Abstract
Nuclear receptors and coregulators orchestrate diverse aspects of biological functions and inappropriate expression of these factors often associates with human diseases. The present study describes a conditional overexpression system consisting of a minigene located at the Rosa26 locus in the genome of mouse embryonic stem (ES) cells. Before activation, the minigene is silent due to a floxed STOP cassette inserted between the promoter and the transgene. Upon cre-mediated excision of the STOP cassette, the minigene constitutively expresses the tagged transgene driven by the ubiquitous CAGGS promoter. Thus, this system can be used to express target gene in any tissue in a spatial and/or temporal manner if respective cre mouse lines are available. Serving as proof of principle, the CAG-S-hCOUP-TFI allele was generated in ES cells and subsequently in mice. This allele was capable of conditionally overexpressing human chicken ovalbumin upstream promoter-transcription factor I (COUP-TFI) in all tissues tested upon activation by cre drivers. This allele was further subjected to address functionality of expressed COUP-TFI and the functional similarity between COUP-TFI and COUP-TFII. Expression of COUP-TFI in COUP-TFII-ablated uterus suppressed aberrant estrogen receptor-α activities and rescued implantation and decidualization defects of COUP-TFII mutants, suggesting that COUP-TFI and COUP-TFII are able to functionally compensate for each other in the uterus. A toolbox currently under construction will contain ES cell lines for overexpressing all 48 nuclear receptors and selected 10 coregulators. Upon completion, it will be a very valuable resource for the scientific community. Several ES cells are currently available for distribution.
Genetically engineered mouse embryonic stem cell lines are able to conditionally express nuclear receptors and coregulators in tissue- and/or temporal-specific manners.
Nuclear receptors (NRs) and coregulators (CoRs) control a wide spectrum of physiological and pathological processes. Physiologically, NRs bind to cis-acting elements in response to endocrine hormone signaling, recruit CoRs to form versatile protein complexes with multiple enzymatic capabilities, and then regulate the transcription of specific sets of hormone-responsive genes (1). Pathologically, deregulation of NR and CoR functions have been linked to many human disorders including cancer, metabolic syndromes, cardiovascular diseases, congenital syndromes, and neurological disorders (2,3,4,5). Few examples among many are chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) genomic locus deletion in Bochdalek-type congenital diaphragmatic hernia, Nur-related protein 1 missense mutations in schizophrenia and manic-depressive disorder, androgen receptor polymorphism in prostate cancer, thyroid hormone receptor β missense mutation in generalized resistance to thyroid hormone, vitamin D receptor mutations in vitamin D-dependent rickets type II, steroid receptor coactivator 3 (SRC-3) amplification and overexpression in prostate and breast cancer, and various E6-associated protein mutations in Angelman syndrome (6,7,8,9,10,11,12,13,14). In the last two decades, new roles of NRs and CoRs emerged with the aid of an array of genetic and proteomic tools, leading to better understanding of their key functions in fields such as reproduction, embryonic development, circadian rhythm, energy metabolism, drug metabolism, and stem cell biology (15,16,17,18,19,20,21,22). The majority of the in vivo analysis of the roles of these NRs relied on gene ablation and loss of function studies.
Whereas gene ablation strategy has been used successfully to dissect the physiological and developmental functions of NRs and CoRs, diseases resulting from gene amplification and overexpression are in need of animal models for further studies in vivo. To mimic such human diseases and to provide better resolution for dissecting the causes in certain tissues, an ideal animal model should permit appropriate levels of expression with tissue specificity and should impart phenotypes of interest. Previous transgenic approaches to overexpress NRs and CoRs in a tissue-specific fashion have met with limited success. Here we report that the combination of the cre-lox technology and a uniformly expressing minigene at a fixed genomic locus appears to generate a reliable in vivo system to target these molecules in a tissue-specific fashion. Given the numerous roles of NRs and CoRs in vivo, an inducible overexpression system with the aforementioned features built in embryonic stem (ES) cells or animals will be an extremely valuable resource for investigators in this field. In this report, we present the construction of a collection of ES cell lines that are able to overexpress NRs and CoRs subsequent to cre recombinase-dependent activation.
To serve as a proof of principle, we have generated ES cell lines to conditionally express the human version of chicken ovalbumin upstream promoter-transcription factor I (COUP-TFI). Mice created from these ES cells successfully demonstrated its capacity of conditionally expressing the human COUP-TFI transgene. COUP-TFI, a member of steroid hormone receptor superfamily capable of transcriptional regulation, is known as a major regulator in controlling neural development and tissue morphogenesis (23,24,25,26). Its paralog, COUP-TFII, also critically modulates cell fate determination, organogenesis, metabolism, and tumorigenesis (16,27,28,29). Structurally, COUP-TFI and COUP-TFII share high similarity across species with nearly identical DNA and ligand-binding domains (30). Functionally, in vitro biochemical studies have shown that these two factors can bind the same DNA response elements of AGGTCA direct repeat with variable spacing in either homo- or heterodimer forms to regulate transcription of target genes (31). Thus, it is reasonable to speculate that COUP-TFI and COUP-TFII may have functional redundancy in vivo. In this report, using the human COUP-TFI conditional expression mice, we provided evidence that COUP-TFI and COUP-TFII, as predicted, are functionally interchangeable in the uterus.
Results
Design of the conditional overexpression system
We have generated ES cell lines that are capable of expressing NRs or CoRs in a temporal and/or spatial manner upon cre recombinase-mediated activation. Each ES cell line harbors a single copy of minigene consisting of a CAGGS promoter, a loxP-STOP-loxP (LSL) cassette, a tagged NR/CoR cDNA sequence, and a polyadenylation signal (Fig. 1A). The CAGGS promoter is active in almost all tissues in vivo (32), whereas the LSL cassette placed 3′ to the CAGGS promoter disrupts the expression of the downstream NR/CoR coding sequence, silencing its expression before activation (33). In the presence of cre recombinase, the LSL cassette is excised, which allows the CAGGS promoter to drive the expression of NR/CoR. Moreover, to ensure uniform levels of expression throughout the animal, the minigene was inserted at the ROSA26 locus of the mouse genome in ES cells.
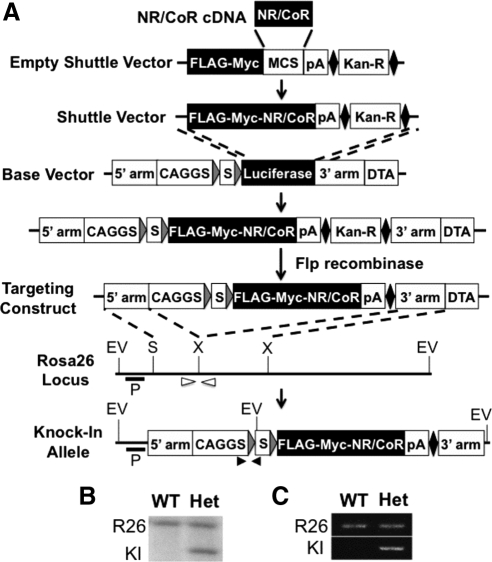
Figure 1.
Generation of the NR/CoR conditional overexpression system. A, General scheme of the production process. The empty shuttle vector contains a FLAG and myc tandem tag, a multiple cloning site, a transcription stop sequence (pA), a FRT-flanked kanamycin-resistant cassette (solid diamond and Kan-R), and two homologous sequences for recombination with the base vector. NR/CoR cDNA is cloned into the shuttle vector at the multiple cloning sites. The base vector contains two ROSA26 genomic sequences for gene targeting (5′- and 3′-arms), a diphtheria toxin A fragment gene (DTA), and a minigene consisting of a CAGGS promoter, a loxP-STOP-loxP (LSL) cassette (gray triangles and S), and luciferase cDNA. The targeting construct is made by homologous recombination between the shuttle vector and the base vector followed by removal of Kan-R with Flp recombinase. The minigene is inserted into the ROSA26 locus as indicated by dashed lines to generate the knock-in allele. P, probe for southern analysis; EV, EcoRV; S, SacII; X, XbaI; open and closed arrowheads, primers for PCR genotyping on unmodified wild-type (R26) and knock-in (KI) alleles, respectively. B, Southern blot of DNA from the wild-type (WT) and the minigene-carrying heterozygous (Het) ES cell lines, digested with EcoRV and probed as indicated in panel A. The 11-kb band at top and the 3.8-kb band at bottom are from the R26 and the KI alleles, respectively. C, PCR genotyping of tail DNA from WT and Het animals by allelic-specific primer sets indicated in panel A.
To streamline the targeting construct production process, a shuttle vector was made to deliver cDNAs for NR/CoR or any gene of interest into the base vector, pCAGGc-LSL-Luciferase (34), by recombineering technology to replace the luciferase (Fig. 1A). The shuttle vector contains a kozak sequence, a translation starting site, a FLAG-myc tandem tag sequence, and multiple cloning sites 3′ to the tag. The NR/CoRs is cloned into the multiple cloning sites in frame with the tag to produce N terminus-tagged protein. After recombineering, the resulting targeting construct then mediates insertion of the minigene at the ROSA26 locus by homologous recombination (35).
Generation of the conditional overexpression allele for human COUP-TFI
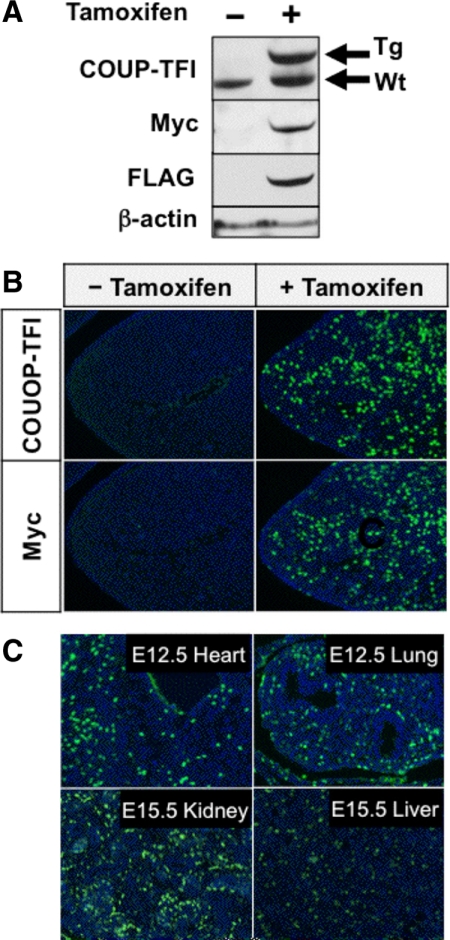
As a proof of principle, a minigene that is able to conditionally express tagged human COUP-TFI protein was made accordingly in ES cell lines. The resulting engineered allele is named CAG-S-hCOUP-TFI allele, and its presence was detected by Southern blotting and PCR assays, respectively (Fig. 1, B and C). To test whether or not the inserted minigene performs as designed, this allele was crossed into the R26creERT2 background, in which cre recombinase activity was induced by treatment with tamoxifen, to generate the R26creERT2/CAG-S-hCOUP-TFI mouse line. Embryonic d 12.5 (E12.5) pharyngeal arches, in which endogenous COUP-TFI expression is not detected, were chosen to validate the induced human COUP-TFI expression. As shown in Fig. 2, A and B, no transgenic protein was observed in the absence of tamoxifen treatment, indicating that this allele remains quiescent. Upon tamoxifen administration, the cre recombinase was activated, rendering the removal of the LSL cassette and subsequent expression of the tagged human COUP-TFI. The resulting product stained positively with both COUP-TFI and the tag-specific antibodies and was nuclear localized (Fig. 2, A and B). Moreover, this minigene gave not only uniform expression across various tissues when temporally induced, but also high-expression efficiency, with an estimated more than 50% of cells expressing the transgene after transient induction of cre activity (Fig. 2C).
Figure 2.
Expression of transgenic human COUP-TFI after cre-mediated activation. Animals that carried both the R26creERT2 and CAG-S-hCOUP-TFI alleles were given oil and tamoxifen for control and activation, respectively. A, Western blot of E15.5 embryonic extracts with and without activation at E12.5. The membrane was blotted with anti-COUP-TFI, anti-myc, and anti-FLAG antibodies to detect transgenic (Tg) or endogenous (Wt) COUP-TFI expression. B, Immunofluorescence staining with anti-COUP-TFI and anti-myc antibodies on tissue sections of E12.5 pharyngeal arches with and without activation at E9.5. C, Immunofluorescence staining with the anti-myc antibody on E12.5 tissue sections of various organs after activation at E9.5.
COUP-TFI overexpression rectified defects in COUP-TFII-deficient uterus
As the system proved able to work as designed, we move forward to test whether or not the expressed human COUP-TFI is functional in vivo. Previous structural and biochemical studies already suggest that COUP-TFI and COUP-TFII are functionally interchangeable. To prove it in vivo, we decided to ectopically express COUP-TFI in a tissue-specific COUP-TFII-deficient mouse and tested whether COUP-TFI could rescue the phenotypes exhibited by the COUP-TFII-deficient mouse. We chose a mouse model using PR-Cre to ablate COUP-TFII in the uterine stroma (36). These mutant mice are infertile due to defective embryo attachment and decidualization (36,37). To investigate whether COUP-TFI can functionally compensate for COUP-TFII in the uterine stroma, we ablated the COUP-TFII gene and activated COUP-TFI expression by crossing together the COUP-TFIIflox/flox, CAG-S-hCOUP-TFI, and PRcre alleles. Expression of the transgenic COUP-TFI was detected in a vast majority of stromal cells in which COUP-TFII is primarily expressed (Fig. 3C and Ref. 36). On the other hand, overexpression in the epithelial compartment appeared patchy in a clonal manner, possibly due to the differential presence of cre activities during development of epithelium.
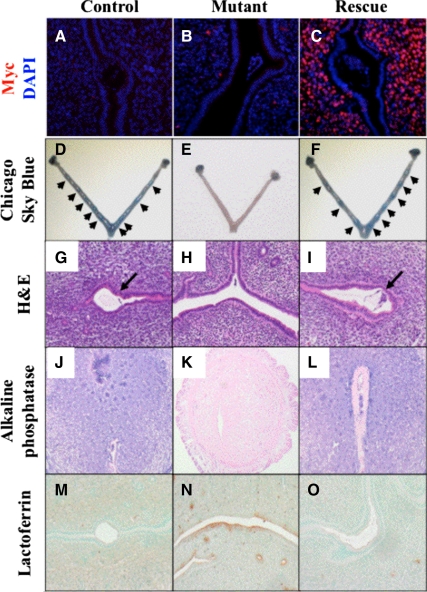
Figure 3.
The expression of COUP-TFI in uterine stroma rescued the implantation defects of the uterine COUP-TFII-deficient mice. Control: PRcre/+;COUP-TFIIflox/+. Mutant: PRcre/+;COUP-TFIIflox/flox. Rescue: PRcre/+;COUP-TFIIflox/flox; CAG-S-hCOUP-TFI. A–C, Immunofluorescence staining of tagged human COUP-TFI on sections of implantation sites by anti-myc antibody with 4′,6-diamidino-2-phenylindole (DAPI) counterstaining. D–F, Gross view of uterine horns with implantation sites (arrows) illustrated by the Chicago sky blue dye. G–I, Hematoxylin and eosin (H&E) staining on sections of the implantation sites (arrows). J–L, Staining of alkaline phosphatase activities (purple) for the decidualization with nuclear fast red counterstaining. M–O, Immunostaining of the estrogen receptor-α downstream gene, lactoferrin. Brown indicates positive signals, whereas methyl green indicates counterstaining.
Three groups of female mice (6 wk of age) were bred with wild-type males (C57BL6 background) to establish pregnancy: PRcre/+;COUP-TFIIflox/+ (control), PRcre/+;COUP-TFIIflox/flox (mutant), and PRcre/+;COUP-TFIIflox/flox; CAG-S-COUP-TFI (rescue) mice. Afterward, females were killed at 4.5 d post coitum and injected with Chicago sky blue dye to facilitate visualization of the implantation sites. Consistent with previous studies, no implantation site was observed in COUP-TFII mutant mice (Fig. 3E). In contrast, overexpression of COUP-TFI in the COUP-TFII-deficient mice not only led to appearance of implantation sites (Fig. 3F) but also restored implantation capacity to a similar level of the control (Fig. 3D). The average number of implantation sites per animal was 7.8 ± 0.83 (n = 6), 0 (n = 3), and 7.25 ± 1.70 (n = 4) for control, mutant, and rescue mice, respectively. Histological analysis also found embryo attachment in both the control and the rescue but not in the mutant mice (Fig. 3, G–I). Furthermore, the rescue mice regained capability to undergo decidualization response after embryo attachment, as indicated by the increase in alkaline phosphatase activities in the stroma (Fig. 3, J–L).
The uterine COUP-TFII deficiency displays aberrantly elevated epithelial estrogen receptor activities that disrupt the window of uterine receptivity, leading to infertility (36). Using lactoferrin, an estrogen-responsive gene, as a marker to score for estrogen receptor activities, mutant epithelium exhibited higher estrogen receptor activities than the control, which is consistent with our previous observation (Fig. 3, M and N). Expression of COUP-TFI restored the lactoferrin expression levels to that of the control mice (Fig. 3O). Taken together, these results strongly suggest that the expressed COUP-TFI is functional, and it can substitute for the loss of COUP-TFII activity in the uterus.
Currently, we have generated eight ES cell lines (Table 1) among which COUP-TFI and steroid receptor coactivator 2 (SRC-2) overexpression alleles have been introduced into mice. Many more ES cell lines will be generated in the near future, and updates on available reagents can be found under the Nuclear Receptor Signaling Atlas (NURSA) web site (www.nursa.org/reagents.cfm?&moltype=embryonic).
Table 1.
Available NR/CoR conditional overexpression ES cell lines
| Molecule | NRNC symbol | Species |
|---|---|---|
| SRC-1 | NCOA1 | Homo sapiens |
| SRC-2 | NCOA2 | Homo sapiens |
| PPARα | NR1C1 | Mus musculus |
| PPARγ2 | NR1C3 | Homo sapiens |
| RXRα | NR2B1 | Homo sapiens |
| COUP-TFI | NR2F1 | Homo sapiens |
| ERα | NR3A1 | Homo sapiens |
| ERRα | NR3B1 | Homo sapiens |
ER, Estrogen receptor; ERR, estrogen-related receptor; nuclear receptor coactivator; PPAR, peroxisome proliferator-activated receptor; RXR, retinoid X receptor; NRNC, nuclear receptor nomenclature committee.
Discussion
Genetically engineered mouse (GEM) models, have served as potent tools to unlock the functions of the NRs and CoRs in vivo. GEM models first offered the ability to ectopically express these molecules by using tissue-specific promoters to direct their expression in transgenic mice. The success of this approach has been limited due to problems inherent with this technology. Expression of NRs/CoRs using the transgenic approach has suffered from variable expression due to influences of the site of integration of the transgene in the murine genome and the inability to control the number of copies of the transgene present. In addition, some promoters cannot recapitulate original tissue-specific expression pattern due to missing regulatory element(s) of the promoter used as well as the influence of integration site. The second GEM technology that has been used to conduct in vivo investigations on the biology of the NRs/CoRs is the use of homologous recombination in ES cells. This approach has been used extensively to ablate the expression of NRs and CoRs either in the whole animal or in a tissue-specific fashion. This technology, when applied correctly, has been more effective in deciphering the functions of the NRs/CoRs in vivo. The present study employed homologous recombination in ES cells to generate mice in which the expression of the nuclear NRs/CoRs can be activated in a spatial- and temporal-specific fashion. This was accomplished by inserting a conditionally inducible NR/CoR transgene into the ROSA26 locus of the murine genome that supports ubiquitous expression of the inserted genes (35). Another advantage to use ROSA locus is the very high efficiency of homologous recombination to this locus, which renders screening of recombination event high throughput to obtain a few correctly recombined ES cells (38,39).
The transgene is engineered to remain quiescent until being activated by the presence of cre recombinase. This design allows conditional expression at any given place and time under the control of spatial/temporal-specific cre recombinase mouse lines. Advantages of this system include: 1) fast in generating recombination construct; 2) no expression in the absence of the cre recombination; 3) a single mouse line to express NRs/CoRs or any gene of interest in any given tissue or cell type of interest with available tissue-specific cre recombinase mouse lines; 4) temporal expression is feasible when inducible expression of cre is employed (e.g. tamoxifen or tetracycline inducible cre); 5) uniform expression levels wherever activated; and 6) limited expression of the inserted gene. The data from works on CAG-S-hCOUP-TFI have provided confidence on employing this system to construct overexpression alleles for other NRs and CoRs.
Applications of this tool can be multiple, including, but not limited to, mimicking human diseases, directing cell differentiation, reprogramming cell identity, and dissecting genetic pathways. For example, SRC-3 is found amplified and overexpressed in breast, prostate, and many hormone-independent cancers in human patients (9,40). Torres-Arzayus et al. (41) were able to recreate the SRC-3 overexpression condition in a mouse model in which high incidence of mammary tumors was observed, supporting the notion of SRC-3 being an oncogene. The SRC-3 conditional overexpression allele could be used to create tissue-specific tumor models, which may be useful to study SRC-3-dependent tumor progression and metastasis without potential system-wide complications. In addition, the attached tags may serve as a tool to dissect proteomic alterations at the cellular level or to track tumor metastasis at the tissue level. Furthermore, in combination with genetic engineered alleles of other genes that pose as cancer risk factors, investigators can study effects of genetic interaction or dissect signal hierarchy between multiple genes. Finally, because the NRs/CoRs conditional overexpression alleles mainly carry human cDNA, the resulting tumor model may be used to screen drugs that directly target a human form of NRs/CoRs, accelerating the development of new therapeutic methods for future treatment.
Because human COUP-TFI and mouse COUP-TFII share high degree of similarity in amino acid sequences in both the DNA-binding and ligand-binding domains, it is reasonable to predict that functions of COUP-TFI and COUP-TFII are interchangeable (30). Indeed, the rescue experiments conducted in the present study provide in vivo evidence to support such a prediction. The pronounced phenotypes exhibited by the COUP-TFI and COUP-TFII null mutants and their distinct, yet overlapping, expression patterns suggest that they are critical regulators of biological activities acting in different regions. In tissues in which both COUP-TFI- and -II are present, such as in eyes, dosage-dependent phenotype was observed depending on the numbers of copies of each gene, suggesting the possibility of functional compensation of COUP-TFI and II in vivo (42). In the uterus, endogenous COUP-TFI was barely detectable, and no alteration in its expression levels was observed in COUP-TFII-deficient uterus (data not shown). Thus, the uterine defects displayed by the COUP-TFII-deficient mice are likely contributed solely by the loss of COUP-TFII in the uterine stroma. In addition, neither lactoferrin level reduction nor sites of embryo attachment were spatially correlated with the ectopic COUP-TFI expression, suggesting that the COUP-TFI-dependent suppression of estrogen activities was primarily the result of stromal derived signals. Taken together, the ability of ectopic expression of COUP-TFI in uterine stroma to rescue the uterine defects of COUP-TFII-deficient mice firmly established that these two family members could functionally compensate for each other's function in the uterus.
In summary, we have demonstrated the capability to generate CAG-S-NR/CoR alleles in ES cell lines. Mice made from the CAG-S-hCOUP-TFI ES cells were successfully tested for activation, expression, and functional analysis as proof of principle. With the support of NURSA, we plan eventually to generate ES cells for all NRs and some of the CoRs. These ES cells, as well as all the constructs generated, will be freely available to scientific community.
Materials and Methods
Generation of the targeting construct
The shuttle vector RfNLIII was constructed in house with a backbone of pBluescript II KS+ that can be grown up by ampicillin selection. The sequence information and illustration of the region of tags and the multiple cloning sites can be found in Supplemental Fig. 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org. The base vector pCAGGc-LSL-Luciferase is a generous gift from Dr. Tyler Jacks at the Massachusetts Institute of Technology.
To generate the pCAGGS-LSL-hCOUP-TFI targeting construct, human COUP-TFI full cDNA was amplified by PCR and inserted into the SpeI site of the RfNLIII vector. The backbone of this shuttle vector was removed through SacI and KpnI double digestion. The backbone free DNA fragment and the base vector were transformed into SW102 cells by electroporation for recombination through recombineering technology (recombineering. ncifcrf.gov). Transformed hosts were cultured on LB plates at 30 C for 48–60 h in the presence of 50 μg/ml ampicillin and 25 μg/ml kanamycin. The successfully and correctly recombined intermediate construct was transformed into 294-Flp cells for removal of the FRT-flanked kanamycin-resistant cassette to finalize the generation of targeting construct. The targeting construct was sequenced twice through the cDNA and junctions of components for verification.
Gene targeting in ES cells and mice
The targeting construct was linearized by PacI digestion. DNA (25 μg) was electroporated into R1 ES cells using previously described methods (43,44). Correctly targeted ES clones were screened by their acquired puromycin resistance (positive selection), enriched by the diphtheria toxin A-mediated elimination of random integration (negative selection) and identified by Southern blot as described previously (45,46). In brief, genomic DNA of the ES clones were digested with EcoRV and subjected to Southern blotting. Correct clones should yield an 11.5-kb hybridization band corresponding to the wild-type locus and a 3.8-kb band that represents the targeted ROSA26 locus.
The production of chimeras from the ES cells was as described by Bradley (47). Germ line transmission of the CAG-S-hCOUP-TFI allele was determined by Southern blotting and PCR assay. The allele-specific primers for PCR genotyping are 5′-GCTTTCTGGCGTGTGACC-3′ (forward) and 5′-ATTAAGGGCCAGCTCATTCC-3′ (reverse) for the knock-in allele with a product size 0.5 kb. PCR primers for the wild-type ROSA26 locus are 5′-GGAGCGGGAGAAATGGATATG-3′ (forward) and 5′-AAAGTCGCTCTGAGTTGTTAT-3′ (reverse) with a product size of 0.5 kb (45). The PCR program ran 35 cycles on 95 C, 1 min, for denaturing; 56 C, 1 min, for annealing; and 72 C, 1 min, for extension.
Generation of PRcre and COUP-TFIIflox alleles was previously described (48,49).
All animal experiments adhered to guidelines of the Institutional Animal Care and Use Committee of the Baylor College of Medicine and conducted within the scope of approved animal protocols.
Histology analysis
Immunostaining, implantation site study, and alkaline phosphatase staining were performed as described elsewhere (36). Primary antibodies used in this study included the mouse monoclonal anti-COUP-TFI (1:3000; Perseus Proteomics, Tokyo, Japan), the mouse monoclonal anti-myc (clone 9E10, 1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and the antilactoferrin (1:5000; Upstate Biotechnology, Inc., Lake Placid, NY).
Supplementary Material
Acknowledgments
We thank Wen Chen, Xuefei Tong, and Wei Qian (Baylor College of Medicine) for their excellent technical assistance.
Footnotes
This work was supported by NURSA Grants U19DK062434 (to M.J.T., S.Y.T. and F.J.D.) and U54HD07495 (to S.Y.T. and F.J.D.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 9, 2010
Abbreviations: CoRs, Coregulators; COUP-TFI and COUP-TFII, chicken ovalbumin upstream promoter-transcription factors I and II; E12.5, embryonic d 12.5; ES cells, embryonic stem cells; GEM, genetically engineered mouse; NRs, nuclear receptor; LSL, loxP-STOP-loxP; PR, progesterone receptor; SRC, steroid receptor coactivator.
References
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM 1995 The nuclear receptor superfamily: the second decade. Cell 83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chute JP, Ross JR, McDonnell DP 2010 Minireview: nuclear receptors, hematopoiesis, and stem cells. Mol Endocrinol 24:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzen SD 2008 Minireview: nuclear receptors and breast cancer. Mol Endocrinol 22:2215–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonard DM, Lanz RB, O'Malley BW 2007 Nuclear receptor coregulators and human disease. Endocr Rev 28:575–587 [DOI] [PubMed] [Google Scholar]
- Sonoda J, Pei L, Evans RM 2008 Nuclear receptors: decoding metabolic disease. FEBS Lett 582:2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman KG, Greer JJ 2007 Development of the diaphragm and genetic mouse models of diaphragmatic defects. Am J Med Genet C Semin Med Genet 145C:109–116 [DOI] [PubMed] [Google Scholar]
- You LR, Takamoto N, Yu CT, Tanaka T, Kodama T, Demayo FJ, Tsai SY, Tsai MJ 2005 Mouse lacking COUP-TFII as an animal model of Bochdalek-type congenital diaphragmatic hernia. Proc Natl Acad Sci USA 102:16351–16356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buervenich S, Carmine A, Arvidsson M, Xiang F, Zhang Z, Sydow O, Jönsson EG, Sedvall GC, Leonard S, Ross RG, Freedman R, Chowdari KV, Nimgaonkar VL, Perlmann T, Anvret M, Olson L 2000 NURR1 mutations in cases of schizophrenia and manic-depressive disorder. Am J Med Genet 96:808–813 [DOI] [PubMed] [Google Scholar]
- Zhou HJ, Yan J, Luo W, Ayala G, Lin SH, Erdem H, Ittmann M, Tsai SY, Tsai MJ 2005 SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res 65:7976–7983 [DOI] [PubMed] [Google Scholar]
- Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R 2003 Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 95:353–361 [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Eccleshall TR, Gross C, Van Maldergem L, Bouillon R, Feldman D 1997 Hereditary vitamin D resistant rickets caused by a novel mutation in the vitamin D receptor that results in decreased affinity for hormone and cellular hyporesponsiveness. J Clin Invest 99:297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P, Lev-Lehman E, Tsai TF, Matsuura T, Benton CS, Sutcliffe JS, Christian SL, Kubota T, Halley DJ, Meijers-Heijboer H, Langlois S, Graham Jr JM, Beuten J, Willems PJ, Ledbetter DH, Beaudet AL 1999 The spectrum of mutations in UBE3A causing Angelman syndrome. Hum Mol Genet 8:129–135 [DOI] [PubMed] [Google Scholar]
- Sakurai A, Takeda K, Ain K, Ceccarelli P, Nakai A, Seino S, Bell GI, Refetoff S, DeGroot LJ 1989 Generalized resistance to thyroid hormone associated with a mutation in the ligand-binding domain of the human thyroid hormone receptor β. Proc Natl Acad Sci USA 86:8977–8981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertelli MA, Scheller A, Brogley M, Robins DM 2006 Replacing the mouse androgen receptor with human alleles demonstrates glutamine tract length-dependent effects on physiology and tumorigenesis in mice. Mol Endocrinol 20:1248–1260 [DOI] [PubMed] [Google Scholar]
- Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ 2008 In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol 19:178–186 [DOI] [PubMed] [Google Scholar]
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY 2005 Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435:98–104 [DOI] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P 2009 Function of retinoic acid receptors during embryonic development. Nucl Recept Signal 7:e002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM 2006 Nuclear receptor expression links the circadian clock to metabolism. Cell 126:801–810 [DOI] [PubMed] [Google Scholar]
- Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S 1997 Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 16:391–396 [DOI] [PubMed] [Google Scholar]
- Chopra AR, Louet JF, Saha P, An J, DeMayo F, Xu J, York B, Karpen S, Finegold M, Moore D, Chan L, Newgard CB, O'Malley BW 2008 Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke's disease. Science 322:1395–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qatanani M, Moore DD 2005 CAR, the continuously advancing receptor, in drug metabolism and disease. Curr Drug Metab 6:329–339 [DOI] [PubMed] [Google Scholar]
- Jeong Y, Mangelsdorf DJ 2009 Nuclear receptor regulation of stemness and stem cell differentiation. Exp Mol Med 41:525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S, Tang K, Iida A, Inoue M, Kodama T, Tsai SY, Tsai MJ, Furuta Y, Watanabe S 2009 The spatial patterning of mouse cone opsin expression is regulated by bone morphogenetic protein signaling through downstream effector COUP-TF nuclear receptors. J Neurosci 29:12401–12411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka H, Nakamura S, Shimazaki T, Okano H 2008 Requirement for COUP-TFI and II in the temporal specification of neural stem cells in CNS development. Nat Neurosci 11:1014–1023 [DOI] [PubMed] [Google Scholar]
- Tang LS, Alger HM, Pereira FA 2006 COUP-TFI controls Notch regulation of hair cell and support cell differentiation. Development 133:3683–3693 [DOI] [PubMed] [Google Scholar]
- Qiu Y, Pereira FA, DeMayo FJ, Lydon JP, Tsai SY, Tsai MJ 1997 Null mutation of mCOUP-TFI results in defects in morphogenesis of the glossopharyngeal ganglion, axonal projection, and arborization. Genes Dev 11:1925–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Takamoto N, Yan J, Tsai SY, Tsai MJ 2009 Chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) regulates growth and patterning of the postnatal mouse cerebellum. Dev Biol 326:378–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xie X, Qin J, Jeha GS, Saha PK, Yan J, Haueter CM, Chan L, Tsai SY, Tsai MJ 2009 The nuclear orphan receptor COUP-TFII plays an essential role in adipogenesis, glucose homeostasis, and energy metabolism. Cell Metab 9:77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Chen X, Xie X, Tsai MJ, Tsai SY 2010 COUP-TFII regulates tumor growth and metastasis by modulating tumor angiogenesis. Proc Natl Acad Sci USA 107:3687–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Tsai MJ 1997 Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): coming of age. Endocr Rev 18:229–240 [DOI] [PubMed] [Google Scholar]
- Cooney AJ, Tsai SY, O'Malley BW, Tsai MJ 1992 Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol Cell Biol 12:4153–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y 1997 ‘Green mice' as a source of ubiquitous green cells. FEBS Lett 407:313–319 [DOI] [PubMed] [Google Scholar]
- Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, Mercer KL, Grochow R, Hock H, Crowley D, Hingorani SR, Zaks T, King C, Jacobetz MA, Wang L, Bronson RT, Orkin SH, DePinho RA, Jacks T 2004 Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 5:375–387 [DOI] [PubMed] [Google Scholar]
- Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG 2005 Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res 33:e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P 1997 Disruption of overlapping transcripts in the ROSA β geo 26 gene trap strain leads to widespread expression of β-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci USA 94:3789–3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY 2007 COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet 3:e102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamoto N, Kurihara I, Lee K, Demayo FJ, Tsai MJ, Tsai SY 2005 Haploinsufficiency of chicken ovalbumin upstream promoter transcription factor II in female reproduction. Mol Endocrinol 19:2299–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Orkin SH 1999 Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci USA 96:5037–5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaas L, Musteanu M, Eferl R, Bauer A, Casanova E 2009 Bacterial artificial chromosomes improve recombinant protein production in mammalian cells. BMC Biotechnol 9:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS 1997 AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965–968 [DOI] [PubMed] [Google Scholar]
- Torres-Arzayus MI, Font de Mora J, Yuan J, Vazquez F, Bronson R, Rue M, Sellers WR, Brown M 2004 High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell 6:263–274 [DOI] [PubMed] [Google Scholar]
- Tang K, Xie X, Park JI, Jamrich M, Tsai SY, Tsai MJ 2010 COUP-TFs regulate eye development by controlling factors essential for optic vesicle morphogenesis. Development:725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC 1993 Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA 90:8424–8428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P, Montgomery C, Geske R, Bradley A 1991 Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64:693–702 [DOI] [PubMed] [Google Scholar]
- Soriano P 1999 Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21:70–71 [DOI] [PubMed] [Google Scholar]
- Yanagawa Y, Kobayashi T, Ohnishi M, Kobayashi T, Tamura S, Tsuzuki T, Sanbo M, Yagi T, Tashiro F, Miyazaki J 1999 Enrichment and efficient screening of ES cells containing a targeted mutation: the use of DT-A gene with the polyadenylation signal as a negative selection maker. Transgenic Res 8:215–221 [DOI] [PubMed] [Google Scholar]
- Bradley A 1987 Production and analysis of chimaeric mice. In Teratocarcinomas and embryonic stem cells: a practical approach. Oxford: IRL Press; 113–151 [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP 2005 Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41:58–66 [DOI] [PubMed] [Google Scholar]
- Takamoto N, You LR, Moses K, Chiang C, Zimmer WE, Schwartz RJ, DeMayo FJ, Tsai MJ, Tsai SY 2005 COUP-TFII is essential for radial and anteroposterior patterning of the stomach. Development 132:2179–2189 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.