Table 4.
Incremental CE ratios for selected regimens (Discounted)
| Pair | Sequence | Incremental Cost | Incremental Effectiveness in weeks | ICER/week | ICER/year |
|---|---|---|---|---|---|
| 1 | C vs. A (benefit of oxaliplatin and irinotecan) | $57,689 | 36.28 | $1,968 | $102,347 |
| 2 | G vs. C (benefit of both antibodies) | $67,313 | 21.54 | $3,286 | $170,896 |
| 3 | I vs. G (benefit of cetuximab/irinotecan in third line) | $44,388 | 8.56 | $4,675 | $243,096 |
Sensitivity Analysis
The CE frontier using generic irinotecan pricing is show in figure 3; as expected, the overall cost of treatments containing front line irinotecan are lower than those containing front line oxaliplatin.
Figure 3.
CE Frontier using Generic Irinotecan (cost and effectiveness)
In addition, we performed one-way analyses on rates of several key parameters for pairs 1, 2 and 3 (Tables 5-8). The “base case” results are shaded, with lower and higher rates of each variable on the left and right, respectively. These results showed that despite improvements in the clinical parameters (toxicity and progression), cost, and length of supportive care, the ICERs for the antibody containing sequences (G and I) are very high. The most significant changes in the ICERs occurred when the parameters for first line treatment were changed. Because we chose to focus on the pair-wise comparison of treatment strategies of lesser or greater effectiveness, the information is best interpreted by analyzing columns, which represent escalating effectiveness of treatment; with the exception of the analysis on second toxicity (Table 7), the ICERs uniformly increase reading down the columns.
Table 5.
Progression
| Pair | 50% | 100% | 150% | |
|---|---|---|---|---|
| First line | 1 | $121,784 | $102,336 | $95,784 |
| 2 | $208,052 | $170,872 | $158,028 | |
| 3 | dominated | $243,100 | $164,372 | |
| Second line | 1 | $93,444 | $102,336 | $108,420 |
| 2 | $175,240 | $170,872 | $169,156 | |
| 3 | $334,776 | $243,100 | $229,892 | |
| Third line | 1 | $102,336 | $102,336 | $102,336 |
| 2 | $157,560 | $170,872 | $176,748 | |
| 3 | $217,776 | $243,100 | $274,404 | |
| All | 1 | $108,576 | $102,336 | $101,192 |
| 2 | $194,480 | $170,872 | $161,876 | |
| 3 | dominated | $243,100 | $160,784 |
Table 8.
Drug Cost
| 25% | 100% | 150% | ||
|---|---|---|---|---|
| First line | 1 | $56,940 | $102,336 | $132,652 |
| 2 | $54,808 | $170,872 | $248,300 | |
| 3 | $145,652 | $243,100 | $308,048 | |
| Second line | 1 | $66,924 | $102,336 | $125,996 |
| 2 | $173,732 | $170,872 | $169,000 | |
| 3 | $307,268 | $243,100 | $200,356 | |
| Third line | 1 | $102,336 | $102,336 | $102,336 |
| 2 | $153,920 | $170,872 | $182,208 | |
| 3 | $101,816 | $243,100 | $337,272 | |
| All | 1 | $21,476 | $102,336 | $156,260 |
| 2 | $40,664 | $170,872 | $257,712 | |
| 3 | $68,536 | $243,100 | $359,476 |
Table 7.
Probability of Second Toxicity
| Pair | 0% | 10% | 50% | |
|---|---|---|---|---|
| First line | 1 | $95,264 | $107,328 | $117,104 |
| 2 | $150,228 | $185,952 | $209,196 | |
| 3 | $123,552 | dominated | dominated | |
| Second line | 1 | $100,516 | $103,896 | $111,332 |
| 2 | $171,496 | $170,456 | $168,740 | |
| 3 | $212,316 | $262,964 | $287,040 | |
| Third line | 1 | $102,336 | $102,336 | $102,336 |
| 2 | $170,664 | $171,132 | $172,588 | |
| 3 | $227,188 | $259,376 | $400,972 | |
| All | 1 | $94,068 | $109,408 | $143,156 |
| 2 | $150,644 | $185,796 | $214,552 | |
| 3 | $123,604 | dominated | $40,092 |
For example, Table 5 shows that a 50% decrease in progression rates (i.e. shorter time on first line treatment) for first line treatment would result in increased ICERS; for the incremental benefit of the most aggressive treatment (Pair 3) would actually be “dominated” and be more costly and less effective. On the other hand, improving the efficacy of front therapy (150% of base case) has the greatest impact on the ICERs, likely because the patients spend the longest time on first line therapy. The results were only modestly sensitive to the cost of the infusion pump, cost or time on supportive care (results not shown).
Monte Carlo Simulation (Figure 4)
Figure 4.
Monte Carlo simulation
The scatter plot shows that there are two distinct “bands” of treatment sequences; the steeper slope in the right upper portion of the figure includes the regimens that incorporate antibodies.
Discussion
We found that survival increases as patients with metastatic colorectal cancer are exposed to all available agents, but this benefit comes at high cost. This cost exceeds commonly accepted cost-effectiveness thresholds.18 Furthermore, our estimates only include drug costs, representing a conservative estimate of total treatment expense. Patients who are treated with 5FU/LV, oxaliplatin, and irinotecan have longer survival compared to 5FU/LV alone, at an ICER of approximately $100,000/DLY. However, treatment with antibody-containing regimens is associated with higher ICERs due to the relatively modest survival benefits and high costs. These results are similar to an analysis of salvage chemotherapy in platinum-refractory ovarian cancer, which found that second line monotherapy came at an ICER of $57,000/DLY, but the benefits of second line doublet therapy and third line monotherapy came at unacceptably high incremental cost.19 Whether the benefits are worth the costs clearly depends on the stakeholder; patients with advanced cancer may perceive greater value than healthy patients, policymakers, insurers, and physicians.8, 9
Our goal was not to compare “competing” regimens such as FOLFOX and FOLFIRI, which are both acceptable first line treatment strategies,20 but instead to study the impact of sequential progress (new chemotherapy and antibodies) on the overall cost of managing metastatic colorectal cancer. We found that similar treatment sequences (F and G or H and I) had similar life expectancies when prices for brand irinotecan were used. This suggests that ICERs are not affected by the sequence in which the drugs are used, but the effectiveness of the overall treatment strategy. Our sensitivity analyses show that the ICERs are most affected by changes in first line therapy, since this is associated with the longest PFS. This is of particular significance as a phase III study is underway that explores the use both bevacizumab and cetuximab in combination with chemotherapy in the front line setting. (Clinicaltrials.gov identifier NCT00265850).
We believe that it is reasonable to use life expectancy, rather than quality adjusted life expectancy as our measure for several reasons. Patients with life threatening diseases may choose treatments associated with a high risk of toxicity but low potential benefit.21, 22 In addition, using life expectancy rather than utilities results in a conservative (lower) estimate for ICERs, since preference weights for patients with advanced cancer are generally less than one.23
Limitations
Decision analysis models are limited by the accuracy of the probability estimates. Toxicity estimates are based on published data from Phase III and multicenter Phase II trials, which were inconsistently reported. We assumed that only grade 3 and 4 toxicities would prompt a dose reduction, delay or change in therapy. However, patients may discontinue or delay treatment for other reasons.24 In addition, the rate of toxicities in clinical trials may not accurately reflect community practice since clinical trial patients may be more robust than an unselected patient population. However, our sensitivity analysis show that changing the toxicity rates (Table 6) does not significantly alter the ICER, since changes in toxicity would also accompanied by changes in drug utilization. As shown in Table 7, only if the probability of second toxicity is 50% in all three lines of treatment, would the ICERs drop significantly.
Table 6.
Toxicity
| Pair | 50% | 100% | 150% | |
|---|---|---|---|---|
| First line | 1 | $101,400 | $1,968 | $1,995 |
| 2 | $165,620 | $170,872 | $176,384 | |
| 3 | $151,736 | $243,100 | $974,792 | |
| Second line | 1 | $102,076 | $102,336 | $103,064 |
| 2 | $171,340 | $170,872 | $170,560 | |
| 3 | $242,996 | $243,100 | $246,532 | |
| third line | 1 | $102,336 | $102,336 | $102,336 |
| 2 | $171,392 | $170,872 | $170,664 | |
| 3 | $240,344 | $243,100 | $250,276 | |
| $0 | $0 | $0 | ||
| All | 1 | $101,192 | $102,336 | $104,624 |
| 2 | $166,452 | $170,872 | $175,760 | |
| 3 | $152,360 | $243,100 | >$ 2 million |
In our model, we assumed that bevacizumab would add 4.4 months to FOLFIRI or FOLFOX based on its benefit seen when added to IFL.4. However, it is possible that the benefit of bevacizumab may be less. In N016966, the magnitude of benefit of the addition of bevacizumab to chemotherapy was shorter than expected (9.4 months vs. 8.0 months).25 The addition of bevacizumab to FOLFIRI in BICC C resulted in a PFS of 11.2 compared to 8.3 months for IFL, though the difference was not statistically significant.26 If our model overestimates the benefit of the addition of bevacizumab to front-line chemotherapy, this will result in a conservative (lower) estimate of the ICER. As our sensitivity analysis shows (Table 6), a 50% decrease in progression in first line therapy results in higher ICERs.
This discrepancy also raises questions about the cost effectiveness of multi-drug regimens. In contrast to the shorter PFS from the addition of bevacizumab to FOLFIRI and FOLFOX (11.2 and 9.4 months) reported in these recent clinical trials, a phase II study of 5FU/LV and bevacizumab alone have found a relatively long median PFS of 9.2 months,27 Therefore, the benefit of adding oxaliplatin or irinotecan to 5FU and bevacizumab needs to be further investigated both from a clinical and cost-effectiveness standpoint.
We only included intravenous regimens in our model. Capecitabine is considered equivalent to intravenous 5FU as a single agent for both localized and metastatic disease. In addition, capecitabine and oxaliplatin was found to be non-inferior to FOLFOX28 and this regimen is also included in the NCCN guidelines. Studies in the adjuvant setting have found that despite its higher drug cost, capecitabine may be cost effective given the reduced drug administration costs and indirect costs (travel time, lost work).29, 30 However, since we only included drug costs in our model capecitabine's high cost would bias the results against capecitabine containing regimens.
We also chose to include only one EGFR inhibitor in our model. Like cetuximab, panitumumab, is indicated as a single agent after failure of prior chemotherapy. However, it is not approved in combination with chemotherapy. In addition, there is no role for sequential use of EGFR inhibitors. Although there are no head to head data comparing panitumumab and cetuximab, the median progression free survival for panitumumab as last line therapy are similar (8 weeks vs. 1.5 months). Since cetuximab can be used as both a single agent and in combination with chemotherapy, we chose to use this in our model. This might result in higher ICERs, since drug costs for panitumumab are lower than cetuximab (Table 2). However, as the sensitivity analyses for drug costs show (Table 8), decreasing the drug costs in the third line setting only results in small changes in the ICERs, given the modest activity of these agents and short duration of therapy for most patients who are treated in these settings. For example, when drug costs are decreased to 25% of the base case for all three lines of treatment, ICERs decrease significantly. However, if only 3rd line drug costs are considered, the ICERs only drop modestly.
We also did not include the costs of managing toxicities such as diarrhea or infection, since they should be small relative to overall drug costs. We do not believe that this would bias the results significantly in favor of one particular sequence since patients on the most effective sequences (F, G, H and I) are exposed to all of the available agents and thus will likely experience similar associated side effects and management costs. In practice, treatment breaks are now commonly employed.24 Several strategies are being explored where patients on FOLFOX or FOLFIRI are offered scheduled periods entirely off chemotherapy or off oxaliplatin or irinotecan.31, 32 If treatment “holidays” for select patients provide similar survival to those patients treated continuously, the ICERs may be improved since such patients will not be accruing comparably high treatment-related costs.
Future Directions
Although our study uses metastatic colorectal cancer as a platform for discussion, these issues are relevant to many cancer types, where advances in translational research have led to the introduction of new targeted therapies. The costs for palliative treatment of will likely increase as patients are treated with sequential therapies until death or toxicity.
Given these high costs, future studies should be designed to address both dose intensity and duration of treatment. For example, although bevacizumab was approved at 10 mg/kg every two weeks in conjunction with FOLFOX, it is unclear if this dose is more beneficial than the previously approved 5 mg/kg every two week dose studied in conjunction with IFL.33 In addition, it is important to establish the role of continuing antibody therapy following failure of first-line chemotherapy. An ongoing NCI-sponsored Intergroup Bevacizumab Continuation Study is randomizing patients with metastatic colorectal cancer who have had progressive disease while on FOLFOX and bevacizumab to irinotecan plus cetuximab alone or with bevacizumab. (Clinicaltrials.gov identifier NCT00499369) It is important to identify which patients are most likely to benefit from these treatments; recent data suggest that patients with mutant KRAS derive no benefit from the addition of cetuximab to FOLFOX or FOLFIRI, while patients with wild-type KRAS demonstrate improved outcomes.34, 35 Other studies have also shown that the efficacy of panitumumab is also confined to patients with wild type K-ras.36
The cost will decrease as generics become available. At the time this article was written, the payment for branded irinotecan was approximately 145% greater than generic versions. As our sensitivity analyses on drug costs shows (Table 8), a 50% decrease in the cost of first line therapy will result in a $1200 decrease in ICER for Pair 3; the most substantial decreases occur when all three lines of treatment occur simultaneously. However, since oxaliplatin, bevacizumab and cetuximab, all remain under patent protection, the overall cost of treatment will still remain very high. Nevertheless, its availability raises an important societal question. When prices for generic irinotecan are used, the results clearly favor using regimens that include FOLFIRI in the front line setting given the lower cost.(Figure 3) This is supported by our finding that ICERs are most sensitivity to the changes in cost in the front line therapy, given the longer duration of treatment. Although FOLFIRI and FOLOX are both acceptable front line regimens, the majority of US physicians choose FOLFOX for front line treatment.37 Although the current reimbursement system for chemotherapy does not favor the use of generic regimens, payors may want to consider this as a mechanism for cost control in cases where similar clinical outcomes are obtained with alternate treatments that vary widely in cost. The potential savings to payers (including taxpayers) will need to be weighed against patients' and physicians' preferences for side effects.
The cost of cancer care will continue to rise as new palliative treatments are introduced, and patients incur costs as they live longer. We can be optimistic that these promising new agents may be truly “cost effective” if they are effective in the adjuvant setting. Cost-effectiveness assessments should be included in studies of new treatments, such that policymakers can make more informed decisions regarding coverage, and patients and oncologists can make the most informed decisions regarding treatment.
Acknowledgments
Dr. Wong was supported by R25 CA057708 as well as a Young Investigator Award through the American Society of Clinical Oncology. This project was also supported by P30 CA006927.
Footnotes
Disclosures: Dr. Wong: Bristol Myers Squibb (Research Funding). Dr. Meropol: Amgen, Genentech, Pfizer, Sanofi-aventis (Consulting) Dr. Sargent : Pfizer, Sanofi Aventis, Genentech, Amgen and Roche (Consulting and Honoraria). Dr. Goldberg: Amgen, Bristol Myers Squibb, Genentech, Pfizer, Sanofi-aventis (Consulting and Honoraria). Dr Beck and William Speier have nothing to disclose.
References
- 1.Goldberg RM, Rothenberg ML, Van Cutsem E, Benson AB, III, Blanke CD, Diasio RB, et al. The Continuum of Care: A Paradigm for the Management of Metastatic Colorectal Cancer. Oncologist. 2007;12(1):38–50. doi: 10.1634/theoncologist.12-1-38. [DOI] [PubMed] [Google Scholar]
- 2.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343(13):905–14. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22(1):23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-Label Phase III Trial of Panitumumab Plus Best Supportive Care Compared With Best Supportive Care Alone in Patients With Chemotherapy-Refractory Metastatic Colorectal Cancer. J Clin Oncol. 2007;25(13):1658–64. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 7.Colorectal Cancer Cancer Version2.2008. Practice Guidelines in Oncology: National Comprehensive Cancer Network. 2007. [Google Scholar]
- 8.Meropol NJ, Schulman KA. Perspectives on the Cost of Cancer Care. J Clin Oncol. 2007;25(2):169–70. doi: 10.1200/JCO.2006.09.6081. [DOI] [PubMed] [Google Scholar]
- 9.Meropol NJ, Schulman KA. Cost of cancer care: issues and implications. J Clin Oncol. 2007;25(2):180–6. doi: 10.1200/JCO.2006.09.6081. [DOI] [PubMed] [Google Scholar]
- 10.Schrag D. The price tag on progress--chemotherapy for colorectal cancer. N Engl J Med. 2004;351(4):317–9. doi: 10.1056/NEJMp048143. [DOI] [PubMed] [Google Scholar]
- 11.Hillner BE, Schrag D, Sargent DJ, Fuchs CS, Goldberg RM. Cost-effectiveness projections of oxaliplatin and infusional fluorouracil versus irinotecan and bolus fluorouracil in first-line therapy for metastatic colorectal carcinoma. Cancer. 2005;104(9):1871–84. doi: 10.1002/cncr.21411. [DOI] [PubMed] [Google Scholar]
- 12.Starling N, Tilden D, White J, Cunningham D. Cost-effectiveness analysis of cetuximab/irinotecan vs active/best supportive care for the treatment of metastatic colorectal cancer patients who have failed previous chemotherapy treatment. Br J Cancer. 2007;96(2):206–12. doi: 10.1038/sj.bjc.6603561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medicare Payment for Irinotecan. Office of the Inspector General, Department of Health and Human Services; 2008. [Google Scholar]
- 14.Beck JR, Pauker SG, Gottlieb JE, Klein K, Kassirer JP. A convenient approximation of life expectancy (the “DEALE”). II. Use in medical decision-making. Am J Med. 1982;73(6):889–97. doi: 10.1016/0002-9343(82)90787-2. [DOI] [PubMed] [Google Scholar]
- 15.Beck JR, Kassirer JP, Pauker SG. A convenient approximation of life expectancy (the “DEALE”). I. Validation of the method. Am J Med. 1982;73(6):883–8. doi: 10.1016/0002-9343(82)90786-0. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham D, Pyrhonen S, James RD, Punt CJ, Hickish TF, Heikkila R, et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet. 1998;352(9138):1413–8. doi: 10.1016/S0140-6736(98)02309-5. [DOI] [PubMed] [Google Scholar]
- 17.Report to Congress: Reforming the Delivery System. Medicare Payment Advisory Council; 2008. [Google Scholar]
- 18.Hidden Costs, Value Lost:Uninsurance in America INSTITUTE OF MEDICINE OF THE NATIONAL ACADEMIES. 2003 [PubMed] [Google Scholar]
- 19.Rocconi RP, Case AS, Straughn JM, Jr, Estes JM, Partridge EE. Role of chemotherapy for patients with recurrent platinum-resistant advanced epithelial ovarian cancer: a cost-effectiveness analysis. Cancer. 2006;107(3):536–43. doi: 10.1002/cncr.22045. [DOI] [PubMed] [Google Scholar]
- 20.Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI Followed by FOLFOX6 or the Reverse Sequence in Advanced Colorectal Cancer: A Randomized GERCOR Study. J Clin Oncol. 2004;22(2):229–37. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 21.Gaskin DJ, Weinfurt KP, Castel LD, DePuy V, Li Y, Balshem A, et al. An exploration of relative health stock in advanced cancer patients. Med Decis Making. 2004;24(6):614–24. doi: 10.1177/0272989X04271041. [DOI] [PubMed] [Google Scholar]
- 22.Gaskin DJ, Kong J, Meropol NJ, Yabroff KR, Weaver C, Schulman KA. Treatment choices by seriously ill patients: the Health Stock Risk Adjustment model. Med Decis Making. 1998;18(1):84–94. doi: 10.1177/0272989X9801800116. [DOI] [PubMed] [Google Scholar]
- 23.CEA Registry, vol 2006: Center for the Evaluation of Value and Risk in Health (CEVR) Tufts-New Enlgand Medical Center. 2006 [Google Scholar]
- 24.Denlinger C, Collins MA, Wong YN, Litwin S, Meropol NJ. Metastatic colorectal cancer (mCRC) patterns of care: Implications for clinical trial design. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I; 2007. Abstract 4079. [Google Scholar]
- 25.Saltz LB, C S, Diaz Rubio E, et al. Bevacizumab (Bev) in cominbatin with XELOX or FOLFOX4: Efficacy results from Xelox-1/NO16966, a randomized phase III trial in the first line treatment of metastatic colorectal cancer (MCRC); Program/Proceedings Gastrointestinal Cancer Symposium; 2007. Abstract 238. [Google Scholar]
- 26.Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, et al. Randomized, Controlled Trial of Irinotecan Plus Infusional, Bolus, or Oral Fluoropyrimidines in First-Line Treatment of Metastatic Colorectal Cancer: Results From the BICC-C Study. J Clin Oncol. 2007;25(30):4779–86. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 27.Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23(16):3697–705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 28.Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Randomized Phase III Study of Capecitabine Plus Oxaliplatin Compared With Fluorouracil/Folinic Acid Plus Oxaliplatin As First-Line Therapy for Metastatic Colorectal Cancer. J Clin Oncol. 2008;26(12):2006–12. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 29.Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe P. The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation. Health Technol Assess. 2006;10(41):iii–iv. xi–xiv, 1–185. doi: 10.3310/hta10410. [DOI] [PubMed] [Google Scholar]
- 30.Twelves CJ. Xeloda in Adjuvant Colon Cancer Therapy (X-ACT) trial: overview of efficacy, safety, and cost-effectiveness. Clin Colorectal Cancer. 2006;6(4):278–87. doi: 10.3816/CCC.2006.n.046. [DOI] [PubMed] [Google Scholar]
- 31.Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, et al. OPTIMOX1: A Randomized Study of FOLFOX4 or FOLFOX7 With Oxaliplatin in a Stop-and-Go Fashion in Advanced Colorectal Cancer--A GERCOR Study. J Clin Oncol. 2006;24(3):394–400. doi: 10.1200/JCO.2005.03.0106. [DOI] [PubMed] [Google Scholar]
- 32.Maindrault-Goebel F, Lledo G, Chibaudel B, Mineur L, Andre T, Bennamoun M, Mabro M, Artru P, Louvet C, De Gramont A. OPTIMOX2, a large randomized phase II study of maintenance therapy or chemotherapy-free intervals (CFI) after FOLFOX in patients with metastatic colorectal cancer (MRC). A GERCOR study. Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings; 2006. p. 3504. Part I. [Google Scholar]
- 33.Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23(15):3502–8. doi: 10.1200/JCO.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 34.Bokemeyer C, B I, Hartmann JT, De Braud FG, Volovat C, Nippgen J, Stroh C, Celik I, Koralewski P. KRAS status and efficacy of first-line treatment of patients with metastatic colorectal cancer (mCRC) with FOLFOX with or without cetuximab: The OPUS experience. J Clin Oncol. 2008;26 Abstract 4000. [Google Scholar]
- 35.Van Cutsemes E, L I, D'haens G, Moiseyenko V, Zaluski J, Folprecht G, Tejpar S, Kisker O, Stroh C, Rougier P. KRAS status and efficacy in the first-line treatment of patients with metastatic colorectal cancer (mCRC) treated with FOLFIRI with or without cetuximab: The CRYSTAL experience. J Clin Oncol. 2008;26(May 20 suppl) Abstract 2. [Google Scholar]
- 36.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-Type KRAS Is Required for Panitumumab Efficacy in Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2008;26(10):1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 37.Treatment of Metastatic Colon Cancer Patterns of Care in Medical Oncology. 2005;2 [Google Scholar]