Abstract
Although the ability of coactivators to enhance the expression of estrogen receptor-α (ERα) target genes is well established, the role of corepressors in regulating 17β-estradiol (E2)-induced gene expression is poorly understood. Previous studies revealed that the silencing mediator of retinoic acid and thyroid hormone receptor (SMRT) corepressor is required for full ERα transcriptional activity in MCF-7 breast cancer cells, and we report herein the E2-dependent recruitment of SMRT to the regulatory regions of the progesterone receptor (PR) and cyclin D1 genes. Individual depletion of SMRT or steroid receptor coactivator (SRC)-3 modestly decreased E2-induced PR and cyclin D1 expression; however, simultaneous depletion revealed a cooperative effect of this coactivator and corepressor on the expression of these genes. SMRT and SRC-3 bind directly in an ERα-independent manner, and this interaction promotes E2-dependent SRC-3 binding to ERα measured by co-IP and SRC-3 recruitment to the cyclin D1 gene as measured by chromatin IP assays. Moreover, SMRT stimulates the intrinsic transcriptional activity of all of the SRC family (p160) coactivators. Our data link the SMRT corepressor directly with SRC family coactivators in positive regulation of ERα-dependent gene expression and, taken with the positive correlation found for SMRT and SRC-3 in human breast tumors, suggest that SMRT can promote ERα- and SRC-3-dependent gene expression in breast cancer.
The SMRT corepressor binds to the SRC-3 coactivator and promotes ERα and SRC-3 interaction thereby stimulating estrogen induced expression of PR and cyclin D1 genes.
Estrogens regulate biological processes including the growth, proliferation, differentiation, and function of diverse tissues (1,2). They also play important roles in pathological processes such as carcinogenesis in the reproductive system (3). The biological functions of estrogens are mediated through two distinct estrogen receptors (ERs), ERα and ERβ, which belong to a superfamily of ligand-activated transcription factors. Classically, in response to binding to the receptor's cognate ligand 17β-estradiol (E2), ERs undergo a series of sequential events: a change in conformation, dimerization, interactions with target genes either directly by binding to specific estrogen-responsive elements (EREs) or indirectly by binding to other DNA-binding proteins such as AP-1 or Sp1, and recruitment of coactivators to gene regulatory regions to activate gene expression (4).
The best-characterized steroid receptor coactivators (SRCs) belong to the p160 SRC family, which consists of three members: SRC-1, the first cloned coactivator in this family (5); SRC-2, also known as glucocorticoid receptor-interacting protein (GRIP1), transcription intermediary factor (TIF2), and NCoA-2 (6); and SRC-3, also known as amplified in breast cancer 1 (AIB1), TRAM-1, p/CIP, and RAC3 (7). Normally, SRCs interact with estrogen-bound ERs and recruit other chromatin-remodeling factors involved in chromatin acetylation [e.g. cAMP response element-binding protein-binding protein (CBP) and p300] (8,9) and methylation (e.g. coactivator-associated arginine methyltransferase 1 and protein arginine methyltransferase 1) (10), and collectively, these factors promote the transcription of ER target genes. Thus, changes in SRC coactivator expression influence ERα-dependent gene expression and consequently modulate cellular processes such as proliferation and apoptosis (11). Just as there is an association of estrogens with carcinogenesis, recent evidence indicates that SRC-3 is an oncogene that is amplified and/or overexpressed in several types of tumors and cancer cell lines (12,13). Studies also demonstrate that high levels of both SRC-3 and the HER-2 receptor tyrosine kinase in breast cancer are associated with poor patient outcome (14,15,16).
Typically, in the absence of hormone, DNA-bound type II nuclear receptors such as thyroid hormone receptor (TR) and retinoic acid receptor (RAR) interact with the corepressor proteins nuclear receptor corepressor (NCoR) and silencing mediator of retinoic acid and thyroid hormone receptor (SMRT) at gene regulatory regions. These corepressors play crucial roles in transcriptional repression of multiple transcription factors (17,18,19). As their name implies, SMRT/NCoR attenuate gene expression via recruitment of additional proteins that includes histone deacetylases (HDACs), transducin-like protein-1 (TBL1) (20,21), TBL1-related protein-1, G protein pathway suppressor 2 (GPS2) (22), and mSin3A (23), and collectively, these corepressor complexes reduce the overall acetylation state of promoters, thereby inhibiting gene expression. In addition to agonists such as E2, ERα can bind to antagonistic ligands including the selective ER modulators (SERMs) 4-hydroxytamoxifen (4HT) and raloxifene, which exert agonist and antagonist effects on ERα transcriptional activity depending on tissue and gene contexts. In SERM antagonist environments, SERM-bound ERα recruits corepressor proteins such as NCoR and SMRT to gene regulatory regions, and gene transcription is blocked (24,25). Chromatin immunoprecipitation (ChIP) assays also suggest an interaction between unliganded ERα, and these corepressors at some ERα target genes. Although these interactions do not appear to be as strong as for antiestrogen-bound ERα, there is some evidence that corepressors may work with unoccupied ERα to inhibit gene expression (26,27,28).
In addition to their role in repressing the activity of unliganded or antagonist-bound nuclear receptors, several recent studies suggested that SMRT and NCoR also play a role in inhibiting agonist-dependent nuclear receptor function (26,29). In some instances, corepressors appear to inhibit coactivator binding to receptors, whereas in others, coactivator and corepressor work together to repress gene expression. In an example of the former, SMRT and NCoR were reported to actively compete with coactivators for binding to agonist-bound androgen receptor (30). SRC-2 also is reported to compete with corepressor binding to glucocorticoid and progesterone receptors (PRs) through its amino-terminal domain (31). In contrast to the competition model, corepressors also can synergize with coactivators to repress target gene expression. For example, the p300 coactivator, as part of a YY1 transcription factor and HDAC3 corepressor complex, serves to down-regulate the transcription of the c-myc gene (32,33).
Although most data suggest that the primary role of corepressors is to inhibit gene expression, a few reports suggest that corepressors and/or their associated proteins also may participate in transcriptional activation. For instance, NCoR can directly interact with SRC-3, and in transient transfection assays, NCoR and SRC-3 cooperatively enhance TRβ transcriptional activity (34). The corepressor-associated proteins TBL1 and TBL1-related protein-1 are reported by some, but not all, laboratories to be required for transcriptional activation by a variety of nuclear receptors (35,36). In addition, HDAC1 and HDAC3, which typically function to remove acetyl groups from histones and thereby repress gene transcription, also have been reported to potentiate glucocorticoid receptor and androgen receptor transcriptional activity (37,38). Finally, our laboratory also recently reported that SMRT stimulates the expression of specific ERα target genes such as cyclin D1, BCL2, and PR, but not pS2, indicating that SMRT coactivates ERα transcriptional activity in a gene-specific manner (26). Just as there are instances where corepressors appear to play a role in stimulating gene expression, there are also examples in which coactivators appear to repress nuclear receptor transcriptional activity. For instance, SRC-2 uses a domain distinct from its two activation domains to repress GR transcriptional activity in a response element-specific manner (39). The coactivator, p300, also plays a role in repressing c-Myc expression in quiescent cells (32). Thus, there is increasing evidence that the functions of coactivators and corepressors cannot be neatly compartmentalized as positive and negative regulators of gene expression.
Our previous work had established that both SMRT and SRC-3 are necessary for full E2-dependent activation of several ERα-regulated genes such as PR, cyclin D1, and Bcl-2 (11,26) and suggested the possibility that SMRT and SRC-3 play cooperative, rather than antagonistic, roles in regulating the activity of E2-bound ERα at these target genes. In this report, we investigate the ability of SMRT and members of the SRC family to synergistically stimulate E2-dependent ERα transcriptional activity. In addition to illustrating functional interactions between p160 coactivators and SMRT, we demonstrate that SMRT directly binds to SRC-3 and plays a role in modulating the transcriptional activity of this coactivator as well as its interaction with ERα target genes. Finally, a positive correlation is observed between SMRT and SRC-3 protein levels in human breast tumors, and this suggests the possibility that SMRT-SRC-3 interactions may amplify estrogen action and thereby play a role in breast carcinogenesis.
Results
SMRT and p160 coactivators cooperatively stimulate ERα transcriptional activity
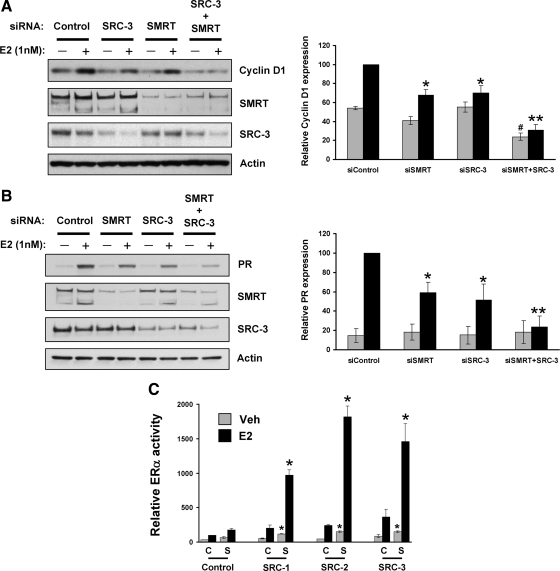
Previous data from this laboratory indicated that SMRT acts as a coactivator for a subset of E2-ERα target genes, because depletion of SMRT significantly decreased E2-induced expression of cyclin D1 and PR in MCF-7 cells in comparison with their expression in E2-treated control cells (26). Similarly, in an independent study designed to understand the role of each member of the p160 family of coactivators to regulate ERα target gene expression, it was observed that SRC-3 but not SRC-1 or SRC-2 was required for maximal E2-dependent cyclin D1 and PR gene expression (11). To determine whether SMRT and SRC-3 acted in concert to regulate these genes, the effect of SMRT or SRC-3 depletion, alone or in combination, on cyclin D1 and PR expression in MCF-7 cells was investigated. MCF-7 cells were treated with control small interfering RNA (siRNA) or siRNA directed against SMRT and/or SRC-3 followed by hormone treatment, and levels of cyclin D1 or PR expression were assessed by Western blot analysis (Fig. 1, A and B). Efficacy of siRNA depletion of SMRT and SRC-3 also were determined. Consistent with our previous work (26), two major splice variants of SMRT were detected in MCF-7 cells. Individual depletion of SMRT or SRC-3 decreased E2-dependent cyclin D1 and PR protein expression, compared with control E2-treated cells. Moreover, concurrent depletion of SMRT and SRC-3 reduced the E2-dependent expression of cyclin D1 and PR to an extent significantly lower than that obtained for cells depleted of either SMRT or SRC-3 alone. This result indicates that SMRT and SRC-3 cooperate to coactivate E2-dependent cyclin D1 and PR gene expression.
Figure 1.
Stimulatory effect of SMRT and SRC coactivators on ERα transcriptional activity and endogenous target gene expression. MCF-7 cells were transfected with either 6 pmol control siRNA or siRNA against SRC-3 (1 pmol) or SMRT (5 pmol) alone or in combination and then treated with vehicle (0.1% ethanol) or 1 nm E2 for 3 h (for cyclin D1) or 24 h (for PR). Subsequently, expression of cyclin D1 (panel A) and PR (panel B) was assessed by Western blotting. Expression of SMRT, SRC-3, and actin also was measured for each set of samples. The experiment was repeated three times, and representative blots are shown. Blots obtained from three independent experiments were quantitated by densitometry, and values normalized to actin are expressed as mean ± sem. Statistical analyses were done by one-way ANOVA. *, Significance at P < 0.05; **, P < 0.001 vs. E2-treated siControl cells; #, significance at P < 0.05 vs. vehicle-treated siControl cells. Panel C, HeLa cells were transfected with 10 ng ERα expression vector and 1 μg ERE-E1b-Luc reporter gene along with the indicated plasmids for empty pCR3.1 (C, control), SMRTτ (S, SMRT) or SRC-1, -2, or -3. Cells were subsequently treated with vehicle or 1 nm E2 for 24 h followed by measurement of luciferase activity. Values represent the average ± sem of three independent experiments. *, P < 0.01 in comparison with the respective vehicle (Veh)- or E2-treated SMRT-transfected cells.
An ERα trans-activation assay was used to determine whether the ability of SMRT to functionally interact with p160 coactivators is limited to SRC-3 or extends to SRC-1 and SRC-2. HeLa cells transfected with an ERα expression vector and reporter gene were cotransfected with expression plasmids for SMRT alone, p160 coactivators alone, or combinations thereof, followed by vehicle or E2 treatment. In each case, SMRT and the coactivators individually stimulated ERα-dependent transcriptional activity, and together, they exerted a synergistic positive effect on ERα transcriptional activity (Fig. 1C). An additive effect of SMRT and SRCs on ERα activity is also observed in the absence of hormone, indicating a ligand-independent combinatorial activity of these two cofactors. The results of this ER trans-activation assay, together with gene expression analyses, clearly suggest that SMRT has the ability to functionally interact with all members of the p160 coactivator family to stimulate ERα transcriptional activity.
E2-dependent recruitment of SMRT or SRC-3 to the ERα regulatory regions of the cyclin D1 and PR genes
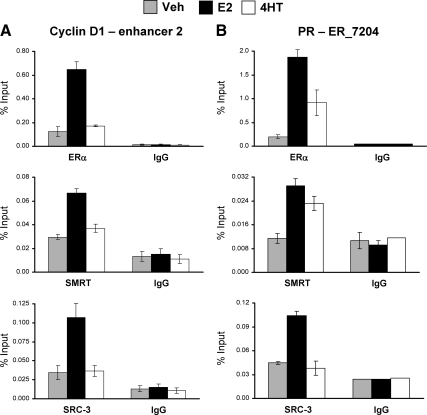
A recent study on estrogen regulation of cyclin D1 gene expression indicated that there are three deoxyribonuclease hypersensitive sites surrounding the cyclin D1 coding region (40), of which a region approximately 2000 bp upstream of the transcriptional start site (referred to as enhancer 1) and a second site approximately 500 bp downstream of the coding region (referred to as enhancer 2) were shown to be the locations of E2-dependent ERα recruitment (for location of enhancers, see Supplemental Fig. 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Stimulation of cyclin D1 expression by E2 depends on the cell-type-specific enhancer 2, which is the primary ERα recruitment site (40). Binding of ERα to this region in E2-treated MCF-7 cells has been confirmed by genome-wide ChIP-chip (41) and ERα-chromatin interaction analysis by paired-end tag sequencing (ChIA-PET) studies (42), with the latter also demonstrating intrachromosomal interaction between ERα at the enhancer 2 region and the 5′ end of the cyclin D1 gene (Supplemental Fig. 1A). In agreement with a previous report (40), our experiments also demonstrated that enhancer 2 is the location of the greatest E2-dependent ERα recruitment to the cyclin D1 gene (Supplemental Fig. 1C). A genome-wide ERα ChIP-chip study identified three ERα interaction sites adjacent to the PR gene (41) (Supplemental Fig. 1B). Intrachromosomal interactions between ER_7206 and ER_7204 sites of the PR gene (42) and the recent demonstration of ER_7206 interaction with the PR promoter (43) are indicative of the importance of these sites for gene expression. Our ChIP-quantitative PCR (qPCR) analyses confirm the strongest binding of ERα to the ER_7204 site (Supplemental Fig. 1D). A set of ChIP experiments was performed to detect SMRT and SRC-3 recruitment to enhancer 2 for cyclin D1 and the ER_7204 site for PR. As for ERα, estrogen-dependent recruitment of SMRT and SRC-3 was noted for enhancer 2 and ER_7204 regions (Fig. 2), indicating that E2 has the ability to induce interaction of both SMRT and SRC-3 to the regulatory regions of cyclin D1 and PR at the same time. The extent of SMRT or SRC-3 recruitment is lower than that obtained for ERα but is significantly greater than for the IgG negative control, indicating specific interactions of these coregulators with the tested genes. Both ERα and SMRT were recruited to the PR ER_7204 sites but not enhancer 2 of the cyclin D1 gene in 4HT-treated cells, indicating that 4HT-dependent recruitment of ERα and SMRT is gene specific.
Figure 2.
Interaction of ERα, SRC-3, and SMRT with enhancer regions of the cyclin D1 and PR genes. Chromatin was prepared from MCF-7 cells treated with vehicle (Veh), 10 nm E2, or 100 nm 4HT for 45 min and then subjected to ChIP assay using antibodies for ERα (top), SMRT (middle), or SRC-3 (bottom) in parallel to the appropriate IgG negative control. Immunoprecipitated DNA was quantitated by qPCR using primers to amplify enhancer-2 of the cyclin D1 gene (A) or the ER_7204 binding site in the PR gene (B). Data represent an average ± sem of three independent experiments.
ERα, SMRT, and SRC-3 interactions in vivo
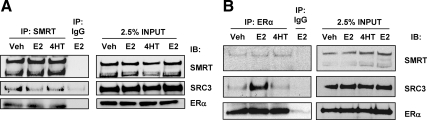
The ChIP experiments raise the possibility of interactions between ERα, SMRT, and SRC-3. To investigate this, a coimmunoprecipitation (co-IP) assay was conducted in which MCF-7 cell lysates were first immunoprecipitated with a SMRT antibody and then subsequently Western blotted for SMRT, SRC-3, and ERα. Results indicate that SMRT interacts with both SRC-3 and ERα [relative molecular mass (Mr) 66 kDa] in vivo in a hormone-independent manner (Fig. 3A). A second assay beginning with ERα IP reveals a constitutive interaction of ERα with SMRT, whereas the expected E2-enhanced interaction between ERα-SRC-3 is observed (Fig. 3B). Taken together, these experiments suggest that endogenous SMRT, SRC-3, and ERα interact with each other in vivo.
Figure 3.
In vivo interaction of ERα, SMRT, and SRC-3 in MCF-7 cells. MCF-7 cells were grown 24 h in phenol red-free DMEM supplemented with 10% sFBS and then treated with vehicle (Veh), 1 nm E2, or 100 nm 4HT for 60 min. Thereafter, cells were harvested, lysed, and immunoprecipitated with antibodies to SMRT (A) or ERα (B) and subsequently Western blotted for SMRT, SRC-3, and ERα (left panels). IPs performed with rabbit IgG were incorporated into each experiment as negative controls, and 2.5% of the cell lysates (INPUT) was assessed for SMRT, SRC-3, and ERα by Western blot. IB, Immunoblot.
SMRT enhances the intrinsic transcriptional activity of SRC family coactivators
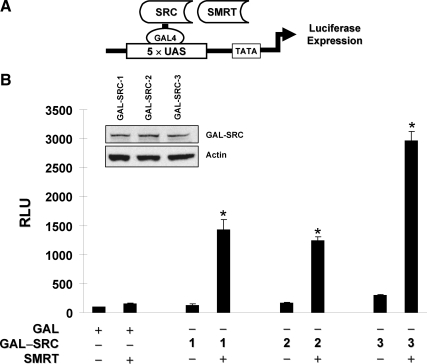
To investigate the functional outcome of SMRT and p160 coactivator interactions, a modified mammalian one-hybrid assay (Fig. 4A) was conducted in ERα-negative HeLa cells to test whether exogenous SMRT could stimulate the intrinsic transcriptional activity of chimeras consisting of the GAL4 DNA-binding domain (GAL) fused to each of the p160 coactivators. As shown in Fig. 4B, measurement of luciferase expression revealed that the intrinsic transcriptional activities of GAL-SRC-1, GAL-SRC-2, and GAL-SRC-3 were significantly enhanced in cells cotransfected with a human SMRT expression vector, indicating a positive functional interaction of SMRT with SRC-1, SRC-2, and SRC-3 that is independent of ERα. Analysis of GAL-SRC fusion protein expression by Western blotting revealed comparable expression of the chimeric proteins and suggests that the difference in extent of interaction is not due to the unequal expression of SRCs (Fig. 4B, inset). There was no significant effect of exogenous SMRT on luciferase expression in cells containing only the GAL4 DNA-binding domain. Consistent with the results of the ERα trans-activation assay above (Fig. 1C), these results further suggest that SMRT has the ability to functionally cooperate with all of the p160 family coactivators. Because the overall transcriptional activity was greatest for SRC-3, this coactivator was employed for subsequent studies.
Figure 4.
SMRT enhancement of intrinsic SRC-3 transcriptional activity is ERα independent. A, Schematic diagram illustrating a modified mammalian one-hybrid assay for evaluating functional interaction of GAL4-SRC fusion proteins with SMRT. B, HeLa cells were transfected with 5×UAS-Luc reporter plasmid, along with expression vectors for the GAL4 DNA-binding domain fused to full-length SRC-1 (1), SRC-2 (2), or SRC-3 (3) in the presence of the pCR3.1 control vector (−) or a SMRTτ expression plasmid (+). Twenty-four hours after transfection, cells were harvested for measurement of luciferase activity. Values represent the average ± sem from three independent experiments. *, P < 0.01 in comparison with the respective control group. Equal expression of GAL-SRC fusion proteins were confirmed by Western blot analysis using an antibody specific for the GAL4 DNA-binding domain. A representative blot of three independent experiments is shown (inset).
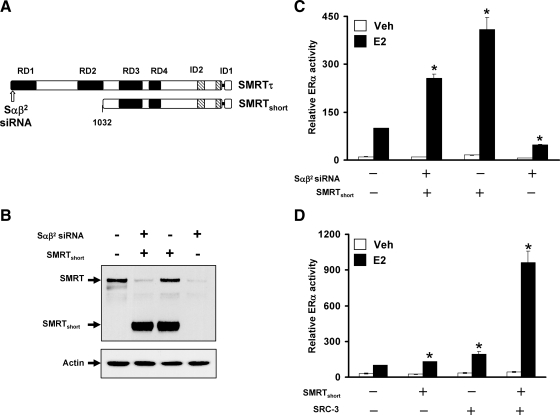
The SMRT N terminus is not required for SRC-3 interaction and synergistic activation of ERα transcriptional activity
To identify the domains of SMRT required for SRC-3 interaction and synergistic coactivation of ERα transcriptional activity, experiments were initiated with a truncated version of SMRT that lacks the first 1031 amino acids and therefore repressor domain (RD) 1 and RD2 (herein termed SMRTshort, Fig. 5A). To ascertain the ability of this SMRT deletion mutant to stimulate ERα activity, a rescue experiment was performed in HeLa cells where endogenous SMRT was first depleted by siRNA targeting the 5′ end of the coding region followed by exogenous expression of SMRTshort. The efficacy of siRNA knockdown and exogenous expression of SMRTshort were verified by Western blotting (Fig. 5B). Measurement of ERα transcriptional activity demonstrated that siRNA depletion of endogenous SMRT reduces ERα transcriptional activity and that exogenous SMRTshort rescues the depletion effect (Fig. 5C), similar to a previous experiment conducted with full-length SMRT (26). The stimulatory action of SMRTshort also was observed for control siRNA-treated cells, where exogenous SMRTshort coactivated ERα transcriptional activity.
Figure 5.
The N terminus of SMRT is dispensable for synergistic coactivation of ERα by SRC-3 and SMRT. A, Schematic representation of SMRTτ and SMRTshort illustrating repression domains (black box) and CoRNR box motifs (striped box). The 47-amino-acid splice deletion in the C-terminal region of both SMRTτ and SMRTshort adjacent to ID1 is represented as a single horizontal line. The arrow indicates the location of the sequence targeted by this SMRT siRNA (Sαβ2). B, Representative Western blot analysis of SMRT (top) or actin (bottom) expression in cells obtained from HeLa cells transfected in parallel. C, HeLa cells were transfected with negative control (−) siRNA or the Sαβ2 (+) siRNA directed against SMRT before transfection with vectors for ERα, ERE-E1b-Luc, and either 500 ng SMRTshort or control (pCR3.1) expression vector followed by treatment with vehicle or 1 nm E2 for 24 h. The Mr of SMRTshort is approximately 175 kDa. Values represent the average ± sem of three independent experiments. *, P < 0.01 in comparison with control E2 values. D, HeLa cells were transfected with 250 ng expression vectors for control, SMRTshort, or SRC-3 alone or in combination in addition to ERα and ERE-E1b-Luc. Cells were subsequently treated with vehicle or E2 for 24 h. Values represent the average ± sem of four independent experiments. *, P < 0.01 in comparison with control E2 values.
To elucidate whether SMRTshort can act cooperatively with SRC-3 to stimulate ERα, a trans-activation assay testing the ability of SMRTshort and SRC-3, alone or in combination, to affect ERα transcriptional activity was conducted in HeLa cells. Under these assay conditions, SMRTshort and SRC-3 individually exerted a modest coactivation effect on ERα transcriptional activity. However, coexpression of both coregulators synergistically stimulated ERα activity (2.9 times greater than the sum of SMRTshort and SRC-3 activities; Fig. 5D), similar to the effect observed for full-length SMRT and SRC-3 coactivation of ERα shown in Fig. 1C (2.7 times greater than the sum of full-length SMRT and SRC-3 activities). Taken together, these results indicate that the N terminus of SMRT containing the RD1 and RD2 domains is dispensable for functional interactions with SRC-3 and synergistic coactivation of ERα transcriptional activity.
Identification of SMRT and SRC-3 interaction domains
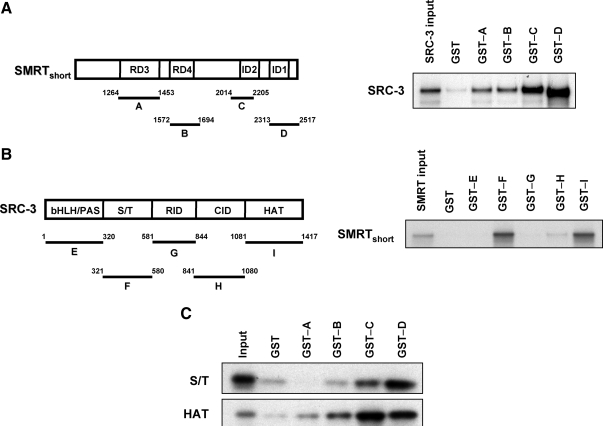
Because the first 1031 amino acids of SMRT are not required for functional interaction with SRC-3, the remainder of the molecule was tested for its ability to directly bind to SRC-3. In the first experiment, chimeras of glutathione S-transferase (GST) fused to known regions of SMRT (see schematic in Fig. 6A) were tested for their ability to bind to in vitro-translated full-length SRC-3 in GST pull-down assays. As depicted in Fig. 6A (right), the two most C-terminal fragments of SMRT encompassing amino acids 2014-2205 (GST-C) and amino acids 2313-2517 (GST-D) showed the greatest interaction with SRC-3 compared with the tested N-terminal fragments or the negative control GST protein. Thus, these data demonstrate a direct interaction of the C-terminal region of SMRT with SRC-3, with a lesser interaction of the SMRT regions encompassing RD3 and RD4; this demonstrates multiple contact points between SMRT and SRC-3.
Figure 6.
SMRT binds directly to SRC-3 in vitro. Panel A, Schematic diagram showing SMRTshort and the GST-tagged fragments of SMRT (A–D) used in the experiment (left). GST pull-down assay demonstrating the interaction of full-length in vitro-transcribed and -translated SRC-3 with GST-SMRT fusion proteins or GST control (right). Panel B, Schematic representation of full-length SRC-3 and the GST-tagged SRC-3 fragments used in this study (left). Interaction of in vitro-transcribed and -translated SMRTshort with GST-SRC-3 fusion proteins or GST control (right). Panel C, Interaction of in vitro-translated S/T or HAT domains of SRC-3 with GST-SMRT fusion proteins or GST control. In each assay, bound 35S-labeled proteins were resolved by SDS-PAGE and detected by autoradiography. Input lanes represent 10% of 35S-labeled full-length SRC-3 or fragments (S/T or HAT) of SRC-3 or 0.5% of SMRTshort proteins used in pull-down assays. Results are representative of three independent experiments.
To identify SRC-3 domains mediating interaction with SMRT, GST fusion proteins encompassing the basic helix-loop-helix/Per-ARNT-Sim, serine/threonine (S/T), receptor-interacting domain (RID), CBP-interacting domain (CID), and histone acetyltransferase (HAT) domains of SRC-3 (fragments E–I, see schematic in Fig. 6B) were tested in GST pull-down assays for binding to in vitro-translated SMRTshort. This form of SMRT was used in this experiment because full-length SMRT did not translate efficiently in vitro, likely due to its large size (Mr ∼270 kDa), and SMRTshort was shown above to work synergistically with SRC-3 to stimulate ERα transcriptional activity. The results demonstrate that the GST-F (amino acids 321-580) and GST-I (amino acids 1081-1417) fragments containing the S/T and HAT domains, respectively, have the greatest binding affinity for SMRT interaction in comparison with other GST fragments or GST protein alone (Fig. 6B, right). To further strengthen this observation, a third set of GST pull-down assays was conducted in which the in vitro-translated S/T or HAT domains of SRC-3 were tested for binding to each of the GST-SMRT fusion proteins (A→D) examined above. These experiments confirmed that the S/T and HAT domains of SRC-3 preferentially bind to the C-terminal region of SMRT (Fig. 6C). In addition, they reveal a minor interaction of SRC-3's HAT domain with the SMRT GST fusion proteins encompassing amino acids 1264-1453 (fragment A) and amino acids 1572-1694 (fragment B), which is consistent with the relatively weak binding of full-length SRC-3 to these regions observed in Fig. 6A. Taken together, these data indicate that SMRT and SRC-3 can bind directly and that the major sites of SMRT-SRC-3 interaction occur via the C-terminal region of SMRT that contains the interaction domains and the S/T and HAT-containing domains of SRC-3.
ERα, SMRT, and SRC-3 exist in a ternary complex at an ERα target gene
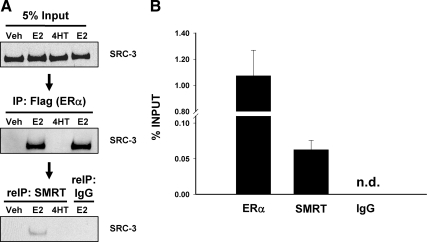
Having established that SMRT, SRC-3, and ERα interact with each other, we examined the ability of these three molecules to exist in a ternary complex. Cell lysates prepared from HeLa cells transfected with SMRTshort and FLAG epitope-tagged ERα expression vectors and treated with vehicle, E2, or 4HT were immunoprecipitated with FLAG-M2 affinity beads. Immune complexes were then eluted with FLAG peptide and immunoprecipitated with either SMRT antibody or normal rabbit IgG. Input samples and material eluted after the first and second IP were analyzed by Western blot using SRC-3 antibody. SRC-3, which was equally present in all input samples (Fig. 7A, top), was found in a complex with ERα only in E2-treated cells (Fig. 7A, top and middle). After elution of those complexes and subsequent IP with SMRT antibody or IgG, SRC-3 was found only in the SMRT-precipitated complex, indicating the presence of an ERα/SRC-3/SMRT ternary complex (Fig. 7A, bottom).
Figure 7.
ERα, SMRT, and SRC-3 form a trimeric complex in vivo. A, HeLa cells were transfected with expression vector for FLAG-tagged ERα and SMRTshort and 24 h thereafter were treated with vehicle (Veh), 1 nm E2, or 100 nm 4HT for 60 min. Five percent of the cell lysate (top panel) was set aside, and remaining lysates were immunoprecipitated with Sigma's EZview Red anti-FLAG M2 affinity gel. Immune complexes were eluted with 3X FLAG peptide, and a portion of this eluant was retained (middle panel), whereas the rest was subjected to a second IP [re-immunoprecipitated (reIP)] with either SMRT antibody or rabbit IgG (bottom panel). Material from the first and second IPs as well as input samples were analyzed by SDS-PAGE and Western blotted with SRC-3 antibody. B, MCF-7 cells were treated with 10 nm E2 for 45 min and first subjected to ChIP with SRC-3 antibody, followed by re-IP with antibodies to ERα, SMRT, or IgG. Chromatin recovered from the second IP was quantitated by qPCR using primers to an amplicon in the enhancer-2 region of the cyclin D1 gene. Data represent an average ± sem of three independent experiments. Signal was not detected (n.d.) in the IgG control.
To test the hypothesis that the synergistic activation of ERα target genes occurs via an ERα/SRC-3/SMRT ternary complex, a sequential ChIP was performed with E2-treated MCF-7 cells for the cyclin D1 gene. The first ChIP was done with SRC-3 antibody, and after elution and a second round of ChIP with antibodies to either ERα, SMRT, or IgG, the resulting DNA was analyzed by qPCR with cyclin D1 enhancer 2-specific primers. Both ERα and SMRT were found in a complex with SRC-3 on enhancer 2 (Fig. 7B). Although the extent of SMRT-SRC-3 interaction is weaker than for ERα-SRC-3, both interactions are highly specific in comparison with the IgG control. It is possible that the relative affinity of the antibodies for ERα and SMRT influences the apparent relative level of interaction detected by PCR. Taken together, the results of these two experiments clearly indicate that ERα, SMRT, and SRC-3 form a ternary complex, and the complex occupies the E2-ERα regulatory region of the cyclin D1 gene in E2-treated MCF-7 cells.
Depletion of SMRT reduces ERα and SRC-3 interactions in vivo
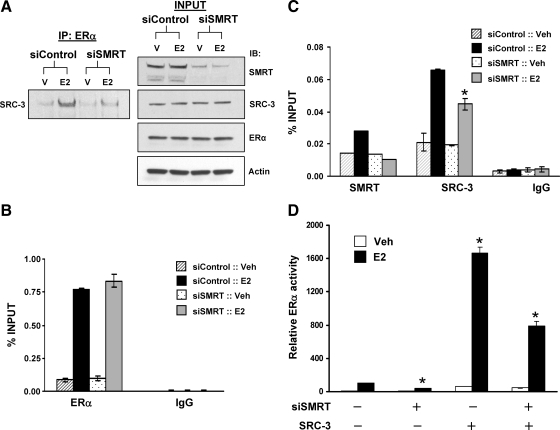
To assess the function of the ERα/SRC-3/SMRT complex in the regulation of transcription, one of the components of the complex, SMRT, was depleted, and the impact of this on the interaction of the two other components of the complex, ERα and SRC-3, was determined. MCF-7 cells transfected with control or SMRT siRNAs were treated with vehicle or E2 and immunoprecipitated with an ERα antibody followed by Western blotting for SRC-3. In control cells, the expected E2 induction of ERα and SRC-3 interaction was observed (Fig. 8A). Depletion of SMRT significantly reduced hormone-induced ERα-SRC-3 complex formation, indicating that SMRT makes a significant contribution to ERα-SRC-3 interactions in vivo.
Figure 8.
Depletion of SMRT inhibits SRC-3 and ERα interaction, recruitment of SRC-3 to cyclin D1 enhancer and ERα transcriptional activity. A, MCF-7 cells were transfected with 20 nm control or SMRT siRNA and 48 h thereafter were treated with either vehicle (V) or 10 nm E2 for 60 min, followed by cell lysis and IP with ERα antibody. Levels of SRC-3 immunoprecipitated with ERα were assessed by Western blot (left). Five percent of the total cell lysates was reserved (INPUT), and Western blots were employed to assess SMRT, SRC-3, ERα, and actin expression (right). The experiment was performed three times, and a representative blot is shown. B and C, MCF-7 cells were transfected with 20 nm control or SMRT siRNA, and 48 h later, cells were treated with either vehicle (Veh) or 10 nm E2 for 45 min and subjected to ChIP assay using antibodies for ERα, SMRT, SRC-3, or IgG. Immunoprecipitated chromatin was quantitated by qPCR using primers for the cyclin D1 enhancer 2. Data represent an average ± sem of two to three independent experiments. D, HeLa cells were transfected with control siRNA or Sαβ2 siRNA before transfection with an ERE-E1b-Luc reporter gene and expression vectors for ERα, SRC-3, or vector control (−; pCR3.1) and treated with vehicle (Veh) or 1 nm E2. Values represent the average ± sem of three independent experiments. *, P < 0.01 in comparison with the respective E2-treated control values.
To ascertain the impact of the ternary complex on recruitment of these factors to chromatin, ChIP assays were performed in SMRT-depleted vs. control cells. Depletion of SMRT did not affect E2-induced recruitment of ERα to the cyclin D1 enhancer 2 (Fig. 8B), and as expected, no E2-dependent SMRT recruitment to enhancer 2 was observed for SMRT-depleted cells (Fig. 8C). Estradiol-dependent recruitment of SRC-3 is significantly reduced in SMRT depleted vs. control cells, indicating that SMRT promotes SRC-3 occupancy of the transcriptional regulatory region of this ERα target gene (Fig. 8C). The effect of SMRT on SRC-3 function also was reflected in ERα trans-activation assays where the level of E2-dependent ERα activity achieved by exogenous SRC-3 is significantly reduced in SMRT-depleted vs. control cells (Fig. 8D). Collectively, these experiments reveal that SMRT, ERα, and SRC-3 form a ternary complex that occupies the regulatory region of the cyclin D1 gene in the presence of E2. Depletion of SMRT reduces the level of ERα-SRC-3 complex in vivo, inhibits recruitment of SRC-3 to this cyclin D1 gene regulatory region, and ultimately negatively impacts E2 regulation of this ERα target gene.
Correlation of SMRT and SRC-3 expression in human breast tumors
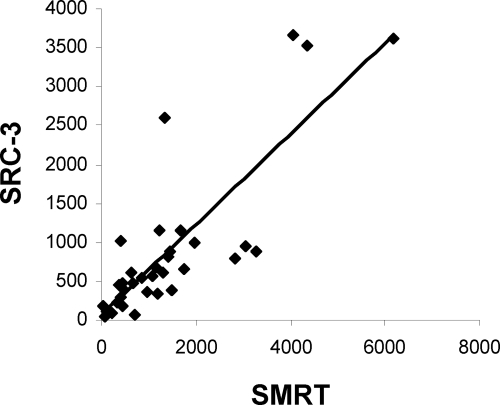
Our previous findings establish a direct interaction between SMRT and SRC-3 both in vitro and at the promoter region of endogenous genes in MCF-7 breast cancer cells. To establish whether there is a correlation between the two coregulators in primary breast cancer, we screened lysates from 34 breast cancer samples by Western blot analysis. Lysates were examined for expression of SRC-3, SMRT, and actin, and protein levels were quantified by densitometry (Fig. 9). Spearman rank correlation analysis shows a positive correlation between SMRT and SRC-3 protein expression in breast tumors (ρ = 0.78; P < 0.0001). This study was not powered to study associations between SRC-3 or SMRT expression and survival.
Figure 9.
SMRT and SRC-3 protein expression are positively correlated in breast cancer. Total protein from tumor lysates of 34 breast cancer patients was analyzed by Western blot. Expression of SMRT, SRC-3, and actin protein was quantitated by densitometry using ImageJ software (http://rsbweb.nih.gov/ij/), and the relative expression of actin-normalized SRC-3 vs. actin normalized SMRT was plotted.
Discussion
In the classical model, antagonist-bound ERα does not bind to coactivators such as SRC-3 but rather recruits SMRT to target genes, and consequently, gene transcription is repressed. However, in a recent report, we demonstrated that SMRT was recruited to a synthetic ERα target gene in E2-treated cells and was required for full expression of a subset of endogenous ERα target genes (26). In this current report, we demonstrate that silencing of SMRT and SRC-3 in combination profoundly inhibited E2-dependent expression of the PR and cyclin D1 genes, compared with the effect obtained upon their individual depletion. Using a variety of approaches including GST pull-down and single or sequential co-IP and ChIP, we show that the SRC-3 coactivator and the SMRT corepressor directly interact with each other, form a trimeric complex with ERα, and occupy target gene regulatory regions in a ligand-dependent fashion. Moreover, we found that down-regulation of SMRT destabilizes ERα and SRC-3 interactions and reduces recruitment of SRC-3 to the regulatory regions of the tested target genes, thereby inhibiting gene expression. To our knowledge, this is the first report of functional cooperation of SMRT with p160 family coactivators in the activation of ERα-mediated gene transcription. A previous ChIP study of transfected 293T cells demonstrated occupancy of target gene promoters by NCoR, SRC-3, and apo-TRβ (34). Upon the addition of T3 ligand, NCoR left the gene, and additional SRC-3 coactivator was detected at the promoter region, leading to the suggestion of coordinate regulation of TRβ target genes by NCoR and SRC-3. However, our data clearly indicate that cooccupancy by SMRT and SRC-3 are important for E2-dependent expression of the studied ERα target genes, thus differentiating our results. Interestingly, the functional cooperation of p160 family coactivators with SMRT is not restricted to SRC-3, because SRC-1 and SRC-2 have a similar ability to synergize with SMRT to regulate ERα transcriptional activity. Taken together, our data indicate that the presence of SMRT as part of a p160 coactivator complex is required for maximal agonist-dependent ERα transcription activity.
Multiple lines of evidence indicate that SMRT, SRC-3, and ERα associate with one another in MCF-7 cells. Although co-IP experiments reveal the expected E2-induced interaction between ERα and SRC-3, interactions of SMRT with either of these two molecules in whole-cell lysates was not stimulated by E2. This contrasts with the ability of E2 to recruit SRC-3 and SMRT to the regulatory regions of the PR and cyclin D1 genes. Based on the ability of E2 to prevent ERα-corepressor interactions in vitro (44,45), it seems likely that E2 promotes SRC-3 recruitment to these genes, and as a consequence, SMRT interaction with the regulatory regions is increased. It also is possible that SMRT can interact with the PR and cyclin D1 genes via interactions with the ligand-independent AF1 domain of ERα (Pace, M. C., and C. L. Smith, personal communication), similar to the ability of SRC coactivators to bind to the ERα AF1 domain in an LXXLL-independent manner (46).
GST pull-down assays suggest that there are multiple, ERα-independent contact points between SMRT and SRC-3. The C-terminal domain of SMRT encompassing the CoRNR box motifs and the RD3 and RD4 domains were able to bind to the S/T and HAT domains of SRC-3. This is similar to a previous study in which it was demonstrated that the latter two thirds of NCoR containing the RD3 and ID domains was capable of binding to SRC-3 in GST pull-down experiments (34). This demonstration of a direct interaction between coactivator and corepressor is consistent with the ability of SMRT to stimulate the intrinsic transcriptional activity of GAL-SRC fusion proteins in an ERα-independent manner. Moreover, the ability of SMRTshort, which lacks the corepressor's first 1031 amino acids, to interact with SRC-3 in co-IP assays as well as synergize with SRC-3 to stimulate ERα transcriptional activity provides further evidence of the importance of C-terminal SMRT interactions with SRC-3 for positive regulation of gene expression. This also suggests that SMRT may be able to stimulate the expression of ERα-independent genes that are positively regulated by SRC-3 coactivation of other transcription factors such as activator protein 1, nuclear factor-κ B, or E2F transcription factor 1 (47,48,49).
A number of previous studies have examined the ability of coactivators and corepressors to compete for regulation of nuclear receptor transcriptional activity. For ERα bound to SERMs such as tamoxifen, the relative interaction of coactivators and corepressors with receptor, and consequently, the ability of the SERM to activate or repress transcription is dependent on the stoichiometric ratio of the coregulators within the cell and cell signaling events that control coregulator interaction with receptor (27,50). For instance, in tamoxifen-sensitive MCF-7 cells, levels of the PAX2 corepressor are sufficiently high to prevent effective binding of SRC-3 to the ERα regulatory region of the erbB2 gene; this is reflected in the inability of tamoxifen to stimulate erbB2 mRNA expression (51). However, in tamoxifen-resistant MCF-7 cells where PAX2 expression is low, SRC-3 occupies the erbB2 regulatory region and the antiestrogen stimulates erbB2 gene expression.
Competition between coactivator and corepressor for binding to agonist-bound ERα also has been documented. The ER-specific corepressor, repressor of estrogen receptor activity (REA), interacts with ERα in the presence of E2 and competes with the SRC-1 coactivator for binding to agonist-bound receptor, thus attenuating ER-dependent gene expression (52,53). A conceptually similar experiment has demonstrated competition between SRC-2 or SRC-3 and SMRT for binding to agonist-bound VDR-RXR heterodimers with down-regulation of corepressor levels by siRNA enhancing 1,25-D-dependent transactivation of gene expression (54). Other studies have demonstrated the ability of coactivator to promote and corepressors to reduce agonist-induced transcriptional activity for MR, GR, and AR (30,55,56), indicating a broad ability of corepressors to attenuate the activity of agonist-bound nuclear receptors. The inhibitory effects of corepressors on nuclear receptor transcriptional activity need not be mediated via direct interaction with the receptors because NCoR appears to attenuate VDR/RXR transcriptional activity via competition with p300 for binding to the VDR coactivator ski-interacting protein (SKIP) (57).
In contrast to these examples of competitive inhibition, our study clearly shows that SMRT and SRC-3 are present simultaneously at ERα target genes in E2-treated cells and that SMRT and SRC-3 work together to stimulate gene expression. Several previous ChIP studies have demonstrated low levels of SMRT present at ERα target genes such as pS2, c-Myc, and cathepsin D before E2 treatment (58,59,60). The role for SMRT, if any, at the promoters of these genes before E2 treatment is not defined, although the demonstration of enhanced E2 induction of pS2 gene expression in SMRT-depleted MCF-7 cells suggests a repressive effect on this gene (26). At least two mechanisms are involved in SMRT and SRC-3 stimulation of cyclin D1 gene expression; SMRT increases the occupancy of the cyclin D1 gene by SRC-3, and SMRT stimulates SRC-3 intrinsic transcriptional activity. Previous studies identified intrinsic transcriptional activity in the C-terminal portion of SRC-3 (61,62), and our work suggests that SMRT interaction with SRC-3 likely contributes to this transcriptional activity. Intriguingly, SMRT interacts with the HAT domain of SRC-3. However, in vitro acetyltransferase assays failed to reveal an impact of the SMRT ID1 domain on the HAT activity of SRC-3 (data not shown). Overall, these findings underscore that the functional output of a given coregulator is context dependent and illustrate a model whereby gene regulation occurs through the positive, coordinated efforts of a corepressor and a coactivator rather than these molecules exerting their effects in a temporally distinct and/or opposing fashion. Indeed, these data in conjunction with other reports detailing the ability of coactivators to inhibit gene expression (39,63,64) suggest a broad potential for coactivators and corepressors to exert overlapping effects on regulation of gene expression.
The role of p160 family of coactivators in breast cancer is well recognized (65). In particular, SRC-3 is an oncogene whose expression is elevated in more than 50% of breast tumors, and amplification of this gene has been detected in approximately 10% of breast cancer patients (66). Moreover, high expression of SRC-3 in conjunction with erbB2 overexpression is correlated with poor disease-free and overall survival (14,67). Comparatively little is known, however, regarding the association of SMRT expression with breast tumorigenesis. Within the framework of the SERM hypothesis, it is envisioned that SMRT, like other corepressors, is required for SERM (e.g. tamoxifen) inhibition of the expression of genes including those required for cell proliferation and survival. Consequently, it has been hypothesized that lower corepressor expression would result in antiestrogen resistance, with little consideration of the role of SMRT in tumorigenesis. Indeed, a reduction in NCoR expression has been correlated with acquired tamoxifen resistance in a mouse xenograft model of human MCF-7 breast cancer (68), and low NCoR expression has been associated with poor relapse-free survival in 99 breast cancer patients who had received tamoxifen adjuvant therapy (69).
With regards to SMRT, there are few to no data suggesting a protective role in breast cancer. Several studies have failed to detect changes in SMRT expression for tamoxifen-resistant breast tumors or estrogen-hypersensitive (long-term estrogen-deprived) MCF-7 cells (70,71). It is, however, intriguing that higher levels of corepressor have been found in scenarios associated with altered responses to ER ligand. For instance, elevated levels of SMRT mRNA were detected in cells resistant to the antiestrogen toremiphene, and increased SMRT and NCoR expressions were observed for estrogen-independent MCF-7 cells (72). Our previous studies also demonstrated a reduction in proliferation of SMRT-depleted MCF-7 cells (26). Interestingly, a recent study investigating the pattern of coregulator expression in a recent large study of 235 breast tumors identified SMRT as an independent prognostic indicator of poor overall patient survival and disease-free interval and was significantly correlated with distant metastases and local recurrence (73). Moreover, they also found a positive correlation (P < 0.0001) between the expression of SMRT and SRC-3, which is in agreement with our study. The correlations between SMRT and SRC-3 expression, and the association with poor prognosis suggests that elevated expression of these coregulators might drive breast tumor progression. Our finding that SMRT and SRC-3 cooperate to promote gene expression suggests a mechanism by which these coregulators may work to promote breast cancer. Further investigations are therefore warranted to establish the links between these two coregulators and patient outcome.
Materials and Methods
Chemicals, siRNAs, and cell culture
E2 and 4HT were obtained from Sigma Chemical Co. (St. Louis, MO). Vehicle for all experiments was 0.1% ethanol. A pan-SMRT siRNA was used in all experiments except for Figs. 5 and 8 where a SMRT siRNA (Sαβ2) targeting the 5′ end of the SMRT coding region was employed. These siRNAs (26) as well as the siRNA for SRC-3 (74) were obtained from Ambion (Austin, TX). Ambion's Silencer #2 negative control was used as a nonspecific control. The HeLa human cervical carcinoma cell line and the MCF-7 human breast cancer cell line used in this study were obtained from American Type Culture Collection (Manassas, VA) and maintained in DMEM supplemented with 10% fetal bovine serum (FBS).
Plasmids
The plasmid pCR3.1-ERα encoding human full-length ERα and the ERE-E1b-Luc reporter gene have been described previously (75,76). The expression plasmid pCR3.1-SMRTshort for human SMRT lacking amino acids 1-1031 was derived by cloning the truncated cDNA isolated from pCMX-SMRTshort (17) into the KpnI and XhoI sites of pCR3.1 (Invitrogen, Carlsbad, CA). GST fusion plasmids for portions of SMRT harboring RD3 (A, amino acids 1264-1453), RD4 (B, amino acids 1572-1694), and ID2 (C, amino acids 2014-2205) were generated by PCR amplification using pCMX-SMRTe, which encodes human full-length SMRTτ, as template (a kind gift from Dr. J. D. Chen, University of Massachusetts Medical School, Worcester, MA) (77). The PCR products were cloned into the pGEX4T-1 vector (GE Healthcare, Piscataway, NJ). The GST expression vector for the SMRT C terminus, harboring the ID1 domain (amino acids 2313-2517) was a kind gift from Dr. M. L. Privalsky (University of California, Davis, CA) (78). The pCR3.1-hSMRTτ plasmid was generated by removing the SMRT cDNA from pCMX-hSMRTe with EcoRI and cloning it into the corresponding site of pCR3.1. The pCR3.1 plasmids for SRC-1, SRC-2, and SRC-3 have been described previously (79,80). The GST expression vectors for the helix-loop-helix (amino acids 1-320), serine/threonine (amino acids 321-580), receptor-interacting domain (amino acids 581-844), CBP-interacting domain (amino acids 841-1080), and HAT domain (amino acids 1081-1417) of SRC-3 were kindly provided by Dr. R. C. Wu (Baylor College of Medicine) (81). The pCR3.1 expression vectors for SRC-3 domains were generated by inserting the cDNAs isolated from pCMX expression vectors for these fragments (82) into the pCR3.1 vector. The pBIND vector encoding the GAL4 DNA-binding domain was purchased from Promega Corp. (Madison, WI), and pBIND expression vectors for GAL-SRC-1A, GAL-SRC-2, and GAL-SRC-3 have been described previously (79).
ERα trans-activation assays
For transient transfections, HeLa cells were plated in six-well culture dishes with phenol red-free DMEM supplemented with 10% charcoal-stripped FBS (sFBS) 24 h before transfection. Plasmid DNAs were transfected into cells using Lipofectamine transfection reagent (Invitrogen). In siRNA knockdown experiments, HeLa cells were transfected first with the indicated siRNA using Oligofectamine (Invitrogen), followed 24 h thereafter by transfection with the desired plasmid DNAs. Twenty-four hours after the final transfection, cells were treated with 0.1% ethanol (vehicle) or 1 nm E2 for an additional 24 h. Cell lysates were prepared for luciferase assay using the Luciferase assay system kit (Promega) and Luminoskan Ascent Thermo Labsystems (Thermo Electron Corp., Milford, MA). Values were normalized to total protein content measured using Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA).
Co-IP assay
MCF-7 cells were grown at least 24 h in phenol red-free DMEM supplemented with 10% sFBS before IP. For siRNA and IP experiments, MCF-7 cells were transfected with control siRNA or siRNA against SMRT using Oligofectamine and allowed to grow 48 h in phenol red-free DMEM containing 10% sFBS before treatment with hormones as indicated in the respective figure legends. Cell lysates were prepared by incubating cells in the IP lysis buffer [20 mm Tris-HCl (pH 7.5), 1% Triton X-100, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 5% glycerol] supplemented with phosphatase inhibitor (Halt phosphatase inhibitor cocktail; Thermo Scientific) and protease inhibitor cocktails (Roche, Indianapolis, IN) for 20 min at 4 C, followed by centrifugation at 14,000 × g for 15 min at 4 C. The protein concentrations of the lysates were determined using the Bio-Rad protein assay reagent. Lysates were precleared with protein A/G Plus-agarose beads (30 μl packed beads; Santa Cruz Biotechnology, Santa Cruz, CA) at 4 C for 1 h, and then approximately 1000 μg cell lysate was immunoprecipitated with antibodies against SMRT (H300) or ERα (H184) or normal rabbit IgG as control (all antibodies from Santa Cruz Biotechnology) overnight at 4 C. Aliquots of cell lysates obtained before IP were used as input control. Immune complexes were precipitated by protein A/G Plus-agarose beads (30 μl packed beads) after a 2-h incubation. Beads were then washed three times with lysis buffer. Precipitated proteins were eluted from beads by resuspending the beads in NuPAGE sample buffer (Invitrogen) and heating them at 75 C for 10 min. The resulting protein from IP or total cell lysates (input) were separated by SDS-PAGE using 3–8% NuPAGE Novex gels (Invitrogen) and analyzed by Western blotting.
Re-IP assay
HeLa cells (1.5 × 106/100-mm plate) were transfected with expression vectors for Flag-tagged ERα and SMRTshort (1 μg each) using TransIT-LT1 following the manufacturer's protocol (Mirus, Madison, WI). After 24 h, cells were treated with hormone for 60 min, lysed in buffer containing protease and phosphatase inhibitor and subjected to IP using EZview red anti-Flag M2 affinity gel (Sigma; 30 μl packed beads) for 3 h at 4 C with constant rotation. Thereafter, beads were washed thrice with IP lysis buffer and eluted with 50 μl 100 μg/ml 3X FLAG peptide (Sigma). Five percent of the eluates were saved, and the rest were diluted 20 times with IP lysis buffer and subjected to a second IP with SMRT antibody (H300; Santa Cruz) overnight at 4 C. Immune complexes were reprecipitated by protein A/G Plus-agarose beads, washed thrice with IP lysis buffer, and eluted by heating the beads in SDS-PAGE loading buffer at 75 C for 10 min. Eluted material from the first and second IP as well as input samples was resolved by SDS-PAGE and Western blotted for SRC-3.
Western blot analyses
Western blot analyses were performed essentially as described previously (11). Briefly, cultured cells were harvested and lysed with extraction buffer [50 mm Tris-Cl (pH 7.4), 5 mm EDTA, 1% Nonidet P-40, 0.2% sarkosyl, 0.4 m NaCl, and complete protease inhibitor cocktail (Roche)] for 30 min at 4 C. The whole-cell lysates were clarified by centrifugation and their protein content determined. Equal amounts of protein were separated by SDS-PAGE using Invitrogen's precast NuPAGE 3–8% or 4–12% gels and transferred to nitrocellulose membrane (Osmonics Inc., Gloucester, MA) by electrotransfer. The transferred membrane was blocked with PBS plus 0.5% Tween 20 (PBST) containing 5% nonfat dry milk for 1 h and then incubated with primary antibody overnight. After washing, the membrane was incubated with horseradish peroxidase-conjugated antimouse or antirabbit secondary antibody (GE Healthcare) as appropriate, and the target proteins were visualized by ECL Plus Western blotting detection system (GE Healthcare). The primary antibodies are as follows: anti-ERα [rabbit polyclonal, HC-20 (Santa Cruz Biotechnology) and mouse monoclonal (Cell Signaling, Beverly, MA)], anti-PR (mouse monoclonal clone 1294; a kind gift from Dr. Dean Edwards, Baylor College of Medicine), anti-cyclin D1 (554180; BD Transduction Laboratories, Lexington, KY), anti-SRC-3 (611105; BD Transduction Laboratories), anti-SMRT (611386; BD Transduction Laboratories), anti GAL4 DNA-binding domain (horseradish peroxidase-conjugated mouse monoclonal; Santa Cruz Biotechnology), and anti-actin (MAB1501R; Millipore, Bedford, MA).
In vitro protein-protein interaction
The GST-tagged fusion proteins were expressed in Escherichia coli BL21 (DE3) cultures (Invitrogen) and purified with a B-PER GST spin purification kit (Pierce, Rockford, IL). Five to 10 μg purified GST fusion protein were incubated with 30 μl immobilized glutathione beads in 125 mm Tris (pH 8.0) buffer containing 150 mm NaCl and complete protease inhibitor at 4 C for 4 h. The SMRTshort, full-length SRC-3 or SRC-3 domains were in vitro translated using a TNT system (Promega) in the presence of [35S]methionine (PerkinElmer, Waltham, MA). Glutathione-Sepharose beads coupled to GST fusion proteins were incubated with radiolabeled proteins in 1 ml NETN buffer [20 mm Tris (pH 8.0), 1 mm EDTA, 50 mm NaCl, 0.5% Nonidet P-40] overnight at 4 C. Unbound proteins were removed by two washes with NETN buffer and two washes with buffer A [2 mm Tris-Cl (pH 7.4), 0.5 mm EDTA, 0.5% Nonidet P-40]. The bound proteins were resolved by SDS-PAGE and visualized by autoradiography.
ChIP and sequential ChIP assay
ChIP assays were performed as described earlier (26). Briefly, MCF-7 cells were grown in phenol red-free DMEM containing 5% sFBS for 48 h and treated with 2.5 μm α-amanitin for 90 min. After removal of α-amanitin, cells were washed twice with PBS and then treated with vehicle (0.1% ethanol), 10 nm E2, or 100 nm 4HT for 45 min before cell fixation with formaldehyde. Chromatin was incubated with antibodies against ERα (HC-20 and H-184), SRC-3 (C-20), SMRT (H300), or normal rabbit/goat IgG (all antibodies from Santa Cruz Biotechnology) and subjected to precipitation using protein A/G Sepharose beads (GE Healthcare) preblocked with salmon-sperm DNA (Invitrogen). Purified, precipitated chromatin was quantified by qPCR using SYBR Green chemistry and normalized against the input chromatin. Primer sequences used in the qPCR are provided in Supplemental Table 1. For the sequential ChIP experiment, chromatin prepared from E2-treated MCF-7 cells were first immunoprecipitated with SRC-3 antibody. The immune complexes were eluted from the beads by heating at 65 C for 10 min in ChIP TE buffer [50 mm Tris (pH 8.1), 10 mm EDTA] containing 1% sodium dodecyl sulfate. Eluted material was diluted 10 times in TSE-I [20 mm Tris-HCl (pH 8.1), 2 mm EDTA, 150 mm NaCl, and 1% Triton X-100] containing 25 μg/ml λ-phage DNA, 5 mg/ml BSA (fraction V; Sigma), 50 μg/ml E. coli tRNA, 4 μg antibody, and 50 μl protein A-Sepharose and incubated for 90 min at room temperature. Thereafter, immunoprecipitated materials were eluted from beads, purified, and quantitated by qPCR.
Analysis of human breast tumor lysates
Thirty-four frozen human tumor specimens were obtained from the Baylor College of Medicine Breast Center tumor bank, which was created more than 20 yr ago from excess tissue after medically indicated procedures and is maintained under the auspices of a local Institutional Review Board-approved protocol. Tissues had been previously solubilized in 5% sodium dodecyl sulfate and stored at −80 C. Forty micrograms of each tumor lysate were separated on a 3–8% Invitrogen precast NuPage gel and transferred to a nitrocellulose membrane (Osmonics Inc., Gloucester, MA) by electrotransfer. Fifteen micrograms of the same MCF-7 cell lysate also were incorporated into each gel experiment to standardize detection between Western blots. The membranes were blocked with PBST containing 3% nonfat dry milk for 1 h at room temperature and then incubated with the appropriate primary antibody overnight at 4 C. Primary antibodies included mouse-antiSMRT (BD Transduction Laboratories; 611387, 1:500 dilution), mouse-antiSRC-3 (BD Transduction Laboratories; 611105, 1:1000 dilution), and mouse anti-actin (Millipore; Mab1501R, 1:50,000 dilution). Membranes were washed with PBST and incubated with IRDye goat antimouse secondary antibody (LI-COR Biosciences, Lincoln, NE; 926-32110, 1:20,000 dilution) for 1 h at room temperature in the dark. Membranes were washed in PBST and protein bands detected using the LI-COR Odyssey infrared imaging system. Final quantification of protein bands was performed using ImageJ software (http://rsbweb.nih.gov/ij/).
Supplementary Material
Acknowledgments
We thank the Breast Center Tumor Bank and Biostatistics Core and Dr. Susan Hilsenbeck, Ryan Hartmaier, and Tao Wang for their assistance with the tumor studies. We also thank Dr. Benita Katzenellenbogen for providing primer sequences for the PR gene, and Bryan Nikolai for bioinformatics assistance. The technical support of Judy Roscoe and Cheryl Parker for cell culture is gratefully acknowledged.
Footnotes
The generation and maintenance of the tumor bank was supported by P01CA030195 (S.O.) and SPORE P50CA058183 (Dr. C. K. Osborne, Director BCM Breast Center). S.K. was supported by a postdoctoral fellowship award (PDF 0707868) from the Susan G. Komen for the Cure foundation. This work was supported by Public Health Service Grant DK53002 to C.L.S.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 14, 2010
Abbreviations: CBP, cAMP response element-binding protein-binding protein; ChIP, chromatin immunoprecipitation; E2, 17β-estradiol; ER, estrogen receptor; ERE, estrogen-responsive element; FBS, fetal bovine serum; GST, glutathione S-transferase; HAT, histone acetyltransferase; HDAC, histone deacetylase; 4HT, 4-hydroxytamoxifen; IP, coimmunoprecipitation; Mr, relative molecular mass; NCoR, nuclear receptor corepressor; PBST, PBS plus 0.5% Tween 20; PR, progesterone receptor; qPCR, quantitative PCR; RD, repressor domain; SERM, selective ER modulator; sFBS, charcoal-stripped FBS; siRNA, small interfering RNA; SMRT, silencing mediator of retinoic acid and thyroid hormone receptor; TBL1, transducin-like protein-1; TR, thyroid hormone receptor.
References
- Katzenellenbogen BS 1996 Estrogen receptors: bioactivities and interactions with cell signaling pathways. Biol Reprod 54:287–293 [DOI] [PubMed] [Google Scholar]
- Sarrel PM, Lufkin EG, Oursler MJ, Keefe D 1994 Estrogen actions in arteries, bone and brain. Sci Am Sci Med 1:44–53 [Google Scholar]
- Henderson BE, Ross R, Bernstein L 1988 Estrogens as a cause of human cancer: the Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res 48:246–253 [PubMed] [Google Scholar]
- Glass CK 1994 Differential recognition of target genes by nuclear receptor monomers, dimers, and heterodimers. Endocr Rev 15: 391–407 [DOI] [PubMed] [Google Scholar]
- Oñate SA, Tsai SY, Tsai MJ, O'Malley BW 1995 Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354–1357 [DOI] [PubMed] [Google Scholar]
- Voegel JJ, Heine MJ, Zechel C, Chambon P, Gronemeyer H 1996 TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J 15:3667–3675 [PMC free article] [PubMed] [Google Scholar]
- Oh Y, Müller HL, Ng L, Rosenfeld RG 1995 Transforming growth factor-β-induced cell growth inhibition in human breast cancer cells is mediated through insulin-like growth factor-binding protein-3 action. J Biol Chem 270:13589–13592 [DOI] [PubMed] [Google Scholar]
- Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin SC, Heyman RA, Rose DW, Glass CK, Rosenfeld MG 1996 A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85:403–414 [DOI] [PubMed] [Google Scholar]
- Chakravarti D, LaMorte VJ, Nelson MC, Nakajima T, Schulman IG, Juguilon H, Montminy M, Evans RM 1996 Role of CBP/P300 in nuclear receptor signalling. Nature 383:99–103 [DOI] [PubMed] [Google Scholar]
- Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR 1999 Regulation of transcription by a protein methyltransferase. Science 284:2174–2177 [DOI] [PubMed] [Google Scholar]
- Karmakar S, Foster EA, Smith CL 2009 Unique roles of p160 coactivators for regulation of breast cancer cell proliferation and estrogen receptor-α transcriptional activity. Endocrinology 150: 1588–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudelist G, Czerwenka K, Kubista E, Marton E, Pischinger K, Singer CF 2003 Expression of sex steroid receptors and their co-factors in normal and malignant breast tissue: AIB1 is a carcinoma-specific co-activator. Breast Cancer Res Treat 78:193–204 [DOI] [PubMed] [Google Scholar]
- List HJ, Reiter R, Singh B, Wellstein A, Riegel AT 2001 Expression of the nuclear coactivator AIB1 in normal and malignant breast tissue. Breast Cancer Res Treat 68:21–28 [DOI] [PubMed] [Google Scholar]
- Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R 2003 Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 95:353–361 [DOI] [PubMed] [Google Scholar]
- Harigopal M, Heymann J, Ghosh S, Anagnostou V, Camp RL, Rimm DL 2009 Estrogen receptor co-activator (AIB1) protein expression by automated quantitative analysis (AQUA) in a breast cancer tissue microarray and association with patient outcome. Breast Cancer Res Treat 115:77–85 [DOI] [PubMed] [Google Scholar]
- Fereshteh MP, Tilli MT, Kim SE, Xu J, O'Malley BW, Wellstein A, Furth PA, Riegel AT 2008 The nuclear receptor coactivator amplified in breast cancer-1 is required for Neu (ErbB2/HER2) activation, signaling, and mammary tumorigenesis in mice. Cancer Res 68:3697–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JD, Evans RM 1995 A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377:454–457 [DOI] [PubMed] [Google Scholar]
- Hörlein AJ, Näär AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Söderström M, Glass CK 1995 Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377:397–404 [DOI] [PubMed] [Google Scholar]
- Jepsen K, Rosenfeld MG 2002 Biological roles and mechanistic actions of co-repressor complexes. J Cell Sci 115:689–698 [DOI] [PubMed] [Google Scholar]
- Li J, Wang J, Wang J, Nawaz Z, Liu JM, Qin J, Wong J 2000 Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J 19:4342–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA, Shiekhattar R 2000 A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev 14: 1048–1057 [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kalkum M, Chait BT, Roeder RG 2002 The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Mol Cell 9:611–623 [DOI] [PubMed] [Google Scholar]
- Nagy L, Kao HY, Chakravarti D, Lin RJ, Hassig CA, Ayer DE, Schreiber SL, Evans RM 1997 Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell 89:373–380 [DOI] [PubMed] [Google Scholar]
- Nettles KW, Greene GL 2005 Ligand control of coregulator recruitment to nuclear receptors. Annu Rev Physiol 67:309–333 [DOI] [PubMed] [Google Scholar]
- Privalsky ML 2004 The role of corepressors in transcriptional regulation by nuclear hormone receptors. Annu Rev Physiol 66:315–360 [DOI] [PubMed] [Google Scholar]
- Peterson TJ, Karmakar S, Pace MC, Gao T, Smith CL 2007 The silencing mediator of retinoic acid and thyroid hormone receptor (SMRT) corepressor is required for full estrogen receptor-α transcriptional activity. Mol Cell Biol 27:5933–5948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, Nawaz Z, O'Malley BW 1997 Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol 11:657–666 [DOI] [PubMed] [Google Scholar]
- Métivier R, Stark A, Flouriot G, Hübner MR, Brand H, Penot G, Manu D, Denger S, Reid G, Kos M, Russell RB, Kah O, Pakdel F, Gannon F 2002 A dynamic structural model for estrogen receptor-α activation by ligands, emphasizing the role of interactions between distant A and E domains. Mol Cell 10:1019–1032 [DOI] [PubMed] [Google Scholar]
- Hodgson MC, Astapova I, Cheng S, Lee LJ, Verhoeven MC, Choi E, Balk SP, Hollenberg AN 2005 The androgen receptor recruits nuclear receptor corepressor (N-CoR) in the presence of mifepristone via its N and C termini revealing a novel molecular mechanism for androgen receptor antagonists. J Biol Chem 280:6511–6519 [DOI] [PubMed] [Google Scholar]
- Yoon HG, Wong J 2006 The corepressors silencing mediator of retinoid and thyroid hormone receptor and nuclear receptor corepressor are involved in agonist- and antagonist-regulated transcription by androgen receptor. Mol Endocrinol 20:1048–1060 [DOI] [PubMed] [Google Scholar]
- Wang D, Wang Q, Awasthi S, Simons Jr SS 2007 Amino-terminal domain of TIF2 is involved in competing for corepressor binding to glucocorticoid and progesterone receptors. Biochemistry 46:8036–8049 [DOI] [PubMed] [Google Scholar]
- Sankar N, Baluchamy S, Kadeppagari RK, Singhal G, Weitzman S, Thimmapaya B 2008 p300 provides a corepressor function by cooperating with YY1 and HDAC3 to repress c-Myc. Oncogene 27:5717–5728 [DOI] [PubMed] [Google Scholar]
- Guidez F, Howell L, Isalan M, Cebrat M, Alani RM, Ivins S, Hormaeche I, McConnell MJ, Pierce S, Cole PA, Licht J, Zelent A 2005 Histone acetyltransferase activity of p300 is required for transcriptional repression by the promyelocytic leukemia zinc finger protein. Mol Cell Biol 25:5552–5566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Kimbrel EA, Kenan DJ, McDonnell DP 2002 Direct interactions between corepressors and coactivators permit the integration of nuclear receptor-mediated repression and activation. Mol Endocrinol 16:1482–1491 [DOI] [PubMed] [Google Scholar]
- Perissi V, Aggarwal A, Glass CK, Rose DW, Rosenfeld MG 2004 A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell 116:511–526 [DOI] [PubMed] [Google Scholar]
- Yoon HG, Choi Y, Cole PA, Wong J 2005 Reading and function of a histone code involved in targeting corepressor complexes for repression. Mol Cell Biol 25:324–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Zhao Y, Becker M, John S, Parekh BS, Huang S, Hendarwanto A, Martinez ED, Chen Y, Lu H, Adkins NL, Stavreva DA, Wiench M, Georgel PT, Schiltz RL, Hager GL 2006 HDAC1 acetylation is linked to progressive modulation of steriod receptor-induced gene transcription. Mol Cell 22:669–679 [DOI] [PubMed] [Google Scholar]
- Welsbie DS, Xu J, Chen Y, Borsu L, Scher HI, Rosen N, Sawyers CL 2009 Histone deacetylases are required for androgen receptor function in hormone-sensitive and castrate-resistant prostate cancer. Cancer Res 69:958–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatsky I, Luecke HF, Leitman DC, Yamamoto KR 2002 Alternate surfaces of transcriptional coregulator GRIP1 function in different glucocorticoid receptor activation and repression contexts. Proc Natl Acad Sci USA 99:16701–16706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhoute J, Carroll JS, Geistlinger TR, Torres-Arzayus MI, Brown M 2006 A cell-type-specific transcriptional network required for estrogen regulation of cyclin D1 and cell cycle progression in breast cancer. Genes Dev 20:2513–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M 2006 Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297 [DOI] [PubMed] [Google Scholar]
- Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, Orlov YL, Velkov S, Ho A, Mei PH, Chew EG, Huang PY, Welboren WJ, Han Y, Ooi HS, Ariyaratne PN, Vega VB, Luo Y, Tan PY, Choy PY, Wansa KD, Zhao B, Lim KS, Leow SC, Yow JS, Joseph R, Li H, Desai KV, Thomsen JS, Lee YK, Karuturi RK, Herve T, Bourque G, Stunnenberg HG, Ruan X, Cacheux-Rataboul V, Sung WK, Liu ET, Wei CL, Cheung E, Ruan Y 2009 An oestrogen-receptor-α-bound human chromatin interactome. Nature 462:58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonéy-Montoya J, Ziegler YS, Curtis CD, Montoya JA, Nardulli AM 2010 Long-range transcriptional control of progesterone receptor gene expression. Mol Endocrinol 24:346–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Wada O, Suzawa M, Yogiashi Y, Yano T, Kato S, Yanagisawa J 2001 The tamoxifen-responsive estrogen receptor α mutant D351Y shows reduced tamoxifen-dependent interaction with corepressor complexes. J Biol Chem 276:42684–42691 [DOI] [PubMed] [Google Scholar]
- Webb P, Nguyen P, Kushner PJ 2003 Differential SERM effects on corepressor binding dictate ERα activity in vivo. J Biol Chem 278:6912–6920 [DOI] [PubMed] [Google Scholar]
- Webb P, Nguyen P, Shinsako J, Anderson C, Feng W, Nguyen MP, Chen D, Huang SM, Subramanian S, McKinerney E, Katzenellenbogen BS, Stallcup MR, Kushner PJ 1998 Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol Endocrinol 12:1605–1618 [DOI] [PubMed] [Google Scholar]
- Werbajh S, Nojek I, Lanz R, Costas MA 2000 RAC-3 is a NF-κB coactivator. FEBS Lett 485:195–199 [DOI] [PubMed] [Google Scholar]
- Louie MC, Zou JX, Rabinovich A, Chen HW 2004 ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Mol Cell Biol 24:5157–5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Yu CT, Ozen M, Ittmann M, Tsai SY, Tsai MJ 2006 Steroid receptor coactivator-3 and activator protein-1 coordinately regulate the transcription of components of the insulin-like growth factor/AKT signaling pathway. Cancer Res 66:11039–11046 [DOI] [PubMed] [Google Scholar]
- Smith CL, O'Malley BW 2004 Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev 25:45–71 [DOI] [PubMed] [Google Scholar]
- Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, Jiang J, Howat WJ, Ali S, Carroll JS 2008 Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature 456:663–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano MM, Ekena K, Delage-Mourroux R, Chang W, Martini P, Katzenellenbogen BS 1999 An estrogen receptor-selective coregulator that potentiates the effectiveness of antiestrogens and represses the activity of estrogens. Proc Natl Acad Sci USA 96:6947–6952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delage-Mourroux R, Martini PG, Choi I, Kraichely DM, Hoeksema J, Katzenellenbogen BS 2000 Analysis of estrogen receptor interaction with a repressor of estrogen receptor activity (REA) and the regulation of estrogen receptor transcriptional activity by REA. J Biol Chem 275:35848–35856 [DOI] [PubMed] [Google Scholar]
- Sánchez-Martínez R, Zambrano A, Castillo AI, Aranda A 2008 Vitamin D-dependent recruitment of corepressors to vitamin D/retinoid X receptor heterodimers. Mol Cell Biol 28:3817–3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Anzick S, Richter WF, Meltzer P, Simons Jr SS 2004 Modulation of transcriptional sensitivity on mineralocorticoid and estrogen receptors. J Steroid Biochem Mol Biol 91:197–210 [DOI] [PubMed] [Google Scholar]
- Szapary D, Huang Y, Simons Jr SS 1999 Opposing effects of corepressor and coactivators in determining the dose-response curve of agonist, and residual agonist activity of antagonists, for glucocorticoid receptor-regulated gene expression. Mol Endocrinol 13: 2108–2121 [DOI] [PubMed] [Google Scholar]
- Leong GM, Subramaniam N, Issa LL, Barry JB, Kino T, Driggers PH, Hayman MJ, Eisman JA, Gardiner EM 2004 Ski-interacting protein, a bifunctional nuclear receptor coregulator that interacts with N-CoR/SMRT and p300. Biochem Biophys Res Commun 315:1070–1076 [DOI] [PubMed] [Google Scholar]
- Métivier R, Penot G, Carmouche RP, Hübner MR, Reid G, Denger S, Manu D, Brand H, Kos M, Benes V, Gannon F 2004 Transcriptional complexes engaged by apo-estrogen receptor-α isoforms have divergent outcomes. EMBO J 23:3653–3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Brown M 2002 Molecular determinants for the tissue specificity of SERMs. Science 295:2465–2468 [DOI] [PubMed] [Google Scholar]
- Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M 2000 Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843–852 [DOI] [PubMed] [Google Scholar]
- Suen CS, Berrodin TJ, Mastroeni R, Cheskis BJ, Lyttle CR, Frail DE 1998 A transcriptional coactivator, steroid receptor coactivator-3, selectively augments steroid receptor transcriptional activity. J Biol Chem 273:27645–27653 [DOI] [PubMed] [Google Scholar]
- Li H, Gomes PJ, Chen JD 1997 RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc Natl Acad Sci USA 94:8479–8484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Takeyama K, Sawatsubashi S, Ito S, Suzuki E, Yamagata K, Tanabe M, Kimura S, Fujiyama S, Ueda T, Murata T, Matsukawa H, Shirode Y, Kouzmenko AP, Li F, Tabata T, Kato S 2009 Corepressive action of CBP on androgen receptor transactivation in pericentric heterochromatin in a Drosophila experimental model system. Mol Cell Biol 29:1017–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvoro A, Tzagarakis-Foster C, Tatomer D, Paruthiyil S, Fox MS, Leitman DC 2006 Distinct roles of unliganded estrogen receptors in transcriptional repression. Mol Cell 21:555–564 [DOI] [PubMed] [Google Scholar]
- Lonard DM, Lanz RB, O'Malley BW 2007 Nuclear receptor coreguators and human disease. Endocr Rev 28:575–587 [DOI] [PubMed] [Google Scholar]
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS 1997 AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965–968 [DOI] [PubMed] [Google Scholar]
- Kirkegaard T, McGlynn LM, Campbell FM, Müller S, Tovey SM, Dunne B, Nielsen KV, Cooke TG, Bartlett JM 2007 Amplified in breast cancer 1 in human epidermal growth factor receptor-positive tumors of tamoxifen-treated cancer patients. Clin Cancer Res 13:1405–1411 [DOI] [PubMed] [Google Scholar]
- Lavinsky RM, Jepsen K, Heinzel T, Torchia J, Mullen TM, Schiff R, Del-Rio AL, Ricote M, Ngo S, Gemsch J, Hilsenbeck SG, Osborne CK, Glass CK, Rosenfeld MG, Rose DW 1998 Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc Natl Acad Sci USA 95:2920–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault I, Lerebours F, Amarir S, Tozlu S, Tubiana-Hulin M, Lidereau R, Bièche I 2003 Expression analysis of estrogen receptor α coregulators in breast carcinoma: evidence that NCOR1 expression is predictive of the response to tamoxifen. Clin Cancer Res 9:1259–1266 [PubMed] [Google Scholar]
- Chan CM, Lykkesfeldt AE, Parker MG, Dowsett M 1999 Expression of nuclear receptor interacting proteins TIF-1, SUG-1, receptor interacting protein 140, and corepressor SMRT in tamoxifen-resistant breast cancer. Clin Cancer Res 5:3460–3467 [PubMed] [Google Scholar]
- Chan CM, Martin LA, Johnston SR, Ali S, Dowsett M 2002 Molecular changes associated with the acquisition of oestrogen hypersensitivity in MCF-7 breast cancer cells on long-term oestrogen deprivation. J Steroid Biochem Mol Biol 81:333–341 [DOI] [PubMed] [Google Scholar]
- Sarvilinna N, Eronen H, Miettinen S, Vienonen A, Ylikomi T 2006 Steroid hormone receptors and coregulators in endocrine-resistant and estrogen-independent breast cancer cells. Int J Cancer 118:832–840 [DOI] [PubMed] [Google Scholar]
- Green AR, Burney C, Granger CJ, Paish EC, El-Sheikh S, Rakha EA, Powe DG, Macmillan RD, Ellis IO, Stylianou E 2008 The prognostic significance of steroid receptor co-regulators in breast cancer: co-repressor NCOR2/SMRT is an independent indicator of poor outcome. Breast Cancer Res Treat 110:427–437 [DOI] [PubMed] [Google Scholar]
- Labhart P, Karmakar S, Salicru EM, Egan BS, Alexiadis V, O'Malley BW, Smith CL 2005 Identification of target genes in breast cancer cells directly regulated by the SRC-3/AIB1 coactivator. Proc Natl Acad Sci USA 102:1339–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz Z, Lonard DM, Smith CL, Lev-Lehman E, Tsai SY, Tsai MJ, O'Malley BW 1999 The Angelman syndrome-associated protein, E6-AP, is a coactivator for the nuclear hormone receptor superfamily. Mol Cell Biol 19:1182–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz Z, Lonard DM, Dennis AP, Smith CL, O'Malley BW 1999 Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci USA 96:1858–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Schroen DJ, Yang M, Li H, Li L, Chen JD 1999 SMRTe, a silencing mediator for retinoid and thyroid hormone receptors-extended isoform that is more related to the nuclear receptor corepressor. Proc Natl Acad Sci USA 96:3519–3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson ML, Jonas BA, Privalsky ML 2005 Alternative mRNA splicing of SMRT creates functional diversity by generating corepressor isoforms with different affinities for different nuclear receptors. J Biol Chem 280:7493–7503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutertre M, Smith CL 2003 Ligand-independent interactions of p160/steroid receptor coactivators and CREB-binding protein (CBP) with estrogen receptor-α: regulation by phosphorylation sites in the A/B region depends on other receptor domains. Mol Endocrinol 17:1296–1314 [DOI] [PubMed] [Google Scholar]
- Lonard DM, Nawaz Z, Smith CL, O'Malley BW 2000 The 26S proteasome is required for estrogen receptor-α and coactivator turn-over and for efficient estrogen receptor-α transactivation. Mol Cell 5:939–948 [DOI] [PubMed] [Google Scholar]
- Wu RC, Qin J, Yi P, Wong J, Tsai SY, Tsai MJ, O'Malley BW 2004 Selective phosphorylations of the SRC-3/AIBI coactivator integrate genomic responses to multiple cellular signaling pathways. Mol Cell 15:937–949 [DOI] [PubMed] [Google Scholar]
- Yi P, Wu RC, Sandquist J, Wong J, Tsai SY, Tsai MJ, Means AR, O'Malley BW 2005 Peptidyl-prolyl isomerase 1 (Pin1) serves as a coactivator of steroid receptor by regulating the activity of phosphorylated steroid receptor coactivator 3 (SRC-3/AIB1). Mol Cell Biol 25:9687–9699 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.