Abstract
Minocycline (an anti-inflammatory drug approved by the FDA) has been reported to be effective in mouse models of amyotrophic lateral sclerosis and Huntington disease. It has been suggested that the beneficial effects of minocycline are related to its ability to influence mitochondrial functioning. We tested the hypothesis that minocycline directly inhibits the Ca2+-induced permeability transition in rat liver mitochondria. Our data show that minocycline does not directly inhibit the mitochondrial permeability transition. However, minocycline has multiple effects on mitochondrial functioning. First, this drug chelates Ca2+ ions. Secondly, minocycline, in a Ca2+-dependent manner, binds to mitochondrial membranes. Thirdly, minocycline decreases the proton-motive force by forming ion channels in the inner mitochondrial membrane. Channel formation was confirmed with two bilayer lipid membrane models. We show that minocycline, in the presence of Ca2+, induces selective permeability for small ions. We suggest that the beneficial action of minocycline is related to the Ca2+-dependent partial uncoupling of mitochondria, which indirectly prevents induction of the mitochondrial permeability transition.
Keywords: Minocycline, Mitochondria, Permeability transition, Bilayer membrane, Channel formation
Introduction
Minocycline (7-dimethylamino-6-dimethyl-6-deoxytetracycline; see Fig. 1) is an FDA approved anti-inflammatory derivative of tetracycline that possesses a number of clinically useful properties. The non-antibiotic properties of this drug have been intensively investigated and have been shown to be beneficial in a number of disparate medical conditions, including rosacea, bullous dermatoses, neutrophilic diseases, pyoderma gangrenosum, sarcoidosis, aortic aneurysms, cancer metastasis, periodontitis, and autoimmune disorders such as rheumatoid arthritis and scleroderma (reviewed in: Sipos et al. 1994; Sapadin and Fleischmajer 2006).
Fig. 1.
Structural formula of Minocycline
It has been reported that some of the favorable effects of minocycline are related to its ability to influence mitochondrial functioning. For example, Lin et al. (2002) showed that minocycline treatment reduces the levels of RNA transcripts of mitochondrion-encoded genes in the protozoan Plasmodium falciparum. Several groups have reported that minocycline prevents, or considerably lowers, pathophysiologically related cytochrome c (cytC) release from mitochondria—a key process leading to apoptosis. Work from Friedlander’s laboratory showed that minocycline inhibits cytC release in rodent models of amyotrophic lateral sclerosis (Zhu et al. 2002), Huntington disease (Wang et al. 2003) and spinal cord injury (Teng et al. 2004). The authors suggested that the cytC release that was inhibited by minocycline is mediated by the mitochondrial permeability transition (MPT) (Zhu et al. 2002; Wang et al. 2003; Teng et al. 2004). Data presented by Matsuki et al. (2003) indicated that minocycline suppresses heat stress-induced release of cytC from mitochondria both in vitro and in vivo. Scarabelli et al. showed that minocycline significantly reduces the expression level of initiator caspases, increases the ratio of X-linked inhibitor of apoptosis protein to Smac/DIABLO at both the messenger RNA and protein level, and prevents release of cytC and Smac/DIABLO from mitochondria (Scarabelli et al. 2004). It was also reported that under stressful or pathological conditions, minocycline protects mitochondria by inducing up-regulation of antiapoptotic Bcl-2 and down-regulation of proapoptotic Bax, Bak, Bid (Wang et al. 2004; Castanares et al. 2005), and Fas (Chu et al. 2005) proteins. An underlying mechanism for this protection was suggested to result from a decrease in membrane damage and cytC release, leading to blockage of downstream caspase activation (Wang et al. 2004; Castanares et al. 2005; Chu et al. 2005). In these reports (Wang et al. 2004; Castanares et al. 2005; Chu et al. 2005), the authors proposed that the beneficial effects of minocycline involve direct or indirect inhibition of MPT in mitochondria.
However, studies that were performed by Jordán’s group on cerebral granular cells using a malonate-induced model of apoptosis showed that minocycline in the concentration range of 10–100 µM is not cytoprotective (Fernandez-Gomez et al. 2005a). The authors presented data indicating that minocycline is not able to prevent Bcl-2 down-regulation by malonate. In the set of experiments conducted by this group on isolated mitochondria, the authors showed that minocycline is protective against Ca2+-induced mitochondrial swelling. The authors suggested that this effect might be mediated through dissipation of the mitochondrial membrane potential (ΔΨ) and inhibition of Ca2+ uptake (Fernandez-Gomez et al. 2005b). Recently, other groups have presented data indicating that the neuroprotective mechanism of minocycline in animals and humans is not related to the inhibition of MPT (Cornet et al. 2004; Mansson et al. 2007). Furthermore, Smith et al. (2003) have suggested that minocycline at high micromolar concentrations might even be toxic rather than protective.
Very recently, Kupsch et al. (2009) suggested that the apparent discrepancy in the effects of minocycline that have been reported by different groups may likely be related to the difference in experimental conditions that were used. The authors showed that in a KCl-based incubation medium, but not in a sucrose-based buffer, minocycline administered directly to the isolated mitochondria triggers mitochondrial swelling and cytC release (Kupsch et al. 2009). The authors also presented data indicating that minocycline, even in low micromolar concentrations in a KCl-based buffer, impairs several energy-dependent functions of mitochondria in vitro such as ΔΨ and substrate-dependent oxygen (O2) uptake (Kupsch et al. 2009). The authors provided strong evidence that the major effects of minocycline on mitochondria are likely related to its ability to deplete endogenous mitochondrial [Mg2+] and induce inner mitochondrial membrane (IMM) permeabilization (Kupsch et al. 2009). The finding that minocycline induces permeabilization of the IMM is undoubtedly an important step forward in the study of this drug. Nevertheless, the question “Is the actual mechanism of such permeabilization related to the MPT or to a phenomenon that is unrelated to the MPT?” still remains to be answered.
It has been shown that some antibiotics of the tetracycline family exhibit ionophoric properties in model membrane systems. For example, Chlortetracycline, which has a close structural similarity to minocycline, was shown to transport calcium ions through an organic solvent phase in a pH-dependent manner (White and Pearce 1982). Based on this information, we suggest that minocycline may induce ion fluxes across the IMM. However, there is no evidence in the literature that minocycline directly interacts with mitochondria. The present study was designed to determine whether minocycline directly interacts with mitochondria and possesses channel-forming abilities. To accomplish these goals, experiments were carried out with isolated rat liver mitochondria (RLM), a planar bilayer lipid membrane (BLM) model and a liposome model.
Materials and methods
Chemicals
β-Alanine, micro Biuret protein assay kit, bovine serum albumin (fatty acid free grade Sigma A-6003), CaCl2, EGTA, gramicidin A, Hepes (ultra grade), minocycline, KCl, KOH, KH2PO4, MES, Ruthenium 360 (Ru360), succinate, sucrose (ultra grade), PBS-Tween buffer, and Tris-base were obtained from Sigma (St. Louis, MO, USA). Cholesterol, dioleoylphosphatidylcholine, dioleoylphosphatidylglycerol, 1,2-diphytanoyl-sn-glycero-3-phosphocholine, diphytanoyl-phosphatidylcholine (DPhPC), egg phosphatidylcholine and Escherichia coli total lipid fraction were obtained from Avanti Polar Lipids (Alabaster, AL, USA). n-Decane was obtained from Merck (Darmstadt, Germany). All reagents used were of the highest purity available.
Recordings of minocycline spectra
Minocycline was dissolved in ethanol and then diluted with water to yield a final concentration of 20 mM in 10% ethanol. Minocycline is unstable in solution if stored at room temperature; when the solution is stored in the refrigerator (4 °C), it is stable for not more than two weeks. Therefore, minocycline solutions were used no later than one week after preparation and storage at 4 °C. Minocycline spectra were recorded in a plate reader SpectraMax 340 system (Molecular Devices Corporation, Downing-town, PA, USA) using untreated, medium binding, Corning 96-well clear flat bottom polystyrene plates (Thomas Scientific Inc., Swedesboro, NJ, USA).
Isolation of rat liver mitochondria and mitochondrial experiments
Mitochondria were isolated from six-month old male/female Fisher 344X Brown Norway F1 rats (fed ad libitum and with full access to water) by the procedure described previously (Krasnikov et al. 2005). All procedures were carried out on ice in a 4 °C cold room. Mitochondria isolated using this procedure and kept on ice were stable (as judged by consistent ADP/O ratio and RCR values) for at least 12 h after isolation. The mitochondrial protein concentration was determined by the micro Biuret method.
Mitochondrial functioning was assessed by using a multi-parameter chamber (for details see Krasnikov et al. 2005), which permits simultaneous measurement of four mitochondrial parameters: O2 uptake using a Clark-type closed electrode; calcium ion flux by means of a Ca2+-selective electrode and a reference electrode; ΔΨ using a TPP+-selective electrode and reference electrode; swelling as reflected by changes in transmittance at 660 nm (by means of a light-emitting and a photo diodes).
The experimental incubation buffer consisted of 300 mM sucrose, 5 mM Hepes, 2.5 mM KH2PO4 supplemented with 5 mM succinate and 1 µM rotenone, pH adjusted to 7.4 with Tris-base. The concentrations of the reagents added to the mitochondria in all experiments presented here were such that the volume (1 ml) was altered by less than 2%. All additions listed in the figure legends are final concentrations.
Experimental design for determination of minocycline binding to mitochondria
RLM protein concentration was varied from 0.25 to 3 mg/ml. Minocycline was used at concentrations of 100 and 200 µM. The concentration of Ca2+ was varied from 5 µM to 2 mM. Mitochondria were incubated with minocycline in 1 ml of sucrose buffer (300 mM sucrose, 3 mM Hepes, 2.5 mM KH2PO4, 5 mM succinate plus 1 µM rotenone, pH 7.4) on ice for either 3 or 5 min (except where noted). Controls were identical except that minocycline was omitted. After incubation, the mitochondria were centrifuged at 14,000 g for 10 min at 4 °C. The supernatant was carefully removed, labeled as the ‘Super’ fraction and saved. The mitochondrial pellet was re-suspended in 1 ml of PBS-Tween buffer (10 mM phosphate-buffered saline, 0.5% (v/v) Tween, pH 7.4). The procedure results in solubilization of most of the mitochondrial pellet. The solubilized sample was centrifuged at 14,000 g for 10 min at 4 °C. The supernatant was removed, labeled as the ‘Pellet’ fraction and saved. A very small amount of insoluble material was discarded.
The ‘Super’ fraction contained minocycline unbound to RLM, whereas the ‘Pellet’ contained minocycline bound to mitochondria. In order to obtain the spectrum of minocycline in the ‘Pellet’ fraction it was necessary to subtract the blank spectrum due to detergent and mitochondrial components in the sample.
We investigated the possibility of using a filtration procedure to rapidly separate suspension buffer from mitochondria. However, we found that variable amounts (up to 30%) of minocycline were bound to the filter membranes (data not shown). Therefore, in all experiments carried out on mitochondria, the incubation buffer was removed by centrifugation.
Planar bilayer lipid membranes
To verify the suggestion that minocycline can induce conductivity in phospholipid membranes, a set of experiments was carried out using the planar BLM model. BLMs were formed from a 2% solutions of DPhPC, 1,2-diphytanoyl-snglycero-3-phosphocholine or E. coli total lipid extract in n-decane by the brush technique (Mueller et al. 1963). Lipid was applied to a 0.55-mm diameter hole in a Teflon partition that separates two compartments of a specially designed cell (see Mueller et al. 1963 for details). Both compartments were filled with buffer containing 100 mM KCl, 10 mM MES, 10 mM Tris, 10 mM β-alanine, pH 8.5 (unless otherwise stated). The electrical current (I) was measured with an amplifier (Keithley 428, Keithley Instruments, Cleveland, OH, USA), digitized by a LabPC 1200 (National Instruments, Austin, TX, USA), and analyzed using WinWCP Strathclyde Electrophysiology Software designed by J. Dempster (University of Strathclyde, UK). A voltage of 60 mV (unless otherwise stated) was applied to the BLM with Ag/AgCl electrodes placed directly into the cell.
Detection of proton transport in pyranine-loaded liposomes
The lumenal pH of the liposomes was assayed with pyranine [a fluorescent dye with a pKa ∼ 7.3 and a λex = 405 nm in its acid form (−3 charge) and a λex=455 nm in its basic form (−4 charge)] by a slight modification of the previously published procedure (Chen et al. 1999). Briefly, to prepare pyranine-loaded liposomes, lipids (5 mg of egg phosphatidylcholine and 1 mg of cholesterol) in a chloroform suspension were dried in a round bottom flask under a stream of nitrogen. The lipids were then resuspended in buffer (100 mM KCl, 20 mM MES, 20 mM MOPS, 20 mM Tricine titrated with KOH to pH 6.0) containing 0.5 mM pyranine. The suspension was vortexed and then freeze-thawed 3 times. Unilamellar liposomes were prepared by extrusion through 0.1-µm-pore size Nucleopore polycarbonate membranes using an Avanti Mini-Extruder. The unbound pyranine was then removed by passage through a Sephadex G-50 coarse column equilibrated with the same buffer solution. Pyranine leakage from the liposomes was less than 1% per day. The liposomes were used within 1 week of preparation. To measure the rate of pH dissipation in liposomes with a lumenal pH 6.0, the liposomes were diluted in a solution buffered to pH 8.0. The emission fluorescence at 510 nm (excitation 455 nm) was monitored with a Panorama Fluorat 02 spectrofluorimeter (Lumex, Russia). At the end of each recording, 1 µM nigericin was added to dissipate the remaining pH gradient.
Leakage assay with carboxyfluorescein-loaded liposomes
Liposomes were prepared from the same lipid mixture as described in the above section in a solution containing 100 mM carboxyfluorescein titrated with Tris-base to pH 8.5. The unloaded carboxyfluorescein was then removed by passage through a Sephadex G-50 coarse column using an eluting buffer containing: 100 mM KCl, 20 mM MES, 20 mM MOPS, and 20 mM Tricine titrated with KOH to pH 8.0. To measure the rate of carboxyfluorescein leakage, the liposomes were diluted in the same buffer. The emitted fluorescence was monitored at 520 nm (excitation 490 nm) as described above. At the end of each recording, 1 µM mellitin was added to complete the leakage process.
All experiments were carried out at room temperature (∼20 °C) unless indicated otherwise. All results presented here are average ± standard deviation and were calculated using SigmaPlot 2000 software; p values were calculated by the paired t-test using Microsoft Excel software.
Results
Determination of an isosbestic point in the minocycline spectrum that is independent of buffer composition, pH and [Ca2+]
The minocycline spectrum was noted to change in the presence of Ca2+, indicating formation of complex(es) between these two components (Fig. 2, A Panels). In this respect, it should be emphasized that correct measurement of minocycline concentrations must take into account possible alterations in the spectrum of the compound in different buffers and under different conditions. To this end, measurement of minocycline concentrations should be determined at the wavelength of an isosbestic point. For the sucrose buffer, the Ca2+-independent minocycline isosbestic point was found to be at ∼358 nm (Fig. 2, B Panels).
Fig. 2.
Determination of isosbestic points for the minocycline spectrum under different experimental conditions. (A Panels) Ca2+-dependent changes in the spectrum of minocycline at three different concentrations (Black, 60 µM; Red, 120 µM; Green, 200 µM) in sucrose buffer at three different [Ca2+]. (B Panels) Determination of the Ca2+-independent isosbestic points for minocycline spectra in sucrose buffer at three concentrations of minocycline (Mino) at four [Ca2+] (Black, no Ca2+; Red, 50 µM; Green, 100 µM; Yellow, 150 µM; Blue, 200 µM). (C Panels) Dependence of the minocycline spectra on the experimental buffer and [Ca2+]. The Hepes buffer contained 3 mM Hepes, pH 7.4; the Sucrose buffer consisted of 300 mM sucrose, 3 mM Hepes, pH 7.4; and the PBS*Tween buffer contained 10 mM phosphate-buffered saline, 0.5 % Tween, pH 7.4. The minocycline concentration was 100 and 200 µM ([Ca2+]: Black, no Ca2+; Red, 20 µM; Green, 50 µM; Yellow, 100 µM). (D Panels) pH dependence (Black, pH 6.6; Red, pH 7.4; Green, pH 8.2) of the minocycline (80 µM) spectrum in sucrose buffer supplemented with 40 and 60 µM Ca2+
Due to the fact that mitochondrial experiments were carried out with different buffers (Hepes, Sucrose, and PBS*Tween) it was also necessary to determine the Ca2+-independent isosbestic points for minocycline in all the buffers used (Fig. 2, C Panels). We also took into account the possibility that a potential change in pH may occur during the mitochondrial experiments. Therefore, isosbestic points for different pH and Ca2+ values were obtained (Fig. 2,D Panels). Finally, we found that the Ca2+-, buffer-, and pH-independent isosbestic points for minocycline were in a very close range of ∼356–360 nm. Thus, under conditions in which Ca2+ or pH might change unpredictably, the concentration of the total minocycline can still be calculated from the absorbance at the isosbestic point (∼358 nm).
In order to calculate the amount of the complex formed between minocycline and Ca2+ (Mino*Ca) the concentration dependence of the free minocycline (Mino) at 340 nm absorbance (peak of the minocycline spectrum; Fig. 3, Panel A) was first determined, and an equation and coefficients of the linear regression were established (Fig. 3, Panel B). Next, a series of titrations with increasing Ca2+ concentrations (0–100 µM) was performed using a single 100 µM concentration of minocycline. The decrease in absorbance at 340 nm corresponded to an increase in the Ca2+-related formation of the Mino*Ca complex (Fig. 3, Panel C). The values of the absorbance for each [Ca2+]at 340 nm were used to calculate the concentration of Mino using the equation and coefficients of the linear regression shown in Panel B. Mino*Ca was calculated as 100 µM minus Mino. Titration of minocycline (100 µM) over the range of 0 to 2 mM Ca2+ was performed. The dependence of the Mino*Ca/Mino ratio on [Ca2+] is presented in Fig. 3, Panels D and E. Panel D shows Ca2+-dependent (sigmoidal) changes in the Mino*Ca/Mino ratio over a wide range of Ca2+ concentrations in sucrose buffer. Panel E of Fig. 3 shows the linear ratio dependence at low concentrations of Ca2+ (0–200 µM). The concentration of Ca2+ required to yield a Mino*Ca/Mino ratio equal to 1 was determined from the linear regression plot shown in Panel E. This value is the Ca2+-binding constant KCa2+ for the Mino*Ca complex and is ∼65 µM (see the “Discussion”).
Fig. 3.
Calculation of the ratio of the complex formed between minocycline and Ca2+ (Mino*Ca) to free minocycline (Mino) in sucrose buffer. (Panel A) Concentration dependence of the minocycline (Mino) spectra. (Panel B) Calibration curve for Mino absorbance at 340 nm versus concentration, showing the equation and coefficients of the linear regression. (Panel C) Ca2+-dependent changes in the spectrum of 100 µM minocycline. The values of the absorbance for each Ca2+ concentration at 340 nm in Panel C were used to calculate the concentration of Mino using the equation of the linear regression from Panel B. [Mino*Ca] was calculated as 100 µM minus [Mino]. (Panel D) The sigmoidal dependence of the Mino*Ca/Mino ratio over a wide range of [Ca2+]. (Panel E) Linearity of Mino*Ca/Mino versus [Ca2+] in the low [Ca2+] range of Panel D. The binding constant (KCa2+) found from Panel E for Ca2+ in the presence of 100 µM minocycline is ∼65 µM as determined from the value of the Mino*Ca/Mino ratio equal to 1
Binding of minocycline to mitochondria
RLM at a total protein concentration of 1 mg/ml were incubated in sucrose buffer containing 200 µM minocycline for 5 min on ice. After incubation the samples were c entrifuged at 14,000 g and the spectrum of minocycline in the supernatant was recorded. The concentration of minocycline was calculated from the absorbance at the isosbestic point (∼358 nm). The minocycline concentrations in the control buffer (lacking mitochondria), and two experimental supernatants (RLM, no Ca2+ added; and RLM, 50 µM Ca2+ added) were: 200, 132±16.5 and 78±10.5 µM, respectively (Fig. 4, Panel A).
Fig. 4.
(Panel A) Decrease of minocycline (200 µM) concentration in sucrose buffer (1 ml) after incubation with RLM (1 mg/ml) for 5 min on ice in the absence or presence of 50 µM Ca2+. N=4. (Panel B) Ca2+-dependence of minocycline (100 µM) binding to mitochondria (1 mg/ml/3min). Gray diamond symbols with error bars are the average ± standard deviation. N=4. White and black diamond symbols represent values from two experiments. The sucrose buffer contained 300 mM sucrose, 3 mM Hepes, 2.5 mM KH2PO4, and 5 mM succinate plus 1 µM rotenone, pH 7.4
The mitochondria-induced decrease in minocycline concentration in the supernatant suggests that this compound binds to these organelles. The Ca2+-induced increase in minocycline binding to RLM was investigated. The concentrations of RLM and minocycline were fixed at 1 mg/ml and 100 µM, respectively, but the concentration of Ca2+ was varied from 0 to 100 µM. After incubation in sucrose buffer on ice for 3 min, the amount of minocycline bound to RLM was determined from the concentration of the minocycline remaining in the supernatant, and is presented as nmol/mg of mitochondrial protein (Fig. 4, Panel B). From the data depicted in this figure, one can calculate that the maximal amount of minocycline bound to RLM is ∼60 nmol/mg of protein. Maximal binding requires the presence of at least 40 µM Ca2+.
The time dependence for minocycline binding to mitochondria was determined in sucrose buffer under the following conditions: 0.5 mg/ml RLM was incubated on ice for 3 min with 100 µM minocycline, followed by an additional period of incubation in the presence or absence of 50 µM Ca2+ (Fig. 5, Panel A). The T1/2 value for minocycline binding in the presence of Ca2+ was shorter (less than 1.5 min) compared to that obtained in the absence of Ca2+ (∼2.5 min).
Fig. 5.
(Panel A) Time-dependence of minocycline (100 µM) binding to RLM (0.5 mg of protein/ml). N=4. (Panel B) Minocycline (200 µM) binding (5 min on ice) to RLM is dependent on RLM concentration and the presence of Ca2+. The values are the average ± standard deviation. N=4, except for a single measurement indicated as ‘$’. Individual sets of experiments in Panels A and B were carried out four months apart, which may have introduced some variability. The experimental buffer conditions were the same as those described in the legend to Fig. 2 and the total volume of the experimental mixture was 1 ml
The dependence of minocycline (200 µM) binding on the concentration of mitochondria was also investigated. Binding was determined after 5 min incubation on ice in a reaction mixture in which the final volume was 1 ml (Fig. 5, Panel B). Minocycline binding was found to be satisfactorily described by the following empirical equation: Y = a(1 − bx). This equation holds true whether or not Ca2+ (50 µM) was present in the buffer. In both cases, regression analysis yielded r2 coefficients of 0.991. The equation predicts a maximum amount of minocycline bound to mitochondria (corresponding to 3 mg of protein) of 170 nmol. Half of the minocycline binding occurred with mitochondrial amounts corresponding to ∼0.5 mg and ∼1.8 mg of protein, respectively, in the presence and absence of Ca2+ (Fig. 5, Panel B).
Effect of minocycline on mitochondrial ion fluxes
The effect of minocycline on Ca2+-induced MPT in RLM was investigated using the multiparameter chamber (Krasnikov et al. 2005). Four parameters were measured simultaneously in the same sample: O2 uptake, Ca2+ flux, ΔΨ and swelling. Representative results are shown in Fig. 6. The experimental conditions were: sucrose buffer, RLM 0.45 mg/ml, additions: Ca2+ = 60 µM, minocycline = 100 µM and Ru360 = 100 µg/ml (Ru360 is a specific inhibitor of the mitochondrial Ca2+ uniporter (Matlib et al. 1998)).
Fig. 6.
Effects of different treatments on four parameters measured simultaneously in RLM. Traces ‘a’, MPT induction by Ca2+; traces ‘b’, effect of minocycline; traces ‘c’, inhibitory effect of Ru360 on MPT. Experimental conditions: sucrose buffer contained 300 mM sucrose, 3 mM Hepes, 2.5 mM KH2PO4, and 5 mM succinate plus 1 µM rotenone, pH 7.4. The RLM concentration was 0.45 mg/ml. Additions of Ca2+, minocycline (M) and Ru360 (Ru) were 60, 100 µM and 100 µg/ml, respectively. Panels A–D: O2 uptake, Ca2+ flux, ΔΨ, and swelling, respectively. (Panel A) The decrease in O2 concentration reflects O2 uptake by mitochondria. (Panel B) The increase in Ca2+ concentration indicates either Ca2+ efflux from mitochondria or absence of Ca2+ influx into mitochondria. (Panel C) The increase in TPP+ concentration reflects ΔΨ dissipation. (Panel D) The decrease in absorbance indicates mitochondrial swelling. The traces are representative of at least four experiments obtained with different mitochondrial preparations
The classical Ca2+-induced MPT in RLM is characterized by: 1) a nonlinear increase of O2 uptake, 2) an initial sequestration of added Ca2+ followed by its release, 3) an initial drop of ΔΨ followed by recovery and further dissipation, and 4) a high-amplitude swelling (e.g. Krasnikov et al. 2005). See also Fig. 6, Panels A–D, traces ‘a’.
Addition of 60 µM Ca2+ to RLM previously incubated with Ru360 for 3 min, resulted in 1) no increase in O2 uptake, 2) no uptake of added Ca2+ (the steady state level of Ca2+ remained at ∼60 µM), 3) no dissipation of ΔΨ, and 4) no swelling (Fig. 6, Panels A–D, traces ‘c’).
We found that the initial addition of minocycline to RLM caused 1) a linear increase in O2 uptake, 2) a significant drop, followed by partial recovery of ΔΨ, and 3) a short (less than 1 min), but rapid, initial decrease in absorbance, followed by a plateau (Fig. 6, Panels A–D, traces ‘b’).
Addition of Ca2+ in the presence of minocycline did not affect the linear increase in O2 uptake. No uptake of added Ca2+ was recorded (Fig. 6, Panel A, trace ‘b’), and the steady state level of the Ca2+ remained at ∼30–35 µM (Fig. 6, Panel B, trace ‘b’). At the time at which Ca2+ was added, the ΔΨ was completely abolished (Fig. 6, Panel C, trace ‘b’), and a short (∼2 min), rapid decrease in absorbance occurred, followed by a trend toward a plateau (Fig. 6, Panel D, trace ‘b’).
We found that in the KCl-based buffer (KCl 150 mM), the minocycline-related decrease in ΔΨ was somewhat stronger and the rate of this decrease was more rapid compared to the relatively slow dissipation of ΔΨ that occurred in sucrose buffer (Fig. 7, Panel A). At pH 8.0 addition of minocycline almost completely abolished ΔΨ, whereas at pH 6.8 the change in ΔΨ was similar to that which occurred at neutral pH (Fig. 7, Panel C). In contrast to the sucrose buffer where addition of Ca2+ completely abolished ΔΨ, partial restoration of the potential was observed after addition of Ca2+ in KCl-based buffer (Fig. 7, Panel A). The level of ΔΨ restoration was dependent on KCl concentration (data not shown).
Fig. 7.
Effects of minocycline on ΔΨ and swelling of mitochondria upon addition of Ca2+. (Panels A and B) Sucrose-(black traces) and 150 mM KCl buffer (gray traces), respectively. (Panels C and D) The effects of minocycline on ΔΨ and swelling of RLM under slightly acidic (pH 6.8, black traces) and slightly basic (pH 8.0, gray traces) conditions in Sucrose buffer, respectively. Additions: RLM, rat liver mitochondria 0.5 mg/ml; M, minocycline 200 µM; Ca2+ 60 µM (enough to induce the MPT-trace not shown)
With regard to the inhibition of Ca2+-induced mitochondrial swelling in KCl-based buffer, minocycline induced short-term low-amplitude initial swelling followed by a steady-state. Addition of Ca2+ did not cause swelling, but rather a slight shrinkage of mitochondria compared to that in sucrose-based buffer (Fig. 7, Panel B). The inhibitory effect of minocycline on mitochondrial swelling was greater at pH 8.0 compared to that at pH 6.8 (Fig. 7, Panel D).Panels A and B in Fig. 7 indicate that the initial effects of minocycline on ΔΨ and swelling are much more pronounced in 150 mM KCl compared to those in sucrose buffer. Panels C and D in Fig. 7 also show that the effects of minocycline on these parameters are greater under slightly basic compared to slightly acidic conditions in sucrose buffer.
To obtain further insights into the mechanism by which minocycline affects mitochondria functioning, experiments were performed on model membranes.
Minocycline induces anion permeability in planar BLM
To test the possibility that minocycline induces ion permeability in BLM the electrical current was measured in this model system. A typical trace of the increase in the current upon the addition of minocycline in the presence of Ca2+ is shown in Fig. 8, Panel A. The increase in the noise during 23–27 min was a result of stirring of the solutions. The experiment was performed at alkaline pH (8.5) because the effect at pH 7.0 was much less pronounced. It is important to note that neither minocycline nor Ca2+ alone was effective in the induction of the electrical current under these conditions (data not shown).
Fig. 8.
(Panel A) Minocycline (20 µM) in the presence of 3 mM Ca2+ induces an electrical current in BLM made from DPhPC. The experimental buffer was 10 mM Tris, 10 mM MES, 100 mM KCl, pH 8.5; the voltage was 30 mV. (Panels B, C) Ion channel activity induced by minocycline (8 µM) in the presence of Ca2+ (3 mM in panel B and 20 µM in panel C) at 50 mV in BLM made from DPhPC. The experimental buffer was 10 mM Tris, 10 mM MES, 1 M KCl, pH 9.0. (A current of 0.7 pA at 50 mV corresponds to a conductance of 14 pS)
The data presented in Panel A of Fig. 8 are more consistent with ion channel activity than with carrier-mediated conductance. To emphasize this point the experiments were carried out at higher sensitivity and lower conductance. A typical trace of minocycline-mediated current under single-channel conditions is shown in Panel B of Fig. 8. The predominant channel conductance was calculated to be 14 pS at 1 M KCl. These data were obtained at a high concentration of Ca2+ (3 mM), which is comparable to total calcium that may be present inside the mitochondrial matrix under normal physiological conditions. However, it should be noted that the channel activity can be observed even at relatively low calcium concentration (i.e. 20 µM, Fig. 8, Panel C).
The concentration-dependence of the minocycline-mediated current is shown in Panel A of Fig. 9.The dependence was strongly nonlinear, indicating that the number of molecules forming the channel varies with concentration. To determine the ion selectivity of minocycline-mediated channels, I-V curves were measured under symmetrical (Fig. 9, Panel B, closed circles) and asymmetrical conditions (Fig. 9, Panel B, open circles). The value of zero-voltage current under asymmetrical conditions (12 mV; with the “plus” sign at the side with higher KCl concentration) corresponds to the ratio of chloride anion to potassium cation permeability, which according to the Goldman-Hodgkin-Katz equation was estimated to be about 9.
Fig. 9.
(Panel A) The dependence of the BLM electrical current on the concentration of minocycline at V=50 mV. (Panel B) I–V curves for minocycline (50 µM)-mediated electrical current under symmetrical (100 mM:100 mM KCl; closed circles) and asymmetrical (190 mM:100 mM KCl; open circles) conditions. BLM was made from DPhPC in the presence of Ca2+ (3 mM). The experimental buffer conditions were the same as described for Fig. 8, Panel A
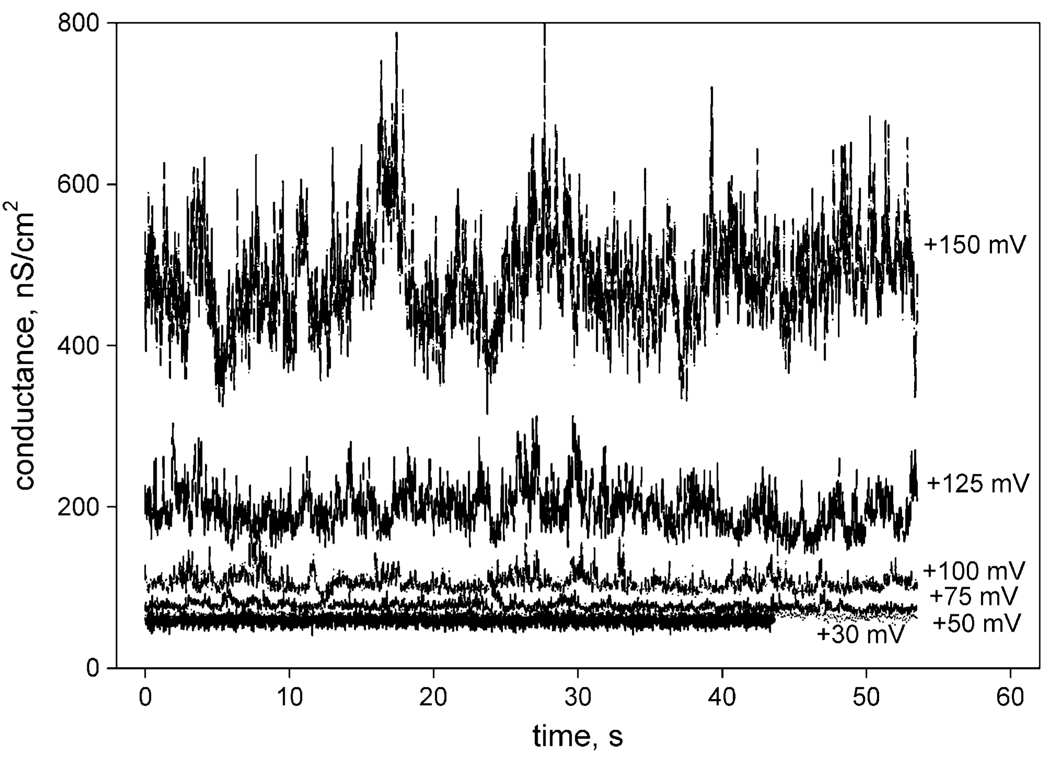
The voltage-dependence of the minocycline-mediated ion channel activity (minocycline-mediated membrane conductance) is illustrated in Fig. 10. The conductance increased several times when voltage was increased from +50 to +150 mV, suggesting formation of additional channels at high voltages. It is important to note that the dependence of the channel activity on the applied voltage in the range from +50 to +150 mV was completely reversible (data not shown).
Fig. 10.
Dependence of the BLM conductance on the voltage applied in the presence of 20 µM minocycline and 3 mM CaCl2. The BLM was made from an E. coli total lipid fraction. The experimental buffer conditions were the same as those described for Fig. 8, Panel A
Minocycline induces proton permeability in liposomes
After establishing that minocycline forms channels in planar BLM, it was important to determine next whether such channels are capable of conducting protons, because normal mitochondrial functioning requires maintenance of a proton gradient. To investigate this possibility, a more sensitive model than the planar BLM model was employed. Lipid vesicles with entrapped pyranine are a very sensitive system for measurement of proton fluxes at pH values of ∼7.0 (Chen et al. 1999). The kinetics of pyranine fluorescence after the addition of minocycline in the presence and absence of 5 mM CaCl2 are shown in Panel A of Fig. 11. Liposomes with a mildly acidic interior (pH 6.0) were placed in a medium buffered at pH 8.0. The increase in fluorescence after the addition of minocycline indicates acceleration of proton equilibration, i.e. induction of proton permeability. Minocycline was ineffective in the absence of Ca2+ (Fig. 11, Panel A, gray curve). As a control, addition of nigericin was used to abolish the pH gradient.
Fig. 11.
(Panel A) Minocycline induces proton permeability in liposomes loaded with pyranine in the presence of Ca2+ (5 mM). Addition of nigericin was used as a positive control. The lipid concentration was 20 µg/ml. Excitation, 455 nm; emission, 505 nm. (Panel B) Minocycline does not induce carboxyfluorescein (CF) leakage from dye-loaded liposomes in the presence or absence of Ca2+ (5 mM). Addition of melittin was used as a positive control. The lipid concentration was 5 µg/ml. Excitation, 488 nm; emission, 520 nm; a.u., arbitrary units
It was also important to be sure that minocycline induces proton permeability without affecting the integrity of the liposomal membranes. To verify this point, a model system consisting of liposomes loaded with carboxyfluorescein was used. This methodology has higher sensitivity compared to that described above for the measurements of proton fluxes. No leakage of carboxyfluorescein was detected in this model either in the presence of minocycline alone or in the presence of minocycline plus Ca2+ (Fig. 11, Panel B). This result indicates that the ion channels formed by minocycline are impermeable to carboxyfluorescein. The antibiotic mellitin, which forms channels (∼3 nm) in phospholipid membranes (Yang et al. 2001), was used as a control to induce carboxyfluorescein egress. This observation is also consistent with the relatively small size of ion channels formed by minocycline measured electrophysio-logically (see above).
Discussion
The data obtained with the planar BLM and liposome models suggest that a combination of minocycline with Ca2+ leads to f ormation of channels for anions and protons. The pH dependence of this process suggests that the deprotonated form of minocycline is involved in formation of this complex. [This conclusion is also supported by the data obtained with RLM under slightly alkaline (pH 8.0) conditions (Fig. 7, Panel D, gray curve).] Even though the exact mechanism of the channel formation is not yet clear, our work shows that this mechanism is different from the carrier-type of ion transport, which is known for some derivatives of tetracycline (White and Pearce 1982). Based on our findings, we hypothesize that minocycline-derived channel formation in BLM is similar to that of the amino-coumarin antibiotic novobiocin. This drug has been shown to form ion channels in both natural (O’Brodovich et al. 1993) and artificial (Feigin et al. 1995) membranes via its oligomerization. No ions are apparently required for formation of these channels. We suggest that minocycline may also form channels by oligomerization. We wish to emphasize that if this is the case, then unlike novobiocin, the process of channel formation with minocycline strongly requires the presence of Ca2+.
The data discussed in the previous paragraph together with those obtained with RLM suggest that the induction of proton conductance in the mitochondrial membrane is likely the mechanism that underlies mitochondrial depolarization and increased O2 uptake by RLM in the presence of minocycline (Fig. 6, Panels A,C). The depolarization was substantially increased after the addition of Ca2+, but the rate of O2 uptake did not change. The data obtained in a KCl-based buffer (Fig. 7, Panel A, gray curve) also suggest that minocycline alone administered to RLM is capable of partial mitochondrial depolarization, which is in accord with the recently reported findings of Kupsch et al. (2009). The combined results on ΔΨ and O2 uptake by mitochondria suggest a phenomenon similar to mild uncoupling.
Addition of Ca2+ (final concentration 60 µM) to RLM in the presence of minocycline resulted in a rapidly established steady-state level of Ca2+ in the incubation buffer of ∼30–35 µM (Fig. 6, Panel B, trace ‘b’). However, in the presence of Ru360, addition of Ca2+ (final concentration 60 µM) resulted in a Ca2+ steady state that was maintained in the range of ∼60 µM (Fig. 6, Panel B, trace ‘c’). The difference in the steady-state concentration of Ca2+ in the presence of minocycline and Ru360 cannot be explained by inhibition of the mitochondrial Ca2+ uniporter by minocy-cline for at least two reasons.
First, we found that minocycline acts as a weak Ca2+ chelator with an apparent binding constant of ∼65 µM Ca2+ (at 100 µM of minocycline, Fig. 3, Panel E). However, at Ca2+ concentrations greater than 200 µM, the response deviates from linearity and becomes sigmoidal (Fig. 3, Panel D). This finding suggests formation of Ca2+-minocycline complexes at ratios of ∼1:2, 1:1, and 2:1. The formation of even weaker complexes at higher ratios is suggested by the sigmoidal shape of the curve in the presence of saturating Ca2+, but the strongest interaction corresponds to a Mino*Ca/Mino ratio of 1 and occurred at ∼65 µM. Formation of the Ca2+-minocycline complex(es) explains the steady-state levels of ∼60 µM Ca2+ in the presence of Ru360, but only ∼30 µM Ca2+ in the presence of minocycline, despite the addition of equal amounts of Ca2+ to the incubation medium in both experiments (Fig. 6, Panel B, ‘b’ and ‘c’ traces, respectively).
Secondly, no change in ΔΨ was noted in the presence of Ru360 and Ca2+ (Fig. 6, Panel C, trace ‘c’). However, a complete collapse of ΔΨ occurred when only both minocycline and Ca2+ were added sequentially in sucrose-(Fig. 6, Panel C, trace ‘b’) or KCl-based buffers (Fig. 7, Panel A). This phenomenon does not take place if only one of the compounds is employed. It is well known that Ca2+ flux through its uniporter is dependent on ΔΨ (Scott et al. 1980; Gunter and Pfeiffer 1990). Once the ΔΨ drops below a certain critical level the uniporter shuts down and Ca2+ influx is no longer possible. Ru360 directly and irreversibly blocks the Ca2+ uniporter. Therefore, in the presence of this inhibitor, Ca2+ influx is prevented, but at the same time ΔΨ remains unaffected. On the other hand, our data show that addition of Ca2+ causes complete dissipation of ΔΨ only in the presence of minocycline, which, in turn, results in transition of the Ca2+ uniporter from an open to a closed state.
The minocycline inhibition of RLM swelling can be interpreted, at first glance, erroneously as inhibition of the Ca2+-induced MPT (Fig. 6, Panel D, traces ‘a’ and ‘b’). However, three other parameters that we measured simultaneously (O2 uptake, ΔΨ, and Ca2+ fluxes) indicate that the effect of minocycline on mitochondria is much more complicated than can be described by a direct MPT inhibition. Our combined data suggest that minocycline prevents Ca2+ influx into mitochondria through the following chain of sequential events: partial chelation of Ca2+ → incorporation of minocycline-Ca2+ complex(es) into mitochondrial membranes → formation of proton and anion selective channels → dissipation of ΔΨ. It is a well accepted point of view that ΔΨ, matrix pH and matrix Ca2+ play key roles in the MPT induction (Petronilli et al. 1993). Therefore, if prior to addition of Ca2+, mitochondria were depolarized (not necessarily even completely, but just enough to close the Ca2+ uniporter), then calcium accumulation will be prevented, and, thus MPT opening will not occur (unless a much higher [Ca2+] is employed (Halestrap et al. 1997)).
Many research groups have studied the effects of minocycline on isolated mitochondria and on cells in culture at concentrations up to 100–200 µM (Zhu et al. 2002; Smith et al. 2003; Wang et al. 2003; Matsuki et al. 2003 ; Cornet et al. 2004; Teng et al. 2004; Fernandez-Gomez et al. 2005a, b; Mansson et al. 2007; Kupsch et al. 2009). Similarly, we used 100–200 µM concentrations in our studies, which were designed to understand the effect of minocycline on isolated mitochondria. Although 100 µM concentrations of minocycline might be difficult to attain in vivo, our work may nevertheless be of clinical relevance. For example, Kupsch et al. (2009) estimated that pharmacological doses of minocycline used in clinical trials on ALS patients resulted in levels in the brain calculated to be about 20 µM. We noted the ability of minocycline to form channels in artificial bilayers even at concentration as low as 8 µM (Fig. 8, Panel C).
As we stated in the Introduction, the purpose of the present study was to determine whether minocycline directly interacts with mitochondria and possesses channel-forming abilities. The possibility was also considered that minocycline may affect the properties of plasma membranes. To investigate this possibility in detail would require a major separate study. However, a few points can be made indicating that minocy-cline is unlikely to have a major effect on plasma membrane properties. First, there are many reports in the literature indicating that minocycline easily permeates the blood-brain barrier (Zhu et al. 2002; Wang et al. 2003; Smith et al. 2003; Teng et al. 2004; Cornet et al. 2004; Chu et al. 2005; Sapadin and Fleischmajer 2006; Mansson et al. 2007). Secondly, we performed a set of experiments using an erythrocyte hemolysis model, indicating that under normal physiological conditions minocycline has no major impact on erythrocyte plasma membranes (data not shown).
Thus, in conclusion, we have provided evidence that mitochondria are the primary target for minocycline. Moreover, the effect of minocycline on mitochondria is likely not related to direct inhibition of the Ca2+-induced MPT, but is related to its ability first, to chelate Ca2+, secondly to bind to RLM membranes, thirdly, to partially uncouple mitochondria by formation of ion channels, and, finally, to prevent Ca2+ accumulation in the mitochondrial matrix.
Acknowledgements
We thank Dr. Bruce S. Kristal for help and encouragement. The project was supported in part by grants from NIH/NIEHS 5RO1 NS38471 and the Russian Fund for Basic Research # 09-04-00890.
Contributor Information
Yuri N. Antonenko, A.N. Belozersky Institute of Physico-Chemical Biology, Moscow State University, Moscow 119992, Russia
Tatyana I. Rokitskaya, A.N. Belozersky Institute of Physico-Chemical Biology, Moscow State University, Moscow 119992, Russia
Arthur J. L. Cooper, Department of Biochemistry and Molecular Biology, New York Medical College, 15 Dana Road, BSB, NYMC, Valhalla, NY 10595, USA
Boris F. Krasnikov, Department of Biochemistry and Molecular Biology, New York Medical College, 15 Dana Road, BSB, NYMC, Valhalla, NY 10595, USA. boris_krasnikov@nymc.edu
References
- Castanares M, Vera Y, Erkkila K, Kyttanen S, Lue Y, Dunkel L, Wang C, Swerdloff RS, Hikim AP. Biochem Biophys Res Commun. 2005;337:663–669. doi: 10.1016/j.bbrc.2005.09.101. [DOI] [PubMed] [Google Scholar]
- Chen Y, Schindler M, Simon SM. J Biol Chem. 1999;274:18364–18373. doi: 10.1074/jbc.274.26.18364. [DOI] [PubMed] [Google Scholar]
- Chu HC, Lin YL, Sytwu HK, Lin SH, Liao CL, Chao YC. Br J Pharmacol. 2005;144:275–282. doi: 10.1038/sj.bjp.0706079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet S, Spinnewyn B, Delaflotte S, Charnet C, Roubert V, Favre C, Hider H, Chabrier PE, Auguet M. Eur J Pharmacol. 2004;505:111–119. doi: 10.1016/j.ejphar.2004.10.039. [DOI] [PubMed] [Google Scholar]
- Feigin AM, Aronov EV, Teeter JH, Brand JG. Biochim Biophys Acta. 1995;1234:43–51. doi: 10.1016/0005-2736(94)00257-p. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gomez FJ, Gomez-Lazaro M, Pastor D, Calvo S, Aguirre N, Galindo MF, Jordán J. Neurobiol Dis. 2005a;20:384–391. doi: 10.1016/j.nbd.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gomez FJ, Galindo MF, Gomez-Lazaro M, González-García C, Ceña V, Aguirre N, Jordán J. Neuroscience. 2005b;133:959–967. doi: 10.1016/j.neuroscience.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Pfeiffer DR. Am J Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Woodfield KY, Connern CP. J Biol Chem. 1997;272:3346–3354. doi: 10.1074/jbc.272.6.3346. [DOI] [PubMed] [Google Scholar]
- Krasnikov BF, Zorov DB, Antonenko YN, Zaspa AA, Kulikov IV, Kristal BS, Cooper AJL, Brown AM. Biochim Biophys Acta. 2005;1708:375–392. doi: 10.1016/j.bbabio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Kupsch K, Hertel S, Kreutzmann P, Wolf G, Wallesch CW, Siemen D, Schönfeld P. FEBS J. 2009;276:1729–1738. doi: 10.1111/j.1742-4658.2009.06904.x. [DOI] [PubMed] [Google Scholar]
- Lin Q, Katakura K, Suzuki M. FEBS Lett. 2002;515:71–74. doi: 10.1016/s0014-5793(02)02437-7. [DOI] [PubMed] [Google Scholar]
- Mansson R, Hansson MJ, Morota S, Uchino H, Ekdahl CT, Elmer E. Neurobiol Dis. 2007;25:198–205. doi: 10.1016/j.nbd.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Matlib MA, Zhou Z, Knight S, Ahmed S, Choi KM, Krause-Bauer J, Phillips R, Altschuld R, Katsube Y, Sperelakis N, Bers DM. J Biol Chem. 1998;273:10223–10231. doi: 10.1074/jbc.273.17.10223. [DOI] [PubMed] [Google Scholar]
- Matsuki S, Iuchi Y, Ikeda Y, Sasagawa I, Tomita Y, Fujii J. Biochem Biophys Res Commun. 2003;312:843–849. doi: 10.1016/j.bbrc.2003.10.191. [DOI] [PubMed] [Google Scholar]
- Mueller P, Rudin DO, Tien HT, Wescott WC. J Phys Chem. 1963;67:534–535. [Google Scholar]
- O’Brodovich H, Wang X, Li C, Rafii B, Correa J, Bear C. Am J Physiol. 1993;264:C1532–C1537. doi: 10.1152/ajpcell.1993.264.6.C1532. [DOI] [PubMed] [Google Scholar]
- Petronilli V, Cola C, Bernardi P. J Biol Chem. 1993;268:1011–1016. [PubMed] [Google Scholar]
- Sapadin AN, Fleischmajer R. J Am Acad Dermatol. 2006;54:258–265. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Scarabelli TM, Stephanou A, Pasini E, Gitti G, Townsend P, Lawrence K, Chen-Scarabelli C, Saravolatz L, Latchman D, Knight R, Gardin J. J Am Coll Cardiol. 2004;43:865–874. doi: 10.1016/j.jacc.2003.09.050. [DOI] [PubMed] [Google Scholar]
- Scott ID, Akerman KE, Nicholls DG. Biochem J. 1980;192:873–880. doi: 10.1042/bj1920873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos EP, Tamargo RJ, Weingart JD, Brem H. Ann NY Acad Sci. 1994;732:263–272. doi: 10.1111/j.1749-6632.1994.tb24741.x. [DOI] [PubMed] [Google Scholar]
- Smith DL, Woodman B, Mahal A, Sathasivam K, Ghazi-Noori S, Lowden PA, Bates GP, Hockly E. Ann Neurol. 2003;54:186–196. doi: 10.1002/ana.10614. [DOI] [PubMed] [Google Scholar]
- Teng YD, Choi H, Onario RC, Zhu S, Desilets FC, Lan S, Woodard EJ, Snyder EY, Eichler ME, Friedlander RM. Proc Natl Acad Sci USA. 2004;101:3071–3076. doi: 10.1073/pnas.0306239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM. Proc Natl Acad Sci USA. 2003;100:10483–10487. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z. J Biol Chem. 2004;279:19948–19954. doi: 10.1074/jbc.M313629200. [DOI] [PubMed] [Google Scholar]
- White JR, Pearce FL. Biochemistry. 1982;21:6309–6312. doi: 10.1021/bi00267a041. [DOI] [PubMed] [Google Scholar]
- Yang L, Harroun TA, Weiss TM, Ding L, Huang HW. Biophys J. 2001;81:1475–1485. doi: 10.1016/S0006-3495(01)75802-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]