Abstract
Cancer pain management can be improved by overcoming patients’ attitudinal barriers to reporting pain and using analgesics. A simple cost-effective barriers intervention designed to reach a large number of persons with cancer has not yet been tested. Such an intervention should be tested against barriers’ assessment-alone, as well as no-treatment control. The purpose of this study was to test the efficacy and the cost-effectiveness of a Tailored Barriers Intervention (TBI), an educational intervention tailored to participants’ attitudinal barriers toward reporting pain and using analgesics. This was a randomized three-group (TBI, assessment-alone, or control) trial with measures at baseline and 28 days later conducted at the NorthCentral and Heartland offices of the Cancer Information Service (CIS), an NCI program that provides information to persons seeking answers to cancer-related questions. Participants (1256 adult CIS callers diagnosed with cancer with moderate to severe pain in the past week) joined the study and were randomized. Of those participants, 970 (77.23%) provided follow up data. The TBI consisted of educational messages tailored to each participant’s attitudinal barriers, delivered orally over the telephone, followed by a printed mailed copy. The outcome measures were attitudinal barriers to pain management, as well as pain outcomes (duration, severity, and interference with life activities). At follow-up the TBI group had significantly lower attitudinal barriers scores compared to assessment-alone and control, but the groups did not differ on the pain outcome variables. TBI and assessment alone had similar cost effectiveness. The TBI needs to be strengthened to achieve reductions in pain severity.
1. Introduction
Unrelieved pain impairs quality of life for many individuals with cancer, despite years of attention to this problem from both clinicians and researchers (13). One reason for this continuing problem is that many persons with cancer under-utilize available analgesic medications (15, 24). This under-utilization often results from negative attitudes about reporting pain and using analgesics, such as exaggerated fear of addiction (24). These attitudes have been termed patient-related barriers to pain management.
A number of patient education interventions have focused on helping patients to report pain and to make optimal use of pharmacologic strategies to manage their cancer pain. Rimer and colleagues (18) tested interactive counseling sessions in which nurses provided rationales for compliance with prescribed regimens, countered myths regarding addiction and tolerance, and discussed ways to implement the prescribed regimen. Since that study, others have shown that overcoming attitudinal barriers can improve pain management, although effect sizes are modest and not all outcomes reveal benefit (2, 4, 7, 16, 22).
Tailored interventions in which content is customized to individuals’ characteristics (such as attitudes) are better remembered, discussed more, and in one-half of trials are more efficacious than are standardized messages (5, 19). The intervention tested in the present study involved educational messages specific to participants’ attitudinal barriers delivered to each participant after a baseline assessment of which barriers they had. Baseline assessment, however, can itself function as an intervention, a problem noted in studies of tailored barriers interventions (11). For example, asking people to think about barriers could motivate them to speak to their health care providers about pain management. A careful study of a tailored intervention requires control for assessment-alone, resulting in the need for a larger sample than if one were only comparing the tailored intervention to a no-treatment control condition.
To recruit a large sample, we turned to the Cancer Information Service (CIS). Heidrich and colleagues (9) have shown that a sizable portion of CIS callers (84%) held one or more attitudinal barriers to pain management, and, although pain was not their expressed reason for calling the CIS, when asked if it was a problem and whether assistance would be useful, they answered “yes”. In considering dissemination of educational interventions through the CIS, cost-effectiveness is a consideration. Cost-effectiveness analysis (CEA) evaluates both the outcomes and costs of alternative health care interventions. There have been very few cost-effectiveness analyses of tailored interventions (3), and there have been no cost-effectiveness studies of tailored interventions designed to improve pain management.
This trial tested the efficacy of a TBI, controlling for potential reactivity to baseline assessment, and evaluated the TBI’s cost-effectiveness. We hypothesized that TBI would be superior to assessment-only, which in turn would be superior to no-baseline-assessment no-intervention, with respect to outcomes that included attitudinal barriers scores, and pain duration, severity and interference with life activities. We also hypothesized that the assessment-only condition would be more cost-effective than TBI.
2. Methods
2.1 Design
We used a randomized three-group design. Participants were blocked by CIS location (Wisconsin versus Kansas) before being randomized with equal allocation (1:1:1) to one of three groups. Group 1 was the no-baseline-assessment no-intervention control group. Group 2 received baseline assessment but no intervention. Group 3 received baseline assessment plus TBI. Outcome data were collected approximately 28 days later. This time frame for follow up was selected because, based on past research, we deemed it long enough for the intervention to have an impact, yet short enough to retain a large number of those who enrolled. All study procedures (described below) were guided by websites that were developed for the study and were designed to guide study staff through all facets of the study from eligibility assessment to outcome data collection.
2.2 Participants
Potential participants were CIS callers who first received service as usual and then were invited to stay on the line to join a study. Self report eligibility criteria were: ability to participate in English, age 18 or older, diagnosed with cancer, and the presence of moderate to severe pain related to cancer in the past week. The item that assessed pain asked about the amount of time the person had spent in moderate to severe pain during the past week with response options of “never”, “sometimes” “often” “almost always” “always”. If someone selected the “never” response they were considered not eligible. Exclusion criteria were having received pain information as part of usual CIS service, or having called previously during the study accrual period. Based on power analysis, our goal was to have approximately 930 participants (310 in each group) with evaluable outcome data, which would yield 0.8 power to detect a small treatment effect on pain duration.
2.3 Settings
The primary function of CIS Contact Centers is to provide information to persons who call seeking answers to cancer-related questions. The CIS receives thousands of calls each year from people with cancer (over 22,000 calls in 2005) and thus can recruit a large number of participants to studies. Participants were initially recruited from two of the fifteen Cancer Information Service (CIS) Contact Centers, the North Central Region located at the University of Wisconsin and covering Iowa, Minnesota, North Dakota, South Dakota, and Wisconsin, and the Heartland Region located at the University of Kansas Medical Center and covering Illinois, Kansas, Missouri, and Nebraska. After the study was running for about 4 months, the CIS reconfigured its network from 15 Contact Centers to four. The North Central CIS Contact Center contract was not renewed and from then on participants were recruited only from Heartland CIS, which then began handling approximately one-fourth of all CIS calls in the US.
This shift in the site of data collection should have negligible, if any, effect on the data because the CIS policies for uniform hiring, training, and monitoring of staff. At the beginning of the study there were eight Information Specialists (IS) at the North Central CIS and seven at the Heartland CIS. All of them had had experience in studies supported by the CIS Research Consortium and most of them were bachelor’s prepared nurses or heath educators. Nonetheless, careful systematic training on every step in the procedure was conducted. The staff read and discussed background information about patient-related barriers to pain management, tailored interventions, and the study protocol. Then they had practice sessions on a training web site with mock callers. With these mock callers, the Specialists logged onto the website and performed all aspects of the study from consent through sign off. This training site mimicked all facets of the real site except that it did not save the test data that were collected. As new IS joined the CIS, they underwent the same training. By the end of the study, 43 staff (8 from North Central and 35 from Heartland) had participated in the study.
2.4 Experimental groups
Group 1 was the control group
Participants randomized to this condition responded to measures of demographics and a single-item pain screening measure at baseline. They did not complete baseline assessment of attitudinal barriers or the other pain measures nor did they receive the intervention. This true no-treatment control group controlled for the fact that some persons in pain may experience symptom improvement simply with the passage of time.
Group 2 was the assessment-alone group
Participants randomized to this condition responded to baseline measures, but did not receive the educational intervention. This group was included so that we could ascertain whether baseline assessment of barriers and pain functioned as an intervention.
Group 3 was the TBI group
Participants randomized to this condition responded to baseline measures and received educational messages tailored to their barriers. A participant’s barriers were determined at baseline by their responses (agree/disagree) to the 8 items on the Barriers Questionnaire Short Form (described below). These 8 attitudinal barriers are: fatalism about cancer pain management, fear of addiction, worry about developing tolerance, concern about side effects, fear of being a complainer, worry about immune system damage, worry about masking changes in disease status, and concern about distracting a physician from focusing on cure. For each barrier, a brief educational message had been prepared and stored in the website. The messages were developed from evidence in published, peer reviewed empirical literature, have been used in previous studies (22, 23), and have been shown to be effective in improving pain management. The IS read the educational messages that corresponded to a given participants’ barriers (see Table 1 for the messages). To reinforce the messages, tailored print material (hard copies of what had been presented orally) was sent to the participants. Thus, as is true in all tailored interventions, the “dose” of the intervention that a given subject received depended on how many of the 8 barriers he/she had, as this determined how many educational messages were read (and sent). The mean (SD) number of barriers was 4.21 (1.86), with a range of 0 to 8. The mean (SD) number of minutes to deliver the TBI was 6.96 (2.64) minutes. The percentage of subjects in group 3 (TBI) who responded “yes” to each barrier at baseline is reported in Table 1.
Table 1.
TBI messages and percentage of subjects in group 3 who responded “yes” to each of the barriers during the baseline assessment
| Barrier | TBI Message |
|---|---|
| Fatalism N = 307 (83%) |
|
| Fear of addiction N = 252 (68%) |
|
| Fear of being a complainer N = 48 (13%) |
|
| Worry about side effects N = 152 (41%) |
|
| Worry about tolerance N = 163 (44%) |
|
| Fear of distracting MD from focus on cure N = 107 (29%) |
|
| Concern about immune system N = 196 (53%) |
|
| Worry about monitoring bodily changes N = 307 (83%) |
|
2.5 Questionnaires
Pain duration
Pain duration was assessed with a single item regarding the amount of time the person had spent in moderate to severe pain during the past week. There were five response options (1 to 5) -- “never”, “sometimes” “often” “almost always” “always”. This item has been used in a number of studies addressing adequacy of pain management as part of the Total Quality Pain Management (TQPM) program (6). Reliability and validity of the item are supported by its moderate positive correlation with the pain severity scales and by its inverse correlation with patient satisfaction with pain management (8). In the present study the item was used in two ways: 1) it was the screening item that determined eligibility (participants must have had at least some moderate to severe pain in the past week); 2) it was one of the outcome measures.
Pain severity
Three intensity items from the Brief Pain Inventory (BPI) were used to assess pain severity (1). Participants were asked to report their worst pain during the past week, least pain during the past week, and pain now. Response options for each item range from 0 to 10. An average of the three items was computed for analyses to enhance reliability and power. The BPI pain severity items have been widely used in cancer research and have demonstrated reliability, validity, and sensitivity to change (1, 20).
Pain interference
The seven pain interference items from the BPI were used (23). The items address the extent to which pain interferes with daily functioning such as sleeping, walking, and working. Response options range from 0 to 10 and a mean of the seven items was used in analyses. These items have been used extensively in pain research and have shown excellent internal consistency and construct validity (20, 23).
Barriers
The short form of the Barriers Questionnaire-II (BQ-II) was used. It is designed to measure whether an individual subscribes to each of eight attitudinal barriers to reporting pain and using analgesics. Response options for each item are agree/disagree. These items have been used previously, including in the pilot test of the present study conducted at the CIS (9, 23). As described above, this questionnaire was used to assess barriers at baseline for purposes of selecting the tailored information provided to participants in Group 3 (TBI). It was also used in the efficacy analyses; a sum of the “agree” responses, ranging from 0 to 8, was used for this purpose. The internal consistency of the short form in a previous study was alpha = .67 (21) and in the present study was alpha = .62.
Cost
A cost analysis based on the model provided by Crane and colleagues (3) was conducted in which all direct costs associated with intervention delivery were identified and calculated. The web program recorded the cost of personnel time (See Table 2). That is, after completing usual service, the IS logged onto the web site, obtained consent and collected demographic data. Then the website clock started. The clock stopped just before the IS asked questions needed for follow up data collection (telephone number, best time to call). Thus, neither the time for usual service nor the time for collecting demographic data and obtaining consent from the participant were included in the calculation. Rather, only the cost of the incremental time spent as a result of the baseline data collection and intervention was identified. Thus, we separated costs of study involvement from costs of delivering the intervention, because it is the latter that is of interest. Personnel cost were determined using the national average salary, prorated fringe rate, and overhead/indirect costs for CIS IS. These data allowed calculation of the mean call time per participant, and the mean personnel cost per participant. Also included in the cost analysis are printing costs and mailing costs for the tailored print material. All costs are presented in U.S. dollars.
Table 2. Procedure followed by the CIS Information Specialists (IS).
|
|
|
|
|
|
|
|
|
|
|
|
|
2.6 Procedure
The study was reviewed and approved by the IRBs at the University of Wisconsin-Madison and the Kansas University Medical Center and participants were recruited from March 2004 to January 2006. Two websites were designed, pilot tested, and refined for the study. One site was used by the CIS in baseline data collection and the other site was used by the University of Wisconsin Carbone Cancer Center Survey Research Shared Service (SRSS) for outcome data collection. For baseline data collection and intervention delivery, the procedures followed by the CIS staff are described in Table 2. In brief, when a potential participant called the CIS, an IS completed service as usual and then invited the caller to stay on the line. The IS logged onto the study website which directed him/her, question by question, through eligibility determination, consent, randomization, baseline assessment, intervention delivery, and the recording of contact information that would allow the participant to be telephoned in approximately 28 days for outcome data collection. Note that this procedure assured concealment of the sequence of allocation of participants to group and further that during baseline data collection the IS would not be aware of whether participants were randomized to assessment-alone versus TBI, although they would quickly see if the participant was in the Group 1 (control). To accomplish randomization, one of the six permutations of the numbers 1, 2, and 3 was selected at random and without replacement using RAN2, so that assignment to the three arms was balanced every three subjects, and the orders of assignment were balanced every eighteen subjects (17).
Staff from the SRSS collected outcome data. Eight staff trained and experienced in conducting survey research over the telephone served as data collectors. Each day the SRSS staff would log on to their website to see which participants were within the calling window (27 to 40 days past study entry), call participants, access the interview text, and record participants’ responses to the questionnaires. These staff were blind to the condition (group) to which the participants had been allocated because that information was not included on their website. The mean (SD) length of time between baseline and follow up data collection was 28.47 (3.39) days with a minimum of 25 and a maximum of 40.
3. Results
3.1 Quality control
Staff performance on telephone lines at the CIS is monitored regularly for quality allowing adherence to the study protocol to be monitored. During the study period, approximately 10% of the calls were monitored by CIS supervisory staff. They listened to determine if potentially eligible participants (callers who self identified as a person diagnosed with cancer) were invited, and if ineligible persons (callers who self identified as not being diagnosed with cancer) were not invited. Data revealed that 68.8% of potentially eligible callers were invited to join the study, and only 1 (0.3%) ineligible caller was invited. Of course, fidelity to the protocol was outstanding because once the participant consented to the study, the words and actions of the CIS staff were completely directed by the text on the web site. The mean (SD) number of participants recruited by each Information Specialist was 29.21 (23.24) with a minimum of 2 and a maximum of 97.
3.2 Recruitment and retention
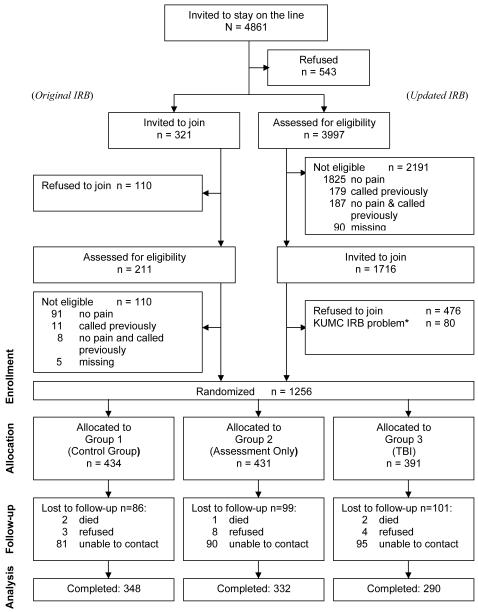
Figure 1 shows that 4861 callers were invited to stay on the telephone to learn about the study, and 4318 (88.83%) did so. Note that Figure 1 reports data for “Original IRB” and “Updated IRB”. This is because for the first 3 months of the study, recruitment was conducted under an IRB requirement that staff invite potential participants to join the study before ascertaining that they were eligible. After 3 months, the IRB approved a request for a change of protocol so that eligibility could be determined before an invitation to join the study was made. As a consequence of having to use the invite-first approach for the first 3 months of the study, it is not possible to calculate separately the percentage of potential participants who declined to join versus were not eligible to join. We know, however, that once the IRB removed the requirement of inviting before assessing eligibility (right column of Figure 1), 1240 out of 1716 (72%) who were eligible joined.
Figure 1.
CONSORT Diagram.
Of the 1256 participants who joined the study and were randomized, 970 (77.23%) were retained and provided follow up data. Retention rates did not differ by group; 80% for Group 1, 77% for Group 2, and 74% for Group 3 [χ2(2, N = 1253) = 2.730, p = .256]. Similarly, comparisons of those who dropped to those who were retained revealed that baseline scores did not differ significantly for any of the study variables. Specifically, the mean (SD) scores for those who dropped versus those who stayed were: for barriers, 4.36 (1.88) vs. 4.17 (1.85) [t (789) = 1.180, p = .238], for pain duration, 3.13 (1.17) vs. 3.00 (1.12) [t (1254) = 1.752, p = .08], for pain severity 3.81 (2.34) vs. 3.59 (2.22) [t (801) = 1.133, p = .258], for pain interference 5.05 (2.90) vs. 4.93 (2.85) [t (794) = 0.503, p = .615].
3.3 Demographics and descriptive data
Demographic data are presented in Table 3. The 1256 participants ranged in age from 39 to 89 with a mean (SD) age of 55.77 (12.72) years. Most participants were female (74.2%) and Caucasian (77.6%) and most had at least some college education (64.5%). For the sake of comparison, all persons with cancer who called the CIS during the study period (N=35,853) had a mean (SD) age of 58.26 (13.87), most were female (65.9%) and Caucasian (72.6%) and most had at least some college education (61.2%).
Table 3. Demographic Variables for Participants at Baseline.
| Participants (N=1256) | ||
|---|---|---|
| Variables | N | % |
| Gender | ||
| Male | 315 | 25.1 |
| Female | 932 | 74.2 |
| Missing | 9 | 0.7 |
| Hispanic or Latino | ||
| Yes | 59 | 4.7 |
| No | 1179 | 93.9 |
| Missing | 18 | 1.4 |
| Race | ||
| American Indian or | ||
| Alaska Native | 38 | 3.0 |
| Asian | 17 | 1.4 |
| Pacific Islander | 5 | 0.4 |
| African American | 69 | 13.5 |
| Caucasian | 959 | 77.6 |
| More than one race | 20 | 1.6 |
| Missing | 48 | 3.8 |
| Education | ||
| Grade School | 24 | 1.9 |
| Some High School | 94 | 7.5 |
| High School | 328 | 26.1 |
| Some College | 458 | 36.5 |
| College Grad | 197 | 15.7 |
| PostGrad | 145 | 11.5 |
| Missing | 10 | 0.8 |
Baseline data for pain duration were available for all three groups and revealed that subjects were often in moderate to severe pain in the past week, with a mean (SD) value of 3.03 (1.13), with a range of 2 to 5. Baseline data for those randomized to Groups 2 and 3 reveal that the mean (SD) number of barriers was 4.21 (1.86), with a range of 0 to 8. These participants were in a mild to moderate amount of pain in that the mean (SD) pain severity score was 3.64 (2.25), and the mean (SD) pain interference score was 4.96 (2.86), with ranges of 0 to 10 for both of these measures.
3.4 Efficacy analyses
Because Groups 2 (assessment only) and 3 (TBI) were tested both at baseline and at post-treatment, the comparisons of these two groups were performed within an analysis of covariance using baseline score as the covariate. Alternatively, comparisons of post-treatment measures of these two groups to Group 1 (control) were accomplished with analysis of variance. Shaffer’s sequentially rejective multiple test procedure was used to test pairwise comparisons among the three treatment group means for the outcome measures (21). The power achieved using the Shaffer approach is superior to that achieved with a planned post-omnibus approach (Fisher LSD), an F test followed by pairwise contrasts. According to Shaffer’s method, the contrast whose p-value was smallest among those associated with the three pairwise comparisons for a particular dependent variable was tested first, assigning to it a Type I error rate equal to one-third of the family-wise error rate allotted the dependent variable. If this first test was statistically significant, the remaining two pairwise comparisons were tested, assigning to each of these comparisons the full family-wise error rate allotted. If the first test was not statistically significant, then all three comparisons for the dependent variable were declared not to be statistically significant. All tests were directional. Table 4 provides mean (SD) scores for the outcome variables.
Table 4. Mean (SD) Value of Outcome Variables at Baseline and follow-up by Group*.
| Baseline | Follow-up | |||||
|---|---|---|---|---|---|---|
| Variable (range) |
Group 1 Control |
Group 2 Assessment only |
Group 3 TBI |
Group 1 Control |
Group 2 Assessment only |
Group 3 TBI |
| Pain duration (1-5) | 3.00(1.13) (n = 434) |
3.06 (1.14) (n = 431) |
3.03 (1.14) (n = 391) |
2.53 (1.31) (n = 361) |
2.59 (1.30) (n = 343) |
2.50 (1.30) (n = 303) |
| Barriers (0-8) | 4.28 (1.86) (n = 421) |
4.13 (1.86) (n = 370) |
4.01 (2.01) (n = 348) |
3.90 (2.00) (n = 332) |
2.98 (1.95) (n = 290) |
|
| Pain severity (0-10) | 3.63 (2.20) (n = 426) |
3.65 (2.30) (n = 377) |
3.26 (2.54) (n = 348) |
3.43 (2.46) (n = 335) |
3.32 (2.36) (n = 292) |
|
| Pain interference (0-10) | 5.03 (2.85) (n = 423) |
4.87 (2.88) (n = 373) |
4.45 (3.09) (n = 361) |
4.56 (3.03) (n = 343) |
4.30 (3.05) (n = 303) |
|
Sample sizes vary slightly due to missing data.
Barriers was allotted a Type I error rate of .05, and the Type I error rate allotted to the most significant contrast test, comparing Groups 1 and 3, was .05/3=.0167. This comparison of means for the two groups revealed that barriers scores were significantly lower in the TBI group than in the control group, [t (619) = 6.519, p = .000], with an effect size of d = 0.52. The comparison of Group 1 (control) to 2 (assessment only) revealed that these two groups did not differ [t (967) = 0.671, p = 0.25]. The comparison of Group 2 (assessment only) to Group 3 (TBI) revealed that barriers scores were significantly lower in the TBI group than in the assessment only group [t (967) = 5.800, p = .000], with an effect size of d = 0.47.
Pain interference was allotted a Type I error rate of .05, and the error rate allotted to the most significant contrast test, in this case comparing Groups 2 and 3, was .05/3=.0167. This contrast revealed no significant difference in pain interference scores, [t (638) = 0.618, p = .268]. Therefore, no further group comparisons were performed for this outcome variable. Pain duration and pain severity, because they are conceptually quite similar, shared a type I error rate of .05 (each was allotted a Type I error rate of .025), and the Type I error rate allotted to the most significant of the tests of each of these variables, in both cases comparing Groups 2 and 3, was .025/3=.00833. There were no significant differences between groups for either pain duration or for pain severity.
In post hoc analyses we examined whether CIS staff expertise in delivering the TBI might have been linked to how well the intervention worked for subjects. We examined the correlations between the number of calls made by the CIS staff person and changes from baseline to follow-up in subjects’ barriers, pain duration, pain severity, and pain interference. This analysis involved only those subjects who had received the TBI (those in Group 3). We found no significant associations.
3.5 Cost-effectiveness analysis (CEA)
Because there were no group differences in pain outcomes, the only outcome (effect) of interest for this analysis is attitude change (barriers change) between baseline and follow up. The barriers scores were dichotomized for this analysis in that we considered a decrease by 1 or more in the barriers scores to be a desired effect. CEA compared the aggregate cost for Group 2 (assessment only) and for Group 3 (TBI) to this outcome. Note there were no analyses involving Group 1 because that group had neither assessment nor TBI. Table 5 describes the analysis. Note that 44.9% of participants in Group 2 (Assessment Only) experienced a decrease in barriers scores of 1 or more, while 60.7% of participants in Group 3 (TBI) experienced this desired outcome. The costs of delivering the assessment and the TBI to all of the participants, regardless of their outcome, were computed. In the final step of the analysis, the total costs were divided by the number of participants in each group with the desired outcome. This resulted in the comparison ratio. The average cost-effectiveness for Group 2 was $7.09 and for Group 3 was $7.18. We conclude that the cost-effectiveness is similar for assessment-alone versus assessment plus TBI.
Table 5.
Cost-Effectiveness Analysis for Decreases in Barriers Scores
| Group 2 (Assessment-only) N=332 |
Group 3 (TBI) N=290 |
|
|---|---|---|
| Number (%) experiencing a desired outcome (a pre to post decrease in number of barriers) |
149 (44.9%) | 176 (60.7%) |
| Mean call time per participant | 5.24 minutes | 6.96 minutes |
| Mean call time per participant with a desired outcome |
(5.24×332)/149 = 11.68 minutes |
(6.96×290)/176 = 11.47 minutes |
| Mean cost§ of printing and mailing tailored print material per participant |
NA -- no print material sent | $0.14 |
| Mean cost of printing and mailing tailored print material per participant with a desired outcome |
NA -- no print material sent | $0.14×290/176 = $0.23 |
| Mean personnel cost* per participant @$36.4/hr |
($36.40/60)×5.24 = $3.18 | ($36.40/60)×6.96 = $4.22 |
| Mean personnel cost per participant with a desired outcome |
($3.18×332)/149 = $7.09 | ($4.22×290)/176 = $6.95 |
| Mean total cost** of delivery per participant |
$3.18 | $4.22+$0.14 = $4.36 |
| Cost effectiveness ratio: Mean total cost of delivery times the number of participants divided by the number of participants with a desired outcome |
($3.18×332)/149 = $7.09 | ($4.36×290)/176 = $7.18 |
NOTE:
Cost of ink: HP LaserJet 4250/4350 smart print cartridge, black (20,000-pages) $224.00 ⇒ $.0112/page. Staff time to retrieve the printed material and place it in an envelope were not included in this calculation.
Cost of paper: HP MultiPurpose™ Paper, 8-1/2 × 11, Case (500 Sheets/Ream ×10) $ 40.00 ⇒ $.0080/page.
Mean number of pages per participant based on Mean number of Barriers @ baseline.
Mean cost of printing per participant ⇒ ($.0112 + $.0080) × 1 = $.0192.
Cost of mailing: 1/3 of CIS accruals receive a .5oz letter/envelope resulting in the need for extra postage to cover the additional weight (source, Amy Gaier 9/2/05).
Mean cost of mailing per participant ⇒ $.37 / 3 = $.1233.
Mean cost of printing and mailing per participant ⇒ $.0192 + $.1233 = $.1425 rounded to $.14.
NOTE:
Personnel cost was computed as the national average wage, fringe, and overhead for CIS Information Specialists -- $36.40/hour. (Linda Squiers, personal communication, 8/11/05).
NOTE:
Total cost is the combined cost of mailing print material and personnel cost.
4. Discussion
The primary findings from this study are that the TBI changed negative attitudes about reporting pain and using analgesics, but not outcomes such as pain severity, and that the TBI and assessment-alone were similar in terms of cost-effectiveness. That is to say, compared to assessment-alone, the TBI was effective in decreasing barriers scores and was also as cost effective. Given the seriousness of the problem of pain related to cancer and its treatment, attitude change is an important beginning step toward improvement. It is important, however, to consider ways to enhance both the intervention itself and the design of a study that will capture its affect on pain severity, duration and interference.
Within the context of the CIS, previous investigators have demonstrated that short proactive communications can be effective in changing both attitudes and behavior, although effects on the latter (behavior) tend to diminish over time (12, 25). The behaviors in question in those two studies, fruit and vegetable consumption (12) and mammography utilization (25), are complex multifaceted behaviors, as is pain management. But they differ from pain management in that they are less emotionally charged and more within the control of the person with cancer, as opposed to being at least partly within the control of clinicians who control access to potent pain medications.
Outside the context of the CIS, a number of patient education interventions for pain management have been evaluated. Many of these interventions have gone beyond attempts to overcome patient-related barriers by also addressing the broader range of system-related and clinician-related barriers that can interfere with optimal pain management (4, 10, 14, 16). These multi-faceted interventions have comprehensively addressed multiple barriers to pain management, have sometimes involved lengthy clinician interaction including home visits, and have shown beneficial effects on patient outcomes, including knowledge, attitudes, and pain outcomes, although none of the interventions show benefit on all outcomes. For example, Oliver and colleagues (16) tested an individualized patient education and coaching intervention that lasted 20 minutes and included information on misconceptions, the World Health Organization pain control guidelines, goal setting, and strategizing to meet those goals. Results revealed that experimental subjects compared to controls had lower average pain severity but the groups did not differ in pain frequency, pain knowledge, or functional impairment. A brief tailored intervention, such as the one tested in the present study, is not analogous to these multi-faceted interventions with respect to focus of the intervention, content, length, and cost. The potential usefulness of a TBI is not as a replacement for other clinically-based interventions but rather as a relatively inexpensive way to intervene in one component of a multifaceted problem. Nonetheless, one should consider that the TBI for pain could be improved by providing the person with cancer with more concrete suggestions as to how to communicate with care providers, given the important role of clinician-related barriers to pain management. Also, based on Oliver’s findings (16), perhaps the TBI should be combined with coaching or communication skills enhancement.
The timing of our outcome measures may be a study limitation. Our selection of the 4-week time frame was based on the rationale that time frames of 2 to 6 weeks are typical in the literature, and it seemed reasonable based on past research to expect the TBI to have an impact in that amount of time. However, changes in pain outcomes require effective ongoing communication between patient and provider; it may be that 4 weeks was too short of a time frame for such communication to occur. A related limitation is that the study did not include measures of some variables that would have helped explain the causal chain from intervention to outcome. We did not have measures of coping that would address what patients actually did about pain management, including talking with clinicians, using higher doses of pain medications, or changing the timing and pattern of their medication use. Such measures were not included in this study because of the near impossibility of collecting valid and reliable data on these variables given the logistics of the study. All of these limitations could be subsumed under an umbrella limitation related to the relatively atheoretical nature of the work. The study was designed to test a simple pragmatic intervention rather than to break new ground with respect to theory regarding attitudes and health behavior. Finally, one should note that this was a motivated sample of subjects who had initiated a call to the CIS and who were relatively well educated. As in true in any convenience sampling situation, the subjects are not representative of the population of all persons with cancer.
For future research, the websites designed for this study could readily be modified to accommodate changes in the intervention protocol, including strengthening it by adding a component to address suggestions for communicating effectively with clinicians. Lengthening the follow-up time could also be accomplished.. Replications with less well educated samples would be useful as the generalizability of study findings is limited to the rather well educated CIS caller.
In conclusion, in this collaborative effort between university investigators, website developers, CIS leaders and staff, and staff from the Survey Research Shared Service at UW, recruitment, retention, and intervention fidelity were outstanding. A major innovation was the utilization of cost-effectiveness analysis of a tailored intervention designed to improve pain management. The data revealed that TBI had a beneficial impact on attitudes that are barriers to effective pain management, but further work needs to be done to strengthen the intervention so that it has an effect on pain severity, duration and interference.
Acknowledgement
Funding for this work was provided by NIH NCI CA 101907. The authors thank Jeff Crucius, B.S. of the University of Wisconsin-Madison Division of Information Technology for designing, developing, and maintaining the study websites. We also thank Kristine Kwekkeboom, PhD, Susan Heidrich, PhD, and Patrick McDonnell, B.A. for careful, thoughtful suggestions that improved the clarity of the manuscript.
Footnotes
* An annual review date at the KUMC site was missed and their IRB required that we drop the 80 participants who had been recruited while the approval was lapsed.
None of the investigators have financial arrangements that could represent a possible conflict of interest.
Contributor Information
Sandra E. Ward, University of Wisconsin-Madison.
Ko Kung Wang, University of Wisconsin-Madison.
Ronald C. Serlin, University of Wisconsin-Madison.
Shelly L. Peterson, University of Missouri-Kansas City.
Mary Ellen Murray, University of Wisconsin-Madison.
References
- [1].Cleeland C, Syrjala K. How to assess cancer pain. In: Turk D, Melzack R, editors. Handbook of Pain Assessment. Guilford Press; New York, NY: 1992. pp. 362–87. [Google Scholar]
- [2].Clotfelter C. The effect of an educational intervention on decreasing pain intensity in elderly people with cancer. Oncol Nurs Forum. 1999;26:27–33. [PubMed] [Google Scholar]
- [3].Crane L, Leakey T, Ehrsam G, Rimer B, Warnecke R. Effectiveness and cost-effectiveness of multiple outcalls to promote mammography among low-income women. Cancer Epi Bio Prevent. 2000;9:923–31. [PubMed] [Google Scholar]
- [4].De Wit R, van Dam F, Zandbelt L, vanBuuren A, van der Heijden K, Leenhouts G, Loonstra S. A pain education program for chronic cancer pain patients: follow up results from a randomized controlled trial. Pain. 1997;73:55–69. doi: 10.1016/s0304-3959(97)00070-5. [DOI] [PubMed] [Google Scholar]
- [5].DeVries H, Brug J. Computer-tailored interventions motivating people to adopt health promoting behaviors: introduction to a new approach. Patient Educ Couns. 1999;36:99–105. doi: 10.1016/s0738-3991(98)00127-x. [DOI] [PubMed] [Google Scholar]
- [6].Einhorn GW. Total quality pain management: a computerized quality assessment tool for postoperative pain management. Analgesia. 1994;5:10–13. [Google Scholar]
- [7].Ferrell BR, Grant M, Chan J, Ahn C, Ferrell BA. The impact of cancer pain education on family caregivers of elderly patients. Oncol Nurs Forum. 1995;22:1211–18. [PubMed] [Google Scholar]
- [8].Gordon DB, Pellino T, Erickson S, Whitman H, McConley R, Schroeder S. How much pain is too much? Examining pain intensity and its relation to other quality indicators of postoperative pain management; Poster presented at the 22nd Annual Scientific Meeting of the Midwest Pain Society; Chicago, IL. Apri1 17, 1998. [Google Scholar]
- [9].Heidrich S, Ward S, Julesberg K, Miller N, Donovan H, Gunnarsdottir S, Davis S, Hughes S, Serlin RC. Conducting intervention research through the Cancer Information Service. Oncol Nurs Forum. 2003;30:131–4. doi: 10.1188/03.ONF.131-134. [DOI] [PubMed] [Google Scholar]
- [10].Kim J, Dodd M, West C, Paul S, Facione N, Schumacher K, et al. The PRO-SELF pain control program improves patients’ knowledge of cancer pain management. Oncol Nurs Forum. 2004;31:1137–43. doi: 10.1188/04.ONF.1137-1143. [DOI] [PubMed] [Google Scholar]
- [11].Lauver D, Settersten L, Henriques J, Kane J. Tailored messages, external barriers, & women’s utilization of professional breast cancer screening over time. Cancer. 2003;97:2724–35. doi: 10.1002/cncr.11397. [DOI] [PubMed] [Google Scholar]
- [12].Marcus A, Heimendinger J, Wolfe Pl, Fairclough D, Rimer B, Morra M, Warnecke R, Himes J, Darrow S, Davis S, Julesburg K, Slevin-Perocchia R, Steelman M, Wooldridge J. A randomized trial of a brief intervention to increase fruit and vegetable intake: a replication study among callers to the CIS. Prev Med. 2001;33:204–16. doi: 10.1006/pmed.2001.0873. [DOI] [PubMed] [Google Scholar]
- [13].Miaskowski C, Cleary J, Burney R, Coyne P, Finley R, Foster R, Grossman S, Janjan N, Ray J, Syrajala K, Weisman S, Zahrbrook C. Guideline for the Management of CancerPain in Adults and Children. American Pain Society; Glenview, IL: 2005. APS Clinical Practice Guidelines Series, No. 3. [Google Scholar]
- [14].Miaskowski C, Dodd MJ, West C, Schumacher K, Paul S, Tripathy D, Koo P. Randomized clinical trial of the effectiveness of a self-care intervention to improve cancer pain management. J Clin Oncol. 2004;22:1713–20. doi: 10.1200/JCO.2004.06.140. [DOI] [PubMed] [Google Scholar]
- [15].Miaskowski C, Dodd MJ, West C, Paul S, Tripathy D, Koo P, Schumacher K. Lack of adherence with the analgesic regimen: a significant barrier to effective cancer pain management. J Clin Oncol. 2001;19:4275–79. doi: 10.1200/JCO.2001.19.23.4275. [DOI] [PubMed] [Google Scholar]
- [16].Oliver J, Kravitz R, Kaplan S, Meyers F. Individualized patient education and coaching to improve pain control among cancer outpatients. J Clin Oncol. 2001;19:2206–12. doi: 10.1200/JCO.2001.19.8.2206. [DOI] [PubMed] [Google Scholar]
- [17].Press WH, Flannery BP, Teukolsky SA, Vetterling WT. Numerical Recipes in Fortran 77: The Art of Scientific Computing. Cambridge University Press; Cambridge: 1992. [Google Scholar]
- [18].Rimer B, Levy M, Keintz M, Fox L, Engstrom P, McElwee N. Enhancing cancer pain control regimens through patient education. Patient Educ Couns. 1987;10:267–77. doi: 10.1016/0738-3991(87)90128-5. [DOI] [PubMed] [Google Scholar]
- [19].Ryan P, Lauver D. The efficacy of tailored interventions. J Nurs Scholarsh. 2002;34:4, 331–37. doi: 10.1111/j.1547-5069.2002.00331.x. [DOI] [PubMed] [Google Scholar]
- [20].Serlin RC, Mendoza T, Nakamura Y, Edwards K, Cleeland C. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–84. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- [21].Shaffer JP. Modified sequentially rejective multiple test procedures. J Am Stat Assoc. 1986;81:826–31. [Google Scholar]
- [22].Ward S, Donovan H, Gunnarsdottir S, Serlin R, Shapiro G, Hughes S. A representational intervention to decrease pain. Health Psych. 2008;27:59–67. doi: 10.1037/0278-6133.27.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ward S, Donovan H, Owen B, Grosen E, Serlin R. An individualized intervention to overcome patient-related barriers to pain management in women with gynecologic cancers. Res Nurs Health. 2000;23:393–405. doi: 10.1002/1098-240x(200010)23:5<393::aid-nur6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- [24].Ward S, Goldberg N, Miller-McCauley V, Mueller C, Nolan A, Pawlik-Plank D, Robbins A, Stormoen D, Wiessman D. Patient-related barriers to management of cancer pain. Pain. 1993;52:319–24. doi: 10.1016/0304-3959(93)90165-L. [DOI] [PubMed] [Google Scholar]
- [25].Williams-Hiehota P, Schneider TR, Pizarro J, Mowad L, Salovey P. Matching health messages to information-processing styles: need for cognition and mammography utilization. Health Commun. 2003;15:375–92. doi: 10.1207/S15327027HC1504_01. [DOI] [PubMed] [Google Scholar]