Abstract
Biologists have long recognized that dramatic bending of a cell sheet may be driven by even modest shrinking of the apical sides of cells. Cell shape changes and tissue movements like these are at the core of many of the morphogenetic movements that shape animal form during development, driving processes such as gastrulation, tube formation and neurulation. The mechanisms of such cell shape changes must integrate developmental patterning information in order to spatially and temporally control force production -- issues that touch on fundamental aspects of both cell and developmental biology and on birth defects research. How does developmental patterning regulate force-producing mechanisms, and what roles do such mechanisms play in development? Work on apical constriction from multiple systems including Drosophila, C. elegans, sea urchin, Xenopus, chick and mouse has begun to illuminate these issues. Here, we review this effort to explore the diversity of mechanisms of apical constriction, the diversity of roles that apical constriction plays in development, and the common themes that emerge from comparing systems.
Keywords: apical constriction, morphogenesis, patterning, cytoskeleton, forces
Introduction
Morphogenesis, the reorganization of cells and tissues into new forms, is an essential part of animal development. Cell and tissue reorganizations are driven by the forces that cells produce both internally and on neighboring cells. These forces are generally provided by the molecular motors that walk on intracellular polymers, the microfilaments and microtubules, or by polymerization and depolymerization of these polymers. How development controls these forces, to accomplish the morphogenetic movements that shape the final form of an animal, is a largely unanswered and yet central issue in developmental biology.
Biologists studying morphogenesis have recognized for over a hundred years that shrinking one side of a cell may result in a dramatic bending of a cell sheet. As early as 1902, Rhumbler proposed that constriction of the apical sides of cells may drive the bending of cell sheets in a variety of developmental systems (Fig. 1) (Rhumbler, 1902). Physical modeling in the 1940s tested the feasibility of this hypothesis, with an epithelial sheet modeled using brass bars and rubber bands (Lewis, 1947). Lewis' model demonstrated that increased tension on one side – produced by Lewis adding more rubber bands to one side of his model – could result in bending.
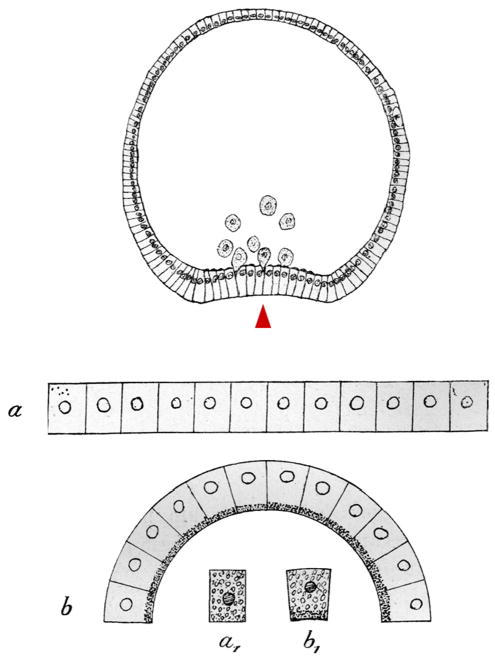
Fig. 1.
Rhumbler's 1902 drawings of cell shape changes driving morphogenesis. Top: A sea urchin embryo undergoing primary invagination. The vegetal-most part of the embryo bends inward (arrowhead). Bottom: “Theoretical gastrulation scheme, to show that invagination (b) of a cell plate (a) necessarily must take place if each cell changes from form a1 (due to higher pressure on the pigmented side) to the form b1. The invagination effect is significant even though the change in cell form from a1 to b1 is very small” (translation of figure legend in Rhumbler, 1902). We have inverted some parts of this figure to match the orientation of tissue bending between drawings.
Animals employ many distinct classes of morphogenetic movements. This review focuses on one class, apical constriction, or the active narrowing of cellular apices. Apical constriction occurs throughout the metazoa, and in many organisms, apical constriction first occurs at early stages of embryogenesis (Fig. 2). This makes apical constriction events valuable candidates for exploring the expected links between early patterning processes, such as cell fate specification or apico-basal cell polarization, and the mechanisms that produce force. Indeed, apical constriction is central to some key cases where we already understand at least an outline of the links between cell fate specification and the forces that drive morphogenesis, such as gastrulation in Caenorhabditis elegans and Drosophila melanogaster and vertebrate neural tube formation (Fig. 3) (Chung and Andrew, 2008; Rohrschneider and Nance, 2009). Apical constriction also may underlie some of the other classes of morphogenetic movements, for example epithelial-to-mesenchymal transition and ingression (Keller and Davidson, 2004).
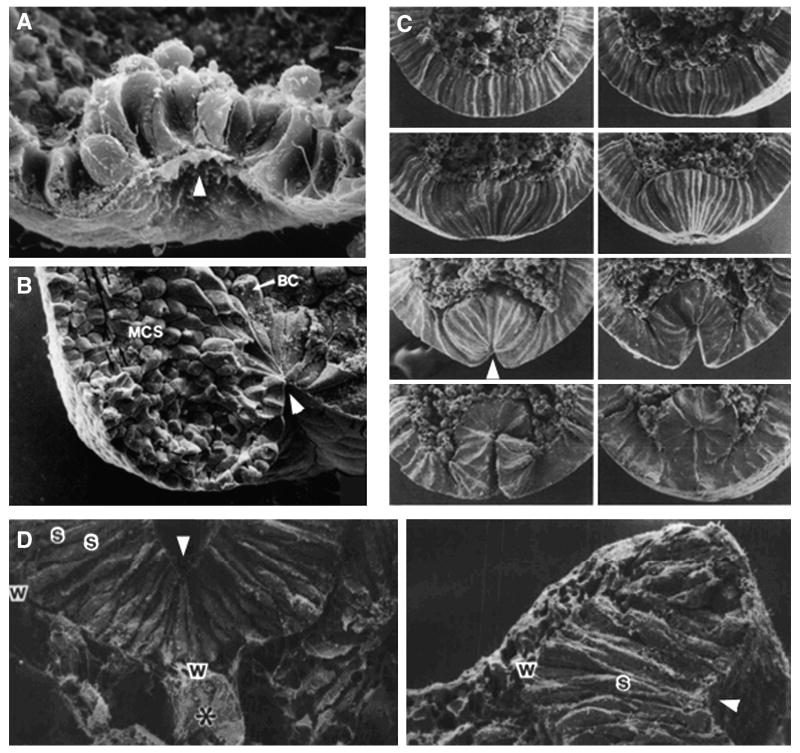
Fig. 2.
Scanning electron micrographs of apically constricting cells in diverse systems. A) Sea urchin vegetal plate (Kimberly and Hardin, 1998), B) X. laevis midsagittal section at early gastrula showing bottle cells (BC) and involuted mesodermal cell stream (MCS) (Keller, 1981) C) Drosophila ventral furrow formation (Sweeton et al., 1991), D) Chick neuroepithelial medial and dorsal lateral hinge points. Scanning electron micrographs of transverse slices through the medial (left) and dorsal lateral (right) hinge points at the future hindbrain level; asterisk, notochord; w, s, wedge- and spindle-shaped cells, respectively (Schoenwolf and Smith 1990). Arrowheads mark bends in epithelia at proposed sites of apical constriction.
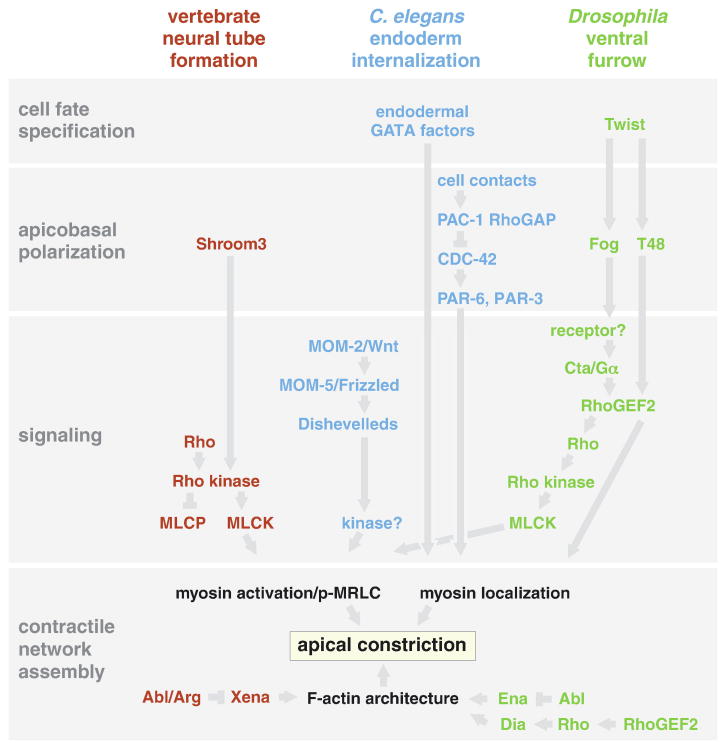
Fig. 3.
Some of the known genetic pathways by which cell fate and cell polarity regulate apical constriction, in three selected systems (Lee et al., 2004; Lecuit, and Lenne, 2007; Lee et al., 2007; Anderson et al., 2008; Chung and Andrew, 2008, and references in text). For simplicity, some other mechanisms for apical constriction are not diagrammed, and important links between contractile actomyosin networks and cell-cell adhesion proteins are omitted here.
How then does apical constriction occur in cells? The most prevalent hypothesis is that apical constriction is driven by contraction of an apical meshwork of filamentous actin (F-actin) by the molecular motor myosin, but other mechanisms are plausible. Morphogenesis has been hypothesized to depend on redundant mechanisms to a large degree (Wieschaus, 1995). Do embryos use multiple, redundant mechanisms to drive apical constriction? Are there general rules by which apical constriction is regulated in diverse animal systems? What mechanical contexts are required for mechanisms to work, and how are these mechanical contexts established? We are also interested in the roles that apical constriction can play in development. Are common processes driven by apical constriction in diverse organisms? Are these processes conserved from ancestral animals of the past to groups of modern animals? These questions have additional importance because human neural tube closure depends on apical constriction, and improper neural tube closure is one of the most common human birth defects (Sadler, 2005). We focus on these issues as we review some historical and well-studied examples of apical constriction.
Sea urchin gastrulation: multiple mechanisms may drive tissue bending
Perhaps surprisingly, given the large repertoire of classes of morphogenetic movements available to embryos, many organisms have evolved a role for apical constriction in gastrulation (Stern, 2004). In gastrulating sea urchin embryos, cells on the vegetal surface of the embryo become columnar, forming the vegetal plate. The surface of this plate bends inward, a process termed primary invagination (Figs. 1 and 2). Primary invagination is accompanied by a number of other movements; here we discuss only the primary invagination, which has been proposed to be driven by apical constriction (for review see Davidson, 1995; Kominami and Takata, 2004).
The cells that undergo primary invagination form the archenteron, or future gut. Computer modeling suggests that apical constriction of cells in the vegetal plate could feasibly drive primary invagination, so long as the extracellular matrix can be deformed easily -- about as easily as the cells can be deformed (Davidson, 1995). In principle then, changes of individual cell shapes can drive tissue bending, although other mechanisms for bending a cell sheet are possible (Davidson, 1995). Forces generated within the vegetal plate are sufficient to drive tissue bending, as invagination can occur normally in a dissected vegetal plate (Moore and Burt, 1939; Ettensohn, 1984). The cells proposed to undergo apical constriction have bands of actin microfilaments associated with apical adherens junctions and also spanning across the inside of each cell's apical surface, as might be expected in cells undergoing apical constriction. But microfilaments are also enriched apically in cells that do not undergo such shape changes. Hence the presence of such an apical microfilament network does not necessarily indicate that it will bend a cell sheet (Ettensohn, 1984).
In certain species of sea urchin, a ring of cells along the edges of the vegetal plate has been recognized to undergo more pronounced apical constriction, as judged by scanning electron micrographs (Nakajima and Burke, 1996, Kimberly and Hardin, 1998, Fig. 2). Cells in this ring have been referred to as bottle cells, a term coined by Ruffini (1907) for amphibian embryonic cells that are shaped like bottles, with dramatically constricted apical sides and enlarged basolateral areas. Bottle cells in sea urchin embryos have a greater enrichment of apical arrays of F-actin than do other cells in the vegetal plate (Nakajima and Burke, 1996). Laser ablation of bottle cells interferes with normal invagination, whereas laser ablation of neighboring cells does not (Kimberly and Hardin, 1998), consistent with the notion that apical constriction may drive primary invagination. RhoA is required for the initiation of primary invagination (Beane et al., 2006), as it is for apical constriction and resulting tissue bending in other systems discussed below. How are specific cells driven to apically constrict during primary invagination? This is not yet clear, although calcium signaling (Nakajima and Burke, 1996), Wnt/Frizzled signaling (Croce et al., 2006), a transcriptional gene regulatory network (Davidson et al., 2002; Wu et al., 2008), and FGF signaling (Röttinger et al., 2008) have all been implicated in regulating primary invagination. The links between these regulators and RhoA activity have yet to be explored.
One key result is at odds with the model that actomyosin-dependent apical constriction is the key driver of primary invagination: cytochalasin treatment, which should depolymerize F-actin networks, fails to fully disrupt primary invagination in sea urchins (Lane et al., 1993). This result suggests the possibility that other mechanisms may provide force, either alone or redundantly with actin-based mechanisms. Interestingly, among the mechanisms proposed to drive apical constriction and tissue bending in sea urchins during primary invagination is one in which vegetal plate cells secrete extracellular matrix components into a multi-layered structure, in a calcium regulated manner (Lane et al., 1993). In this model, later-deposited matrix, secreted into a layer between the cells and the earlier layers of matrix, swells as it hydrates, driving bending of the matrix andh ence the attached epithelial sheet. This is similar to the way in which the thermal expansion of a layer of metal in a thermostat's bimetallic strip can bend the entire strip. In Lane et al.'s model, the proposed source of force is extracellular, driving cell shape changes by bending of the matrix, rather than mediated by intrinsic cell shape changes, an interesting departure from traditional models. As an experimental model, sea urchin primary invagination leaves a variety of possible mechanisms for tissue shape change and some valuable tools for dissecting the contributions to forces made by each.
Bottle cells in Xenopus gastrulation: roles for microfilaments and microtubules
The amphibian archenteron also includes bottle cells at the site where invagination begins (Holtfreter, 1943). Early embryologists believed that amphibian bottle cells functioned in gastrulation because of the cells' unique shapes (Fig. 2). Rhumbler (1902) suggested the possibility that these cells were actively migrating toward the interior of the embryo. Experiments by Holtfreter were consistent with this hypothesis, as isolated bottle cells could stretch in a polarized manner on a glass substrate, similar to migrating cells (Holtfreter, 1944). While no live imaging evidence exists for the active migration of these bottle cells in vivo, vital dye tracings demonstrate that these cells do migrate to the interior of the embryo in Ambystoma mexicanum (Lundmark, 1986). In addition, cell tracing experiments in which labeled bottle cells from Xenopus laevis were grafted into unlabeled host embryos demonstrate that bottle cells spread out and form the anterior of the archenteron (Hardin and Keller, 1988).
Xenopus laevis bottle cells (Fig. 4) are a potentially valuable model for studying mechanisms of cell shape change in morphogenesis, as the cells are large and readily treated with inhibitors. These cells can be manipulated in culture much as sea urchin cells can be, and the potential exists to identify key molecular players using genetic screens in the model frog Xenopus tropicalis. Blastopore initiation begins and proceeds on schedule in explants that include the bottle cells (Hardin and Keller, 1988; Lee and Harland, 2007). When bottle cells are removed from X. laevis embryos, a truncated archenteron still forms, and involution of the mesoderm cells still occurs, but archenteron length is compromised (Keller, 1981). Therefore, bottle cells appear to initiate blastopore formation and to contribute to the full extension of the archenteron in X. laevis.
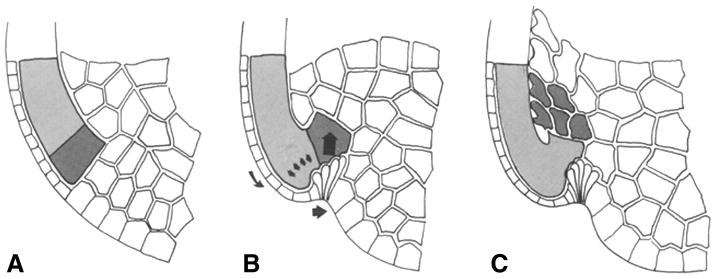
Fig. 4.
Schematic diagrams of bottle cell formation. All images approximate midsagittal views. A) Prior to gastrulation, the prospective anterior mesoderm (darker shading) and posterior mesoderm (lighter shading) comprise the deep marginal zone. B) The bottle cells have undergone apical constriction. Arrows indicate movements hypothesized to result. C) This causes reorientation of the vegetal edge of the marginal zone (anterior mesoderm) such that it is now leading the movement into the blastocoel (Hardin and Keller, 1988).
A number of distinct mechanisms control cell shape in X. laevis bottle cells. In vivo, the apical surfaces of these cells shrink while the apicobasal sides lengthen. Isolated, cultured bottle cells contract uniformly around the entire cell surface, suggesting that contraction is an intrinsic behavior but that the apicobasal elongation seen in vivo depends on contact with surrounding cells (Hardin and Keller, 1988). This likely reflects a cellular mechanism that distinguishes the basolateral and apical sides of bottle cells, or surfaces contacting other cells and free surfaces, perhaps similar to a mechanism that has been outlined in C. elegans, discussed below. F-actin and activated myosin accumulate at the apical surfaces of bottle cells just before the apical surfaces narrow, consistent with a role for F-actin and myosin in apical constriction (Lee and Harland, 2007). Furthermore, pharmacological inhibitors of F-actin or myosin demonstrate that they are both required for bottle cell formation. Interestingly, treatment with a microtuble depolymerizing drug, nocodazole, prevents full apical constriction of bottle cells and invagination without affecting apicobasal cell lengthening, and without apparent effects on F-actin or activated myosin distribution (Lee and Harland, 2007). This result suggests that microtubules may have an as yet undefined role in apical constriction in Xenopus bottle cells.
C. elegans gastrulation: cell manipulations and genetics meet to identify key regulators
Unlike gastrulation in sea urchins or Xenopus, where entire cell sheets are internalized, gastrulation in C. elegans involves the internalization of many cells or groups of cells at distinct times. C. elegans gastrulation begins at the 26-cell stage when two endodermal precursor cells move from the perimeter to the inside of the embryo (Fig. 5). This event is followed later by internalization of mesoderm and germline precursors (Sulston et al., 1983; Nance and Priess, 2002). Internalization of the endodermal precursors has been most thoroughly studied and is the focus of our discussion here.
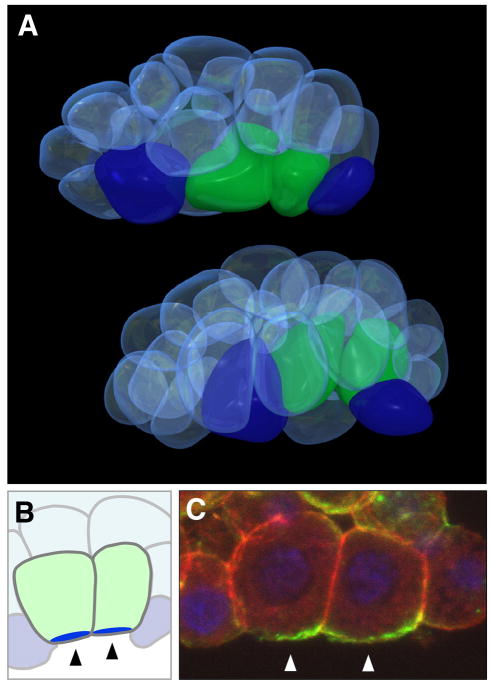
Fig. 5.
C. elegans gastrulation. A) Illustrations of embryos just before (top) and during (bottom) endodermal internalization. Green, endodermal progenitors. Two neighboring cells are marked in purple. Renderings by J. Iwasa based on confocal sections of phalloidin-stained embryos (Lee et al., 2006). B) Diagram showing where apical constriction occurs (arrowheads). C) Myosin is activated in the apical cortex of the internalizing cells. Phospho-regulatory myosin light chain staining is in green (Lee et al., 2006).
Cell movements associated with C. elegans gastrulation can occur in vitro, allowing mechanisms to be explored by cell manipulation experiments as in sea urchins and Xenopus (Lee and Goldstein, 2003). One revealing finding from such studies is that very few cells are required for the movements of C. elegans gastrulation to occur: even a line of embryonic cells in culture arranged in single file will fold at the time of gastrulation (Lee and Goldstein, 2003). This makes clear that mechanisms requiring large numbers of cells to work in concert, such as multicellular purse string mechanisms, are not essential for cell movements in C. elegans gastrulation. Some of the strengths of this system lie in the ability to combine such manipulations with live cell microscopy and genetics, and to study mechanisms of morphogenesis at the level of individual cells, in a developmental system where spatial patterning is so thoroughly studied.
Apical constriction plays a key role in C. elegans gastrulation. Just before endodermal precursor cells internalize, the cell surface that faces the perimeter of the embryo on each of these cells (the apical surface) flattens, and myosin II becomes enriched at this surface (Nance and Priess, 2002). Although the apical surfaces become smaller until they disappear at the time of cell internalization, these cells do not become noticeably bottle-shaped. Contraction of apical cell surfaces was revealed by tracking the movements of fluorescent, microscopic beads placed on the surfaces of the endodermal precursor cells (Lee and Goldstein, 2003). The observed surface movements exclude the possibility that shrinking of the apical surface reflects only a flow of apical surface to lateral positions -- a possibility that is difficult to exclude in many systems. Myosin has been implicated in driving apical constriction because pharmacological inhibitors of myosin activity prevent the endodermal precursors from internalizing (Lee and Goldstein, 2003). In addition, apical myosin becomes activated near the time that gastrulation begins: apically-localized myosin regulatory light chain is phosphorylated at a residue that in other systems unkinks myosin heavy chains, allowing myosin complexes to bundle into bipolar filaments, which can bind to and walk on actin filaments (Lee et al., 2006; Somlyo and Somlyo, 2003). These results suggest that local activation of myosin shrinks the apical actin mesh. Actin architecture is likely to be important as well. Indeed, the Arp2/3 actin-nucleating complex has been reported to localize to the cell cortex in gastrulating embryos, and depletion of this complex results in failure of endodermal precursor cells to internalize on schedule (Severson et al. 2002, Roh-Johnson and Goldstein, in press).
Do neighboring cells contribute to internalization of the endoderm precursors? When neighboring cells were removed and reassociated with endodermal precursor cells in various orientations, the neighboring cells still moved in a direction consistent with the hypothesis that apical constriction in endodermal precursors drives the movement of the neighboring cells, suggesting that neighboring cell polarity is not important for the bulk of their movement (Lee and Goldstein, 2003). However, short, actin-rich extensions form on three of the six neighboring cells of the ring that closes beneath the endoderm precursors, and Arp2/3-depleted embryos that fail to gastrulate also fail to produce these extensions, raising the possibility that the extensions might contribute to completion of endodermal internalization in vivo (Nance and Priess, 2002; Roh-Johnson and Goldstein, in press).
C. elegans genetics has identified multiple regulatory inputs that are important for gastrulation, including inputs that specify which cells should enrich myosin to one side, inputs that specify to which side of a cell this enrichment should occur, as well as a signaling input that directs activation of myosin. Cell fate specification genes including genes encoding endodermal GATA factors are necessary for early cell internalization, and embryos with ectopically specified endoderm have ectopic early cell internalization, suggesting that endoderm fate is both necessary and sufficient for early cell internalization (Lee et al., 2006). One aspect of endodermal cell fate is a gap phase uniquely introduced to the cell cycle of endodermal progenitors one cell cycle after the endoderm precursor cell is born, which is near the time of cell internalization (Sulston, 1983; Edgar and McGhee, 1988). This pause is required for internalization, possibly because it delays a reorganization of the actomyosin cytoskeleton that normally accompanies cell division (Lee et al., 2006; Oegema and Hyman, 2006).
For the endodermal precursor cells to accumulate myosin near their apical surfaces, an apical surface must be established. PAR proteins function in anteroposterior polarization of the embryo first, and are known to become apicobasally polarized later, starting at the four cell stage (see Goldstein and Macara 2007 for review). To test whether PAR proteins function in apicobasal polarization, Nance and colleagues devised a clever method for degrading the polarity proteins PAR-3 or PAR-6 specifically in somatic cells, adding a motif from another protein that becomes degraded in somatic cells. They demonstrated in this way that PAR-3 and PAR-6 are required for apical flattening, apical myosin enrichment, and timely cell internalization (Nance et al., 2003). Elegant cell manipulation experiments revealed that these PAR proteins' localization depends on where cells contact each other: only contact-free membranes accumulate apical PAR proteins, establishing an apical domain at the contact-free surface (Nance et al., 2003). Myosin later accumulates at apical domains, and this is dependent on apical PAR proteins (Nance et al., 2003). Once myosin becomes enriched apically, it becomes activated downstream of a Wnt-Frizzled-Dishevelled signaling pathway that causes regulatory light chain phosphorylation, through an unidentified kinase (Lee et al., 2006, Fig. 3).
These results paint the outlines of a potentially generalizable mechanism for cell internalization by apical constriction: Among cells that polarize PAR proteins apicobasally, the cells with the right cell fate specification machinery enrich myosin where the apical PAR proteins become localized -- at contact-free surfaces. Activation of myosin can then result in shrinking the myosin-enriched, contact-free surfaces of any such cells, pulling neighboring cells across the free surfaces and, as a result, displacing the apically constricting cells toward the interior. The ability to shrink any exterior surface of specific cells could, in theory, make it possible for a cell to internalize regardless of which specific surfaces initially contact other cells.
How then do certain PAR proteins become enriched only apically in response to cell contacts? Anderson et al. (2008) screened for genes required for cell contact-dependent PAR protein localization and identified a key intermediate, a RhoGAP domain-containing protein, PAC-1. PAC-1 localizes to the cell cortex at cell-cell contact zones, where it has been proposed to inactivate CDC-42 at these zones, potentially restricting the active form of CDC-42 to contact-free cell surfaces. Active CDC-42 interacts with a semi-CRIB domain in PAR-6, and through this interaction is thought to establish apical localization of PAR-6 and PAR-6 complex members in these cells. PAC-1 localization to contact zones is therefore the earliest known step in recognizing contact zones as unique, spatial information that is critical to PAR protein and myosin localization. How PAC-1 becomes localized to contact zones is an interesting topic for future study.
Drosophila melanogaster gastrulation: links from cell fate to the cytoskeletal machinery that provides force
The initiation of morphogenesis in fruit flies begins with the internalization of the future mesoderm at the ventral furrow, forming a tube in the interior of the embryo (Leptin and Grunewald 1990). Ventral furrow formation is perhaps the most well studied example of apical constriction, and the cellular shape changes that occur have been thoroughly described. First, a stripe of cells 18 cells wide and 60 cells long, spanning most of the embryo's ventral midline (6% to 86% egg length), begins to apically flatten. Within this stripe, after flattening, cells of the midventral domain, 12 cells in width, begin to reduce the diameter of their apical surfaces. As these ventral midline cells apically constrict, small blebs form on the apical membrane surfaces, possibly aiding in the reduction of apical surface area (Turner and Mahowald 1977; Costa et al., 1994). Other rearrangements can be seen as ventral midline cells' apical surfaces begin to shrink. For instance, cytoplasm and the nuclei of the midline cells shift basally (Leptin and Grunewald, 1990). The cells also elongate along their apicobasal axes up to 1.7 times their original lengths, and then expand their basal surfaces (Leptin and Grunewald, 1990; Sweeton et al., 1991). After reaching their maximum lengths, the ventral furrow cells begin to shorten back to their original length, while remaining constricted apically Sweeton et al., 1991). Shortening of each cell results in a wedge shape that may help to move the ventral furrow beneath the epidermis (Costa et al., 1993). Completing the process, the lateral epidermis covers the tube of mesoderm, pinching it off from the overlying ectoderm (Fig. 6) (Poulson, 1950; Sonnenblick, 1950; Leptin and Grunewald, 1990).
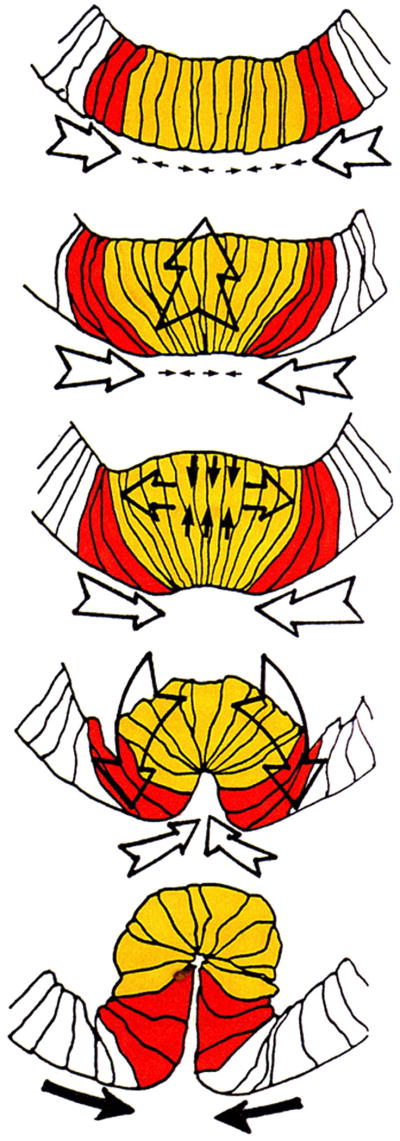
Fig. 6.
Forces driving Drosophila ventral furrow invagination. Tracings of transversely fractured scanning electron micrographs. Cells that apically constrict are colored yellow. Adjacent cells in the ventral plate that develop flattened apical surfaces but do not constrict apically are colored red-orange. Small arrows outside or within cells represent the presumed vectors of forces within these cells as a result of apical constriction or cell elongation or shortening. Larger arrows indicate cumulative forces predicted from the combined forces of individual cells (Costa et al., 1993).
The power of Drosophila as a genetic model system is illustrated by a pathway that spans from cell fate specification to the force-producing mechanisms that drive apical constriction in gastrulation (Fig. 3). Determination of mesodermal fate is governed by the maternal transcription factor Dorsal, which activates ventral expression of the zygotic transcription factors Snail and Twist (Simpson 1983; Nusslein-Volhard et al., 1984; Thisse et al., 1987). Loss of Snail and Twist prevents furrow invagination (Leptin and Grunewald, 1991; Sweeton et al., 1991) and expands lateral cell fates toward the ventral midline (Costa et al., 1993). No targets of Snail that function in ventral furrow formation have been identified thus far, but targets of Twist with specific roles in cell shape changes have been identified. Loss of the Twist target Folded Gastrulation (Fog), a secreted protein, leads to uncoordinated constriction during gastrulation, in which cell shape changes are initiated in many cells at the correct time, but some cells fail to undergo apical constriction (Sweeton et al., 1991; Costa et al., 1994). Loss of the Gα protein Concertina (Cta) results in a similar phenotype, and Cta acts genetically downstream of Fog (Morize et al., 1998). Presumably, there is a G-coupled receptor that links Fog signaling to Cta; to date, it remains unknown. Interestingly, the secretion of Fog protein is apically polarized, and Fog is both necessary and sufficient to target myosin to the apical side of the cell (Dawes-Hoang et al., 2005). A second downstream target of Twist, the transmembrane protein T48, functions in parallel to Fog-Cta signaling (Kolsch et al., 2007). Loss of both Cta and T48, each of which have weak gastrulation phenotypes, i.e. uncoordinated apical constriction, results in failure to make a furrow, suggesting that these two pathways act partially redundantly (Kolsch et al., 2007).
How do these proteins result in force generation? Both the Fog-Cta and T48 pathways converge on the localization of RhoGEF2, a regulator of the Rho family GTPases (Barrett et al., 1997; Rogers et al., 2004; Kolsch et al., 2007). In RhoGEF2 mutants, apical constriction does not occur and the ventral furrow never forms, as in the Cta and T48 double mutants (Barrett et al., 1997; Hacker and Perrimon, 1998, Kolsch et al., 2007). Both T48, which directly binds RhoGEF, and Cta mildly affect the localization of RhoGEF2, but if both proteins are absent, RhoGEF2 does not become apically localized (Kolsch et al., 2007). Disruption of RhoGEF2 in ventral furrow cells disrupts myosin accumulation and localization, and the cells are unable to constrict (Nikolaidou and Barrett, 2004). A similar, albeit not as dramatic, myosin mislocalization phenotype has been observed in cta mutants (Nikolaidou and Barrett, 2004; Fox and Peifer, 2007). RhoGEF2 most likely functions through activation of Rho1, since dominant-negative Rho1 results in ventral furrow defects (Barrett et al., 1997). Both the myosin II regulatory light chain Spaghetti Squash (Sqh) and the myosin II heavy chain Zipper (Zip) become relocalized from the basal side of the cell to the apical side, along with RhoGEF2, which fly cell culture has demonstrated to be bound to microtubule tips via EB1 (Rogers et al., 2004; Nikolaidou and Barrett, 2004). Interestingly, activated Cta is also required to unload RhoGEF2 from microtubule tips to the plasma membrane (Rogers et al., 2004), by an unknown mechanism. Together, these findings build a pathway that links cell fate through signaling components to cytoskeletal regulators (Fig. 3).
If myosin activation and localization is key, then a specific F-actin organization would be predicted to be important as well. Early work proposed that the apical F-actin cytoskeleton was required for internalization of the ventral furrow cells (Young et al., 1991). Further work has explored just how the actomyosin meshwork must be organized and dynamically regulated for apical constriction to occur. Abelson (Abl), a non-receptor tyrosine kinase, is required for the correct localization of F-actin within the ventral furrow cells (Fox and Peifer, 2007). abl mutants also have uncoordinated apical constriction at the ventral furrow, like fog and cta mutants. cta mutants do not have mislocalized F-actin, but RhoGEF2 mutants do, suggesting that Abl functions in parallel to RhoGEF2 in regulation of F-actin localization. Abl targets F-actin organization through a known target, the actin regulator Enabled (Ena). Within the ventral furrow cells, Abl regulates Ena localization, restricting it from the apical end (Fox and Peifer, 2007). One other actin regulator implicated in ventral furrow formation is the formin protein Diaphanous (Dia), which along with Rho kinases is a Rho effector, suggesting that actin regulation and myosin regulation might be coordinated by multiple Rho effectors upon Rho activation (Homem and Peifer, 2008). RhoGEF2 and Dia are also necessary during cellularization for the correct assembly of actin filaments that are required for the proper infolding of the plasma membrane (Grosshans et al., 2005).
The actomyosin network of each ventral furrow cell spans beneath the entire apical surface of the cell and also forms circumferential belts at the apical boundaries with neighboring cells, at adherens junctions (Costa et al., 1993; Martin et al., 2008). Martin et al. have proposed that apical constriction is driven by pulsed coalescences of the actomyosin meshwork across the entire apical surface, rather than being driven by the circumferential belts of actin (Martin et al., 2008). Each pulse of actomyosin coalescence appears to shrink the apical surface, and, in general, each coalescence does not retreat, suggesting the existence of a ratchet-like mechanism limiting expansion of the apical cytoskeleton after each constriction. Interestingly, differential roles for the mesoderm specification proteins Snail and Twist were found: Snail promotes contraction, whereas Twist is necessary to prevent relaxation after each contraction, suggesting that the proposed ratchet involves one or more Twist targets (Martin et al., 2008). Interestingly, invagination of the furrow can be rescued in snail mutants by mechanical deformation of the mesodermal cells, as long as the Twist target Fog is still present (Pouille et al., 2009). This is consistent with the hypothesis that Snail's role is in producing mechanical deformation, or contraction, and that Twist-dependent ratcheting is important to maintain contracted states. Mechanical deformation can induce Twist expression, suggesting an intriguing feedback loop between gene expression and deformation of cells that may serve to coordinate cells and increase the robustness of the system (Farge 2003; Desprat et al., 2008). Such feedback between gene expression and deformation of cells has not been explored similarly in other systems for apical constriction, to our knowledge.
These coordinated cell shape changes occur within a tissue in which cells are mechanically coupled. The actomyosin coalescences that ratchet the apical surfaces together are attached to adherens junctions at discrete sites. As each cell pulls the plasma membrane inward, connected to its neighbors, the result is the coordinated apical constriction across the epithelial sheet (Martin et al., 2008). In fact, if the adherens junctions are disrupted, myosin II coalesces into a ball detached from the cell contact zones, in the apex of each cell, and cells fail to change shape (Dawes-Hoang et al., 2005). Therefore, the adherens junctions provide mechanical links between the apical actomyosin network and the plasma membranes at cell contacts. Interestingly, apical localization of adherens junction components depends on Bazooka, a homolog of C. elegans PAR-3. PAR-3 functions in C. elegans gastrulation as well, but in apical enrichment of myosin rather than of adherens junction complex members, suggesting that similar apicobasal cell polarity inputs can function differently in the two systems (Müller and Wieschaus, 1996; Nance et al., 2003; Harris and Peifer, 2004).
Although the F-actin meshwork is connected to the adherens junctions, this connection is not thought to be a direct link from F-actin to the adherens junction proteins alpha catenin, beta catenin, and cadherin (Weis and Nelson, 2005). Instead, other adhesion proteins may provide a mechanical link to the adherens junction complex. For instance in fly gastrulation, the afadin homolog Canoe, a scaffolding protein, aids in connecting the adherens junction to F-actin (Sawyer et al., 2009). The GTPase Rap1 regulates this interaction, and in both Rap1 and Canoe mutants, actomyosin coalesces into apical balls (Sawyer et al., 2009).
The internalization of the endoderm of the posterior midgut is also completed by an apical actomyosin constriction. At the posterior pole, a population of cells under and near the pole cells forms a cup-shaped invagination (Costa et al., 1993). Similar to ventral furrow formation, formation of the posterior midgut invagination begins with apical flattening (Sweeton et al., 1991). As the apices constrict, the nuclei move basally, and the cells increase their apicobasal lengths and expand their basal widths (Turner and Mahowald, 1977; Sweeton et al., 1991; Costa et al., 1993). Many of the proteins used in ventral furrow formation also drive posterior midgut invagination, but there are some interesting differences that demonstrate that the redundancy of mechanisms can vary between tissues. In the ventral furrow, mutations in Fog and Cta only partially disrupt invagination, whereas in posterior midgut invagination, loss of either of these two proteins completely prevents invagination (Parks and Wieschaus, 1991; Sweeton et al., 1991). RhoGEF2 is also required for posterior midgut invagination, again most likely via Rho1 (Barrett et al., 1997), and cytoplasmic myosin is localized apically in posterior midgut cells (Young et al., 1991). Unlike ventral furrow formation, two other morphogenetic movements besides apical constriction contribute to posterior midgut invagination: dorsal retraction and germband elongation (Costa et al., 1993). Also, Canoe does not seem to be essential for posterior midgut invagination, though Rap1 may still play a role (Sawyer et al., 2009). The model developed for Drosophila gastrulation, in both ventral furrow formation and posterior midgut invagination, has close parallels in other morphogenetic events in Drosophila, as discussed below.
Drosophila eye morphogenetic furrow: a traveling wave of cell shape changes
Patterning of the Drosophila eye is accompanied by a wave of apical constriction (Fig. 7) that passes across a sheet of epithelial cells, the eye imaginal disc. This wave is driven by a wave of cell-cell signaling events. In the posterior margin of the eye imaginal disc, some of the epithelial cells differentiate as photoreceptor neurons. Once differentiated, these cells secrete a Hedgehog ligand, which activates a signaling pathway in the anterior neighboring cells (Heberlein et al., 1993). Responding to this pathway, the latter cells enter cell cycle arrest, followed by apical constriction, resulting in the formation of a dorso-ventral groove known as the morphogenetic furrow (Ready et al., 1976; Tomlinson, 1985). Most of the cells in the furrow soon re-enter the cell cycle, relax, and resurface. Some of the cells undergo cell shape changes and differentiate, becoming the next group of photoreceptors. These newly differentiated cells then induce a new wave of Hedgehog-dependent furrow induction, received by the next row of anterior epithelial neighbors. Thus, over the course of a few hours, there is a wave of morphogenetic furrow progression from posterior to anterior, followed by a synchronized process of neuronal differentiation (Ready et al., 1976).
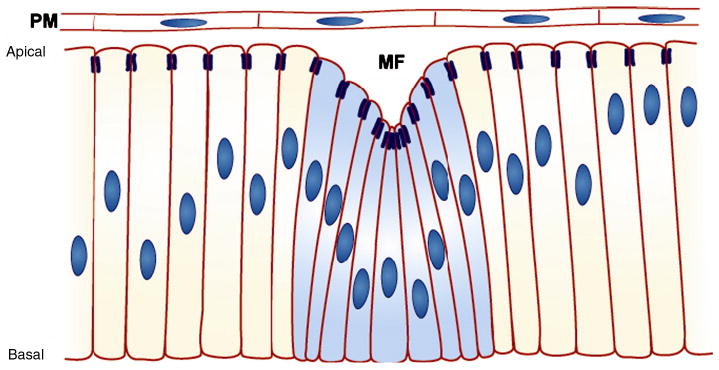
Fig. 7.
Apicobasal shortening of cells within the morphogenetic furrow. Schematic of a cross section through the eye imaginal disc. Columnar cells of the eye imaginal disc epithelium are apically constricted and shorter within the morphogenetic furrow (MF, blue cells). Dark blue lines indicate zonula adherens. A layer of squamous cells, the peripodial membrane (PM), overlies the columnar cells (Schlichting and Dahmann, 2008).
Morphogenetic furrow progression in the eye imaginal disc probably contributes to the planar polarity of the disc epithelium (Chanut and Heberlein, 1995), but it is not completely clear whether apical constriction is needed for cells to properly differentiate. One hypothesis yet to be tested is that apical constriction may result in accumulation of receptors, such as Notch or EGFR, on the apical membrane, allowing cell communication and successive rounds of differentiation to take place (Wolff and Ready, 1991).
Apical constriction in the eye furrow involves some of the same proteins used by ventral furrow cells to achieve apical constriction during gastrulation. F-actin and myosin II accumulate at the apical cortex, and activation of myosin II allows the actomyosin apical network to contract, promoting constriction. Myosin activation is mediated through phosphorylation of its regulatory light chains by a Rho-dependent kinase (ROK) and negatively regulated by a myosin regulatory light chain phosphatase (Lee and Treisman, 2004; Corrigall et al., 2007; Escudero et al., 2007). Although ROK activates myosin II, there are likely to be other inputs to myosin activation, as phosphomimetic myosin but not constitutively active ROK is sufficient for formation of ectopic morphogenetic furrows (Corrigall et al., 2007; Escudero et al., 2007). Microtubules also play a role here, in normal actin organization: apical accumulation of the actomyosin network is accompained by apical accumulation of stabilized microtubules, and severing of microtubules by expression of the severing protein Spastin results in failure of the cells to apically constrict (Corrigall et al., 2007).
One significant difference between the eye morphogenetic furrow and the ventral furrow lies in the regulation of the actomyosin network. Unlike the ventral furrow, genetic evidence suggests that there is no role for RhoGEF2 in regulating Rho1 to promote apical constriction in the eye morphogenetic furrow (Corrigall et al., 2007). In addition, there is no genetic evidence to date for regulation of F-actin reorganization by Abl or Ena, which function in ventral furrow formation (Corrigall et al., 2007; Fox and Peifer, 2007). There is at least one actin regulator that functions in both systems: the formin Diaphanous (Dia) is needed for apical accumulation of F-actin and myosin and for apical constriction to take place in both the eye morphogenetic furrow and the ventral furrow (Grosshans et al., 2005; Corrigall et al., 2007; Homem and Peifer, 2008). The stories diverge significantly when it comes to the molecular pathways that govern cell fates, determining which cells will apically constrict. Unlike mesodermal cells in the ventral furrow, Twist and Snail do not have a role in eye patterning. Instead, Hedgehog signaling acts with the BMP homolog Decapentaplegic (Dpp), regulating microtubule stabilization, F-actin apical accumulation, myosin regulatory light chain phosphorylation, and Cad86C expression, which in concert lead to apical constriction and formation of the morphogenetic furrow (Corrigall et al., 2007; Schlichting and Dahmann, 2008; Vrailas and Moses, 2006; Escudero et al., 2007). Hence, different fate regulators and intracellular signals can function upstream of common cytoskeletal players to drive apical constriction in different tissues.
Drosophila trachea and salivary glands and the chick inner ear: formation of tubes and vesicles
Tube formation is another morphogenetic process that involves apical constriction. Two well-studied cases, the tracheal tubes and the salivary glands in Drosophila, give us some insights into the cellular and molecular mechanisms that govern apical constriction during tube formation. Tube formation in both cases starts with cells at the embryonic surface apically constricting and invaginating (Fig. 8). Further branching and elongation by cell migration and convergent extension results in tubular structures with diverse functions (Myat, 2005).
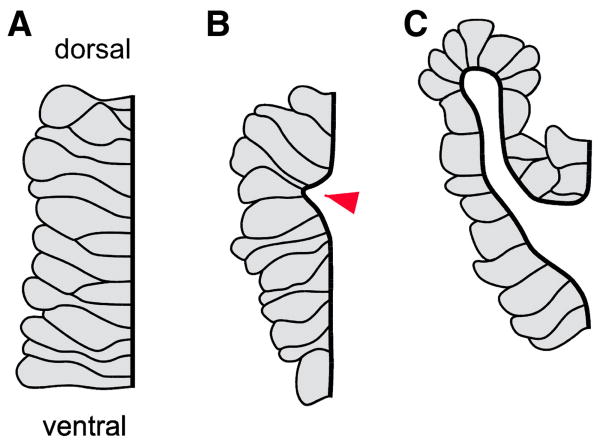
Fig. 8.
Schematic of cell shape changes during tracheal invagination. The dark line delineates the apical surfaces of the cells. Before invagination (left), cells form a flat epithelium. At the onset of invagination (middle), a small group of cells have apically constricted (red arrowhead). The invagination lengthens into a tube that turns dorsally (right) (after Brodu and Casanova, 2006).
The tracheal system is an interconnected network of branched epithelial tubes, responsible for gas transport. Trachea form from clusters of cells, each called a tracheal placode (Myat, 2005). During embryogenesis, ten placodes invaginate on each side of the Drosophila embryo. The onset of each of these invaginations is marked by apical constriction of about six cells in each placode. As the invagination deepens, it appears to turn (Fig. 8), resulting in the formation of a finger-like invagination turned dorsally below the embryo surface (Brodu and Casanova, 2006). As in other systems, apical constriction is preceded by an accumulation of F-actin and myosin II at the apical cortex of each constricting cell (Brodu and Casanova, 2006). Myosin and F-actin enrichment are interdependent, as mutant isoforms of myosin that cannot bind to actin fail to localize apically and, conversely, F-actin is not apically enriched in myosin mutants (Brodu and Casanova, 2006).
Tracheal apical constriction is regulated by upstream patterning genes. Cell fate is governed by the Trachealess bHLH/PAS transcription factor. Trachealess expression defines a region of cells that will later invaginate, positively regulating actomyosin accumulation and apical constriction by activating the EGFR signaling pathway through transcription of the EGF regulator Rhomboid (Affolter and Caussinus, 2008; Brodu and Casanova 2006; Nishimura et al., 2006). This regulation of actomyosin accumulation is mediated by Rho activity, by the function of the RhoGAP Crossveinless and its downstream target Rho1 (Brodu and Casanova, 2006).
The Drosophila salivary glands originate from two ventrolateral, ectodermal placodes (Myat and Andrew, 2000). The cells found in these placodes apically constrict and invaginate in a sequential manner during embryogenesis, starting from the dorsal-posterior portion of the placodes and progressing to other regions. F-actin and myosin accumulate at the apical cortex of constricting cells (Nikolaidou and Barrett 2004), and ROK-dependent phosphorylation of myosin contributes to apical constriction (Xu et al., 2008). Similar to the eye morphogenetic furrow, ROK mutants show only partial defects, suggesting that other kinases act redundantly with ROK to activate myosin contraction. ROK is again regulated here by Fog, Cta, RhoGEF2 and Rho1 (Nikolaidou and Barrett, 2004, Xu et al., 2008), as well as by 18wheeler, a Toll receptor protein that promotes Rho signaling, possibly through inhibition of the RhoGAP crossveinless (Nikolaidou and Barrett, 2007; Kolesnikov and Beckendorf, 2007). Interestingly, comparing phalloidin staining to anti-actin labeling in these cells shows that while total actin is evenly distributed along the cortex, filamentous actin is enriched specifically at the apical domains of the constricting cells. The kinase Tec29 maintains the imbalance between filamentous and monomeric actin (Chandrasekaran and Beckendorf, 2005).
During formation of the chick inner ear, otic ectodermal cells apically constrict, forming a vesicular otocyst within the head mesenchyme (Meier, 1978; Alvarez and Navascues, 1990). Elegant experiments involving extracted chicken tissues treated with various compounds suggested that the invagination of the ectodermal cells does not rely solely on cell-autonomous apical constriction, but probably involves forces exerted from the surrounding mesenchyme (Hilfer et al., 1989). Interestingly, apical constriction of the otic ectodermal cells involves an unconventional mechanism for F-actin localization. Instead of co-localizing with F-actin at the apical cell cortex, phosphorylated myosin accumulates at the basal domains of the cells, and its activity leads to local F-actin depletion and the resulting enrichment of F-actin at the apical domain (Sai and Ladher, 2008). Myosin-dependent F-actin depletion has been shown previously both in vitro (Haviv et al., 2008) and in vivo (Medeiros et al., 2006), but the mechanism(s) behind this are not yet clear. It is interesting to note that reciprocal localization of myosin II and F-actin is also detected during early neural tube formation (Sai and Ladher, 2008).
Drosophila dorsal closure and Xenopus wound healing: apical constriction contributes to sealing openings
Dorsal closure occurs halfway through Drosophila embryogenesis, when a pair of lateral epithelial sheets migrate from each side of the embryo, closing a hole on the dorsal side (Campos-Ortega and Hartenstein, 1985). Prior to this, the hole is transiently filled by an extra-embryonic epithelium, the amnioserosa.
The forces that drive dorsal closure have been dissected extensively by examining movements that occur as immediate responses to cutting specific tissues using a laser. Forces produced by both the amnioserosal cells and the cells of the epidermis regulate dorsal closure. The leading edge of the advancing epidermis forms a supracellular actin cable whose contraction contributes forces for dorsal closure (Kiehart et al., 2000). Initially, it was thought that the lateral epidermis migrated over passive amnioserosal cells (Campos-Ortega and Hartenstein, 1985). However, transmission electron microscopy has revealed that the amnioserosal cells shift from a squamous to a columnar cell shape, constricting their apical surfaces during dorsal closure (Rugendorff et al., 1994). Amnioserosal cells also drop out of the plane of the surface of the embryo (Kiehart et al., 2000) by apical constriction coupled to apoptosis (Toyoma et al., 2008). When amnioserosal cells were severed by laser cutting, or selectively killed by expressing ricin in these cells, dorsal closure was impaired (Kiehart et al., 2002; Scuderi and Letsou, 2005). These results indicate that the amnioserosal cells contract, contributing to closure forces.
The amnioserosal cell forces can act redundantly with the supracellular purse string in producing forces that drive dorsal closure (Kiehart et al., 2000; Hutson et al., 2003). When amnioserosal cells are cut with a laser, the leading edge recoils, but ultimately dorsal closure completes. The same is true for the leading edge – when the leading edge is severed, dorsal closure still completes. However, when both the amnioserosal cells and supracellular purse string are severed, dorsal closure is impaired (Kiehart et al., 2000), indicating that either tissue is able to compensate for cuts in the other. The recoil seen after cutting either tissue indicates that both tissues are under tension. Before dorsal closure begins, amnioserosal cells undergo cycles of apical constriction and retraction, and tension in the amnioserosa appears to feed back on this cycling behavior, as cutting amnioserosal cells arrests or weakens neighboring cells' contraction cycles (Solon et al., 2009).
Actin and myosin regulators have been identified as players in amnioserosal movements (Jacinto et al., 2002), including a Rac GTPase that functions specifically in apical constriction of the amnioserosal cells (Harden et al., 2002). Overexpression of a constitutively active form of Rac leads to overconstriction of the amnioserosal cells, and the cells then begin to pull away from the leading edge of the epidermis. Interestingly, in contrast to these studies, it has also been found that Rac triple mutant embryos do not have defects in amnioserosal cell contraction (Woolner et al., 2005). Thus the role of Rac signalling in amnioserosal cells is still unclear. Further studies using a myosin heavy chain (Zip) mutant reveals that amnioserosal cells that do not express this myosin II fail to apically constrict, remaining rounded (Franke et al., 2005). Rho1 and Dia also play roles in amnioserosal cell constriction, both stabilizing F-actin and activating myosin (Homem and Peifer, 2008). Dpp signaling through the Type I receptor thick veins (tkv) activates this contraction (Fernandez et al, 2007), although how it does so is not yet clear. Integrins are also required to adhere the epidermis and the amnioserosal cells during this movement (MacKrell et al., 1988; Hutson et al., 2003).
Wound healing is a process that requires cell shape changes and coordinated cell movements. Like dorsal closure, wound healing requires the spreading and fusion of epithelial sheets. Wound healing involves forces provided by a contractile, supracellular purse string (Redd et al., 2004; Martin and Parkhurst, 2004; Clark et al., 2009), but a clear, primary role for apical constriction of cells in the wound exists, at least in one system (Davidson et al., 2002). During wound healing in the animal cap of Xenopus embryos, F-actin accumulates in a purse string around the wound margin. Davidson et al. have performed elegant experiments to test whether the purse string or apical constriction of deep cells drives wound closure in Xenopus (Davidson et al., 2002). If a supracellular actin purse string mechanism provides the force, there would be at least two predictions. First, if a square wound is generated, with sharp corners, then as the wound shrinks, purse string forces should cause the wound profile to become rounded. Second, the wound margin should be under tension as it closes. To test the first prediction, the authors made square and rectangular wounds. As the wounds healed, they maintained squared corners, and a triangular wound even closed through a Y-shaped intermediate. Second, a wound was created, allowed to heal for 15 minutes, and then two nicks were made across the purse string cable. Perhaps surprisingly, no recoil was observed, and the rate of wound closure was unaffected. If a purse string does not provide the force for closure, what does? Davidson et al. propose that contraction of the apical surfaces of cells deep in the wound provides a driving force for wound closure (Fig. 9).
Fig. 9.
Apical constriction of deep cells during epithelial wound healing. Schematic of embryonic wound healing in the Xenopus laevis animal cap ectoderm. An excisional wound was made that removed only the outer cell layer of the two cell-layered animal cap ectoderm. Apical constriction drives reduction in wound size (Davidson et al., 2002)
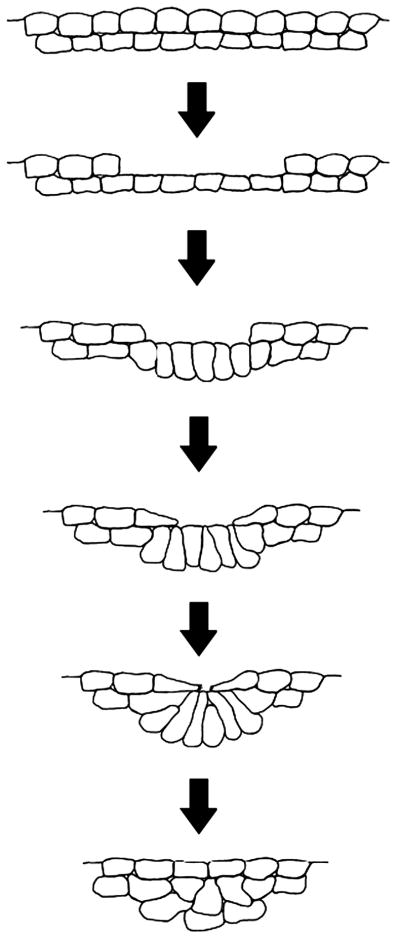
Vertebrate neural tube formation: hingepoint cells bend a sheet
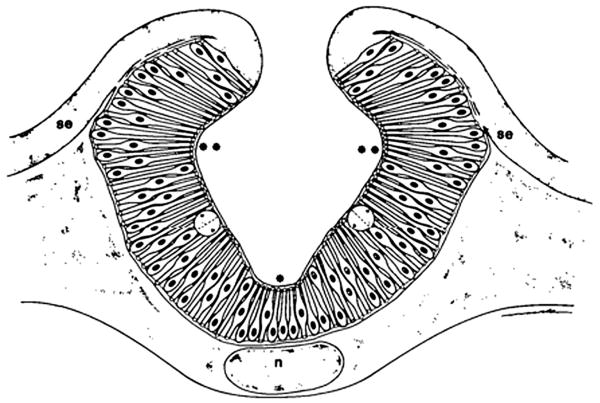
Formation of the neural tube is a complex morphogenetic process that involves a diverse collection of cell movements and cell shape changes, both extrinsic and intrinsic to the neuroepithelium (reviewed in Sadler, 1998). There are two mechanisms by which the neural tube forms, known as primary and secondary neurulation. Primary neurulation occurs in the brain and future trunk region, and refers to the folding of the neuroepithelium into a tube. Secondary neurulation occurs in the posterior neural tube and refers the condensation of mesenchymal cells into a solid rod, followed by an epithelial transition into a tube (Lowery and Sive, 2004). Mechanisms of neural tube formation are of added interest because failure of the neural tube to close is a leading cause of congenital birth defects (Detrait et al., 2005; Harris and Juriloff, 2007). Of particular interest to this review is primary neurulation, in which a group of cells in the neuroepithelium, known as hingepoints cells, apically constrict, aiding in the bending of the neural plate. There are two types of hingepoint cells in the neural tube: the medial hingepoint and the paired dorsal lateral hingepoints. The medial hingepoint is established in the ventral neural tube, and forms the neural groove (Schoenwolf and Smith, 1990) (Fig. 2, Fig. 10). Paired dorsal lateral hingepoints are found at the base of the neural folds (Fig. 2), where the neural plate bends around the dorsal lateral hingepoints and the neural folds converge (Schoenwolf and Smith, 1990) (Fig. 10).
Fig. 10.
Medial and dorsal lateral hingepoint cells in vertebrate neurulation. Schematic representation of a transverse section through the future hindbrain level of a chick embryo, illustrating the characteristics of neurepithelial cells in the medial hingepoints (asterisk), dorsolateral hingepoints (double asterisks) and lateral neural plate between the hinge points; n, notochord; se, surface ectoderm (Schoenwolf and Smith 1990).
Although the spatio-temporal development of hingepoint cells vary between model systems, hingepoint cells share a common description: these cells undergo a distinct change in cell shape in which cells become wedged, and the apical surfaces narrow. This occurs in a variety of vertebrate systems, including amphibians (Baker and Schroeder, 1967; Burnside, 1971; Schroeder, 1970), birds (Karfunkel, 1972; Schoenwolf and Franks, 1984) and mammals (Moore et al., 1987; Morriss-Kay, 1981; Shum and Copp, 1996). Patterns of bending in the neural tube have been shown to correlate with regions of apical constriction in the neuroepithelium (Bush et al., 1990; Nagele and Lee, 1987). Dense distributions of microfilaments have been observed under apical surfaces of neuroepithelial cells, leading to the long-standing hypothesis that a contractile network is responsible for hingepoint cell apical constriction (Baker and Schroeder, 1967; Schroeder, 1970; Freeman, 1972; Burnside, 1973; Schroeder, 1973; Nagel and Lee, 1980). In fact, early studies that disrupted the actin cytoskeleton by cytochalasins (Karfunkel, 1972; Morriss-Kay and Tuckett, 1985; Morriss-Kay, 1981) or by increased hydrostatic pressure (Messier and Seguin, 1978) resulted in disruption of neural tube closure. More recent work has built a molecular pathway supporting the hypothesis that a contractile network drives apical constriction. Similar to some examples of apical constriction from other systems, discussed above, key proteins that play roles in actomyosin contraction are localized apically, including Rho, Rho-kinase (ROCK), and the motor protein myosin IIB (Hildebrand, 2005; Kinoshita et al., 2008; Nishimura and Takeichi, 2008). Importantly, the myosin II motor complex is not only localized apically, but is also active at the apical surface of the neuroepithelium, as observed by phosphorylation of the myosin II light chain (p-MLC) (Kinoshita et al., 2008; Nandadasa et al., 2009; Nishimura and Takeichi, 2008). Other cytoskeletal regulators, including Abl/Arg (Koleske, et al., 1998), Mena/Vasp (Menzies et al., 2004), and MARCKS (Zolessi and Arruti, 2001), are known to function in neural tube closure, but whether these function specifically in apical constriction is unclear (Harris and Juriloff, 2007).
Studies of the protein Shroom3 have been valuable in demonstrating a conserved vertebrate regulator of apical constriction in the neural tube. Shroom3 was first identified in mouse as a mutation that prevented the convergence of neural folds predominantly but not exclusively in the cranial region, leaving neural folds “mushroomed” away from the midline (Hildebrand and Soriano, 1999). In mice, the expression of Shroom3 is dynamic in the neuroepithelium, and it is expressed in several other tissues including the somites, ventral body wall, heart, and gut (Hildebrand and Soriano, 1999). In X. laevis, Xshroom3 RNA expression is initiated in the anterior neural plate and extends posteriorly (Haigo et al., 2003). Within the neural plate, Xshroom3 is expressed in the superficial layer (Lee et al., 2009), where cells undergo apical constriction. Shroom3 protein expression overlaps with F-actin at both stress fibers and adherens junctions in primary neural tube cells (Hildebrand and Soriano, 1999) and localizes to the apical junctions of the neural epithelium (Hildebrand, 2005; Nishimura and Takeichi, 2008).
Shroom3 functions as an apical determinant, required for the apical accumulation of F-actin, myosin IIB, Rock1, and pMLC in the neural tube (Haigo et al., 2003; Hildebrand, 2005; Hildebrand and Soriano, 1999; Nishimura and Takeichi, 2008). In addition, Shroom3 can induce a redistribution of the microtubule regulator γ-tubulin, and is required for the assembly of apically localized parallel microtubule arrays required to drive apicobasal elongation of neural tube cells (Lee et al., 2007). However, Shroom3 is not required for apical ZO-1 localization, indicating that Shroom3 is not essential for all aspects of apicobasal cell polarity (Hildebrand, 2005; Lee et al., 2007; Nishimura and Takeichi, 2008). Shroom3 expression is sufficient to drive apical constriction in undifferentiated and transcriptionally quiescent polarized blastula cells in X. laevis (Haigo et al., 2003), and induces wedge-shaped cells in MDCK cell cultures (Haigo et al., 2003; Hildebrand, 2005). Interestingly, it is likely that Shroom3 expression alone does not determine the identity of hingepoint cells in the neuroepithelium as expression of Shroom3 in mouse and chick does not appear to be restricted to cells undergoing apical constriction (Hildebrand, 2005; Nishimura and Takeichi, 2008).
How does Shroom3 drive apical constriction? Shroom3 binds to and recruits ROCKs to the apical junctions (Nishimura and Takeichi, 2008). When the interaction between Shroom3 and ROCK was antagonized, pMLC failed to accumulate apically and neural tube closure was disrupted (Nishimura and Takeichi, 2008). Rhos are known activators of ROCKs (reviewed in Riento and Ridley, 2003), and thus a reasonable hypothesis is that Rho is required for Shroom3-mediated apical constriction. However, dominant negative constructs that block Rho signaling do not affect Shroom3-mediated apical constriction (Haigo et al., 2003; Hildebrand, 2005). Instead, Rap1 and possibly Ras are required for Shroom3 dependent apical constriction (Haigo et al., 2003). These studies do not exclude a role for Rho in hingepoint apical constriction. Rho is, in fact, found apically in the neuroepithelium, and may show a slight accumulation at the hingepoints (Kinoshita et al., 2008). When Rho signaling was blocked by the addition of C3 toxin, the myosin II motor complex was not active, and the neural tube failed to close (Kinoshita et al., 2008). Additional studies will be necessary to resolve the function of Rap1 and Rho signaling during actomyosin contraction in neuroepithelial cells, and to further define the role of Rap1 in Shroom3-mediated apical constriction.
Despite evidence for a contractile actomyosin network regulating apical constriction in the neural tube, studies have shown that the requirement for F-actin during neural tube closure is not a strict one. In chick embryos treated with cytochalasin D, wedging of dorsolateral neuroepithelial cells and convergence of neural folds were blocked, but medial hingepoints were unaffected in the absence of apical microfilaments (Schoenwolf et al., 1988). Similarly, in mouse embryonic cultures, cytochalasin D treatment prevented neural tube closure at the cranial region, but the formation of medial hingepoints and dorsal lateral hingepoints continued in the spinal region, and spinal neurulation proceeded (Ybot-Gonzalez and Copp, 1999). Thus, the formation of hingepoints and bending of the neuroepithelia in cytochalasin D treated embryos suggests that apical constriction in some hingepoint cells may be actomyosin independent. Alternative mechanisms for creating hingepoints cells have been proposed, including expansion of the basal membrane through nuclear movement. Cells at the medial hingepoint progress through the cell cycle, but there is an accumulation of cells with longer cell cycles and shorter mitotic stages (Smith and Schoenwolf, 1988). Using the observation that mitosis occurs at the apex of the neural plate, Smith and Schoenwolf suggest a model in which cells in the medial hingepoint have lengthened cell cycles, thus the nuclei are positioned basally for longer periods (Smith and Schoenwolf, 1988). This basal expansion may function to narrow the apical surface in relation to the basal surface, but it remains unclear whether this contributes to the forces that result in hingepoint formation and bending of the neuroepithelium.
Many interesting questions remain concerning both the molecular and cellular mechanisms of hingepoint formation. There is some evidence that indicates F-actin is localized basally before apical enrichment: F-actin is more concentrated at the basal sides in mouse when the cranial neural tube is in a biconvex morphology (Sadler et al., 1982), and in chick at the prospective medial hingepoint (Zolessi and Arruti, 2001). During later stages of chick neural tube formation, F-actin and pMLC show a reciprocal pattern, in that F-actin is apically localized and pMLC is predominantly basal. As neural tube formation persists, pMLC becomes apically localized (Sai and Ladher, 2008). Interestingly, myosin II regulatory light chain also becomes relocalized from the basal side to the apical side in Drosophila during apical constriction of the ventral furrow cells (Nikolaidou and Barrett, 2004). The function of this cytoskeletal reorganization in the neural tube is currently unclear. In X. laevis, myosin heavy chain B (MHC-B) is found cortically, with a concentration at the basal surface in neuroepithelial cells (Rolo et al., 2009). Knockdown of MHC-B disrupted apical F-actin accumulation and apical constriction of the neuroepithelial cells (Rolo et al., 2009). Depleting MHC-B increased deformability of the neural tissue, possibly by interfering with myosin IIB's role in cortical integrity (Rolo et al., 2009).
Adhesion, both at cell-cell junctions and at cell-matrix junctions, is likely to be important in apical constriction, and adhesion proteins and regulators have been identified as important players in apical constriction in the neural tube. Mutations in p190 RhoGAP, a mediator of integrin-dependent adhesion, result in excess basal accumulation of F-actin in the neuroepithelium, and apical constriction and neural tube closure are affected (Brouns et al., 2000). The X. laevis homolog of Enabled (Xena) is enriched at cell-cell junction complexes, and is required for apical F-actin accumulation, as well as for apical constriction in the neuroepithelium and cell adhesion (Roffers-Agarwal et al., 2008). Depletion of N-cadherin in the neural plate causes neural tube closure defects in X. laevis; however, cell adhesion is not obviously affected, possibly due to the presence of C-cadherin in the neural plate (Nandadasa et al., 2009). In neural plate cells with diminished N-cadherin, apical F-actin and phospho-myosin regulatory light chain distributions were disrupted and the apical surface areas increased, suggesting a loss of cortical tension (Nandadasa et al., 2009).
Further studies are needed to understand the pathways leading to hingepoint formation and apical constriction in the neuroepithelium, and the interplay between actomyosin contraction and cell adhesion. Cell fate is likely to have a role in determining precisely which cells in the neuroepithelium will apically constrict. Studies have shown that the secreted signal Sonic hedgehog (Shh), emanating from the notochord, and BMP, expressed in the surface ectoderm overlying the spinal neural folds, can inhibit the formation of dorsal lateral hingepoints, while the BMP antagonist Noggin induces dorsal lateral hingepoint bending (Ybot-Gonzalez et al., 2002; Ybot-Gonzalez et al., 2007). In zebrafish, the ventral expression border of zic2a, a transcription factor, appears to predict the location of the dorsal lateral hingepoints (Nyholm et al., 2009). An important area of future research will be to determine what factors cause hingepoint cells to apically constrict or prevent the apical constriction of neighboring cells.
Conclusions
Cell and developmental biologists have come a long way toward building an understanding of apical constriction, from the observations and hypotheses of Rhumbler in 1902, through physical and chemical perturbations, to building genetic pathways and dissecting protein functions, and into an age in which such findings can be integrated with biochemical mechanism and an understanding of force production. This kind of integration is likely to be important to gain a real understanding of the mechanisms by which development regulates the morphogenetic forces that shape animals. The connections established between patterning and morphogenesis are valuable steps toward defining the general rules by which forces are spatially regulated by developmental programs.
What can we conclude so far about common themes and variations? One commonly-demonstrated mechanism for the cell shape change of apical constriction is the localization and activation of myosin on an F-actin meshwork on the apical sides of cells. Mechanisms of spatial regulation of this common mechanism appear to vary widely between organisms and between tissues within an organism. A highly contractile actomyosin network can be localized to the apical side of a cell based on diverse sources of apicobasal polarity information, such as the apically-polarized secretion of Fog protein in Drosophila, apicobasal PAR protein localization in C. elegans, or apical Shroom localization in Xenopus, mouse and chick. Some proteins identified to date seem unlinkely to play conserved roles across the metazoa, as large groups of animals may lack key proteins. For example, Shroom is not yet known to exist outside of deuterostomes and arthropods (Dietz et al., 2006), and Fog appears to be a Drosophila-specific protein (Costa et al., 1994). Which cells will undergo apical constriction can also be determined by diverse sources of cell fate information, often involving transcriptional regulation, for example by GATA factor proteins in C. elegans gastrulation, bHLH and zinc finger proteins in Drosophila gastrulation, and a Drosophila bHLH/PAS protein in trachea formation. These findings suggest that common cytoskeletal mechanisms driving apical constriction are regulated by a variety of patterning mechanisms (Fig. 2).
Apical constriction can play central roles in diverse morphogenetic movements, including the internalization of small numbers of cells, the bending a tissue into a folded sheet, and the initiation of tube formation. One common theme is that apical constriction is used frequently in gastrulation. Of course, other classes of morphogenetic movements are often used in gastrulation as well. Given the diverse regulators identified to date, it will be difficult to estimate the extent to which gastrulation in systems like C. elegans and Drosophila are conserved modifications of an ancestral mechanism, as opposed to independent co-option of apical constriction mechanisms, until mechanisms are compared in relatives of these organisms.
Contraction of an apical microfilament network is not the only way apical constriction can take place. A shrinking of the apical side of a cell may also be driven by basolateral lengthening or expansion, movement of apical surface to lateral domains, or by extracellular forces, as discussed. The forces that can drive apical constriction do so in a mechanical context that can result in shrinking of only apical surfaces, rather than causing cell columnarization, for example, and such mechanical contexts have been explored only rarely (Davidson et al., 1999; Ma et al., 2009, for example). Thus, many questions remain. How much do these other processes act as primary drivers of apical constriction, and how much do they participate alongside constriction of an apical actomyosin meshwork? Forces from multiple cells can also contribute to a morphogenetic process, such as dorsal closure in flies. How are multiple forces coordinated to drive morphogenesis? What determines the degree of redundancy used to drive a morphogenetic event? Redundancy is a theme developmental biologists are increasingly able to address with new tools. Despite the apparent simplicity of apical constriction, redundant mechanisms are often involved. New computer models, building on the brass bar and rubber band models of Lewis, may be able to incorporate redundant mechanisms that may drive morphogenetic movements, and this may become increasingly important for testing the feasibility of hypotheses and for suggesting key experiments in the future. Likewise, more sophisticated experimental analyses of the cellular and multicellular mechanics will lead to better and more accurate models.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Affolter M, Caussinus E. Tracheal branching morphogenesis in Drosophila: new insights into cell behaviour and organ architecture. Development. 2008;135:2055–64. doi: 10.1242/dev.014498. [DOI] [PubMed] [Google Scholar]
- Alvarez IS, Navascues J. Shaping, invagination, and closure of the chick embryo otic vesicle: scanning electron microscopic and quantitative study. Anat Rec. 1990;228:315–26. doi: 10.1002/ar.1092280311. [DOI] [PubMed] [Google Scholar]
- Anderson DC, Gill JS, Cinalli RM, Nance J. Polarization of the C. elegans embryo by RhoGAP-mediated exclusion of PAR-6 from cell contacts. Science. 2008;320:1771–4. doi: 10.1126/science.1156063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PC, Schroeder TE. Cytoplasmic filaments and morphogenetic movement in the amphibian neural tube. Dev Biol. 1967;15:432–50. doi: 10.1016/0012-1606(67)90036-x. [DOI] [PubMed] [Google Scholar]
- Barrett K, Leptin M, Settleman J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell. 1997;91:905–15. doi: 10.1016/s0092-8674(00)80482-1. [DOI] [PubMed] [Google Scholar]
- Beane WS, Gross JM, McClay DR. RhoA regulates initiation of invagination, but not convergent extension, during sea urchin gastrulation. Dev Biol. 2006;292:213–25. doi: 10.1016/j.ydbio.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Blanchard GB, Kabla AJ, Schultz NL, Butler LC, Sanson B, Gorfinkiel N, Mahadevan L, Adams RJ. Tissue tectonics: morphogenetic strain rates, cell shape change and intercalation. Nat Methods. 2009;6:458–64. doi: 10.1038/nmeth.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodu V, Casanova J. The RhoGAP crossveinless-c links trachealess and EGFR signaling to cell shape remodeling in Drosophila tracheal invagination. Genes Dev. 2006;20:1817–28. doi: 10.1101/gad.375706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouns MR, Matheson SF, Hu KQ, Delalle I, Caviness VS, Silver J, Bronson RT, Settleman J. The adhesion signaling molecule p190 RhoGAP is required for morphogenetic processes in neural development. Development. 2000;127:4891–903. doi: 10.1242/dev.127.22.4891. [DOI] [PubMed] [Google Scholar]
- Burnside B. Microtubules and microfilaments in newt neuralation. Dev Biol. 1971;26:416–41. doi: 10.1016/0012-1606(71)90073-x. [DOI] [PubMed] [Google Scholar]
- Burnside B. Microtubules and microfilaments in amphibian neurulation. Am Zool. 1973;13:989–1006. [Google Scholar]
- Bush KT, Lynch FJ, DeNittis AS, Steinberg AB, Lee HY, Nagele RG. Neural tube formation in the mouse: a morphometric and computerized three-dimensional reconstruction study of the relationship between apical constriction of neuroepithelial cells and the shape of the neuroepithelium. Anat Embryol (Berl) 1990;181:49–58. doi: 10.1007/BF00189727. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA, Hartenstein V. The embryonic development of Drosophila melanogaster. Springer-Verlag; Berlin: 1985. [Google Scholar]
- Chandrasekaran V, Beckendorf SK. Tec29 controls actin remodeling and endoreplication during invagination of the Drosophila embryonic salivary glands. Development. 2005;132:3515–24. doi: 10.1242/dev.01926. [DOI] [PubMed] [Google Scholar]
- Chanut F, Heberlein U. Role of the morphogenetic furrow in establishing polarity in the Drosophila eye. Development. 1995;121:4085–94. doi: 10.1242/dev.121.12.4085. [DOI] [PubMed] [Google Scholar]
- Chung S, Andrew DJ. The formation of epithelial tubes. J Cell Sci. 2008;121:3501–4. doi: 10.1242/jcs.037887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AG, Miller AL, Vaughan E, Yu HY, Penkert R, Bement WM. Integration of single and multicellular wound responses. Curr Biol. 2009;19:1389–95. doi: 10.1016/j.cub.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall D, Walther RF, Rodriguez L, Fichelson P, Pichaud F. Hedgehog signaling is a principal inducer of Myosin-II-driven cell ingression in Drosophila epithelia. Dev Cell. 2007;13:730–42. doi: 10.1016/j.devcel.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Costa M, Sweeton D, Wieschaus E. Gastrulation in Drosophila: Cellular Mechanisms of Morphogenetic Movements In The Development of Drosophila melanogaster. Vol. 1. Cold Spring Harbor Laboratory; New York: 1993. pp. 425–66. [Google Scholar]
- Costa M, Wilson ET, Wieschaus E. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell. 1994;76:1075–89. doi: 10.1016/0092-8674(94)90384-0. [DOI] [PubMed] [Google Scholar]
- Croce J, Duloquin L, Lhomond G, McClay DR, Gache C. Frizzled5/8 is required in secondary mesenchyme cells to initiate archenteron invagination during sea urchin development. Development. 2006;133:547–57. doi: 10.1242/dev.02218. [DOI] [PubMed] [Google Scholar]
- Davidson EH, Rast JP, Oliveri P, Ransick A, Calestani C, Yuh CH, Minokawa T, Amore G, Hinman V, Arenas-Mena C, Otim O, Brown CT, Livi CB, Lee PY, Revilla R, Schilstra MJ, Clarke PJ, Rust AG, Pan Z, Arnone MI, Rowen L, Cameron RA, McClay DR, Hood L, Bolouri H. A provisional regulatory gene network for specification of endomesoderm in the sea urchin embryo. Dev Biol. 2002;246:162–90. doi: 10.1006/dbio.2002.0635. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Ezin AM, Keller R. Embryonic wound healing by apical contraction and ingression in Xenopus laevis. Cell Motil Cytoskeleton. 2002;53:163–76. doi: 10.1002/cm.10070. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Koehl MA, Keller R, Oster GF. How do sea urchins invaginate? Using biomechanics to distinguish between mechanisms of primary invagination. Development. 1995;121:2005–18. doi: 10.1242/dev.121.7.2005. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Oster GF, Keller RE, Koehl MA. Measurements of mechanical properties of the blastula wall reveal which hypothesized mechanisms of primary invagination are physically plausible in the sea urchin Strongylocentrotus purpuratus. Dev Biol. 1999;209:221–38. doi: 10.1006/dbio.1999.9249. [DOI] [PubMed] [Google Scholar]
- Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF. folded gastrulation, cell shape change and the control of myosin localization. Development. 2005;132:4165–78. doi: 10.1242/dev.01938. [DOI] [PubMed] [Google Scholar]
- Desprat N, Supatto W, Pouille PA, Beaurepaire E, Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell. 2008;15:470–7. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Detrait ER, George TM, Etchevers HC, Gilbert JR, Vekemans M, Speer MC. Human neural tube defects: developmental biology, epidemiology, and genetics. Neurotoxicol Teratol. 2005;27:515–24. doi: 10.1016/j.ntt.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz ML, Bernaciak TM, Vendetti F, Kielec JM, Hildebrand JD. Differential actin-dependent localization modulates the evolutionarily conserved activity of Shroom family proteins. J Biol Chem. 2006;281:20542–54. doi: 10.1074/jbc.M512463200. [DOI] [PubMed] [Google Scholar]
- Edgar LG, McGhee JD. DNA synthesis and control of embryonic gene expression in C. elegans. Cell. 1988;53:589–99. doi: 10.1016/0092-8674(88)90575-2. [DOI] [PubMed] [Google Scholar]
- Escudero LM, Bischoff M, Freeman M. Myosin II regulates complex cellular arrangement and epithelial architecture in Drosophila. Dev Cell. 2007;13:717–29. doi: 10.1016/j.devcel.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Ettensohn CA. Primary invagination of the vegetal pate during sea urchin gastrulation. Amer Zool. 1984;24:571–588. [Google Scholar]
- Ettensohn CA. Gastrulation in the sea urchin embryo is accompanied by the rearrangement of invaginating epithelial cells. Dev Biol. 1985;112:383–90. doi: 10.1016/0012-1606(85)90410-5. [DOI] [PubMed] [Google Scholar]
- Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–77. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- Fernandez BG, Arias AM, Jacinto A. Dpp signalling orchestrates dorsal closure by regulating cell shape changes both in the amnioserosa and in the epidermis. Mech Dev. 2007;124:884–97. doi: 10.1016/j.mod.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Fox DT, Homem CC, Myster SH, Wang F, Bain EE, Peifer M. Rho1 regulates Drosophila adherens junctions independently of p120ctn. Development. 2005;132:4819–31. doi: 10.1242/dev.02056. [DOI] [PubMed] [Google Scholar]
- Fox DT, Peifer M. Abelson kinase (Abl) and RhoGEF2 regulate actin organization during cell constriction in Drosophila. Development. 2007;134:567–78. doi: 10.1242/dev.02748. [DOI] [PubMed] [Google Scholar]
- Franke JD, Montague RA, Kiehart DP. Nonmuscle myosin II generates forces that transmit tension and drive contraction in multiple tissues during dorsal closure. Curr Biol. 2005;15:2208–21. doi: 10.1016/j.cub.2005.11.064. [DOI] [PubMed] [Google Scholar]
- Freeman BG. Surface modifications of neural epithelial cells during formation of the neural tube in the rat embryo. J Embryol Exp Morphol. 1972;28:437–48. [PubMed] [Google Scholar]
- Gates J, Mahaffey JP, Rogers SL, Emerson M, Rogers EM, Sottile SL, Van Vactor D, Gertler FB, Peifer M. Enabled plays key roles in embryonic epithelial morphogenesis in Drosophila. Development. 2007;134:2027–39. doi: 10.1242/dev.02849. [DOI] [PubMed] [Google Scholar]
- Gates J, Nowotarski SH, Yin H, Mahaffey JP, Bridges T, Herrera C, Homem CC, Janody F, Montell DJ, Peifer M. Enabled and Capping protein play important roles in shaping cell behavior during Drosophila oogenesis. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates J, Peifer M. Can 1000 reviews be wrong? Actin, alpha-Catenin, and adherens junctions. Cell. 2005;123:769–72. doi: 10.1016/j.cell.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–22. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorfinkiel N, Arias AM. Requirements for adherens junction components in the interaction between epithelial tissues during dorsal closure in Drosophila. J Cell Sci. 2007;120:3289–98. doi: 10.1242/jcs.010850. [DOI] [PubMed] [Google Scholar]
- Gorfinkiel N, Blanchard GB, Adams RJ, Martinez Arias A. Mechanical control of global cell behaviour during dorsal closure in Drosophila. Development. 2009;136:1889–98. doi: 10.1242/dev.030866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevengoed EE, Fox DT, Gates J, Peifer M. Balancing different types of actin polymerization at distinct sites: roles for Abelson kinase and Enabled. J Cell Biol. 2003;163:1267–79. doi: 10.1083/jcb.200307026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevengoed EE, Loureiro JJ, Jesse TL, Peifer M. Abelson kinase regulates epithelial morphogenesis in Drosophila. J Cell Biol. 2001;155:1185–98. doi: 10.1083/jcb.200105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans J, Wenzl C, Herz H, Bartoszewski S, Schnorrer F, Vogt N, Schwarz H, Müller H. RhoGEF2 and the formin Dia control the formation of the furrow canal by directed actin assembly during Drosophila cellularisation. Development. 2005;132:1009–20. doi: 10.1242/dev.01669. [DOI] [PubMed] [Google Scholar]
- Hacker U, Perrimon N. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev. 1998;12:274–84. doi: 10.1101/gad.12.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigo SL, Hildebrand JD, Harland RM, Wallingford JB. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol. 2003;13:2125–37. doi: 10.1016/j.cub.2003.11.054. [DOI] [PubMed] [Google Scholar]
- Harden N. Signaling pathways directing the movement and fusion of epithelial sheets: lessons from dorsal closure in Drosophila. Differentiation. 2002;70:181–203. doi: 10.1046/j.1432-0436.2002.700408.x. [DOI] [PubMed] [Google Scholar]
- Hardin J, Keller R. The behaviour and function of bottle cells during gastrulation of Xenopus laevis. Development. 1988;103:211–30. doi: 10.1242/dev.103.1.211. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Juriloff DM. Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res A Clin Mol Teratol. 2007;79:187–210. doi: 10.1002/bdra.20333. [DOI] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J Cell Biol. 2004;167:135–47. doi: 10.1083/jcb.200406024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haviv L, Gillo D, Backouche F, Bernheim-Groswasser A. A cytoskeletal demolition worker: myosin II acts as an actin depolymerization agent. J Mol Biol. 2008;375:325–30. doi: 10.1016/j.jmb.2007.09.066. [DOI] [PubMed] [Google Scholar]
- Heberlein U, Wolff T, Rubin GM. The TGF beta homolog dpp and the segment polarity gene hedgehog are required for propagation of a morphogenetic wave in the Drosophila retina. Cell. 1993;75:913–26. doi: 10.1016/0092-8674(93)90535-x. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD. Shroom regulates epithelial cell shape via the apical positioning of an actomyosin network. J Cell Sci. 2005;118:5191–203. doi: 10.1242/jcs.02626. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, Soriano P. Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell. 1999;99:485–97. doi: 10.1016/s0092-8674(00)81537-8. [DOI] [PubMed] [Google Scholar]
- Hilfer SR, Esteves RA, Sanzo JF. Invagination of the otic placode: normal development and experimental manipulation. J Exp Zool. 1989;251:253–64. doi: 10.1002/jez.1402510213. [DOI] [PubMed] [Google Scholar]
- Holtfreter J. A study of the mechanics of gastrulation. Part I. J Exp Zool. 1943;94:261–318. [Google Scholar]
- Holtfreter J. A study of the mechanics of gastrulation. Part II. J Exp Zool. 1944;95:171–212. [Google Scholar]
- Homem CC, Peifer M. Diaphanous regulates myosin and adherens junctions to control cell contractility and protrusive behavior during morphogenesis. Development. 2008;135:1005–18. doi: 10.1242/dev.016337. [DOI] [PubMed] [Google Scholar]
- Homsy JG, Jasper H, Peralta XG, Wu H, Kiehart DP, Bohmann D. JNK signaling coordinates integrin and actin functions during Drosophila embryogenesis. Dev Dyn. 2006;235:427–34. doi: 10.1002/dvdy.20649. [DOI] [PubMed] [Google Scholar]
- Hutson MS, Tokutake Y, Chang MS, Bloor JW, Venakides S, Kiehart DP, Edwards GS. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science. 2003;300:145–9. doi: 10.1126/science.1079552. [DOI] [PubMed] [Google Scholar]
- Jacinto A, Woolner S, Martin P. Dynamic analysis of dorsal closure in Drosophila: from genetics to cell biology. Dev Cell. 2002;3:9–19. doi: 10.1016/s1534-5807(02)00208-3. [DOI] [PubMed] [Google Scholar]
- Karfunkel P. The activity of microtubules and microfilaments in neurulation in the chick. J Exp Zool. 1972;181:289–301. doi: 10.1002/jez.1401810302. [DOI] [PubMed] [Google Scholar]
- Keller RE. An experimental analysis of the role of bottle cells and the deep marginal zone in gastrulation of Xenopus laevis. J Exp Zool. 1981;216:81–101. doi: 10.1002/jez.1402160109. [DOI] [PubMed] [Google Scholar]
- Keller RE, Davidson L. Cell Motility: From Molecules to Organisms. John Wiley; New York: 2004. Cell crawling, cell behavior and biomechanics during convergence and extension. [Google Scholar]
- Kiehart DP, Franke JD. Actin dynamics: the arp2/3 complex branches out. Curr Biol. 2002;12:R557–9. doi: 10.1016/s0960-9822(02)01053-9. [DOI] [PubMed] [Google Scholar]
- Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol. 2000;149:471–90. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly EL, Hardin J. Bottle cells are required for the initiation of primary invagination in the sea urchin embryo. Dev Biol. 1998;204:235–50. doi: 10.1006/dbio.1998.9075. [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Sasai N, Misaki K, Yonemura S. Apical accumulation of Rho in the neural plate is important for neural plate cell shape change and neural tube formation. Mol Biol Cell. 2008;19:2289–99. doi: 10.1091/mbc.E07-12-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleske AJ, Gifford AM, Scott ML, Nee M, Bronson RT, Miczek KA, Baltimore D. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21:1259–72. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- Kolesnikov T, Beckendorf SK. 18 wheeler regulates apical constriction of salivary gland cells via the Rho-GTPase-signaling pathway. Dev Biol. 2007;307:53–61. doi: 10.1016/j.ydbio.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolsch V, Seher T, Fernandez-Ballester GJ, Serrano L, Leptin M. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science. 2007;315:384–6. doi: 10.1126/science.1134833. [DOI] [PubMed] [Google Scholar]
- Kominami T, Takata H. Gastrulation in the sea urchin embryo: a model system for analyzing the morphogenesis of a monolayered epithelium. Dev Growth Differ. 2004;46:309–26. doi: 10.1111/j.1440-169x.2004.00755.x. [DOI] [PubMed] [Google Scholar]
- Lane MC, Koehl MA, Wilt F, Keller R. A role for regulated secretion of apical extracellular matrix during epithelial invagination in the sea urchin. Development. 1993;117:1049–60. doi: 10.1242/dev.117.3.1049. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–44. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- Lee A, Treisman JE. Excessive Myosin activity in mbs mutants causes photoreceptor movement out of the Drosophila eye disc epithelium. Mol Biol Cell. 2004;15:3285–95. doi: 10.1091/mbc.E04-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Le MP, Wallingford JB. The shroom family proteins play broad roles in the morphogenesis of thickened epithelial sheets. Dev Dyn. 2009;238:1480–91. doi: 10.1002/dvdy.21942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Scherr HM, Wallingford JB. Shroom family proteins regulate γ-tubulin distribution and microtubule architecture during epithelial cell shape change. Development. 2007;134:1431–41. doi: 10.1242/dev.02828. [DOI] [PubMed] [Google Scholar]
- Lee JY, Goldstein B. Mechanisms of cell positioning during C. elegans gastrulation. Development. 2003;130:307–20. doi: 10.1242/dev.00211. [DOI] [PubMed] [Google Scholar]
- Lee JY, Harland RM. Actomyosin contractility and microtubules drive apical constriction in Xenopus bottle cells. Dev Biol. 2007;311:40–52. doi: 10.1016/j.ydbio.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Marston DJ, Walston T, Hardin J, Halberstadt A, Goldstein B. Wnt/Frizzled signaling controls C. elegans gastrulation by activating actomyosin contractility. Curr Biol. 2006;16:1986–97. doi: 10.1016/j.cub.2006.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leptin M. Gastrulation in Drosophila: the logic and the cellular mechanisms. Embo J. 1999;18:3187–92. doi: 10.1093/emboj/18.12.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leptin M, Grunewald B. Cell shape changes during gastrulation in Drosophila. Development. 1990;110:73–84. doi: 10.1242/dev.110.1.73. [DOI] [PubMed] [Google Scholar]
- Lewis WH. Mechanics of invagination. Anat Rec. 1947;97:139–56. doi: 10.1002/ar.1090970203. [DOI] [PubMed] [Google Scholar]
- Lowery LA, Sive H. Strategies of vertebrate neurulation and a re-evaluation of teleost neural tube formation. Mech Dev. 2004;121:1189–97. doi: 10.1016/j.mod.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Lundmark C. Role of bilateral zones of ingressing superficial cells during gastrulation of Ambystoma mexicanum. J Embryol Exp Morphol. 1986;97:47–62. [PubMed] [Google Scholar]
- Ma X, Lynch HE, Scully PC, Hutson MS. Probing embryonic tissue mechanics with laser hole drilling. Phys Biol. 2009;6:036004. doi: 10.1088/1478-3975/6/3/036004. [DOI] [PubMed] [Google Scholar]
- Macara IG. Par proteins: partners in polarization. Curr Biol. 2004;14:R160–2. [PubMed] [Google Scholar]
- MacKrell AJ, Blumberg B, Haynes SR, Fessler JH. The lethal myospheroid gene of Drosophila encodes a membrane protein homologous to vertebrate integrin beta subunits. Proc Natl Acad Sci USA. 1988;85:2633–7. doi: 10.1073/pnas.85.8.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–9. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–34. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- Maybeck V, Roper K. A targeted gain-of-function screen identifies genes affecting salivary gland morphogenesis/tubulogenesis in Drosophila. Genetics. 2009;181:543–65. doi: 10.1534/genetics.108.094052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol. 2006;8:215–26. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- Mena and vasodilator-stimulated phosphoprotein are required for multiple actin-dependent processes that shape the vertebrate nervous system.
- Menzies AS, Aszodi A, Williams SE, Pfeifer A, Wehman AM, Goh KL, Mason CA, Fassler R, Gertler FB. J Neurosci. 2004;24:8029–38. doi: 10.1523/JNEUROSCI.1057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S. Development of the embryonic chick otic placode. I. Light microscopic analysis. Anat Rec. 1978;191:447–58. doi: 10.1002/ar.1091910405. [DOI] [PubMed] [Google Scholar]
- Messier PE, Seguin C. The effects of high hydrostatic pressure on microfilaments and microtubules in Xenopus laevis. J Embryol Exp Morphol. 1978;44:281–95. [PubMed] [Google Scholar]
- Moore AR, Burt AS. On the locus and nature of the forces causing gastrulation in the embryos of Dendraster excentricus. J Exp Zool. 1939;82:159–71. [Google Scholar]
- Moore DC, Stanisstreet M, Evans GE. Morphometric analyses of changes in cell shape in the neuroepithelium of mammalian embryos. J Anat. 1987;155:87–99. [PMC free article] [PubMed] [Google Scholar]
- Morriss-Kay G, Tuckett F. The role of microfilaments in cranial neurulation in rat embryos: effects of short-term exposure to cytochalasin D. J Embryol Exp Morphol. 1985;88:333–48. [PubMed] [Google Scholar]
- Morriss-Kay GM. Growth and development of pattern in the cranial neural epithelium of rat embryos during neurulation. J Embryol Exp Morphol. 1981;65(Suppl):225–41. [PubMed] [Google Scholar]
- Morize P, Christiansen AE, Costa M, Parks S, Wieschaus E. Hyperactivation of the folded gastrulation pathway induces specific cell shape changes. Development. 1998;125:589–97. doi: 10.1242/dev.125.4.589. [DOI] [PubMed] [Google Scholar]
- Müller HA, Wieschaus E. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J Cell Biol. 1996;134:149–63. doi: 10.1083/jcb.134.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro E, Nance J, Priess JR. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev Cell. 2004;7:413–24. doi: 10.1016/j.devcel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Myat MM. Making tubes in the Drosophila embryo. Dev Dyn. 2005;232:617–32. doi: 10.1002/dvdy.20293. [DOI] [PubMed] [Google Scholar]
- Myat MM, Andrew DJ. Fork head prevents apoptosis and promotes cell shape change during formation of the Drosophila salivary glands. Development. 2000;127:4217–26. doi: 10.1242/dev.127.19.4217. [DOI] [PubMed] [Google Scholar]
- Myat MM, Andrew DJ. Organ shape in the Drosophila salivary gland is controlled by regulated, sequential internalization of the primordia. Development. 2000;127:679–91. doi: 10.1242/dev.127.4.679. [DOI] [PubMed] [Google Scholar]
- Myat MM, Isaac DD, Andrew DJ. Early genes required for salivary gland fate determination and morphogenesis in Drosophila melanogaster. Adv Dent Res. 2000;14:89–98. doi: 10.1177/08959374000140011501. [DOI] [PubMed] [Google Scholar]
- Nagele RG, Lee HY. Studies on the mechanisms of neurulation in the chick: microfilament-mediated changes in cell shape during uplifting of neural folds. J Exp Zool. 1980;213:391–98. [Google Scholar]
- Nagele RG, Lee HY. Studies on the mechanisms of neurulation in the chick: morphometric analysis of the relationship between regional variations in cell shape and sites of motive force generation. J Exp Zool. 1987;241:197–205. doi: 10.1002/jez.1402410206. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Burke RD. The initial phase of gastrulation in sea urchins is accompanied by the formation of bottle cells. Dev Biol. 1996;179:436–46. doi: 10.1006/dbio.1996.0273. [DOI] [PubMed] [Google Scholar]
- Nance J. PAR proteins and the establishment of cell polarity during C. elegans development. Bioessays. 2005;27:126–35. doi: 10.1002/bies.20175. [DOI] [PubMed] [Google Scholar]
- Nance J, Lee JY, Goldstein B. Gastrulation in C. elegans. WormBook. 2005:1–13. doi: 10.1895/wormbook.1.23.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance J, Munro EM, Priess JR. C. elegans PAR-3 and PAR-6 are required for apicobasal asymmetries associated with cell adhesion and gastrulation. Development. 2003;130:5339–50. doi: 10.1242/dev.00735. [DOI] [PubMed] [Google Scholar]
- Nance J, Priess JR. Cell polarity and gastrulation in C. elegans. Development. 2002;129:387–97. doi: 10.1242/dev.129.2.387. [DOI] [PubMed] [Google Scholar]
- Nandadasa S, Tao Q, Menon NR, Heasman J, Wylie C. N- and E-cadherins in Xenopus are specifically required in the neural and non-neural ectoderm, respectively, for F-actin assembly and morphogenetic movements. Development. 2009;136:1327–38. doi: 10.1242/dev.031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaidou KK, Barrett K. A Rho GTPase signaling pathway is used reiteratively in epithelial folding and potentially selects the outcome of Rho activation. Curr Biol. 2004;14:1822–6. doi: 10.1016/j.cub.2004.09.080. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Inoue Y, Hayashi S. A wave of EGFR signaling determines cell alignment and intercalation in the Drosophila tracheal placode. Development. 2007;134:4273–82. doi: 10.1242/dev.010397. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Takeichi M. Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development. 2008;135:1493–502. doi: 10.1242/dev.019646. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E, Kluding H. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. I. Zygotic loci on the second chromosome. Wilhelm Roux's Arch Dev Biol. 1984;193:267–82. doi: 10.1007/BF00848156. [DOI] [PubMed] [Google Scholar]
- Nyholm MK, Abdelilah-Seyfried S, Grinblat Y. A novel genetic mechanism regulates dorsolateral hinge-point formation during zebrafish cranial neurulation. J Cell Sci. 2009;122:2137–48. doi: 10.1242/jcs.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates AC, Gorfinkiel N, Gonzalez-Gaitan M, Heisenberg CP. Quantitative approaches in developmental biology. Nat Rev Genet. 2009;10:517–30. doi: 10.1038/nrg2548. [DOI] [PubMed] [Google Scholar]
- Oegema K, Hyman AA. Cell division. WormBook. 2006:1–40. doi: 10.1895/wormbook.1.72.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks S, Wieschaus E. The Drosophila gastrulation gene concertina encodes a G alpha-like protein. Cell. 1991;64:447–58. doi: 10.1016/0092-8674(91)90652-f. [DOI] [PubMed] [Google Scholar]
- Pouille PA, Ahmadi P, Brunet AC, Farge E. Mechanical signals trigger myosin II redistribution and mesoderm invagination in Drosophila embryos. Science Signaling. 2009;2:1–8. doi: 10.1126/scisignal.2000098. [DOI] [PubMed] [Google Scholar]
- Poulson DF. Biology of Drosophila. John Wiley; New York: 1950. Histogenesis, organogenesis, and differentiation in the embryo of Drosophila melanogaster; pp. 168–274. [Google Scholar]
- Ready DF, Hanson TE, Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976;53:217–40. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Redd MJ, Cooper L, Wood W, Stramer B, Martin P. Wound healing and inflammation: embryos reveal the way to perfect repair. Philos Trans R Soc Lond Biol Sci. 2004;359:777–84. doi: 10.1098/rstb.2004.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhumbler L. Zur Mechanik des Gastrulationsvorganges, insbesondere der Invagination. Arch Entw mech. 1902;14:401. [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behavior. Nat Rev Mol Cell Biol. 2003;4:446–56. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Roffers-Agarwal J, Xanthos JB, Kragtorp KA, Miller JR. Enabled (Xena) regulates neural plate morphogenesis, apical constriction, and cellular adhesion required for neural tube closure in Xenopus. Dev Biol. 2008;314:393–403. doi: 10.1016/j.ydbio.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SL, Wiedemann U, Hacker U, Turck C, Vale RD. Drosophila RhoGEF2 associates with microtubule plus ends in an EB1-dependent manner. Curr Biol. 2004;14:1827–33. doi: 10.1016/j.cub.2004.09.078. [DOI] [PubMed] [Google Scholar]
- Roh-Johnson M, Goldstein B. In vivo roles for Arp2/3 in cortical actin organization during C. elegans gastrulation. J Cell Sci. 2009 doi: 10.1242/jcs.057562. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider MR, Nance J. Polarity and cell fate specification in the control of Caenorhabditis elegans gastrulation. Dev Dyn. 2009;238:789–96. doi: 10.1002/dvdy.21893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolo A, Skoglund P, Keller R. Morphogenetic movements driving neural tube closure in Xenopus require myosin IIB. Dev Biol. 2009;327:327–38. doi: 10.1016/j.ydbio.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röttinger E, Saudemont A, Duboc V, Besnardeau L, McClay D, Lepage T. FGF signals guide migration of mesenchymal cells, control skeletal morphogenesis [corrected] and regulate gastrulation during sea urchin development. Development. 2008;135:353–65. doi: 10.1242/dev.014282. [DOI] [PubMed] [Google Scholar]
- Royou A, Field C, Sisson JC, Sullivan W, Karess R. Reassessing the role and dynamics of nonmuscle myosin II during furrow formation in early Drosophila embryos. Mol Biol Cell. 2004;15:838–50. doi: 10.1091/mbc.E03-06-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffini A. Contributo alle conoscenza della ontogenesi degli anfibi urodelied anuri. Anat Anz. 1907;31:448. [Google Scholar]
- Rugendorff A, Younossi-Hartenstein A, Hartenstein V. Embryonic origin and differentiation of the Drosophila heart. Roux's Arch Dev Biol. 1994;203:266–80. doi: 10.1007/BF00360522. [DOI] [PubMed] [Google Scholar]
- Sadler TW. Mechanisms of neural tube closure and defects. Science. 1998;215:172–4. [Google Scholar]
- Sadler TW. Embryology of Neural Tube Development. American Journal of Medical Genetics Part C (Semin Med Genet) 2005;135C:2–8. doi: 10.1002/ajmg.c.30049. [DOI] [PubMed] [Google Scholar]
- Sadler TW, Greenberg D, Coughlin P, Lessard JL. Actin distribution patterns in the mouse neural tube during neurulation. Science. 1982;215:172–4. doi: 10.1126/science.7031898. [DOI] [PubMed] [Google Scholar]
- Sai X, Ladher RK. FGF signaling regulates cytoskeletal remodeling during epithelial morphogenesis. Curr Biol. 2008;18:976–81. doi: 10.1016/j.cub.2008.05.049. [DOI] [PubMed] [Google Scholar]
- Sawyer JK, Harris NJ, Slep KC, Gaul U, Peifer M. The Drosophila afadin homologue Canoe regulates linkage of the actin cytoskeleton to adherens junctions during apical constriction. J Cell Biol. 2009;186:57–73. doi: 10.1083/jcb.200904001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting K, Dahmann C. Hedgehog and Dpp signaling induce cadherin Cad86C expression in the morphogenetic furrow during Drosophila eye development. Mech Dev. 2008;125:712–28. doi: 10.1016/j.mod.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Folsom D, Moe A. A reexamination of the role of microfilaments in neurulation in the chick embryo. Anat Rec. 1988;220:87–102. doi: 10.1002/ar.1092200111. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Franks MV. Quantitative analyses of changes in cell shapes during bending of the avian neural plate. Dev Biol. 1984;105:257–72. doi: 10.1016/0012-1606(84)90284-7. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Smith JL. Mechanisms of neurulation: traditional viewpoint and recent advances. Development. 1990;109:243–70. doi: 10.1242/dev.109.2.243. [DOI] [PubMed] [Google Scholar]
- Schroeder TE. Neurulation in Xenopus laevis. An analysis and model based upon light and electron microscopy. J Embryol Exp Morphol. 1970;23:427–62. [PubMed] [Google Scholar]
- Schroeder TE. Cell constriction: contractile role of microfilaments in division and development. Mental Retardation and Developmental Disabilities Research Reviews. Amer Zool. 1973;4:247–53. [Google Scholar]
- Scuderi A, Letsou A. Amnioserosa is required for dorsal closure in Drosophila. Dev Dyn. 2005;232:791–800. doi: 10.1002/dvdy.20306. [DOI] [PubMed] [Google Scholar]
- Severson AF, Baillie DL, Bowerman B. A formin homology protein and a profilin are required for cytokinesis and Arp2/3-independent assembly of cortical microfilaments in C. elegans. Curr Biol. 2002;12:2066–75. doi: 10.1016/s0960-9822(02)01355-6. [DOI] [PubMed] [Google Scholar]
- Shum AS, Copp AJ. Regional differences in morphogenesis of the neuroepithelium suggest multiple mechanisms of spinal neurulation in the mouse. Anat Embryol (Berl) 1996;194:65–73. doi: 10.1007/BF00196316. [DOI] [PubMed] [Google Scholar]
- Simpson P. Maternal-Zygotic Gene Interactions during Formation of the Dorsoventral Pattern in Drosophila Embryos. Genetics. 1983;105:615–632. doi: 10.1093/genetics/105.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Schoenwolf GC. Role of cell-cycle in regulating neuroepithelial cell shape during bending of the chick neural plate. Cell Tissue Res. 1988;252:491–500. doi: 10.1007/BF00216636. [DOI] [PubMed] [Google Scholar]
- Solon J, Kaya-Copur A, Colombelli J, Brunner D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell. 2009;137:1331–42. doi: 10.1016/j.cell.2009.03.050. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Sonnenblick BP. Biology of Drosophila. John Wiley; New York: 1950. The early embryology of Drosophila melanogaster; pp. 62–167. [Google Scholar]
- Stern C. Gastrulation: From Cells to Embryos. Cold Spring Harbor Laboratory; New York: 2004. [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Sweeton D, Parks S, Costa M, Wieschaus E. Gastrulation in Drosophila: the formation of the ventral furrow and posterior midgut invaginations. Development. 1991;112:775–89. doi: 10.1242/dev.112.3.775. [DOI] [PubMed] [Google Scholar]
- Thisse B, el Messal M, Perrin-Schmitt F. The twist gene: isolation of a Drosophila zygotic gene necessary for the establishment of dorsoventral pattern. Nucleic Acids Res. 1987;15:3439–53. doi: 10.1093/nar/15.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson A. The cellular dynamics of pattern formation in the eye of Drosophila. J Embryol Exp Morphol. 1985;89:313–31. [PubMed] [Google Scholar]
- Toyama Y, Peralta XG, Wells AR, Kiehart DP, Edwards GS. Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science. 2008;321:1683–6. doi: 10.1126/science.1157052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner FR, Mahowald AP. Scanning electron microscopy of Drosophila melanogaster embryogenesis. II. Gastrulation and segmentation. Dev Biol. 1977;57:403–16. doi: 10.1016/0012-1606(77)90225-1. [DOI] [PubMed] [Google Scholar]
- Vrailas AD, Moses K. Smoothened, thickveins and the genetic control of cell cycle and cell fate in the developing Drosophila eye. Mech Dev. 2006;123:151–65. doi: 10.1016/j.mod.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wieschaus E. From Molecular Patterns to Morphogenesis: The Lessons of Drosophila. Nobel Lecture 1995 [Google Scholar]
- Woolner S, Jacinto A, Martin P. The small GTPase Rac plays multiple roles in epithelial sheet fusion--dynamic studies of Drosophila dorsal closure. Dev Biol. 2005;282:163–73. doi: 10.1016/j.ydbio.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Wu SY, Yang YP, McClay DR. Twist is an essential regulator of the skeletogenic gene regulatory network in the sea urchin embryo. Dev Biol. 2008;319:406–15. doi: 10.1016/j.ydbio.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Keung B, Myat MM. Rho GTPase controls invagination and cohesive migration of the Drosophila salivary gland through Crumbs and Rho-kinase. Dev Biol. 2008;321:88–100. doi: 10.1016/j.ydbio.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Cogram P, Gerrelli D, Copp AJ. Sonic hedgehog and the molecular regulation of mouse neural tube closure. Development. 2002;129:2507–17. doi: 10.1242/dev.129.10.2507. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Copp AJ. Bending of the neural plate during mouse spinal neurulation is independent of actin microfilaments. Dev Dyn. 1999;215:273–83. doi: 10.1002/(SICI)1097-0177(199907)215:3<273::AID-AJA9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Gaston-Massuet C, Girdler G, Klingensmith J, Arkell R, Greene ND, Copp AJ. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of Bmp signalling. Development. 2007;134:3203–11. doi: 10.1242/dev.008177. [DOI] [PubMed] [Google Scholar]
- Young PE, Pesacreta TC, Kiehart DP. Dynamic changes in the distribution of cytoplasmic myosin during Drosophila embryogenesis. Development. 1991;111:1–14. doi: 10.1242/dev.111.1.1. [DOI] [PubMed] [Google Scholar]
- Zolessi FR, Arruti C. Apical accumulation of MARCKS in neural plate cells during neurulation in the chick embryo. BMC Dev Biol. 2001;1:7. doi: 10.1186/1471-213X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]