Abstract
The direct glutamatergic projection from the medial prefrontal cortex (mPFC) to the nucleus accumbens plays a critical role in mediating the reinstatement of cocaine-seeking behavior. The mPFC also sends glutamatergic projections to the pedunculopontine tegmental nucleus (PPTg)/laterodorsal tegmental nucleus (LDT), which in turn sends glutamatergic and cholinergic efferents to the ventral tegmental area (VTA) where they synapse on dopaminergic cells that innervate limbic structures including the nucleus accumbens. The goal of these experiments was to examine a potential role for the PPTg/LDT in the reinstatement of cocaine seeking. All rats were trained to self-administer cocaine (0.25 mg, i.v.) on a fixed-ratio 5 (FR5) schedule of reinforcement. Cocaine self-administration behavior was extinguished and a series of subsequent pharmacological experiments were performed to assess the potential role of the mPFC, PPTg/LDT and VTA in the reinstatement of cocaine seeking. Administration of the D1-like dopamine receptor agonist SKF-81297 (1.0 μg) directly into the mPFC produced a small, but statistically significant, increase in cocaine-seeking behavior. Furthermore, microinjection of the ionotropic glutamate receptor antagonist CNQX (0.3 μg) into the PPTg/LDT attenuated the reinstatement of drug seeking induced by a priming injection of cocaine (10 mg/kg, i.p.). Intra-VTA administration of CNQX, the nicotinic receptor antagonist mecamylamine (10.0 μg) or the muscarinic receptor antagonist scopolamine (24.0 μg) also blocked cocaine seeking. Taken together, these results suggest that cocaine priming-induced reinstatement of drug seeking is mediated in part by a serial polysynaptic limbic subcircuit encompassing the mPFC, PPTg/LDT and VTA.
Keywords: medial prefrontal cortex, ventral tegmental area, nucleus accumbens, dopamine, glutamate, acetylcholine
Introduction
A growing literature indicates that afferents from the medial prefrontal cortex (mPFC) play critical roles in priming-induced reinstatement of cocaine seeking (Kalivas et al., 2005; Schmidt et al., 2005). For example, activating the cortico-accumbal glutamatergic pathway reinstates cocaine seeking (Park et al., 2002; McFarland et al., 2003). The mPFC may also influence the activity of the dopaminergic projections from the VTA to the nucleus accumbens, which are critically involved in the reinstatement of cocaine seeking (see Anderson & Pierce, 2005; Schmidt et al., 2005). However, the regulation of the mesoaccumbens dopaminergic projections by the mPFC must be indirect since glutamatergic afferents from the mPFC to the VTA do not synapse on dopaminergic cells that project to the nucleus accumbens (Carr & Sesack, 2000).
The mesopontine tegmentum, which is composed of the rostral pedunculpontine tegmental nucleus (PPTg) and the more caudal laterodorsal tegmental nucleus (LDT), receives excitatory input from limbic sites in the forebrain including the mPFC (Sesack et al., 1989; Semba & Fibiger, 1992) and sends glutamatergic and cholinergic afferent projections to the VTA, where they synapse on dopaminergic neurons some of which project to the nucleus accumbens (Hallanger & Wainer, 1988; Cornwall et al., 1990; Semba & Fibiger, 1992; Steininger et al., 1992; Futami et al., 1995; Charara et al., 1996; Oakman et al., 1999; Omelchenko & Sesack, 2005). Thus, the PPTg/LDT is positioned anatomically to receive information from the mPFC and relay it to dopaminergic cells in the VTA. Consistent with this notion, stimulation of the PPTg/LDT activates dopaminergic cells in the VTA (Kelland et al., 1993; Floresco et al., 2003; Lodge & Grace, 2006b; a) and leads to increased dopamine release in the striatum/accumbens (Floresco et al., 2003; Forster & Blaha, 2003). Moreover, activation of the mPFC increased extracellular dopamine in the accumbens, an effect that was blocked by administration of glutamate receptor antagonists into the VTA but not the nucleus accumbens (Murase et al., 1993; Taber et al., 1995; Karreman et al., 1996). Collectively, these results suggest that the mPFC modulates the activity of the mesoaccumbal dopaminergic pathway indirectly via a polysynaptic circuit encompassing the PPTg/LDT.
Several lines of evidence indicate that the PPTg/LDT processes information related to both drug and natural reinforcers (Winn, 2006). Thus, lesioning the PPTg attenuated the conditioned rewarding properties of drugs of abuse (Bechara & van der Kooy, 1989; Olmstead et al., 1998) as well as natural reinforcers including food (Bechara & van der Kooy, 1992) and sex (Kippen & van der Kooy, 2001). Lesions of the PPTg (Alderson et al., 2003) or the LDT (Nelson et al., 2007) also attenuated the development of behavioral sensitization to d-amphetamine. Moreover, pharmacological inactivation of the PPTg reduced cocaine self-administration maintained on fixed ratio and progressive ratio schedules of reinforcement (Corrigall et al., 1999; Corrigall et al., 2002). It seems plausible, therefore, that the PPTg/LDT complex might also play an important role in the reinstatement of cocaine seeking, an animal model of relapse, but this hypothesis has not yet been examined.
The current experiments used pharmacological methodologies to assess a potential role for the PPTg/LDT, as well as its afferent and efferent connections, in cocaine priming-induced reinstatement of drug seeking. Our results are consistent with the activation of a serial circuit encompassing the mPFC, PPTg/LDT, VTA and nucleus accumbens playing a critical role in the reinstatement of cocaine seeking.
Materials and Methods
Animals and Housing
Male Sprague Dawley rats (Rattus norvegicus) weighing 250–300 g were obtained from Taconic Laboratories (Germantown, N.Y., USA). Animals were single-housed with food and water available ad libitum (rats undergoing food reinstatement experiments were placed on restricted diets, as outlined below). All animals were housed in a colony maintained on a 12-hr/12-hr light/dark cycle with the lights on at 7:00 a.m. All experimental procedures were performed during the light phase. All experimental protocols were in accordance with the guidelines set forth by the National Institutes of Health and were approved by the Boston University School of Medicine Institutional Animal Care and Use Committee.
Materials
All behavioral experiments were conducted in ventilated, sound attenuating operant chambers purchased from Med-Associates Inc. (East Fairfield, VT). Each operant chamber was equipped with both inactive and active response levers, a food pellet dispenser as well as an automated injection pump for administering drug or vehicle solutions intravenously.
Surgery
Rats were allowed one week to acclimate to their home cages upon arrival. Prior to surgery, the rats were anesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine (Sigma/RBI, St. Louis, MO). An indwelling catheter (CamCaths; Cambridge, UK) was inserted into the right, external jugular vein and sutured securely in place. The catheter was connected to a mesh backmount, which was implanted subcutaneously above the shoulder blades. In order to prevent infection and to maintain patency, catheters were flushed daily with 0.3 ml of a solution of the antibiotic Timentin (0.93 mg/ml) dissolved in heparinized saline. When not in use, the catheters were sealed with plastic obturators.
Immediately following implantation of the indwelling catheter, some rats were mounted in a stereotaxic apparatus (Kopf Instruments, CA) and bilateral guide cannulae (14 mm 24 gauge tubing, Small Parts Inc., Roanoke, VA) were implanted 2 mm dorsal to the mPFC, 1 mm dorsal to the PPTg/LDT or 1 mm dorsal to the VTA according to the following stereotaxic coordinates from the atlas of Paxinos and Watson (1997): mPFC: +2.5 mm anteroposterior (A/P, relative to bregma), ±0.5 mm mediolateral (M/L, relative to bregma) and −2.0 mm dorsoventral (D/V, relative to dura): PPTg/LDT: −7.8 mm A/P, ±2.0 mm M/L and −6.2 mm D/V: VTA: −5.8 mm A/P,±0.5 mm M/L and −7.0 mm D/V. Guide cannulae were cemented in place by affixing dental acrylic to three stainless steel screws fastened to the skull. Obturators (14 mm, 33 gauge stainless steel wire, Small Parts Inc., Roanoke, VA) were inserted into each guide cannula in order to prevent occlusion.
Cocaine Self-Administration
After surgery, rats were allowed seven days to recover before behavioral testing commenced. Initially, rats were placed in operant chambers daily and allowed to lever press for intravenous cocaine (0.25 mg cocaine/59 μl saline, infused over a 5 sec period) on a fixed-ratio 1 (FR1) schedule of reinforcement. Each session began with the i.v. administration of 59 μl cocaine (0.25 mg) to fill the catheter. Rats were allowed to self-administer a maximum of 30 injections per 120-minute operant session. Stable responding on the FR1 schedule was defined as less than 15% variation in response rates over three consecutive self-administration days. After stable responding was achieved, animals were switched to a fixed-ratio 5 (FR5) schedule of reinforcement. The maximum number of injections was again limited to 30 per daily self-administration session under the FR5 schedule. For both the FR1 and FR5 schedules, a 20 second time-out period followed each cocaine infusion, during which time active lever responses were tabulated but had no scheduled consequences. Responses made on the inactive lever, which had no scheduled consequences, were also recorded during both the FR1 and FR5 training sessions.
Extinction and reinstatement of cocaine seeking
Following approximately 21 days of daily cocaine self-administration sessions, drug-seeking behavior was extinguished by replacing the cocaine with 0.9% saline. Daily two-hour extinction sessions continued until responding on the active lever was <15% of the response rate maintained by cocaine self-administration under the FR5 schedule of reinforcement. Typically, it took approximately 7 days for rats to meet this criterion.
The FR5 schedule of reinforcement was used throughout the reinstatement phase of the experiment. When an animal met the response requirement (five presses on the active lever) an intravenous infusion of saline was administered. Using a between-session reinstatement paradigm, each daily reinstatement session was followed by extinction days until responding was less than 15% of the maximum number of responses maintained by cocaine self-administration. In general, it took 1–2 days of extinction for each animal to reach criterion between reinstatement sessions. Using this experimental design, subjects underwent a series of extinction and reinstatement sessions that lasted approximately 16 days. During this period, animals may lose the ability to reinstate active lever responding following a priming injection of cocaine. However, we have previously shown that reinstatement of cocaine seeking persists for at least 20 days after the initial extinction of cocaine self-administration (Park et al., 2002; Anderson et al., 2003). Moreover, we were able to assess the magnitude of reinstatement by randomly administering priming injections of cocaine throughout the reinstatement phase of the experiment. All animals displayed stable drug seeking, which was operationally defined as greater than 30 active lever responses per 2-hr operant session, during the reinstatement phase of the experiment.
Once self-administration behavior was extinguished, the ability of a priming injection of cocaine (10 mg/kg, i.p.) or its vehicle (0.9% saline) to reinstate cocaine seeking was assessed. On subsequent test days, the selective D1-like dopamine receptor agonist R(+)-SKF-81297 hydrobromide (1.0 μg/0.5 μl, Sigma/RBI, St. Louis, MO) or its vehicle (0.9% saline) was microinjected into the mPFC in order to test its ability to reinstate cocaine-seeking behavior. Animals were placed into the operant chambers immediately following the intra-mPFC microinfusion of SKF-81297 and the 2-hr reinstatement session began. Additional experiments assessed the ability of an intra-PPTg/LDT microinjection of an ionotropic glutamate receptor antagonist or an intra-VTA microinjection of a nicotinic acetylcholine receptor antagonist, muscarinic acetylcholine receptor antagonist or ionotropic glutamate receptor antagonist to attenuate reinstatement of drug seeking elicited by a priming injection of cocaine (10 mg/kg, i.p.). The ionotropic glutamate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt (CNQX; 0.03 or 3.0 μg/0.5 μl, Tocris, Ellisville, MS) was administered directly into the PPTg/LDT ten minutes prior to a priming injection of cocaine (10 mg/kg, i.p.). Furthermore, CNQX (0.03 or 3.0 μg/0.5 μl), (−)-scopolamine hydrochloride (2.4 or 24.0 μg/0.5 μl, Sigma/RBI, St. Louis, MO) or mecamylamine hydrochloride (1.0 or 10.0 μg/0.5 μl, Sigma/RBI, St. Louis, MO) was microinjected into the VTA ten minutes prior to a systemic priming injection of cocaine (10 mg/kg, i.p.) in order to assess their ability to attenuate cocaine priming-induced reinstatement.
The dose ranges for each of the aforementioned pharmacological compounds were based on the following rat microinjection experiments: SKF-81297 (Beyer & Steketee, 2002; Cools et al., 2002; Schmidt et al., 2006), CNQX (Cornish et al., 1999; See et al., 2001; Park et al., 2002), (−)scopolamine hydrochloride (Ikemoto & Goeders, 2000; Corrigall et al., 2002; See et al., 2003; Pratt & Kelley, 2005) and mecamylamine hydrochloride (Sziraki et al., 2002; Forster & Blaha, 2003; Sharf & Ranaldi, 2006).
Microinjection Procedures
Obturators were removed from the guide cannulae and 33 gauge stainless steel microinjectors (Small Parts Inc.) were inserted. Microinjectors were cut to a length that extended 2 mm (for microinjections targeting the mPFC) or 1 mm (for microinjections targeting the PPTg/LDT or VTA) below the ventral end of the guide. Bilateral infusions were performed simultaneously over a 120 second time period in a total volume of 0.5 μl per side. Microinjectors were left in place for 60 seconds following the microinfusions, in order to allow the drug solution or vehicle to diffuse away from the tips of the cannulae, before they were removed. Animals were placed immediately into the operant chambers following microinjection of SKF-81297 or its vehicle (0.9 % saline) and the reinstatement session began without delay. Animals pretreated intra-cranially with CNQX, scopolamine, mecamylamine or their vehicle (0.9% saline) 10 minutes prior to a priming injection of cocaine (10 mg/kg, i.p.) were placed in the operant chambers immediately following the cocaine priming injection.
The goal of the experimental design was to have each animal serve as its own control and receive up to six microinjections per brain region (i.e. two doses plus vehicle for two drugs for a maximum of 6 microinjections per brain region). However, we were frequently forced to deviate from this experimental design when technical difficulties (i.e. blocked microinjection cannulae or loss of catheter patency) made it impossible to test all doses of a compound plus vehicle in an entire cohort of subjects. In every case, however, an animal received a minimum treatment of one drug dose and its vehicle. In order to control for potential rank order effects of drug or vehicle treatments, the agonist, antagonists and vehicle were counterbalanced across reinstatement sessions and within cohorts of animals. Using this experimental design, no order effects of drug treatments were observed.
Food Reinstatement
Potential nonspecific rate-suppressing effects of the pharmacological compounds tested were evaluated by assessing the influence of intra-PPTg/LDT CNQX or intra-VTA CNQX, scopolamine or mecamylamine on reinstatement of food-reinforced responding. Rats were trained initially to lever press for sucrose pellets (Research Diets, Inc., New Brunswick, NJ) on a FR1 schedule of food reinforcement during 1-hour operant sessions. Once animals achieved stable responding for food (defined as <15% variation in responding over two consecutive days) on the FR1 schedule of reinforcement, the response requirement was increased to an FR5 schedule of reinforcement. Animals were limited to 30 sucrose pellets within a 1-hour operant session and were food restricted to four pellets of lab chow (Harlan Teklad, Wilmington, DE) in their home cages for the duration of the experiment.
After two weeks of food-maintained responding on the FR5 schedule of reinforcement, responding was extinguished by inactivating the food dispenser so that every 5 lever presses had no scheduled consequences. Once lever responding decreased to <15% of the maximum number of responses completed during food self-administration, animals proceeded to reinstatement testing. Bilateral microinjections of CNQX (0.3 μg/0.5 μl) or vehicle into the PPTg/LDT or scopolamine (24.0 μg/0.5 μl), mecamylamine (10.0 μg/0.5 μl), CNQX (0.3 μg/0.5 μl) or vehicle into the VTA were administered ten minutes prior to the beginning of the reinstatement session. The experimenter remotely administered one sucrose pellet every 2 minutes for the first 10 minutes of the reinstatement session. A between-session paradigm was used so that each daily 1-hour reinstatement session was followed by an extinction session the following day until responding was again <15% of the response rate maintained by food.
Histology and Verification of Cannulae Placements
After the microinjection experiments were concluded, animals were overdosed with a systemic injection of pentobarbital (100 mg/kg) and perfused intracardially with 60 ml of 0.9% saline followed by 60 ml of 10% formalin. The brains were removed and stored in 10% formalin. Subsequently, 100–150 μm thick coronal sections were taken at the level of the mPFC, PPTg/LDT or VTA with a Vibratome (Technical Products Int., St. Louis, MO.). These sections were mounted on gel-coated slides and stained with Cresyl violet. An individual blind to the animals’ behavioral responses determined cannulae placements as well as potential drug- or cannula-induced neuronal damage. Light microscopy was used to determine cannulae placements as well as the presence and extent of cell death and associated gliosis.
Behavioral Data Analyses
Total active and inactive lever responses during the reinstatement phase of experiments with SKF-81297 in the mPFC were analyzed with unpaired t-tests. For the experiments utilizing CNQX in the PPTg/LDT, and CNQX, scopolamine and mecamylamine in the VTA, the total mean active and inactive lever responses during the reinstatement phase were analyzed with one-way analyses of variance (ANOVAs) or unpaired t-tests. Pairwise comparisons were made with Tukey’s HSD (P<0.05).
Drugs
Cocaine was obtained from the National Institutes of Drug Abuse (NIDA) and dissolved in sterile 0.9% saline. R(+)-SKF-81297 hydrobromide (1.0 μg/0.5 μl), (−)-scopolamine hydrochloride (2.4 or 24.0 μg/0.5 μl), and mecamylamine hydrochloride (1.0 or 10.0 μg/0.5 μl) were purchased from Sigma/RBI (St. Louis, MO) and dissolved in sterile 0.9% saline. CNQX disodium salt (0.03 or 3.0 μg/0.5 μl) was purchased from Tocris (Ellisville, MS) and dissolved in sterile 0.9% saline.
Results
The mean (±S.E.M.) total active and inactive lever responses during the last day of cocaine self-administration for all subjects used in the following experiments are included in Table 1.
Table 1.
Total active and inactive lever responses during the last day of cocaine self-administration for all subjects receiving microinfusions of vehicle or drug directly into discrete nuclei of the brain during reinstatement trials in the present study.
| Brain Region | Treatment | n/treatment | Total Active Lever Responses (mean ± S.E.M.) | Total Inactive Lever Responses (mean ± S.E.M.) |
|---|---|---|---|---|
| mPFC | Vehicle & 1.0 μg SKF-81297 | 7–9 | 125.00 ±7.04 | 3.89 ±2.47 |
| PPTg/LDT | Vehicle, 0.03 & 0.3 μg CNQX | 6–9 | 114.92 ±9.23 | 9.17 ±3.16 |
| VTA | Vehicle, 0.03 & 0.3 μg CNQX | 10 | 135.87 ±9.17 | 8.25 ±2.94 |
| VTA | Vehicle, 2.4 & 24.0 μg Scopolamine | 8–10 | 121.79 ±8.27 | 9.36 ±3.91 |
| VTA | Vehicle, 1.0 & 10.0 μg Mecamylamine | 10 | 142.00 ±9.48 | 5.08 ±2.44 |
Microinjection of the D1-like dopamine receptor agonist SKF-81297 into the medial prefrontal cortex reinstated cocaine seeking in rats
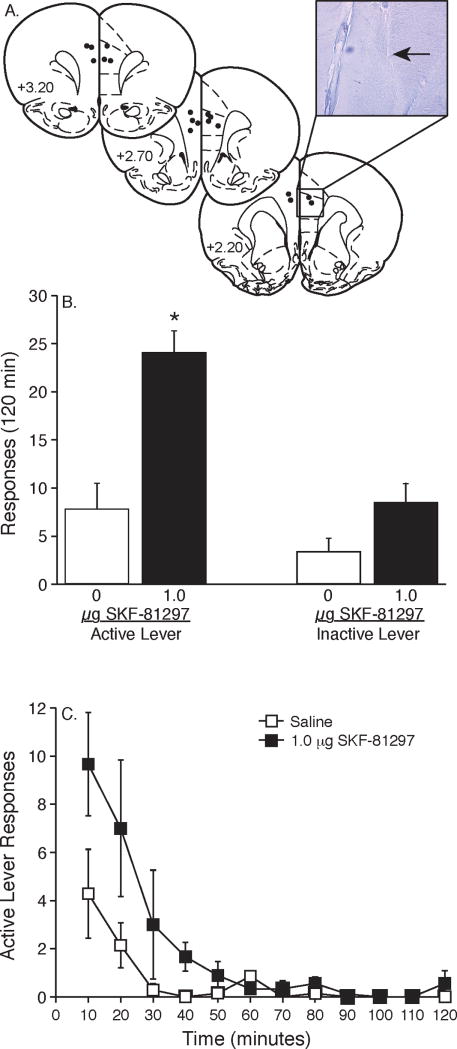
The cannulae placements in this experiment are presented in Figure 1A. Microinjections were targeted to the dorsal mPFC including the dorsal prelimbic cortex and the anterior cingulate cortex. The data depicted in Figure 1B show the cumulative number of responses (mean±S.E.M.) on both the active and inactive levers during the reinstatement phase of the experiment. Animals were administered saline or 1.0 μg SKF-81297 into the medial prefrontal cortex prior to the start of the reinstatement session. Active lever data were analyzed with an unpaired t-tests and a significant difference was found between the saline and 1.0 μg SKF-81297 treatments [t(14)=4.642, p<0.0004]. No significant difference was found between saline and 1.0 μg SKF-81297 treatments in terms of inactive lever responding [t(14)=1.995, p<0.0658]. Figure 1B displays responses made on the active lever during each of the 10-minute components of the 2-hr operant session for the intra-mPFC saline and 1.0 μg SKF-81297 treatments.
Figure 1. Microinjection of the D1-like dopamine receptor agonist SKF-81297 into the medial prefrontal cortex reinstated cocaine seeking in rats.
(A) Ventral tips of the microinjection cannulae, as indicated by the closed circles, targeted the dorsal mPFC. Numbers on the left side of each coronal section denote distance from bregma in the anteroposterior direction. The inset displays a representative coronal section stained with 0.1% cresyl violet and viewed under 4x magnification using a light microscope. The arrow indicates a microinfusion site within the dorsal mPFC. (B) Total number of responses (mean±S.E.M.) on the active and inactive levers during the reinstatement session following intra-mPFC administration of saline (n=7) or 1.0 μg SKF-81297 (n=9). The asterisk denotes a significant difference on active lever responding between 1.0 μg SKF-81297 and saline treatments (unpaired t-test, P<0.0004). (C) The time courses of active lever responses (mean±S.E.M.) for the data summarized in panel B.
Microinjection of the AMPA/kainate receptor antagonist CNQX into the PPTg/LDT dose-dependently attenuated cocaine priming-induced reinstatement of drug seeking but had no influence on the reinstatement of food-seeking behavior in rats
Animals were administered saline (n = 9), 0.03 (n = 6) or 0.3 μg (n = 9) CNQX directly into the PPTg/LDT prior to a priming injection of cocaine (10 mg/kg i.p.). The total active lever responses (mean±S.E.M.) during the reinstatement session are shown in Figure 2A. These data were analyzed with a one-way ANOVA, which revealed a significant main effect of treatment [F(2,21)=17.208, p<0.0001]. Subsequent pairwise analyses (Tukey’s HSD, P<0.05) showed total active lever responses were significantly different between the saline and both the 0.03 μg CNQX as well as 0.3 μg CNQX treatments. Inactive lever responses (mean±S.E.M.) following intra-PPTg/LDT administration of saline, 0.03 or 0.3 μg CNQX prior to a priming injection of cocaine (10 mg/kg, i.p.) were analyzed with a one-way ANOVA and plotted in Figure 2A. No significant differences in responses made on the inactive lever induced by a 10 mg/kg priming injection of cocaine were revealed following microinjection of saline, 0.03 or 0.3 μg CNQX into the PPTg/LDT [F(2,21)=1.493, p<0.2477]. The active lever responses (mean±S.E.M.) made during each of the 10-minute components in the intra-PPTg/LDT saline + cocaine (10 mg/kg, i.p.), 0.03 μg CNQX + cocaine (10 mg/kg, i.p.) and 0.3 μg CNQX + cocaine (10 mg/kg, i.p.) treatments are shown in Figure 2B.
Figure 2. Microinjection of the AMPA/kainate receptor antagonist CNQX into the PPTg/LDT dose-dependently attenuated reinstatement of drug seeking induced by a priming injection of cocaine.
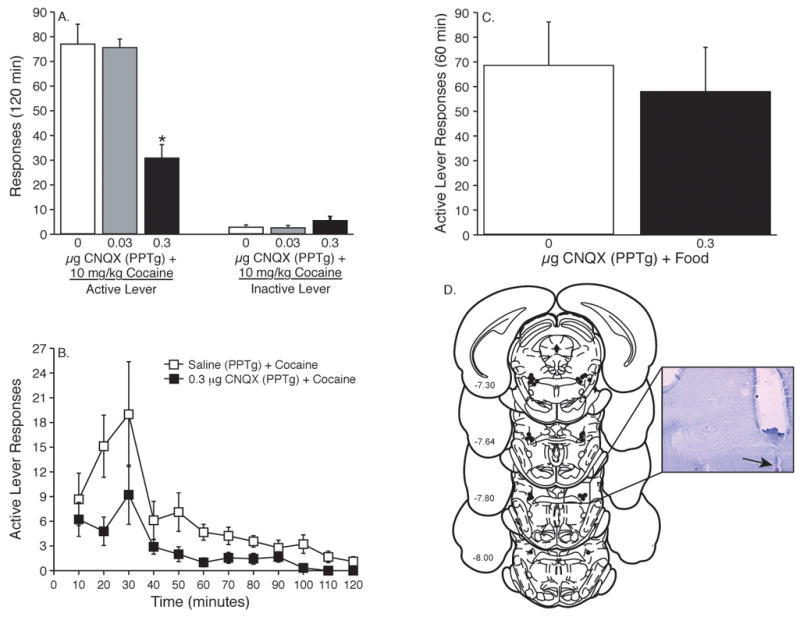
(A) Data in panel A depict the total responses (mean±S.E.M.) on the active and inactive levers following a systemic priming injection of cocaine (10 mg/kg, i.p.) in animals pretreated with microinfusions of saline (n = 9), 0.03 (n = 6) or 0.3 μg (n = 9) CNQX into the PPTg/LDT. The asterisk represents a significant difference between responses on the active lever following 0.3 μg CNQX and saline or 0.03 μg CNQX pretreatments (Tukey’s HSD, P<0.05). No significant differences in responding on the inactive lever (mean±S.E.M.) were found during the reinstatement phase of the experiment when saline, 0.03 or 0.3 μg CNQX were administered into the PPTg/LDT 10 minutes prior to a systemic injection of cocaine (10 mg/kg, i.p.). (B) The time courses of active lever responding (mean±S.E.M.) following intra-PPTg/LDT administration of saline (n = 9) or 0.3 μg CNQX (n = 9) prior to a systemic priming injection of cocaine (10 mg/kg i.p.). (C) Intra-PPTg/LDT administration of CNQX did not affect food-seeking behavior in rats. Animals microinjected with saline (n = 6) or 0.3 μg CNQX (n = 6) directly into the PPTg/LDT prior to the reinstatement of food seeking displayed no difference in total active lever responses between treatments. (D) Coronal sections depicting microinjection sites, as indicated by the closed circles, targeting the PPTg/LDT. Numbers on the left side of the coronal sections denote distance from bregma in the anteroposterior direction. The insert displays a magnified (4x) microinfusion site within the PPTg (the ventral tip of the microinjector is indicated by the arrow).
When using receptor antagonists that decrease reinstatement of cocaine-seeking behavior, such as CNQX, general behavioral suppression is a concern. In the present experiments, two measures were used to evaluate potential nonspecific rate suppressing effects of intra-cranial microinjections of CNQX. First, each operant chamber was equipped with an inactive lever, responses on which are often used as a measure of nonspecific alterations in lever responding. While intra-PPTg/LDT administration of CNQX had no significant effect on inactive lever responding, one could argue that responses were uniformly too low to meaningfully assess potential rate suppressant effects of drug treatment. Therefore, we also assessed the ability of intra-PPTg/LDT microinjections of CNQX to alter food reinstatement, where noncontingent administration of food reinstates responding previously maintained by food reinforcement. Intra-PPTg/LDT infusion of the same dose of CNQX that attenuated cocaine priming-induced reinstatement did not affect food reinstatement (see Figure 2C). Animals were administered 0.3 μg CNQX (n = 6) or saline (n = 6) directly into the PPTg/LDT 10 minutes prior to a 1 hour food reinstatement test session. The active lever responses (mean±S.E.M.) obtained following microinjection of saline or 0.3 μg CNQX into the PPTg/LDT are depicted in Figure 2C. These data were analyzed with an unpaired t-test, which did not reveal a significant difference between saline or 0.3 μg CNQX treatments [t(10)=0.425, p<0.6801]. These data suggest that attenuation of drug seeking induced by a priming injection of cocaine was not due to general motor impairment.
The location of all the cannula placements in the PPTg/LDT are shown in Figure 2D. The PPTg/LDT is located in the mesopontine tegmentum adjacent to the following nuclei: deep mesencephalic nucleus, microcellular tegmentum nucleus, retrorubral nucleus, pontine reticular nucleus, paralemniscal nucleus, subpendencular tegmental nucleus, epirubrospinal nucleus, reticulotegmental nucleus and the lateral tegmentum nucleus (Paxinos & Watson, 1997). Given the small size of the PPTg/LDT, it is conceivable that drug solutions microininjected into the PPTg/LDT diffused into these areas. Repeated microinjections into the PPTg/LDT resulted in no cases of excessive mechanical damage or cell death.
Microinjection of the AMPA/kainate receptor antagonist CNQX into the VTA dose-dependently attenuated cocaine priming-induced reinstatement of drug seeking
Total active lever responses (mean±S.E.M.) following intra-VTA administration of CNQX prior to a 10 mg/kg priming injection of cocaine during the reinstatement phase of the experiment are plotted in Figure 3A. The active lever data from Figure 3A were analyzed using a one-way ANOVA, which revealed a significant main effect of treatment [F(2,27)=34.711, p<0.0001]. Pairwise analyses revealed a significant difference in responding on the active lever between the saline (n=10) or 0.03 μg (n=10) and 0.3 μg (n=10) CNQX treatments (Tukey’s HSD, p<0.05). Total inactive lever responses (mean±S.E.M.) following a systemic priming injection of cocaine (10 mg/kg i.p.) in animals pretreated with intra-VTA administration of saline (n = 10), 0.03 (n = 10) or 0.3 (n = 10) μg CNQX are plotted in Figure 3A. These data were analyzed using a one-way ANOVA. No significant difference on inactive lever responding was found between treatments [F(2,27)=1.362, p<0.2723]. The active lever response rate for each 10-minute component of the operant session during the reinstatement phase of the experiment for intra-VTA saline, 0.03 and 0.3 μg CNQX treatments is shown in Figure 3B.
Figure 3. Intra-VTA administration of the AMPA/kainate receptor antagonist CNQX dose-dependently attenuated cocaine priming-induced reinstatement of drug seeking.
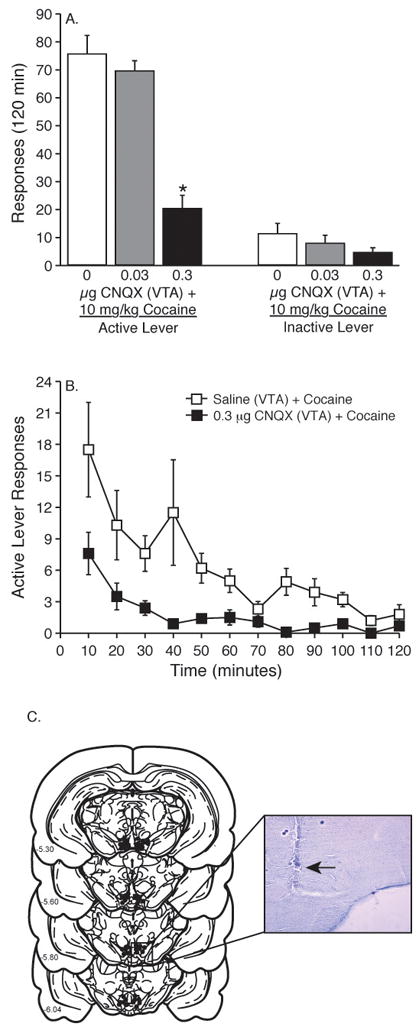
(A) Total number of responses (mean±S.E.M.) on the active lever during the reinstatement session following a 10 mg/kg priming injection of cocaine in animals pretreated with saline (n =10), 0.03 (n = 10) or 0.3 (n = 10) μg CNQX into the VTA. The asterisk indicates a significant difference between 0.3 μg CNQX and 0.03 μg CNQX or saline treatments with regards to total active lever responses (Tukey’s HSD, P<0.05). Total number of responses (mean±S.E.M.) on the inactive lever during the reinstatement phase of the experiment following a systemic priming injection of cocaine (10 mg/kg i.p.) in animals pretreated with animals receiving intra-VTA administration of saline (n =10), 0.03 (n = 10) or 0.3 (n = 10) μg CNQX. No significant difference in responding on the inactive lever was found between treatments. (B) The time courses of active lever responses (mean±S.E.M.) for animals given a priming injection of cocaine (10 mg/kg i.p.) following intra-VTA administration of saline (n = 10) or 0.3 μg CNQX (n = 10). (C) Closed circles denote cannula placements for all the microinjections into the VTA (from Figure 3 & 4). Numbers on the left side of each coronal section indicate distance from bregma in the anteroposterior direction. The arrow displayed within the insert designates a representative microinfusion site within the VTA.
Ten minutes prior to a 1 hour food reinstatement test session animals received intra-VTA microinfusions of 0.3 μg CNQX (n=6) or saline (n=6). The active lever responses (mean±S.E.M.) obtained following microinjection of saline or 0.3 μg CNQX into the VTA are depicted in Table 2. These data were analyzed with an unpaired t-test, which did not reveal a significant difference between saline or 0.3 μg CNQX treatments [t(10)=1.192, p<0.2607]. This finding is consistent with a previous study, which demonstrated that intra-VTA microinfusions of the non-selective ionotropic glutamate receptor antagonist kynurenate did not affect food-seeking behavior (Sun et al., 2005). Collectively, these data indicate that attenuation of cocaine seeking by intra-VTA CNQX was not due to general motor impairment. The cannula placements for all of the microinjections into the VTA (from Figures 3 and 4) are shown in Figure 3C.
Table 2. Intra-VTA administration of CNQX, mecamylamine, or scopolamine does not affect food-seeking behavior in rats.
Animals microinjected with 3.0 μg CNQX, 10.0 μg mecamylamine or 24.0 μg scopolamine directly into the VTA prior to the reinstatement of food seeking displayed no significant difference in total active lever responses relative to the saline control treatment.
| Intra-VTA Treatment | n | Total Active Lever Responses (mean ± S.E.M.) |
|---|---|---|
| Saline | 6 | 95.50 ± 10.19 |
| 0.3 μg CNQX | 6 | 113.00 ± 11.70 |
| 10.0 μg Mecamylamine | 6 | 93.33 ± 14.00 |
| 24.0 μg Scopolamine | 6 | 86.50 ± 12.21 |
Figure 4. Microinjection of the muscarinic acetylcholine receptor antagonist scopolamine or the nicotinic acetylcholine receptor antagonist mecamylamine into the VTA dose-dependently attenuated reinstatement of drug seeking elicited by a priming injection of cocaine.
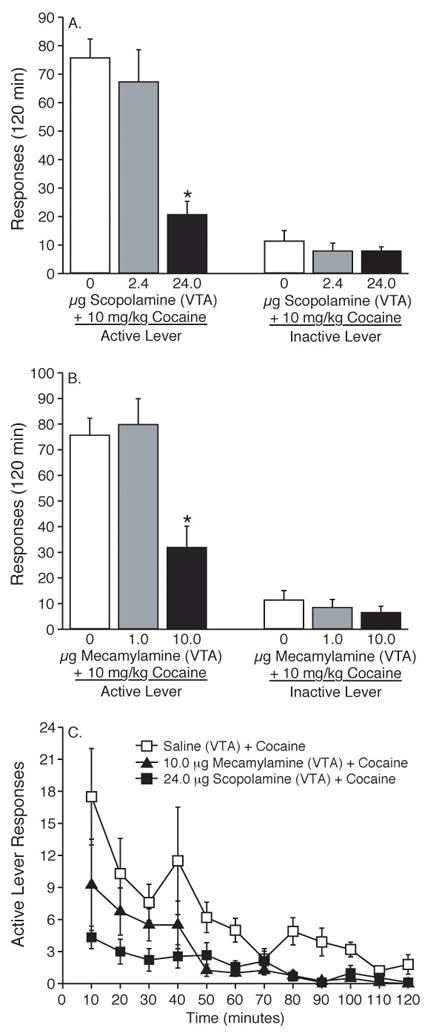
(A) Total number of responses (mean±S.E.M.) on the active and inactive levers during the reinstatement session following a 10 mg/kg priming injection of cocaine in animals pretreated with saline (n =10), 2.4 (n = 8) or 24.0 (n = 9) μg scopolamine into the VTA. The asterisk indicates a significant difference between 24.0 μg scopolamine and 2.4 μg scopolamine or saline treatments with regards to total active lever responses (Tukey’s HSD, P<0.05). (B) Data in panel B depict the total responses (mean±S.E.M.) on the active lever following a systemic priming injection of cocaine (10 mg/kg, i.p.) in animals pretreated with microinfusions of saline (n = 10), 1.0 (n = 10) or 10.0 μg (n = 8) mecamylamine into the VTA. The asterisk represents a significant difference between the 10.0 μg mecamylamine and saline or 1.0 μg mecamylamine treatments (Tukey’s HSD, P<0.05). Total responses (mean±S.E.M.) on the inactive lever following a priming injection of cocaine (10 mg/kg i.p.) in animals pretreated with saline, 1.0 or 10.0 μg mecamylamine into the VTA. No significant difference was found between treatments with regard to inactive lever responses during the 2-hour reinstatement session. (C) The time courses of active lever responses (mean±S.E.M.) for data collected from animals pretreated with intra-VTA microinfusions of saline, 24.0 μg scopolamine or 10.0 μg mecamylamine prior to a priming injection of cocaine.
Microinjection of mecamylamine or scopolamine into the VTA dose-dependently attenuated cocaine priming-induced reinstatement of drug seeking
Total active lever responses (mean±S.E.M.) following intra-VTA administration of the muscarinic acetylcholine receptor antagonist scopolamine prior to a 10 mg/kg priming injection of cocaine during the reinstatement phase of the experiment are plotted in Figure 4A. A one-way ANOVA was used to analyze the active lever data, which revealed a significant main effect of treatment [F(2,24)=15.223, p<0.0001]. Further pairwise analyses revealed a significant difference in responding on the active lever between the saline (n=10) or 2.4 μg (n=8) and 24.0 μg (n=9) scopolamine treatments (Tukey’s HSD, P<0.05). Total inactive lever responses (mean±S.E.M.) following a systemic priming injection of cocaine (10 mg/kg i.p.) in animals pretreated with microinfusions of saline (n = 10), 2.4 (n = 8) or 24.0 (n = 9) μg scopolamine in the VTA also are plotted in Figure 4A. These data were analyzed using a one-way ANOVA. No significant difference on inactive lever responding was found between saline, 2.4 or 24.0 μg scopolamine treatments [F(2,24)=0.499, p<0.613].
Animals were administered saline (n=10), 1.0 (n=10) or 10.0 μg (n=8) of the nicotinic receptor antagonist mecamylamine directly into the VTA prior to a priming injection of cocaine (10 mg/kg i.p.). The total active lever responses (mean±S.E.M.) during the reinstatement session are shown in Figure 4B. These data were analyzed with a one-way ANOVA, which revealed a significant main effect of treatment [F(2,25)=9.003, p<0.0011]. Subsequent pairwise analyses (Tukey’s HSD, P<0.05) showed total active lever responses were significantly different between the saline or 1.0 μg mecamylamine and 10.0 μg mecamylamine treatments. Inactive lever responses (mean±S.E.M.) following intra-VTA administration of saline, 1.0 or 10.0 μg mecamylamine prior to a priming injection of cocaine (10 mg/kg, i.p.) were analyzed using a one-way ANOVA and plotted in Figure 4B. No significant difference was found on inactive lever responding following a 10 mg/kg priming injection of cocaine in animals pretreated with microinjections of saline, 1.0 or 10.0 μg mecamylamine into the VTA [F(2,25)=0.551, p<0.5834).
The active lever response rate for each 10-minute component of the operant session during the cocaine (10 mg/kg, i.p.) priming-induced reinstatement phase of the experiment for animals pretreated with intra-VTA saline, 10.0 μg mecamylamine or 24.0 μg scopolamine is shown in Figure 4C. The cannula placements for all of the microinjections into the VTA (from Figures 3 and 4) are shown in Figure 3C. Repeated microinjections into the VTA did not cause excessive mechanical damage or cell death in any cases.
Ten minutes prior to a 1 hour reinstatement test session, where reinstatement of lever pressing maintained by food reinforcement was initiated by noncontingently administered sucrose pellets, animals received intra-VTA microinfusions of 24.0 μg scopolamine (n=6) or saline (n=6). The active lever responses (mean±S.E.M.) obtained following microinjections of saline or 24.0 μg scopolamine into the VTA are depicted Table 2. These data were analyzed with separate unpaired t-tests, which did not reveal a significant difference between saline or 24.0 μg scopolamine treatments [t(10)= 0.503, p<0.6257] or between saline or 10.0 μg mecamylamine treatments [t(10)= 0.067, p<0.9476]. This data suggests that attenuation of cocaine seeking by intra-VTA administration of acetylcholine antagonists was not due to general motor impairment.
The VTA is a heterogeneous structure composed of at least two anatomically (Swanson, 1982; German & Manaye, 1993; Olson et al., 2005) and functionally (Ikemoto et al., 1997a; Ikemoto et al., 1997b; 1998; Carlezon et al., 2000; Olson et al., 2005) distinct subregions differentiated across the rostral-caudal axis. As shown in Figure 3C, the microinjections in these experiments tended to be in more rostral region of the VTA, although there were many microinjections in the caudal VTA as well.
Discussion
While a growing body of evidence indicates that the mPFC, VTA and nucleus accumbens are critically involved in cocaine seeking (Kalivas et al., 2005; Schmidt et al., 2005), a potential role for the PPTg/LDT region in the reinstatement of cocaine seeking has received relatively little attention to date. The present results include three novel findings: i) administration of a D1-like dopamine receptor agonist into the mPFC reinstated cocaine seeking behavior; ii) intra-PPTg/LDT administration of the AMPA/kainate receptor antagonist CNQX dose-dependently attenuated the ability of a priming injection of cocaine to reinstate drug seeking; and iii) administration of AMPA, nicotinic or muscarinic receptor antagonists into the VTA dose-dependently attenuated cocaine priming-induced reinstatement of drug seeking. Collectively, these results indicate that increases in glutamatergic transmission in the PPTg/LDT and enhancement of glutamatergic and cholinergic release in the VTA promote cocaine-priming induced reinstatement of drug seeking. Taken together, the present findings suggest that the PPTg/LDT region may function as an intermediate nucleus relaying information from the mPFC to the mesoaccumbal dopamine system during the reinstatement of cocaine seeking.
The role of medial prefrontal cortex dopamine in cocaine priming-induced reinstatement of drug seeking
Previous microinjection studies demonstrated that transient pharmacological inactivation of the dorsal mPFC (anterior cingulate and dorsal prelimic cortex), but not the ventral mPFC (i.e. ventral prelibic and infralimbic cortices), with GABAergic agonists, tetrodotoxin or dopamine receptor antagonists attenuated cocaine priming-induced reinstatement of drug seeking in rats (McFarland & Kalivas, 2001; Park et al., 2002; Capriles et al., 2003; Sun & Rebec, 2005). Moreover, administration of cocaine, amphetamine or dopamine into the dorsal mPFC reinstated cocaine seeking (McFarland & Kalivas, 2001; Park et al., 2002). The present results showed that administration of the D1-like dopamine receptor agonist, SKF-81297, into the dorsal mPFC reinstated cocaine seeking (albeit at relatively low levels compared to responding maintained on the last day of cocaine self-administration). Previous studies have demonstrated that the mPFC regulates cocaine-seeking behavior through extralimbic circuits encompassing the basolateral amygdala and dorsal hippocampus (Fuchs et al., 2007) and that different subregions of the mPFC play critical roles in drug seeking depending upon the type of contextual cue present during cocaine self-administration (Di Pietro et al., 2006). The low levels of cocaine seeking following administration of SKF-81297 directly into the mPFC may highlight the importance of this brain region in regulating cocaine-associated contextual cues (the present study did not incorporate contextual cues in its design) and/or the weak ability of mPFC D1-like dopamine receptors to modulate information processing through amygdala- and hippocampus-dependent circuits during cocaine-priming induced reinstatement. Therefore, the complete contribution of stimulating D1-like dopamine receptors in the mPFC on cocaine reinstatement cannot be determined by the present study because a full dose-response curve for intra-mPFC SKF-81297 was not determined. Moreover, a trend toward increased responding on the inactive lever was observed following intra-mPFC administration of SKF-81297, which makes it difficult to establish a firm conclusion based on these findings. However, these results are consistent with previous studies demonstrating a role for prefrontal cortex D1-like dopamine receptors in cocaine reinstatement (Sun & Rebec, 2005) (however see, Capriles et al., 2003). Taken together, these results indicate that increases in dopamine transmission in the dorsal mPFC play a critical role in cocaine-priming induced reinstatement of drug seeking.
A role for the PPTg/LDT in cocaine priming-induced reinstatement of drug seeking
As reviewed above, the mPFC efferents include a glutamatergic projection to the PPTg/LDT region (Sesack et al., 1989; Semba & Fibiger, 1992). The current results demonstrated that microinjection of the AMPA/kainate receptor antagonist CNQX into the PPTg/LDT attenuated the ability of a priming injection of cocaine to reinstate drug seeking. The mesopontine tegmentum consists of the rostral PPTg and the more caudal LDT, which are considered morphologically and physiologically homogenous nuclei (Steriade et al., 1990; Clements et al., 1991; Semba & Fibiger, 1992). Both the PPTg and LDT receive glutamatergic projections from the mPFC and send cholinergic and glutamatergic projections that synapse on dopaminergic neurons in the VTA (Clements et al., 1991; Semba & Fibiger, 1992; Charara et al., 1996; Oakman et al., 1999; Omelchenko & Sesack, 2005). Projections from the mesopontine tegmentum to the midbrain are topographically organized in the rodent brain, such that the LDT and caudal PPTg primarily innervate the VTA, whereas more rostral regions of the PPTg innervate the more medial substantia nigra (Oakman et al., 1995; Blaha et al., 1996; Forster & Blaha, 2000; Maskos, 2008). Although the microinjections of CNQX in the present study did not discriminate among subregions of the mesopontine tegmentum, it seems likely that the attenuation of the reinstatement of cocaine seeking was due to the modulation of activity in the VTA by afferents from the LDT, which receives inputs from the PPTg (Semba & Fibiger, 1992).
The role of AMPA/kainate glutamate receptors in the VTA in cocaine priming-induced reinstatement of drug seeking
A growing body of evidence indicates that the stimulation of dopaminergic neurons in the VTA reinstates cocaine seeking. For example, intra-VTA administration of compounds known to increase the firing rates of dopaminergic neurons promotes cocaine-seeking behavior (Stewart, 1984; Vorel et al., 2001; Placenza et al., 2004). Moreover, microinjection of the ionotropic gluamate receptor antagonist, kynurenate, into the VTA attenuated the reinstatement of drug seeking induced by a priming injection of cocaine as well as cocaine-associated cues (Sun et al., 2005). Consonant with these findings, the present results showed that intra-VTA administration of an AMPA/kainate receptor antagonist dose-dependently attenuated cocaine priming-induced reinstatement of drug seeking. Taken together, these findings demonstrate that increased glutamate transmission in the VTA promotes the reinstatement of cocaine seeking, presumably by increasing dopamine transmission in projection regions such as the nucleus accumbens (Schmidt et al., 2005; Schmidt & Pierce, 2006b; a).
A recent in vitro study demonstrated that stimulation of the PPTg increases acetylcholine release in the VTA and modulates activity of VTA neurons, in part, through an AMPA receptor-mediated mechanism of action (Good & Lupica, 2009). Moreover, PPTg-evoked synaptic currents in the VTA are decreased by an alpha7-nicotinic acetylcholine receptor (α7-nAchR) antagonist, suggesting that stimulation of the PPTg increases acetylcholine release in the VTA, which activates presynaptic α7-containing nicotinic acetylcholine autoreceptors and thereby decreases extracellular levels of glutamate in the VTA (Good & Lupica, 2009). Furthermore, tonic input from the LDT is required for glutamate-mediated burst firing in VTA dopamine neurons (Lodge & Grace, 2006b). These results are consistent with the present findings demonstrating that administration of AMPA or nicotinic acetylcholine receptor antagonists directly into the VTA attenuates cocaine priming-induced reinstatement, effects that are likely mediated by postsynaptic and presynaptic receptors, respectively.
Although the VTA receives glutamatergic afferents from several nuclei, few of these projections synapse directly onto dopaminergic neurons. Indeed, the primary glutamatergic afferents to dopaminegic neurons in the VTA arise from the PPTg/LDT (Charara et al., 1996; Maskos, 2008) and the bed nucleus of the stria terminalis (BNST) (Georges & Aston-Jones, 2001; 2002). While the role of the BNST in cocaine-primed reinstatement has not been determined, the BNST does play a role in stress-induced reinstatement of cocaine seeking (Erb & Stewart, 1999; McFarland et al., 2004). Together with the present results, these findings suggest that cocaine priming-induced reinstatement is mediated in part by glutamatergic projections from the PPTg/LDT, and perhaps the BNST, that synapse directly on dopaminergic neurons in the VTA.
The role of VTA acetylcholine in cocaine priming-induced reinstatement of drug seeking
Cholinergic projections that synapse on dopaminergic neurons in the VTA arise mainly from the caudal PPTg and LDT (Hallanger & Wainer, 1988; Oakman et al., 1995; Omelchenko & Sesack, 2005). Consistent with these anatomical findings, increased cholinergic transmission in the VTA was shown to activate dopaminergic neurons and promote dopamine release in the nucleus accumbens (Westerink et al., 1996; Forster & Blaha, 2000; Gronier et al., 2000). Therefore, we next wanted to assess the influence of nicotinic and muscarinic acetylcholine receptors, which are abundantly expressed on dopaminergic neurons in the VTA (Weiner et al., 1990; Klink et al., 2001), on the reinstatement of cocaine seeking. Our results indicate that administration of nicotinic (mecamylamine) or muscarinic (scopolamine) acetylcholine antagonists into the VTA attenuated the drug seeking induced by a cocaine priming injection. These results suggest that cocaine priming-induced reinstatement of drug seeking is mediated in part by increased cholinergic transmission in the VTA, which may lead to increased dopamine transmission in the nucleus accumbens. To our knowledge, our results are the first to implicate VTA acetylcholine in the reinstatement of cocaine seeking, which is surprising given that mecamylamine has been shown to reduce drug craving in human cocaine addicts (Reid et al., 1999).
Recent evidence indicates that the PPTg/LDT is comprised of distinct subpopulations of cholinergic, glutamatergic and GABAergic neurons (Wang & Morales, 2009). While a growing literature suggests that PPTg/LDT cholinergic and glutamatergic afferents regulate activity of dopamine neurons in the VTA (Overton & Clark, 1997; Forster & Blaha, 2000; Floresco & Grace, 2003; Lodge & Grace, 2006b), the precise role of PPTg/LDT GABAergic afferents on VTA dopamine neurons remains unknown. The present results indicate that both cholinergic and glutamatergic subpopulations of projection neurons in the PPTg/LDT play critical roles in the reinstatement of cocaine seeking.
Summary and Conclusions
The present data indicate a novel role for the PPTg/LDT in the circuitry mediating cocaine priming-induced reinstatement of drug seeking. As reviewed above, the PPTg/LDT receives excitatory glutamatergic projections from the mPFC and in turn sends excitatory glutamatergic and cholinergic projections to the VTA where they synapse on dopaminergic neurons. Our results indicate that increased dopamine transmission in the mPFC, enhanced glutamate transmission in the PPTg/LDT and augmented glutamate and acetylcholine release in the VTA collectively promote cocaine-seeking behavior in rats. However, future studies using more sophisticated disconnection experiments are required to determine the precise role of glutamatergic and cholinergic projections to the VTA from the PPTg and other nuclei mediating cocaine-seeking behavior. Collectively, these findings suggest that during the reinstatement of cocaine seeking the PPTg/LDT relays information from the mPFC to the mesoaccumbens dopaminergic system.
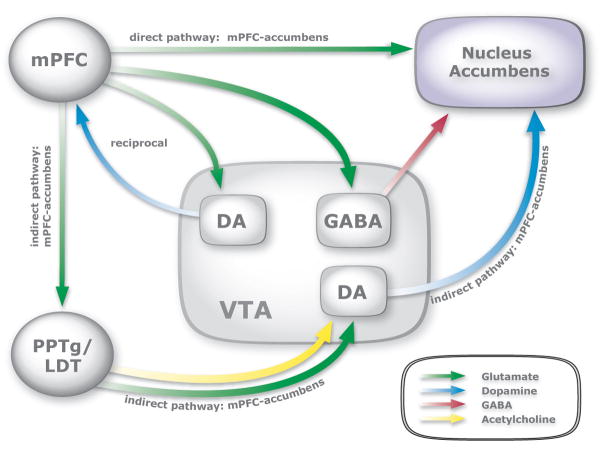
A simplification of the limbic circuitry underlying the reinstatement of cocaine seeking is presented in Figure 5. There is strong evidence supporting a role for the direct glutamatergic projection from the mPFC to the nucleus accumbens in the reinstatement of cocaine seeking (McFarland & Kalivas, 2001; Park et al., 2002; McFarland et al., 2003). The reciprocal connections between the mPFC and VTA also have been implicated in this process (Sun et al., 2005). The present findings further suggest that a serial polysynaptic subcircuit encompassing the mPFC, PPTg/LDT, VTA and nucleus accumbens plays a critical role in cocaine priming-induced reinstatement of drug seeking.
Figure 5. In addition to a direct glutamatergic projection, the mPFC modulates dopamine transmission in the nucleus accumbens through a serial polysynaptic circuit involving the PPTg/LDT and VTA.
The mPFC plays a critical role in cocaine reinstatement through direct glutamatergic projections to the nucleus accumbens and reciprocal connections with the VTA. Here we show that the mPFC also modulates dopamine transmission in the nucleus accumbens through a limbic circuit comprised of the PPTg/LDT and VTA. Thus, the mPFC plays a pivotal role in the limbic circuitry underlying cocaine reinstatement through both direct projections to the nucleus accumbens and VTA as well as an indirect, polysynaptic limbic circuit involving the PPTg/LDT and VTA.
Acknowledgments
This research was sponsored by research grants from the National Institutes of Health to R.C.P. (R01 DA15214, K02 DA18678). H.D.S. was supported in part by a National Research Service Award (NRSA) from the NIH (F31 DA16824). K.R.F. was partially supported by an NIH NRSA (F30 DA19304), as well as an NIH training grant (T32 GM008541). The authors also extend a note of gratitude to Adam Schmidt for sharing his expertise in multimedia design, Dr. Ruth Reeves for her contributions to this project, Judy Yee for her technical assistance and Audrey Pierce-Bancroft for her administrative assistance. The author(s) declare that there are no potential conflicts of interest relating to this study.
Abbreviations
- mPFC
medial prefrontal cortex
- PPTg
pedunculopontine tegmental nucleus
- LDT
laterodorsal tegmental nucleus
- FR
fixed ratio
- VTA
ventral tegmental area
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- i.v.
intravenous
- ANOVA
analysis of variance
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- BNST
bed nucleus of stria terminalis
References
- Alderson HL, Faulconbridge LF, Gregory LP, Latimer MP, Winn P. Behavioural sensitisation to repeated d-amphetamine: effects of excitotoxic lesions of the pedunculopontine tegmental nucleus. Neuroscience. 2003;118:311–315. doi: 10.1016/s0306-4522(03)00152-0. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Bechara A, van der Kooy D. The tegmental pedunculopontine nucleus: a brain-stem output of the limbic system critical for the conditioned place preferences produced by morphine and amphetamine. J Neurosci. 1989;9:3400–3409. doi: 10.1523/JNEUROSCI.09-10-03400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, van der Kooy D. A single brain stem substrate mediates the motivational effects of both opiates and food in nondeprived rats but not in deprived rats. Behav Neurosci. 1992;106:351–363. doi: 10.1037//0735-7044.106.2.351. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Steketee JD. Cocaine sensitization: modulation by dopamine D2 receptors. Cereb Cortex. 2002;12:526–535. doi: 10.1093/cercor/12.5.526. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Allen LF, Das S, Inglis WL, Latimer MP, Vincent SR, Winn P. Modulation of dopamine efflux in the nucleus accumbens after cholinergic stimulation of the ventral tegmental area in intact, pedunculopontine tegmental nucleus-lesioned, and laterodorsal tegmental nucleus-lesioned rats. J Neurosci. 1996;16:714–722. doi: 10.1523/JNEUROSCI.16-02-00714.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Haile CN, Coppersmith R, Hayashi Y, Malinow R, Neve RL, Nestler EJ. Distinct sites of opiate reward and aversion within the midbrain identified using a herpes simplex virus vector expressing GluR1. J Neurosci. 2000;20:RC62. doi: 10.1523/JNEUROSCI.20-05-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charara A, Smith Y, Parent A. Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: Phaseolus vulgaris-leucoagglutinin anterograde labeling combined with postembedding glutamate and GABA immunohistochemistry. J Comp Neurol. 1996;364:254–266. doi: 10.1002/(SICI)1096-9861(19960108)364:2<254::AID-CNE5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Clements JR, Toth DD, Highfield DA, Grant SJ. Glutamate-like immunoreactivity is present within cholinergic neurons of the laterodorsal tegmental and pedunculopontine nuclei. Adv Exp Med Biol. 1991;295:127–142. doi: 10.1007/978-1-4757-0145-6_5. [DOI] [PubMed] [Google Scholar]
- Cools AR, Lubbers L, van Oosten RV, Andringa G. SKF 83959 is an antagonist of dopamine D1-like receptors in the prefrontal cortex and nucleus accumbens: a key to its antiparkinsonian effect in animals? Neuropharmacology. 2002;42:237–245. doi: 10.1016/s0028-3908(01)00169-1. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Cornwall J, Cooper JD, Phillipson OT. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull. 1990;25:271–284. doi: 10.1016/0361-9230(90)90072-8. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL, Chow BL. Manipulations of mu-opioid and nicotinic cholinergic receptors in the pontine tegmental region alter cocaine self-administration in rats. Psychopharmacology (Berl) 1999;145:412–417. doi: 10.1007/s002130051075. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Zhang J, Adamson L. Pharmacological manipulations of the pedunculopontine tegmental nucleus in the rat reduce self-administration of both nicotine and cocaine. Psychopharmacology (Berl) 2002;160:198–205. doi: 10.1007/s00213-001-0965-2. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Grace AA. Gating of hippocampal-evoked activity in prefrontal cortical neurons by inputs from the mediodorsal thalamus and ventral tegmental area. J Neurosci. 2003;23:3930–3943. doi: 10.1523/JNEUROSCI.23-09-03930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 2000;12:3596–3604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- Forster GL, Blaha CD. Pedunculopontine tegmental stimulation evokes striatal dopamine efflux by activation of acetylcholine and glutamate receptors in the midbrain and pons of the rat. Eur J Neurosci. 2003;17:751–762. doi: 10.1046/j.1460-9568.2003.02511.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Futami T, Takakusaki K, Kitai ST. Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neurosci Res. 1995;21:331–342. doi: 10.1016/0168-0102(94)00869-h. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, Manaye KF. Midbrain dopaminergic neurons (nuclei A8, A9, and A10): three-dimensional reconstruction in the rat. J Comp Neurol. 1993;331:297–309. doi: 10.1002/cne.903310302. [DOI] [PubMed] [Google Scholar]
- Good CH, Lupica CR. Properties of distinct ventral tegmental area (VTA) synapses activated via pedunculopontine or VTA stimulation in vitro. J Physiol. 2009 doi: 10.1113/jphysiol.2008.164194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronier B, Perry KW, Rasmussen K. Activation of the mesocorticolimbic dopaminergic system by stimulation of muscarinic cholinergic receptors in the ventral tegmental area. Psychopharmacology (Berl) 2000;147:347–355. doi: 10.1007/s002130050002. [DOI] [PubMed] [Google Scholar]
- Hallanger AE, Wainer BH. Ascending projections from the pedunculopontine tegmental nucleus and the adjacent mesopontine tegmentum in the rat. J Comp Neurol. 1988;274:483–515. doi: 10.1002/cne.902740403. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Goeders NE. Intra-medial prefrontal cortex injections of scopolamine increase instrumental responses for cocaine: an intravenous self-administration study in rats. Brain Res Bull. 2000;51:151–158. doi: 10.1016/s0361-9230(99)00214-2. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Kohl RR, McBride WJ. GABA(A) receptor blockade in the anterior ventral tegmental area increases extracellular levels of dopamine in the nucleus accumbens of rats. J Neurochem. 1997a;69:137–143. doi: 10.1046/j.1471-4159.1997.69010137.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Self-infusion of GABA(A) antagonists directly into the ventral tegmental area and adjacent regions. Behav Neurosci. 1997b;111:369–380. doi: 10.1037//0735-7044.111.2.369. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Regional differences within the rat ventral tegmental area for muscimol self-infusions. Pharmacol Biochem Behav. 1998;61:87–92. doi: 10.1016/s0091-3057(98)00086-0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Karreman M, Westerink BH, Moghaddam B. Excitatory amino acid receptors in the ventral tegmental area regulate dopamine release in the ventral striatum. J Neurochem. 1996;67:601–607. doi: 10.1046/j.1471-4159.1996.67020601.x. [DOI] [PubMed] [Google Scholar]
- Kelland MD, Freeman AS, Rubin J, Chiodo LA. Ascending afferent regulation of rat midbrain dopamine neurons. Brain Res Bull. 1993;31:539–546. doi: 10.1016/0361-9230(93)90121-q. [DOI] [PubMed] [Google Scholar]
- Kippen TE, van der Kooy D. Blockade of sexually-rewarded conditioned place preference by tegmental pedunculopontine nucleus lesions. Behav Pharmacol. 2001;12:S52. [Google Scholar]
- Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006a;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci U S A. 2006b;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskos U. The cholinergic mesopontine tegmentum is a relatively neglected nicotinic master modulator of the dopaminergic system: relevance to drugs of abuse and pathology. Br J Pharmacol. 2008;153 Suppl 1:S438–445. doi: 10.1038/bjp.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci Lett. 1993;157:53–56. doi: 10.1016/0304-3940(93)90641-w. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Wetter JB, Milovanovic M, Wolf ME. The laterodorsal tegmentum contributes to behavioral sensitization to amphetamine. Neuroscience. 2007;146:41–49. doi: 10.1016/j.neuroscience.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Cozzari C, Hartman BK. Characterization of the extent of pontomesencephalic cholinergic neurons' projections to the thalamus: comparison with projections to midbrain dopaminergic groups. Neuroscience. 1999;94:529–547. doi: 10.1016/s0306-4522(99)00307-3. [DOI] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Munn EM, Franklin KB, Wise RA. Effects of pedunculopontine tegmental nucleus lesions on responding for intravenous heroin under different schedules of reinforcement. J Neurosci. 1998;18:5035–5044. doi: 10.1523/JNEUROSCI.18-13-05035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ, Hughes T, Self DW, Neve RL, Nestler EJ. Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. J Neurosci. 2005;25:5553–5562. doi: 10.1523/JNEUROSCI.0345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol. 2005;483:217–235. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1997. [DOI] [PubMed] [Google Scholar]
- Placenza FM, Fletcher PJ, Rotzinger S, Vaccarino FJ. Infusion of the substance P analogue, DiMe-C7, into the ventral tegmental area induces reinstatement of cocaine-seeking behaviour in rats. Psychopharmacology (Berl) 2004;177:111–120. doi: 10.1007/s00213-004-1912-9. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Kelley AE. Striatal muscarinic receptor antagonism reduces 24-h food intake in association with decreased preproenkephalin gene expression. Eur J Neurosci. 2005;22:3229–3240. doi: 10.1111/j.1460-9568.2005.04489.x. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Berger SP. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology. 1999;20:297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 Dopamine Receptors in the Shell, but Not the Core, of the Nucleus Accumbens Reinstates Cocaine-Seeking Behavior in the Rat. European Journal of Neuroscience. 2006;23:219–228. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience. 2006a;142:451–461. doi: 10.1016/j.neuroscience.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Systemic administration of a dopamine, but not a serotonin or norepinephrine, transporter inhibitor reinstates cocaine seeking in the rat. Behav Brain Res. 2006b;175:189–194. doi: 10.1016/j.bbr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- See RE, McLaughlin J, Fuchs RA. Muscarinic receptor antagonism in the basolateral amygdala blocks acquisition of cocaine-stimulus association in a model of relapse to cocaine-seeking behavior in rats. Neuroscience. 2003;117:477–483. doi: 10.1016/s0306-4522(02)00665-6. [DOI] [PubMed] [Google Scholar]
- Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol. 1992;323:387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sharf R, Ranaldi R. Blockade of muscarinic acetylcholine receptors in the ventral tegmental area disrupts food-related learning in rats. Psychopharmacology (Berl) 2006;184:87–94. doi: 10.1007/s00213-005-0235-9. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Rye DB, Wainer BH. Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I. Retrograde tracing studies. J Comp Neurol. 1992;321:515–543. doi: 10.1002/cne.903210403. [DOI] [PubMed] [Google Scholar]
- Steriade M, Pare D, Datta S, Oakson G, Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci. 1990;10:2560–2579. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. Reinstatement of heroin and cocaine self-administration behavior in the rat by intracerebral application of morphine in the ventral tegmental area. Pharmacol Biochem Behav. 1984;20:917–923. doi: 10.1016/0091-3057(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Sun W, Akins CK, Mattingly AE, Rebec GV. Ionotropic glutamate receptors in the ventral tegmental area regulate cocaine-seeking behavior in rats. Neuropsychopharmacology. 2005;30:2073–2081. doi: 10.1038/sj.npp.1300744. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005;177:315–323. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Sziraki I, Sershen H, Hashim A, Lajtha A. Receptors in the ventral tegmental area mediating nicotine-induced dopamine release in the nucleus accumbens. Neurochem Res. 2002;27:253–261. doi: 10.1023/a:1014844823534. [DOI] [PubMed] [Google Scholar]
- Taber MT, Das S, Fibiger HC. Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. J Neurochem. 1995;65:1407–1410. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci U S A. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink BH, Kwint HF, deVries JB. The pharmacology of mesolimbic dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. J Neurosci. 1996;16:2605–2611. doi: 10.1523/JNEUROSCI.16-08-02605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn P. How best to consider the structure and function of the pedunculopontine tegmental nucleus: evidence from animal studies. J Neurol Sci. 2006;248:234–250. doi: 10.1016/j.jns.2006.05.036. [DOI] [PubMed] [Google Scholar]