Abstract
Vitamin D receptor (VDR) is activated by natural ligands, 1α, 25-dihydroxy-vitamin D3 [1α,25(OH)2-D3] and lithocholic acid (LCA). Our previous study shows that VDR is expressed in human hepatocytes, and VDR ligands inhibit bile acid synthesis and transcription of the gene encoding cholesterol 7α-hydroxylase (CYP7A1). Primary human hepatocytes were used to study LCA and 1α,25(OH)2-D3 activation of VDR signaling. Confocal immunofluorescent microscopy imaging and immunoblot analysis showed that LCA and 1α, 25(OH)2-D3 induced intracellular translocation of VDR from the cytosol to the nucleus and also plasma membrane where VDR colocalized with caveolin-1. VDR ligands induced tyrosine phosphorylation of c-Src and VDR and their interaction. Inhibition of c-Src abrogated VDR ligand-dependent inhibition of CYP7A1 mRNA expression. Kinase assays showed that VDR ligands specifically activated the c-Raf/MEK1/2/extracellular signal-regulated kinase (ERK) 1/2 pathway, which stimulates serine phosphorylation of VDR and hepatocyte nuclear factor-4α, and their interaction. Mammalian two-hybrid assays showed a VDR ligand-dependent interaction of nuclear receptor corepressor-1 and silencing mediator of retinoid and thyroid with VDR/retinoid X receptor-α (RXRα). Chromatin immunoprecipitation assays revealed that an ERK1/2 inhibitor reversed VDR ligand-induced recruitment of VDR, RXRα, and corepressors to human CYP7A1 promoter. In conclusion, VDR ligands activate membrane VDR signaling to activate the MEK1/2/ERK1/2 pathway, which stimulates nuclear VDR/RXRα recruitment of corepressors to inhibit CYP7A1 gene transcription in human hepatocytes. This membrane VDR-signaling pathway may be activated by bile acids to inhibit bile acid synthesis as a rapid response to protect hepatocytes from cholestatic liver injury.
In human hepatocytes, lithocholic acid activates membrane VDR signaling to inhibit CYP7A1 gene transcription via the MEK1/2/ERK1/2 MAPK pathway.
Bile acids are physiological agents that are essential for the digestion and absorption of fats and nutrients in the digestive system and disposal of drug and metabolites. Bile acids also are versatile signaling molecules that activate nuclear receptors and cellular signaling pathways and play critical roles in lipid, glucose, drug, and energy metabolism (1,2,3,4). Bile acid synthesis is under the negative feedback regulation by bile acids returning to the liver to inhibit the gene encoding cholesterol 7α-hydroxylase (CYP7A1), the initial and rate-limiting enzyme in the classic bile acid biosynthetic pathway to synthesize two primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA) in human livers (1). Bile acids are known to activate a nuclear receptor, farnesoid X receptor (FXR, NR1H4), which plays a critical role in the regulation of bile acid synthesis and transport and glucose and lipoprotein metabolism (3). In the liver FXR induces a negative nuclear receptor, small heterodimer partner (NR0B2) to inhibit CYP7A1 gene transcription. In the intestine, FXR induces a fibroblast growth factor 15 (FGF15, or human FGF19), which activates the hepatic FGF receptor 4 signaling pathway to inhibit CYP7A1 and bile acid synthesis (5,6). Lithocholic acid (LCA), a highly hydrophobic and toxic bile acid derived from CDCA by intestinal bacteria action, is an efficacious endogenous ligand of vitamin D receptor (VDR, NR1I1) (7) and pregnane X receptor (NR1I2) (8). These two xenobiotic sensors induce drug-metabolizing cytochrome P450 3A4 (CYP3A4) and sulfotransferase 2A1 and may play a role in detoxification of drugs and bile acids in the liver and intestine (9,10).
VDR is widely expressed in most tissues and cells (11). However, it has been reported that VDR is not expressed in mouse livers (12). In rat liver, VDR is expressed mainly in the nonparenchymal and biliary epithelial cells (13). Recently, we reported that VDR protein and mRNA were expressed in HepG2 and human primary hepatocytes (14). LCA and 1α,25-dihydroxyvitamin D3 [1α, 25(OH)2-D3] increased VDR protein expression in the nucleus of hepatocytes and strongly inhibited CYP7A1 mRNA expression and bile acid synthesis in primary human hepatocytes. Furthermore, small interfering RNA knockdown of VDR mRNA blocked the inhibitory effect of VDR ligands on CYP7A1 mRNA expression in human hepatocytes. The VDR and retinoid X receptor α (RXRα) heterodimer binds to the bile acid response elements (BAREs) to block hepatocyte nuclear factor 4α (HNF4α) binding and reduce coactivator occupancy but increases corepressor recruitment to CYP7A1 promoter and results in inhibiting CYP7A1 gene transcription (14).
The active hormone 1α,25(OH)2-D3 regulates various physiological functions including calcium and phosphate homeostasis, cell growth and differentiation, and gene transcription in many tissues (11). Upon binding and activation by 1α,25(OH)2-D3, the VDR/RXRα heterodimer binds to the positive or negative VDR response elements to regulate target gene transcription. The positive regulation of VDR target genes by VDR signaling has been studied extensively. However, the mechanism underlying the negative effect of VDR on gene transcription remains unclear. Furthermore, more recent studies have unveiled the potential nongenomic action of 1α,25(OH)2-D3 in tissues and cells expressing VDR (15). It appears that the rapid response action of 1α,25(OH)2-D3 is mediated through the membrane-bound VDR, which is present in the caveolae-enriched plasma membrane (16). It has been reported that 1α,25(OH)2-D3 stimulates tyrosine phosphorylation of VDR and a cytosolic tyrosine kinase, c-Src, in muscle cells (17,18). This VDR-dependent signaling activates several kinases including cAMP-dependent protein kinase A (PKA), phosphoinositol-3 kinase (PI3K), protein kinase C (PKC), and MAPKs in muscle, keratinocytes, osteoblasts, and kidney (16,19). The presence of VDR in plasma membrane of hepatocytes has not been reported, and the VDR signaling pathway has not been elucidated in human hepatocytes.
We have studied 1α,25(OH)2-D3 and LCA-activated intracellular signaling in primary human hepatocytes, the best model for studying bile acid synthesis and human CYP7A1 gene regulation. Our results show that 1α,25(OH)2-D3 and LCA induce intracellular translocation of VDR to both the plasma membrane and nucleus of human hepatocytes. Membrane VDR signaling stimulates tyrosine phosphorylation of VDR and induces VDR interaction with c-Src. The membrane VDR signaling specifically activates the c-Raf/mitogen-activated extracellular kinase 1/2 (MEK1/2)/MAKP/ERK kinase (ERK)1/2 pathway. In the nucleus, VDR signaling stimulates phosphorylation of VDR, RXRα, and HNF4α and induces VDR/RXRα heterodimer recruitment of corepressors to inhibit CYP7A1 gene transcription. This membrane VDR signaling pathway may be a rapid response to cholestasis to protect the liver from cholestatic injury.
Results
VDR ligands induce intracellular translocation of VDR to the plasma membrane and nucleus in human hepatocytes
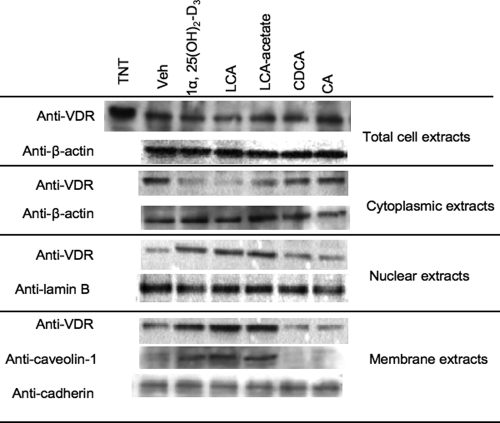
Our previous study has shown that 1α,25(OH)2-D3 increases VDR in nuclear extracts isolated from human hepatocytes (14). To study whether ligands induce VDR nuclear translocation or increase VDR expression, immunoblot assays were used to detect VDR protein in total cellular protein extracts, cytosol, nuclear and membrane fractions isolated from primary human hepatocytes pretreated with an endogenous VDR ligand, 1α, 25(OH)2-D3 (50 nm), or LCA (10 μm). We also treated hepatocytes with LCA-acetate (10 μm), which is a nontoxic, highly efficacious and selective VDR agonist (20). We also treated hepatocytes with CDCA (20 μm) or cholic acid (CA, 20 μm). These two bile acids are endogenous ligands of FXR but not VDR. Figure 1 shows that none of these reagents had effects on VDR protein expression levels in total cell extracts. However, 1α,25(OH)2-D3, LCA, and LCA-acetate markedly decreased VDR protein in the cytosol but increased VDR in the nuclear and plasma membranes (Fig. 1). Interestingly, 1α,25(OH)2-D3, LCA, or LCA-acetate increased membrane caveolin-1 levels, which parallels the increase of VDR levels in the membrane fractions. Caveolin-1 is a marker of caveolae, a membrane subdomain enriched with cholesterol, sphingolipids, and membrane receptors involved in cell signaling. CDCA and CA are weak VDR ligands and had little effect on intracellular localization of VDR.
Figure 1.
Immunoblot analysis of VDR expression in primary human hepatocytes. Human primary hepatocytes were treated with vehicle (Veh, EtOH), 1α,25(OH)2-D3 (50 nm), LCA (10 μm), LCA-acetate (10 μm), CDCA (20 μm), or CA (20 μm) for 6 h. Cytoplasmic, nuclear, and membrane fractions (40 μg) were isolated from human primary hepatocytes for immunoblot analysis. A rabbit antihuman VDR antibody was used to detect VDR. β-Actin and Lamin B were monitored as the internal loading controls of cytosol and nucleus, respectively. Caveolin-1 was monitored as a marker of caveolae membrane subdomain. Anti-pan-cadherin was used to assay cadherin as a marker of plasma membrane protein. Data represent one of three separate experiments using primary human hepatocytes isolated from different liver donors (HH1472, HH1483, and HH1493).
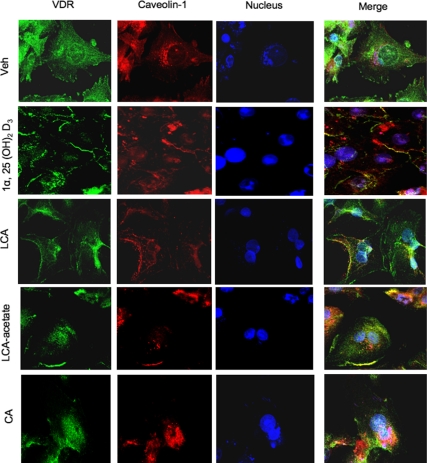
To further study the effect of 1α,25(OH)2-D3, LCA, or LCA-acetate on intracellular translocation of VDR, we used confocal immunofluorescent microscopy imaging to assay intracellular translocation of VDR in primary human hepatocytes. Figure 2 shows that without adding a ligand, VDR (stained with Fluor Alexa and scanned at 488 nm, shown in green) is predominantly located in the cytosol (top row). Caveolin-1 (stained with Fluor Alexa and scanned at 546 nm, shown in orange-red) is localized in the plasma membrane and in the perinucleus surface (purple). Cell nuclei were stained with TO-PRO-3 (blue). Merging of three images confirmed predominant localization of unliganded VDR in the cytosol. After treatment with 1α,25(OH)2-D3, LCA, or LCA-acetate, VDR and caveolin-1 were predominantly localized in the plasma membrane and some were colocalized in the same region (yellow). VDR was also translocated into the nucleus (aqua), and some VDR and caveolin-1 were colocalized in the perinucleus surface (purple). In contrast, CA treatment caused translocation of some VDR to the nuclei, but clearly not to the membrane. These results revealed a ligand-induced intracellular translocation of VDR from the cytosol to both the nucleus and plasma membrane, and that caveolin-1 and VDR may be translocated together to the caveolae in the plasma membrane. These confocal fluorescent imaging data are consistent with the data from immunoblot analysis shown in Fig. 1.
Figure 2.
Confocal immunofluorescent microscopy analysis of effect of VDR ligands on the intracellular localization of VDR in primary human hepatocytes. Caveolin-1 antibody and the secondary antibody conjugated with red fluorescence were used to detect caveolin-1. VDR antibody and the secondary antibody conjugated with green fluorescence were used to detect VDR. Cell nuclei were stained by TOPRO-3 iodide 642/661 nm. The merges of three images are shown in the right column. The cells were treated with vehicle (Veh, EtOH), 50 nm 1α,25(OH)2-D3, 10 μm LCA, 10 μm LCA-acetate, or 25 μm CA for 6 h as indicated.
LCA-acetate and 1α,25(OH)2-D3 activate tyrosine phosphorylation of c-Src and membrane VDR
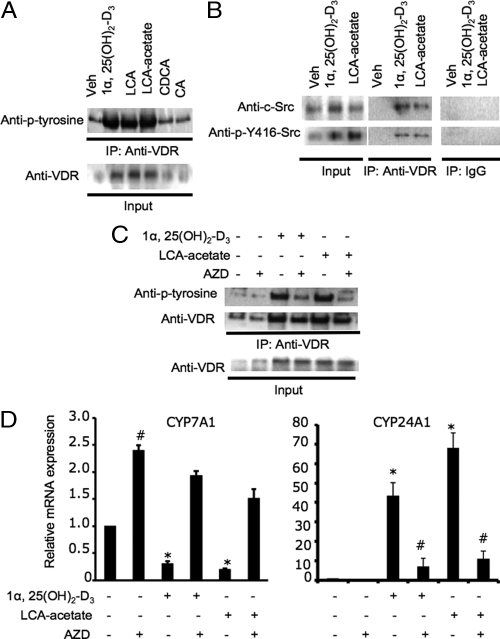
It has been reported that 1α,25(OH)2-D3 stimulated tyrosine phosphorylation of VDR and activated c-Src in skeletal muscle cells, and the tyrosine-phosphorylated VDR forms a complex with c-Src (21,22). Human c-Src has two major phosphorylation sites. Phosphorylation of tyrosine-419 (Y419 in human or Y416 in chicken c-Src) and dephosphorylation of tyrosine 530 (Y530 in human or Y527 in chicken c-Src) activates c-Src. To study the effect of VDR ligands on tyrosine phosphorylation of VDR and c-Src in human hepatocytes, we used an anti-VDR antibody to precipitate VDR from membrane fractions and used antiphosphotyrosine antibody (anti-p-tyrosine) to detect tyrosine-phosphorylated VDR. Figure 3A shows that 1α,25(OH)2-D3, LCA, and LCA-acetate, but not CDCA and CA, markedly increased the amount of tyrosine-phosphorylated VDR in plasma membrane. To test whether c-Src is associated with VDR in the plasma membrane, we used anti-c-Src antibodies to detect c-Src that might be coimmunoprecipitated with VDR. Figure 3B shows that anti-c-Src and anti-phospho-Y416 antibodies detected c-Src and tyrosine-phosphorylated c-Src coimmunoprecipitated with VDR in the plasma membrane fractions isolated from LCA-acetate or 1α,25(OH)2-D3 treated human hepatocytes. To further confirm that 1α,25(OH)2-D3 signaling involves c-Src, we treated primary human hepatocytes with a specific c-Src inhibitor, AZD0530, and performed immunoprecipitation assay. Figure 3C shows that AZD0530 significantly decreased the amount of tyrosine-phosphorylated VDR in the immunoprecipitants isolated from cells treated with 1α,25(OH)2-D3 or LCA-acetate. These coimmunoprecipitation (co-IP) assays suggest that 1α,25(OH)2-D3 signaling stimulates tyrosine phosphorylation of VDR and translocation of VDR and c-Src to the plasma membrane. To test the role of c-Src in regulation of CYP7A1 by membrane VDR signaling, we used quantitative RT-PCR assay to study the effect of AZD0530 on CYP7A1 mRNA expression levels in primary human hepatocytes. Figure 3D (left panel) shows that AZD0530 treatment alone increased CYP7A1 mRNA expression levels by 2-fold indicating that c-Src was constitutively active in inhibiting basal level expression of CYP7A1 in hepatocytes. AZD0530 completely blocked inhibition of CYP7A1 mRNA expression in primary human hepatocytes by 1α,25(OH)2-D3 or LCA-acetate. To further confirm the involvement of c-Src in VDR signaling in hepatocytes, we studied the effect of AZD0530 on the expression of a well-characterized VDR-induced gene CYP24A1, which inactivates 1α,25(OH)2-D3 by 24-hydroxylation (23). Figure 3D (right panel) shows that 1α, 25(OH)2-D3 and LCA-acetate strongly induced CYP24A1 mRNA expression in human primary hepatocytes, and AZD0530 strongly abrogated the induction of CYP24A1 mRNA by 1α,25(OH)2-D3 or LCA-acetate. As a negative control these VDR ligands had no effect on mRNA expression of sterol 27-hydroxylase (CYP27A1), which is involved in bile acid synthesis in hepatocytes (data not shown). These results support that c-Src is involved in membrane VDR signaling in regulation of CYP7A1 and CYP24A1 gene expression in human hepatocytes.
Figure 3.
Effect of VDR ligands on tyrosine phosphorylation and interaction of VDR and c-Src, and CYP7A1 mRNA expression levels in human primary hepatocytes. A, IP assay of tyrosine phosphorylation of VDR in the plasma membrane. Primary human hepatocytes were treated with vehicle (Veh, EtOH),1α, 25(OH)2-D3 (50 nm), LCA (10 μm), LCA-acetate (10 μm), CDCA (20 μm), or CA (20 μm) for 6 h. A rabbit anti-VDR antibody was used to immunoprecipitate VDR from cell membrane extracts (300 μg). A mouse antiphosphotyrosine (Anti-p-Tyr) was used to detect phosphotyrosines in VDR. Cell extract (10%) was set aside as input. B, Co-IP assay of VDR interaction with c-Src. Primary human hepatocytes were treated with Veh, 1α,25(OH)2-D3 (50 nm), or LCA-acetate (10 μm) for 6 h. Cell membrane extracts (300 μg) were treated with a mouse anti-VDR antibody, and immunoprecipitates were used for immunoblot analysis of coprecipitated c-Src using rabbit anti-c-Src and anti-p-Y416/Y419-Src antibodies. Mouse nonimmune IgG was used as a negative control. Cell extract (10%) was set aside as input. C, Effect of a c-Src inhibitor on tyrosine phosphorylation of VDR in the plasma membrane. Primary human hepatocytes were treated with Veh, 1α,25(OH)2-D3 (50 nm), LCA-acetate (10 μm), and/or the c-Src inhibitor AZD0530 (AZD) (5 μm) for 6 h. A rabbit anti-VDR antibody was used to immunoprecipitate VDR from cell membrane extracts (300 μg). A mouse antiphosphotyrosine (Anti-p-Tyr) was used to detect phosphotyrosines in VDR. A mouse anti-VDR was used to detect immunoprecipitated VDR. Cell extract (10%) was set aside as input. D, Q-RT PCR assay of the effects of a c-Src inhibitor on CYP7A1 (left panel) and CYP24A1 (right panel) mRNA expression in primary human hepatocytes. Primary human hepatocytes were treated with Veh, 1α,25(OH)2-D3 (50 nm), LCA-acetate (10 μm), and/or AZD (5 μm) for 16 h. #, Statistically significant difference (P < 0.05; n = 3), AZD-treated vs. vehicle control, or AZD and 1α,25(OH)2-D3 (50 nm) or LCA-acetate (10 μm) treated vs. 1α,25(OH)2-D3 (50 nm) or LCA-acetate (10 μm). *, Statistically significant difference (P < 0.05; n = 3), 1α,25(OH)2-D3 (50 nm) or LCA-acetate (10 μm) treated vs. vehicle control. Data represent one of three separate experiments using primary human hepatocytes from different liver donors (HH1479, HH1483, and HH1493).
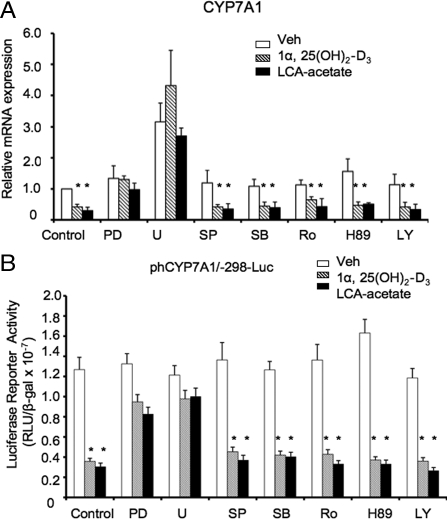
ERK1/2 inhibitors attenuate LCA-acetate and 1α,25(OH)2-D3 inhibition of human CYP7A1 in human hepatocytes
We have reported previously that LCA-acetate and 1α,25(OH)2-D3 inhibited CYP7A1 gene transcription in human hepatocytes (14). It has been reported that the 1α, 25(OH)2-D3 signaling rapidly activates PKC, PKA, PI-3-Kinase, AKT, and MAPK pathways in several tissues including bone, intestine, muscle, epidermal, and cancer cells (16,19). To study 1α,25(OH)2-D3 signaling in the liver and to identify which kinase(s) is/are involved in mediating 1α,25(OH)2-D3 signaling in regulation of the CYP7A1 gene in human hepatocytes, several protein kinase inhibitors were used to investigate their effects on down-regulation of CYP7A1 mRNA expression levels by LCA-acetate or 1α,25(OH)2-D3 in human hepatocytes. Figure 4A shows that among all inhibitors tested, only PD98059 (ERK1/2 inhibitor) and U0126 (MEK1/2 inhibitor), attenuated the inhibitory effect of LCA-acetate or 1α,25(OH)2-D3 on human CYP7A1 mRNA expression in primary human hepatocytes. Transient transfection assays of a human CYP7A1 promoter/luciferase reporter were used to study the effect of VDR ligands and kinase inhibitors on the reporter activity. Figure 4B shows that pretreatment with PD98059 and U0126, but not other inhibitors, weakened the inhibitory effects of LCA-acetate and 1α,25(OH)2-D3 on human CYP7A1 reporter activities. As a negative control, none of these inhibitors and VDR ligands had any effects on human CYP27A1 reporter activity (data not shown). These results suggest that ligand-activated VDR specifically activates the MEK1/2/ERK1/2 pathway to inhibit CYP7A1 transcription in human hepatocytes.
Figure 4.
Identification of 1α,25(OH)2-D3 and LCA-activated protein kinase involved in inhibition of CYP7A1 gene transcription in human hepatocytes. A, Real-time PCR analysis of relative CYP7A1 mRNA expression in primary human hepatocytes treated with kinase inhibitors. Primary human hepatocytes were pretreated with each inhibitor for 1 h, followed by the treatment with 1α,25(OH)2-D3 (50 nm) or LCA-acetate (10 μm) for 24 h. Protein kinase inhibitors used are ERK1/2 inhibitors, PD98059 (PD, 40 μm), and MEK1/2 U0126 (U, 20 μm); JNK inhibitor, SP600125 (SP, 25 μm); p38 inhibitor, SB203580 (SB, 20 μm); PKC inhibitor, Ro318220 (Ro, 2.5 μm); PKA inhibitor, H89 (10 μm); and PI3K inhibitor, LY294002 (LY, 50 μm). B, Transient transfection assay of the effect of kinase inhibitors on CYP7A1 promoter/luciferase reporter activity in HepG2 cells. A human CYP7A1/luciferase reporter, phCYP7A1/-298-Luc, was transfected into HepG2 cells pretreated with PD (40 μm), U (20 μm), SP (25 μm), SB (20 μm), Ro (2.5 μm), H89 (10 μm), or LY (50 μm) for 1 h, followed by treatment with 1α,25(OH)2-D3 (5 nm), or LCA-acetate (5 μm) for 16 h. The luciferase activity was normalized by β-gal activity. Each experiment was done in duplicate, and the same experiment was repeated four times. Ethanol was used as a vehicle control. Data were pooled from assays using three donor hepatocytes (nos. HH1467, HH1471, and HH1472). *, Statistically significant difference (P < 0.05; n = 3), treated vs. vehicle control. RLU, Relative light units.
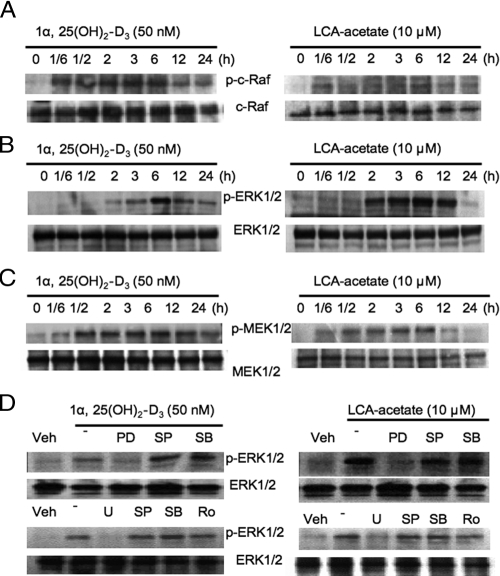
VDR ligands activate c-Raf, MEK, and ERK1/2 in human hepatocytes
We then used immunoblot analysis to study the effect of 1α,25(OH)2-D3 and LCA-acetate on c-Raf, MEK1/2, and ERK1/2 phosphorylation in primary human hepatocytes. c-Raf is an upstream serum kinase of the MEK1/2. Figure 5A shows that both LCA-acetate and 1α,25(OH)2-D3 caused rapid serine phosphorylation of c-Raf in 10 min and reached the maximum after 6 h treatment. Similarly, both LCA-acetate and 1α,25(OH)2-D3 time-dependently activated ERK1/2 by phosphorylation (Fig. 5B). Figure 5C shows that 1α,25(OH)2-D3 or LCA-acetate stimulated phosphorylation of MEK1/2, an upstream kinase of ERK1/2. Furthermore, LCA-acetate and 1α,25(OH)2-D3-induced phosphorylation of ERK1/2 was abolished by a specific inhibitor for ERK1/2 (PD98059) and MEK1/2 (U0126), but not for JNK (SP600125), p38 (SB203580), and PKC (Ro318220) (Fig. 5D). These results demonstrated that VDR signaling specifically activated the c-Raf/MEK1/2/ERK1/2 pathway in human hepatocytes.
Figure 5.
Effects of LCA-acetate and 1α,25(OH)2-D3 phosphorylation of c-Raf, MEK1/2, and MAPKs in primary human hepatocytes. Primary human hepatocytes were treated with 1α,25(OH)2-D3 (50 nm) or LCA-acetate (10 μm) for the time indicated, and total cellular protein extracts were isolated for immunoblot analysis. A, Immunoblot assay of the effects of LCA-acetate and 1α,25(OH)2-D3 on serine phosphorylation of c-Raf. Phosphorylated c-Raf and c-Raf were detected using rabbit anti-p-c-Raf and anti-c-Raf antibodies. B, Immunoblot assay of the effects of 1α,25(OH)2-D3 and LCA-acetate on MAPKs ERK1/2. Total and phosphorylated ERK1/2 were detected by their respective antibodies. C, Immunoblot assay of the effects of 1α,25(OH)2-D3 and LCA-acetate on phosphorylation of MEK. Phosphorylated MEK and total MEK were detected by their respective antibodies. D, Effect of MAPK inhibitors on 1α,25(OH)2-D3 and LCA-acetate activation of ERK1/2. Primary human hepatocytes were pretreated with ERK1/2 inhibitors, PD (40 μm) and U (20 μm): JNK inhibitor, SP (25 μm); p38 inhibitor, SB (20 μm); or PKC inhibitor, Ro318220 (Ro, 2.5 μm) for 1 h, followed by treatment with 1α,25(OH)2-D3 (50 nm) or LCA-acetate (10 μm) for 6 h. Total cellular protein extracts were isolated for immunoblot analysis using an antibody against total or phosphorylated ERK1/2. Data represent one of four separate experiments using different donor hepatocytes (HH1467, HH1471, HH1472, and HH1479).
ERK1/2 phosphorylates VDR and RXRα
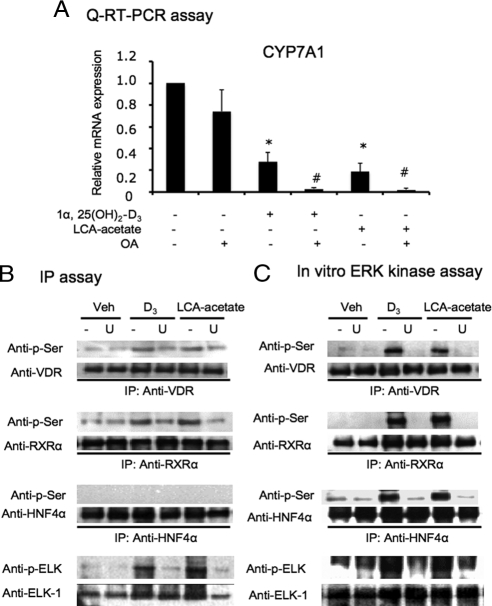
To further confirm that the VDR ligand-dependent phosphorylation is involved in LCA-acetate and 1α, 25(OH)2-D3 inhibition of human CYP7A1 mRNA expression, a serine phosphatase inhibitor, okadaic acid (OA), was used to treat primary human hepatocytes together with LCA-acetate and 1α,25(OH)2-D3. Whereas OA alone had no effect on CYP7A1 mRNA expression, the OA treatment significantly enhanced LCA-acetate and 1α,25(OH)2-D3 inhibition of CYP7A1 mRNA expression in primary hepatocytes (Fig. 6A). These data provided further evidence that serine phosphorylation was involved in mediating VDR signaling in human hepatocytes. We also performed immunoprecipitation (IP) assay and in vitro kinase assay to investigate the possibility that the VDR/ERK1/2 signaling pathway may phosphorylate VDR, RXRα, and HNF4α in human primary hepatocytes. HNF4α is a major transcription factor involved in basal transcription of the CYP7A1 gene. Cells were pretreated with U0126, followed by the treatment with LCA-acetate or 1α,25(OH)2-D3. Serine phosphorylation was detected by a mouse monoclonal antiphosphorylated serine antibody (anti-p-serine). Figure 6B shows that LCA-acetate or 1α,25(OH)2-D3 induced serine phosphorylation of VDR and RXRα, but not HNF4α, and U0126 blocked phosphorylation in this cell-based assay. We also performed a highly sensitive in vitro ERK1/2 assays. The same protein extracts isolated from primary hepatocytes were incubated with the beads coated with an antibody against phospho-ERK1/2 to immobilize activated p-ERK1/2 in cell extracts. The beads containing activated ERK1/2 were used to perform in vitro phosphorylation of in vitro synthesized VDR, RXRα, and HNF4α. Immunoblot analysis using an antiphosphoserine antibody shows that VDR and RXRα were phosphorylated by active ERK1/2, and U0126 blocked the phosphorylation (Fig. 6C). In contrast to IP assay, this ERK assay showed that ERK1/2 was able to phosphorylate HNF4α. The ERK1/2 assay used activated-ERK1/2 in hepatocytes to phosphorylate HNF4α. This method is much more sensitive than the IP assay. HNF4α is known to be phosphorylated by many kinases including PKA, PKB, PKC, and AMP-activated protein kinase, and phosphorylated HNF4α has lower DNA binding, dimerization, and transactivation activity (review in Ref. 24). These results demonstrated that LCA-acetate and 1α,25(OH)2-D3 induced serine phosphorylation of nuclear VDR, RXRα, and HNF4α in human hepatocytes.
Figure 6.
Effects of VDR ligands on CYP7A1 mRNA expression and phosphorylation of VDR, RXRα, and HNF4α in human hepatocytes. A, Q-RT PCR assay of the effects of a protein phosphatase inhibitor, OA, on CYP7A1 mRNA expression in primary human hepatocytes. Primary human hepatocytes were treated with vehicle (Veh), 1α,25(OH)2-D3 (50 nm), LCA-acetate (10 μm), and OA (40 nm) as indicated for 16 h. Data were pooled from three donor hepatocytes (HH1479, HH1483, and HH1493). *, Statistically significant difference between 1α,25(OH)2-D3 or LCA-acetate-treated and untreated; P < 0.05; n = 3. #, Statistically significant difference between OA and 1α,25(OH)2-D3- or LCA-acetate-treated and untreated control, P < 0.05; n = 3. B, IP assay of serine phosphorylation of VDR, RXRα, and HNF4α. Primary human hepatocytes were pretreated with U (20 μm) for 1 h followed by 1α,25(OH)2-D3 (50 nm) or LCA-acetate (10 μm) for 6 h. Rabbit anti-VDR, rabbit anti-RXRα, and goat anti-HNF4α antibodies were used to immunoprecipitate VDR, RXRα, and HNF4α from total cell protein extracts (300 μg), respectively. Antibodies against total and serine-phosphorylated VDR, RXRα, and HNF4α were used for immunoblot analysis. An ERK1/2 target, ELK-1, was monitored as a positive control. C, In vitro ERK assay. Primary human hepatocytes were pretreated with U (20 μm) for 1 h followed by treatment with 1α,25(OH)2-D3 (50 nm) or LCA-acetate (10 μm) for 6 h. Beads coated with an antibody against phospho-ERK1/2 were incubated with cell extracts (300 μg) to immobilize phosphorylated ERK1/2 at 4 C overnight. The beads were used to phosphorylate in vitro synthesized VDR, RXRα, and HNF4α. Antibodies against VDR, RXRα, and HNF4α were used to immunoprecipitate respective nuclear receptors. Antibodies against total and phosphorylated VDR, RXRα, and HNF4α were used for immunoblot analysis. ERK1/2 phosphorylation of ELK-1 was monitored as a positive control. Data represent one of three separate experiments using different donor hepatocytes (HH1479, HH1482, and HH1483). Q-RT-PCR, Quantitative real-time PCR; U, U0126.
VDR ligand-dependent interaction of VDR and HNF4α and effect of coactivators and corepressors
We reported previously that VDR interacted with HNF4α (14). To further test the role of ERK1/2 in mediating VDR interaction with HNF4α, primary hepatocytes were incubated with U0126 for 1 h followed by treatment with LCA-acetate or 1α,25(OH)2-D3 for 6 h and then immunoprecipitated with anti-HNF4α or anti-VDR antibodies. Figure 7A shows that VDR and HNF4α were coimmunoprecipitated in the cell extracts isolated from primary hepatocytes pretreated with LCA-acetate or 1α,25(OH)2-D3. The interaction was blocked by pretreatment with U0126, suggesting that ERK1/2-mediated phosphorylation of VDR and HNF4α may stimulate VDR and HNF4α interaction. We used mammalian two-hybrid assays to further study VDR interaction with coactivators and corepressors. Figure 7B shows that Gal4-VDR and VP16-HNF4α interaction was VDR ligand dependent. Peroxisomal proliferator-activated receptor-γ coactivator 1α (PGC-1α) and steroid receptor coactivator 1 (SRC-1) strongly stimulated the interaction, whereas nuclear receptor corepressor 1 (NCoR-1) and silencing mediator of retinoid and thyroid hormone receptor (SMRT) strongly inhibited VDR and HNF4α interaction in the presence of VDR ligands. Figure 7C shows that Gal4-VDR did not interact with VP16-NCoR-1 or VP16-SMRT. Interestingly, when RXRα was cotransfected, Gal4-VDR interacted with VP16-NCoR-1 and VP16-SMRT in a VDR ligand-dependent manner. These data demonstrated that VDR did not interact with NCoR-1 and SMRT, and that NCoR-1 or SMRT inhibited the interaction of VDR with HNF4α, PGC-1α, and SRC-1 in a VDR ligand-dependent manner.
Figure 7.
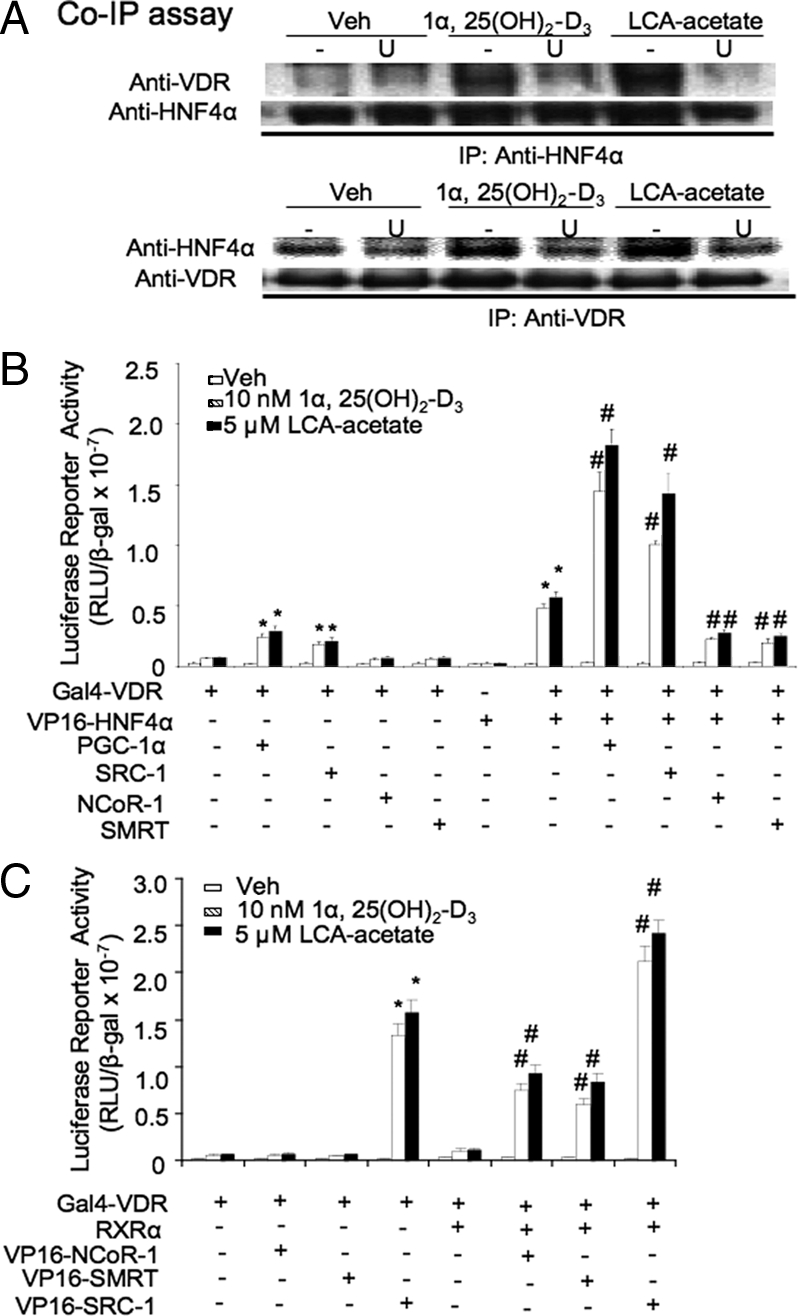
VDR ligand-dependent interaction of VDR with HNF4α, RXRα, coactivators, and corepressors. A, Effect of an ERK1/2 pathway inhibitor on VDR and HNF4α interaction. Primary human hepatocytes were pretreated with U (20 μm) for 1 h followed by treatment with 1α,25(OH)2-D3 (50 nm) or LCA-acetate (10 μm) for 6 h. An anti-HNF4α or anti-VDR antibody was used to immunoprecipitate VDR and HNF4α in the cell extracts (300 μg). Coimmunoprecipitated VDR and HNF4α were detected by immunoblot assay. B, Mammalian two-hybrid assay of VDR and HNF4α interaction and effect of coactivators and corepressors. The Gal4 reporter construct p5XUAS-TK-Luc (0.2 μg) was transfected with 0.1 μg of Gal4-VDR with VP16 empty vector, pcDNA3.1, VP16-HNF4α, PGC-1α, SRC-1, NCoR-1, and/or SMRT, as indicated, into HEK293 cells. After transfected for 24 h, cells were treated with vehicle (EtOH), 1α,25(OH)2-D3 (10 nm), or LCA-acetate (5 μm) for 16 h before harvesting. The experiment was done in duplicate, and the same experiment was repeated three times. *, Statistically significant difference (P < 0.05, n = 3) Gal4-VDR cotransfected with PGC-1α, SRC-1, or VP16-HNF4α vs. Gal4-VDR cotransfected with VP16 empty vector or pcDNA3.1. #, Statistically significant difference (P < 0.05; n = 3) Gal4-VDR and VP16-HNF4α cotransfected with PGC-1α, SRC-1, SMRT, or NCo-R-1 vs. Gal4-VDR and VP16-HNF4α cotransfected with pcDNA3.1. C, Mammalian two-hybrid assays of effect of RXRα on VDR interaction with NCoR-1, SMRT, or SRC-1. The GAL4 reporter construct p5XUAS-TK-Luc (0.2 μg) was cotransfected with 0.1 μg Gal4-VDR, RXRα, VP16 empty vector, pcDNA3.1, VP16-NCoR-1, VP16-SMRT, and/or VP16-SRC-1, as indicated, into HEK293 cells. After transfection for 24 h, cells were treated with vehicle (Veh, EtOH), 1α,25(OH)2-D3 (10 nm), or LCA-acetate (5 μm) for 16 h before harvesting. *, Statistically significant difference (P < 0.05, n = 3) Gal4-VDR cotransfected with VP16-SRC-1 vs. Gal4-VDR cotransfected with VP16 empty vector. #, Statistically significant difference (P < 0.05, n = 3), Gal4-VDR and RXRα cotransfected with VP16-NCoR-1, VP16-SMRT, or VP16-SRC-1 vs. Gal4-VDR and RXRα cotransfected with VP16 empty vector. All transfection experiments were done in duplicate, and the same experiment was repeated three times. RLU, Relative light units; U, U0126.
VDR is a positive regulator of CYP24A1 gene transcription in human hepatocytes
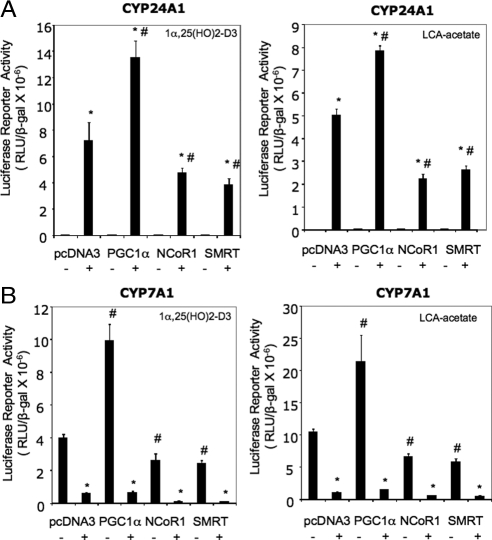
Previously we reported that ligand-activated VDR induced CYP24A1 mRNA expression in human hepatocytes (14). To further confirm that VDR can positively regulate a known VDR-induced gene in human hepatocytes, we performed transient transfection assay of CYP24A1 reporter activity in HepG2 cells. Figure 8A shows that activation of VDR by LCA-acetate or 1α,25(OH)2-D3 strongly stimulated the human CYP24A1/Luc reporter activity. Cotransfection with PGC-1α induced CYP24A1 reporter activity by 2-fold because PGC-1α is a coactivator of VDR. On the other hand, cotransfection with NCoR-1 or SMRT reduced reporter activity by more than 60%. It should be noted that both coactivator and corepressor effect on VDR induction of CYP24A1 is dependent on VDR ligands. In contrast, ligand-activated VDR/RXRα repressed CYP7A1/Luc reporter activity (Fig. 8B). PGC-1α induced CYP7A1 reporter activity in the absence of VDR ligands. PGC-1α is a potent coactivator of HNF4α, which is a critical positive regulator of CYP7A1. NCoR-1 and SMRT strongly inhibited CYP7A1 reporter activity only when VDR was activated by its ligands. These results suggest that VDR is a positive regulation of CYP24A1 gene transcription in human hepatocytes.
Figure 8.
Transient transfection assay of effects of coactivators and corepressors on CYP24A1 and CYP7A1 promoter reporter activity. A, Reporter assays of human CYP24A1 promoter/luciferase reporter, phCYP24A1/-1200-Luc. B, Reporter assays of human CYP7A1 promoter/luciferase reporter, ph-1887Luc. Reporter plasmid (0.2 μg) was cotransfected with VDR (0.1 μg) and RXRα (0.1 μg) expression plasmids or control plasmid (pcDNA3.1), and with expression plasmid (0.1 μg) for PGC-1α, NCoR-1, and SMRT, as indicated, into HepG2 cells for 24 h, followed by treatment with 1α,25(OH)2-D3 (5 nm), or LCA-acetate (10 μm), or vehicle (EtOH) for an additional 24 h. The luciferase activities were assayed as described in Materials and Methods. Each experiment was done in triplicate and expressed as mean ± sd. *, Statistically significant difference (P < 0.05, n = 3) vs. vehicle control with the same cotransfection. #, Statistically significant difference (P < 0.05, n = 3) vs. pcDNA3 cotransfected sample with the same treatment. RLU, Relative light units.
A MEK1/2/ERK1/2 inhibitor blocked VDR interaction with HNF4α and coregulator recruitment to CYP7A1 promoter
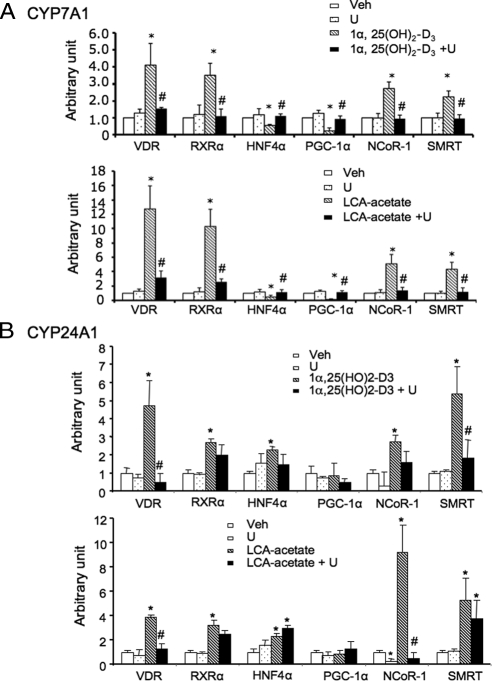
We reported previously that LCA or 1α,25(OH)2-D3 stimulated VDR, NCoR-1, and SMRT occupancy but inhibited HNF4α, PGC1α, and GRIP-1 occupancy to CYP7A1 promoter (14). Here we further studied the effect of U0126 on VDR ligand-induced alteration of CYP7A1 chromatin structure to confirm the role of the MEK1/2/ERK1/2 pathway in mediating VDR signaling. Figure 9A shows that 1α,25(OH)2-D3 increased VDR and RXRα occupancy to human CYP7A1 promoter by about 4-fold but decreased HNF4α occupancy by about 50%. LCA-acetate increased VDR and RXRα occupancy by about 10-fold but decreased HNF4α occupancy by more than 50%. Both 1α,25(OH)2-D3 and LCA-acetate increased recruitment of corepressors NCoR-1 and SMRT by about 2- to 4-fold but decreased recruitment of a coactivator PGC-1α by about 80–90%. U0126 significantly reduced 1α,25(OH)2-D3 or LCA-acetate-stimulated recruitment of VDR, RXRα, NCoR-1, and SMRT and increased HNF4α and PGC-1α recruitment to CYP7A1 promoter. These results confirmed that the MEK1/2/ERK1/2 pathway was involved in VDR signaling to promote VDR/RXRα heterodimer recruitment of corepressors to displace VDR/HNF4α and coactivator complexes on CYP7A1 promoter.
Figure 9.
ChIP assay of nuclear receptors and cofactors association with human CYP7A1 promoter (A) and CYP24A1 promoter (B). Primary human hepatocytes were pretreated with U0126 (U) (20 μm) for 1 h followed by treatment with 1α,25(OH)2-D3 (50 nm) or LCA-acetate (10 μm) for 16 h. Rabbit anti-VDR, anti-RXRα, and anti-HNF4α antibodies, and goat anti-PGC-1α, anti-NCoR-1, and anti-SMRT antibodies were used to immunoprecipitate the chromatin fragments. Nonimmune IgG was used as a negative control. Cell extract (5%) was set aside as input. A, Taqman primer set probes were designed for Q-PCR to detect human CYP7A1 promoter region containing BARE-II and BARE-II (HNF4α and VDR/RXRα binding site) and intron 5 (+9127 to +9193) (a negative control), as described in Materials and Methods. B, Tagman primer set probes were designed for Q-PCR to detect VDRE in the proximal region of human CYP24A1 promoter. Data were pooled from assays using three donor hepatocytes (HH1460, HH1479, and HH1483). *, Statistically significant difference (P < 0.05; n = 3) vs. vehicle (Veh) control. #, Statistically significant difference (P < 0.05; n = 3) vs. LCA-acetate or 1α, 25(OH)2-D3 treatment.
We also performed a similar chromatin immunoprecipitation (ChIP) assay to detect VDR and coactivator binding to CYP24A1 promoter. Figure 9B shows that 1α,25(OH)2-D3 or LCA-acetate increased VDR and RXRα occupancy to human CYP24A1 promoter. In contrast to CYP7A1, these VDR ligands increased HNF4α occupancy to CYP24A1 promoter. We performed adenovirus-mediated gene transduction of HNF4α in human primary hepatocytes and found that Adeno-HNF4α did not affect CYP24A1 mRNA but strongly induced CYP7A1 mRNA expression (data not shown). It is likely that HNF4α interacts with VDR as a coactivator as we reported previously (14). Both 1α,25(OH)2-D3 and LCA-acetate increased recruitment of corepressors NCoR-1 and SMRT to CYP24A1 promoter but did not have much effect on PGC-1α binding. Interestingly, U0126 had no effect on recruitment of VDR, RXR, HNF4α, PGC-1α, NCoR-1, and SMRT to CYP24A1 promoter but significantly reduced recruitment of NCoR-1 and SMRT to CYP24A1 promoter. Thus the membrane VDR/MEK1/2/ERK1/2 MAPK signaling pathway also is involved in VDR recruitment of corepressors to inhibit CYP24A1 gene transcript. In conclusion, these results demonstrated that ligand-activated VDR can either positively or negatively regulate its target gene transcription in human hepatocytes by recruiting coactivators and corepressors to VDR/RXR heterodimer. However, different mechanisms are involved in induction of CYP24A1 and inhibition of CYP7A1 (see Discussion).
Discussion
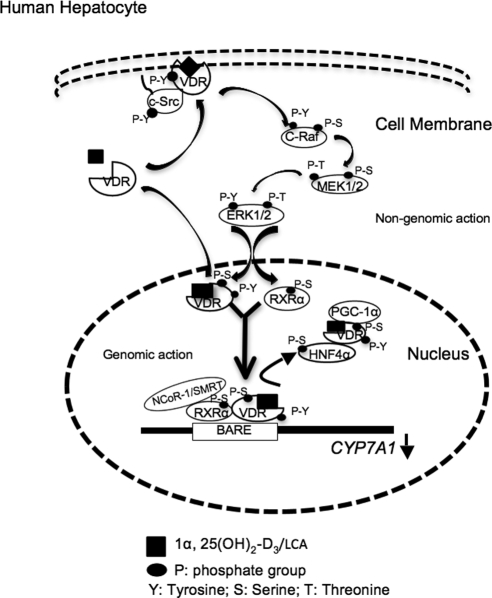
In this study, we identified a novel membrane VDR-signaling pathway that might be involved in the inhibition of CYP7A1 gene transcription by bile acids in human hepatocytes. As in other tissues and cells, VDR has both genomic and nongenomic actions in human liver cells. The nongenomic action of membrane VDR signaling is a very rapid response (in miniseconds or seconds) to cellular stimuli to activate cell-signaling pathways, whereas the genomic action of VDR is a relatively slower response (minutes to hours) to hormonal ligands by dimerization of VDR with RXR and recruitment of coactivators and/or corepressors to gene promoters to modulate the rate of target gene transcription (25). Figure 10 illustrates the proposed molecular mechanism of the coordinated genomic and nongenomic actions of VDR signaling pathway that inhibits CYP7A1 gene expression in human hepatocytes. The VDR ligand 1α,25(OH)2-D3 or LCA stimulates tyrosine phosphorylation of VDR and activation of c-Src. The tyrosine-phosphorylated VDR forms a complex with activated c-Src, which is translocated from the cytosol to the caveolae subdomain of the plasma membrane. Ligand-activated VDR is also translocated to the nucleus independently of tyrosine kinase pathways. The VDR/c-Src activates a G protein, Ras, and c-Raf (Raf-1) likely through the Shc/Grb2/Sos complex (19). The c-Raf activates the MEK1/2/ERK1/2 MAPK pathway, which then phosphorylates the nuclear VDR, RXRα, and HNF4α. In this model the ligand-activated VDR/RXRα recruits corepressors NCo-R1 and SMRT to displace HNF4α and coactivators PGC-1α and SRC-1 complexes from binding to the BARE and results in inhibiting CYP7A1 gene transcription. It has been well established that the HNF4α homodimer binds to the BARE and interacts with PGC-1α and SRC-1 on CYP7A1 promoter to stimulate CYP7A1 gene transcription (1). Phosphorylation of HNF4α is known to reduce its dimerization and DNA binding activity (24), and thus allows VDR/RXR/NCoR-1/SMRT to bind to the BARE. It is also possible that VDR and HNF4α may compete for coactivators, or VDR may interact with HNF4α to prevent HNF4α from dimerization and binding to the BARE. All these mechanisms may inhibit CYP7A1 gene transcription.
Figure 10.
A cartoon illustrates genomic and nongenomic actions of VDR signaling in inhibition of human CYP7A1 gene transcription in human hepatocytes. VDR ligands, 1α,25(OH)2-D3, or LCA stimulate intracellular translocation of VDR to both the plasma membranes. VDR ligands induce tyrosine (Y) phosphorylation of VDR and activate c-Src. VDR interacts with c-Src in the plasma membrane. Membrane VDR signaling activates c-Raf, probably through activation of the Shc/Grb2/Sos complex and Ras (data not shown), which then activates c-Raf and subsequently the MEK1/2/ERK1/2 MAPK pathway. VDR is also translocated to the nucleus through interaction with a heat shock protein 70, independent of the tyrosine kinase pathway. ERK1/2 is translocated to the nucleus and subsequently phosphorylates the serine/threonine (S/T) residues on nuclear VDR, RXRα, and HNF4α. The VDR/RXRα recruits corepressor NCoR-1 or SMRT to the BARE to displace the HNF4α/VDR/PGC-1α or SRC-1 complex and results in inactivating the CYP7A1 gene. It should be noted that NCoR-1/SMRT is recruited to RXRα that dimerizes with ligand-activated VDR. VDR interacts with HNF4α and prevents HNF4α from dimerization and binding to the BARE.
It is well established that 1α,25 (OH)2-D3 exerts rapid biological responses by activating tyrosine phosphorylation-signaling pathways that activate intracellular Ca2+-activated protein kinase cascades leading to cell proliferation, differentiation, apoptosis, muscle growth, and the immune response (19). The Src family of tyrosine kinases plays a critical role in control of various cellular processes including cell adhesion, proliferation, survival, and motility (26). The tyrosine kinase activity of c-Src is mainly regulated by phosphorylation of two major tyrosine phosphorylation sites on human c-Src: one is in the tyrosine kinase domain (Y419) and another one is in the C terminus (Y530). Src is maintained in an inactive conformation with the SH2 domain engaged with Y530. Dephosphorylation of Y530 disrupts the intramolecular interaction between the SH2 domain and Y530 and results in autophosphorylation of Y419 and activation of c-Src. Binding of c-Src to growth factor receptor tyrosine kinase via the SH2 domain results in displacement of Y530 to allow Y419 phosphorylation and activation of c-Src. In this study, we demonstrated that LCA-acetate or 1α,25 (OH)2-D3 was able to stimulate tyrosine phosphorylation of VDR. Binding of VDR to c-Src may cause autophosphorylation of Y419 on human c-Src and activate c-Src. We also provided the evidence that VDR and c-Src interacted in the plasma membrane. Furthermore, a specific c-Src inhibitor AZD0530 reduced VDR phosphorylation and the inhibitory effect of LCA-acetate and 1α, 25(OH)2-D3 on CYP7A1 mRNA expression and abrogated their stimulatory effect on CYP24A1 expression in human hepatocytes. These results are consistent with the involvement of c-Src in membrane VDR signaling in human hepatocytes as in muscle and tumor cells. VDR signaling is known to stimulate several signaling pathways including AKT, ERK1/2, p38, and JNK1/2 kinases in bone, intestine, and muscle cells (18,19,27,28). Interestingly, our results show that membrane VDR signaling only activates the MEK1/2/ERK1/2, but not PKA, PKB, PKC, p38, and JNK pathways in human hepatocytes. It appears that VDR signaling in human hepatocytes is highly specific and may have functions limited to cell proliferation and differentiation in hepatocytes.
Nuclear receptors recruit coactivators and corepressors to regulate target gene transcription. CYP24A1 is a well-characterized VDR target gene highly induced by 1α,25 (OH)2-D3. Agonists or ligands bind to VDR and induce a conformational change in the ligand-binding domain to expose the coactivator-binding surface to allow coactivator binding. Ligand-activated VDR forms a heterodimer with RXR and binds to the canonical DR3 (direct repeat spaced by three nucleotides)-type VDR response elements (VDREs) to induce CYP24A1 gene transcription (29,30). However, the transrepression mechanism of VDR is unclear and controversial. VDR has been shown to interact with corepressors, such as NCoR-1, SMRT, Alien, and Hairless (31,32,33). It has been reported that unliganded VDR interacts with a bipartite nuclear receptor interaction domain on SMRT and binds to the VDRE to inhibit CY24A1 gene transcription, whereas binding of a ligand to VDR abolishes VDR recruitment of SMRT to the VDREs and recruits coactivators to stimulate CYP24A1 gene transcription (33). This mechanism is in contrast to a recent report that binding of an agonist or ligand to VDR enhances VDR dimerization with RXR and recruitment of both corepressors and coactivators to the VDRE on CYP24A1 promoter (34). The relative amount of coactivators and corepressors present in different tissues may determine the expression levels of VDR target gene expression in different tissues.
This study shows that VDR functions as a repressor of the CYP7A1 gene and an activator of CYP24A1 gene in human hepatocytes. Different from the CYP24A1 gene, the CYP7A1 promoter does not have a DR3-type VDRE. Instead the 1α,25(OH)2-D3 or LCA-activated VDR/RXRα binds to BARE-I and BARE-II (also an HNF4α site) in human CYP7A1 proximal promoter (14). In this study, we showed that VDR interacted with NCoR-1 or SMRT only when RXRα and 1α,25(OH)2-D3 or LCA were present (Fig. 7C). This is consistent with the model that the RXRα, but not VDR, recruits corepressors to the VDR/RXRα heterodimer when activated by a VDR agonist (34). It was demonstrated that a VDR agonist induced a conformational change on helix 12 of VDR and subsequently altered the conformation of the AF2 domain on RXRα to expose the corepressor-binding surface. This active repression mechanism is different from that involved in 1α,25 (OH)2-D3 feedback inhibition of 1α-hydroxylase (CYP27B1), an enzyme involved in activation of 1α,25(OH)2-D3. The negative VDRE identified in the CYP27B1 promoter has an E-box sequence that binds a basic helix-loop-helix protein named VDR-interacting receptor, which recruits ligand-activated VDR/RXR, corepressors, and William Syndrome transcription factor to inhibit CYP27B1 gene transcription (35). It appears that VDR binds to different VDREs to regulate CYP7A1, CYP24A1, and CYP27B1 gene expression by different mechanisms. Furthermore, 1α,25(OH)2-D3 signaling may regulate gene transcription by epigenetic mechanisms involving histone acetylation and DNA methylation (35,36).
Bile acids are known to activate several cell signaling pathways in hepatocytes, such as FGF receptor 4, Fas receptor, epidermal growth factor receptor, Gαi protein-coupled receptor, and TNF receptor (2). These signaling pathways activate JNK, ERK, and p38 MAPK pathways to regulate bile acid and glucose metabolism. In this study we identified a novel bile acid-activated membrane receptor, VDR, which may cross talk with bile acid-activated growth factor receptor signaling via the c-Src/ERK pathway. Recently, a Gαs protein-coupled receptor TGR5 (M-BAR) has been identified as a membrane bile acid receptor (37). Although TGR5 binds to various free and conjugated bile acids, LCA and taurine-conjugated LCA are the most efficacious activators of TGR5 (37,38). The TGR5 and VDR signaling activate distinct pathways and have different physiological functions. TGR5 activates cAMP/PKA signaling that regulates energy metabolism in brown adipose tissues (39). TGR5 is widely expressed in many tissues but not in hepatocytes (38).
In conclusion, this study identifies a novel bile acid-activated VDR-signaling pathway that inhibits CYP7A1 gene transcription in human hepatocytes by both genomic and nongenomic actions. Ligand-activated VDR is translocated from the cytosol to the plasma membrane and nucleus. The VDR-dependent signaling specifically activates the c-Raf/MEK1/2/ERK1/2 pathway to inhibit CYP7A1 gene transcription in human hepatocytes. LCA activation of the membrane VDR-signaling pathway may be a rapid response to sudden increase of cholestatic bile acids in human hepatocytes during cholestasis to inhibit bile acid synthesis and protect the liver from cholestatic injury. LCA derivatives may be selective VDR modulators that have therapeutic potential for treating liver diseases (40).
Materials and Methods
Materials
Human CYP7A1 reporter, phCYP7A1/-298, and phCYP7A1-1887, and human CYP27A1 reporter, phCYP27A1/-1774, were constructed as previously described (41,42,43). Human CYP24A1/luciferase reporter, phCYP24A1/-1200-Luc (nucleotides −1200 to +120), was kindly provided by Dr. P. MacDonald (Case Western Reserve University School of Medicine, Cleveland, OH).
Expression plasmid for human VDR (pcDNA3.1/VDR) was provided by Dr. Y.C. Li (University of Chicago, Chicago, IL). LCA, CA, and CDCA were purchased from Sigma-Aldrich (St. Louis, MO). LCA-acetate was from Steraloids, Inc. (Newport, RI). Specific inhibitors for ERK1/2 (PD98059), MEK1/2 (U0126), JNK (SP600125), p38 (SB203580), PKC (Ro318220), PKA (H89), and PI3K (LY294002) were obtained from EMD Chemicals, Inc. (Gibbstown, NJ). The c-Src inhibitor, AZD0530, was purchased from Selleck Chemicals LLC (Shanghai, China). A protein phosphatase inhibitor, okadaic acid, was purchased from Sigma-Aldrich. Rabbit (sc-1009) and mouse (sc-13133) anti-VDR, rabbit (sc-533) and mouse (sc-46659) anti-RXRα, rabbit (sc-8981) and goat (sc-6556) anti-HNF4α, goat anti-PGC-1α, goat anti-β-actin, goat anti-NCoR-1, rabbit anti-SMRT, goat antilamin B, rabbit anticaveolin-1, and mouse antiphosphorylated tyrosine antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Mouse anti-pan-cadherin was purchased from Abcam, Inc. (Cambridge, MA). Mouse antiphosphorylated serine antibody was purchased from GE Healthcare Bio-Sciences Corp. (Piscataway, NJ). Rabbit antiphosphorylated ERK1/2 (Thr202/Tyr204), JNK (Thr183/Tyr185), p38 (Thr180/Tyr182), MEK1/2 (Ser217/221), c-Raf (Ser338), c-Src (Tyr416), and ELK-1 (Ser383) antibodies were purchased from Cell Signaling Technology, Inc. (Lake Placid, NY).
Cell culture
Human hepatoblastoma cell line HepG2 (American Type Culture Collection, Manassas, VA) was cultured as described previously (44). Primary human hepatocytes were isolated from human donors and were obtained through the Liver Tissue and Cells Distribution System of National Institutes of Health (Dr. S. Strom, University of Pittsburgh, Pittsburgh, PA). Cells were maintained in hepatocyte maintenance medium as described previously (44).
Immunofluoresence microscopy
Primary human hepatocytes were treated with vehicle (EtOH), 1α,25(OH)2-D3 (50 nm), LCA (10 μm), LCA-acetate (10 μm), or cholic acid (25 μm) for 6 h. Cells were fixed with 4% paraformaldehyde and permeable with 0.2% Triton X-100. Rabbit anti-VDR antibody (Santa Cruz) and Alexa Fluor 488-conjugated antirabbit IgG antibody (Molecular Probes, Carlsbad, CA) were used for detection of VDR. Mouse anticaveolin-1 antibody (Abcam, Inc.) and Alexa Fluor 546-conjugated antimouse IgG antibody (Molecular Probes) were used for detection of caveolin-1. Cell nuclei were stained with TO-PRO-3 (Molecular Probes). Stained cells were imaged under the confocal microscope Olympus Fluoview FV300 (Olympus, Center Valley, PA).
Transient transfection assay
HepG2 cells were grown to about 80% confluence in 24-well tissue culture plates, and treated with LCA-acetate (Steraloids) or 1α,25(OH)2-D3 (Cayman Chemicals, Ann Arbor, MI). Luciferase reporters and expression plasmids were transfected into HepG2 cells using Tfx-20 reagent (Promega Corp., Madison, WI) following manufacturer's instructions. Luciferase reporter assays were performed as previously described (41). Assays were performed in duplicate, and each experiment was repeated at least four times. Data were expressed as means ± sd.
RNA isolation and quantitative real-time PCR
Primary human hepatocytes were maintained in serum-free media overnight. Cells were treated with 1α,25(OH)2-D3 or LCA-acetate in the amounts and times indicated. Total RNA was isolated using Tri-Reagent (Sigma-Aldrich). Reverse-transcription reactions were performed using RETROscript kit (Ambion, Austin, TX). Real-time quantitative PCR (Q-PCR) assays of relative mRNA expression were performed as previously described (44) using an ABI PRISM 7500 sequence detector (Applied Biosystems, Foster City, CA). TaqMan PCR primers and probes were ordered from TaqMan Gene Expression Assays (Applied Biosystems). Data were analyzed using the Sequence Detector version 1.7 software (Applied Biosystems). Relative mRNA expression levels were calculated by the ΔΔCt method recommended by Applied Biosystems (User Bulletin no. 2, 1997). All PCRs were done in duplicate, and each reaction was repeated at least four times.
Protein extraction and immunoblot assay
To isolate total cell protein extracts, primary human hepatocytes (in T25 flasks) were lysed in modified radioimmunoprecipitation assay buffer (50 mm Tris-HCl, 1% Nonidet P-40, 0.25% deoxycholate, 150 mm NaCl, 1 mm EDTA) containing protease inhibitors (1 mm phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml pepstatin; Sigma-Aldrich) for 30 min. Cytoplasmic and nuclear fractions were isolated using a Nuclear Extraction kit (Millipore Corp., Billerica, MA) following manufacturer's instructions. Plasma membrane fractions were isolated using a Plasma Membrane Protein Extraction kit (Biovision, Inc., Mountain View, CA) following manufacturer's instructions. Protein samples were subjected to SDS-PAGE for immunoblot analysis using specific antibodies and detected by enhanced chemiluminescence detection kit (GE Healthcare Bio-Sciences Corp.).
IP and co-IP assays
Primary human hepatocytes in T25 flasks were maintained in serum-free media overnight and treated with various compounds as indicated. For IP assays, cellular protein extracts (300 μg) were incubated with 10 μg rabbit anti-VDR, rabbit anti-RXRα, or goat anti-HNF4α (Santa Cruz Biotechnology, Inc.) at 4 C with rotation overnight, followed by an additional incubation with Protein A agarose beads for 2 h. The beads were then washed three times with cold 1× PBS, boiled in 2× protein loading buffer for 5 min, and subjected to SDS-PAGE, followed by immunoblot analysis with mouse antiphosphoserine, mouse antiphosphotyrosine (GE Healthcare Bio-Sciences Corp.), mouse anti-VDR, mouse anti-RXRα, and rabbit anti-HNF4α antibodies (Santa Cruz Biotechnology, Inc.). For co-IP assays of VDR interaction with HNF4α, rabbit anti-VDR or goat anti-HNF4α antibody was used to coimmunoprecipitate VDR and HNF4α in cellular protein extracts. Mouse anti-VDR or rabbit anti-HNF4α antibody was used to detect coprecipitated VDR or HNF4α. For co-IP assay of VDR interaction with c-Src, anti-VDR was used to coimmunoprecipitate VDR and c-Src in the membrane protein extracts, and rabbit anti-c-Src, and antiphospho-Src (Y416/Y419) antibodies were used to detect c-Src and p-Y416/Y419-c-Src, respectively, in the immunoprecipitants.
In vitro ERK1/2 assay
The p44/42 (ERK1/2) MAPK Assay kit (Cell Signaling Technology, Inc.) was used to study in vitro serine phosphorylation of VDR, RXRα, and HNF4α by ERK1/2 according to the manufacturer's instruction. Briefly, 15 μl of beads coated with antiphospho-ERK1/2 antibody were incubated with 300 μg of total cell extracts to immobilize phospho-ERK1/2. After gentle rocking at 4 C overnight, the immobilized beads containing active p-ERK1/2 were pelleted at 5000 × g for 30 sec, followed by washing with cold 1× cell lysis buffer twice and with 1× kinase buffer twice. The washed beads were suspended in 50 μl 1× kinase buffer supplemented with 200 μm ATP and then incubated with in vitro synthesized VDR, RXRα, or HNF4α using the transcription and translation (TNT) lysate system (Promega) for 30 min at 30 C. The kinase phosphorylation reaction was terminated by adding 25 μl 3× SDS sample buffer. VDR, RXRα, and HNF4α were immunoprecipitated by their respective antibodies (Santa Cruz Biotechnology, Inc.) for another 6 h. Phosphorylation of the serine residues in VDR, RXRα, or HNF4α was detected by mouse antiphosphoserine antibody (GE Healthcare Bio-Sciences Corp.). The purified ELK-1 included in the ERK1/2 assay kit was assayed as a positive control.
Mammalian two-hybrid assays
The reporter plasmid p5xUAS-TK-Luc containing five copies of the upstream activating sequence (UAS) fused to the upstream of the thymidine kinase (TK) promoter and the luciferase reporter gene was used for mammalian two-hybrid assays as reported previously (14). Gal4-VDR was cotransfected with VP16 empty vector, pcDNA3.1, VP16-HNF4α, VP16-NCoR-1, VP16-SMRT, VP16-SRC-1, RXRα, PGC-1α, SRC-1, SMRT, or NCoR-1 as indicated, into human embryonic kidney (HEK)293 cells. Reporter activity was assayed as described above.
ChIP assay
Primary human hepatocytes in T25 flasks were maintained in serum-free media overnight and then incubated with or without ERK1/2 inhibitor (U) for 1 h followed by treatment with vehicle (EtOH), 1α,25(OH)2-D3 (50 nm), or LCA-acetate (10 μm). ChIP assays were performed using ChIP assay kit (Millipore Corp.) following the manufacturer's protocol. Cells were cross-linked in 1% formaldehyde and sonicated. Protein-DNA complexes were precipitated using rabbit anti-VDR, rabbit anti-RXRα, rabbit anti-HNF4α, goat anti-PGC-1α, goat anti-NCoR-1, or rabbit anti-SMRT antibody (Santa Cruz Biotechnology, Inc.). Rabbit nonimmune IgG was added as a negative control. Q-PCR was used to quantify the CYP7A1 promoter region BARE-I+BARE-II, which was previously identified as a HNF4α-binding site that also binds VDR/RXRα (14), according to a method described previously (45). TaqMan real-time PCR primer set (Applied Biosystems) for CYP7A1BARE-II promoter: forward primer, 5-GGTCTCTGATTGCTTTGGAACC; reverse primer, 5-AAAAGTGGTAGTAACTGGCCTTGAA; TaqMan probe: TTCTGATACCTGTGGACTTA; intron 5 primer set (nucleotide 8127-8195), forward primer, 5-TTTCTTCTGGGAACCCTTCTCTC, reverse primer, 5-TCCTATCCTGCTTGAACGATTAGTT; TaqMan probe: CTAGCTCTGCCTGACTAA.
For ChIP assay of VDRE binding to the human CYP24A1 promoter, Q-PCR was designed to detect a region containing two well-defined VDREs (-285-AGTTCACCGGGTGTG-299 and -164AGGTCAGCGAGGGCG-178) (34). TaqMan real-time PCR primer set for CYP24A1 promoter: forward primer: −30, ATGGTCCCTGGGCATAGGA.
Reverse primer: +24, GCTGGCGCTGCGTTTC; MGB probe: CATGGAGAGGGACAGGA.
Statistical analysis
All results were expressed as mean ± sd. Q-PCR and reporter assay data were analyzed with Student's t test. P values of <0.05 were considered as statistically significant differences between treated and untreated control.
Acknowledgments
Human primary hepatocytes were provided by the Liver Tissue and Cells Distribution System of National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (#N01-DK-7-0004/HHSN267200700004C).
Footnotes
This research was supported by National Institute of Health Grants DK44442 and DK58379 (to J.Y.L.C.), and DK92310 (to S.S.).
Disclosure Summary: The authors have confirmed the originality and disclosed no conflict of interest of this study.
First Published Online April 6, 2010
Abbreviations: BAREs, Bile acid response elements; CA, cholic acid; CDCA, chenodeoxycholic acid; co-IP, coimmunoprecipitation; CYP7A1, cholesterol 7α-hydroxylase; CYP24A1, sterol 24-hydroxylase; CYP27B1, sterol 1α-hydroxylase; CYP27A1, sterol 27-hydroxylase; ERK, extracellular signal-regulated kinase; FXR, farnesoid X receptor; HEK, human embryonic kidney; HNF, hepatocyte nuclear factor; IP, immunoprecipitation; LCA, lithocholic acid; MEK, MAPK/ERK kinase; NCoR-1, nuclear receptor corepressor-1; OA, okadaic acid; 1α,25(OH)2-D3, 1α,25-dihydroxyvitamin D3; PGC, peroxisomal proliferator-activated receptor-γ coactivator; PI3K, phosphoinositol-3 kinase; PKA, protein kinase A; PKC, protein kinase C; Q-PCR, real-time quantitative PCR; RXRα, retinoid X receptor α; SMRT, silencing mediator of retinoid and thyroid; SRC-1, steroid receptor coactivator-1; TK, thymidine kinase; UAS, upstream activating sequence; VDR, vitamin D receptor; VDRE, VDR response element.
References
- Chiang JY 2009 Bile acids: regulation of synthesis. J Lipid Res 50:880–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P 2009 Bile acids as regulatory molecules. J Lipid Res 50:1509–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B 2009 Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 89:147–191 [DOI] [PubMed] [Google Scholar]
- Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K 2008 Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov 7:678–693 [DOI] [PubMed] [Google Scholar]
- Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Donahee M, Wang DY, Mansfield TA, Kliewer SA, Goodwin B, Jones SA 2003 Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev 17:1581–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA 2005 Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2:217–225 [DOI] [PubMed] [Google Scholar]
- Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ 2002 Vitamin D receptor as an intestinal bile acid sensor. Science 296:1313–1316 [DOI] [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA 2001 The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA 98:3369–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drocourt L, Ourlin JC, Pascussi JM, Maurel P, Vilarem MJ 2002 Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J Biol Chem 277:25125–25132 [DOI] [PubMed] [Google Scholar]
- Seo YK, Chung YT, Kim S, Echchgadda I, Song CS, Chatterjee B 2007 Xenobiotic- and vitamin D-responsive induction of the steroid/bile acid-sulfotransferase Sult2A1 in young and old mice: the role of a gene enhancer in the liver chromatin. Gene 386:218–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AW 2006 Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology 147:5542–5548 [DOI] [PubMed] [Google Scholar]
- Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ 2006 Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon-Barré M, Demers C, Mirshahi A, Néron S, Zalzal S, Nanci A 2003 The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology 37:1034–1042 [DOI] [PubMed] [Google Scholar]
- Han S, Chiang JY 2009 Mechanism of vitamin D receptor inhibition of cholesterol 7α-hydroxylase gene transcription in human hepatocytes. Drug Metab Dispos 37:469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AW, Mizwicki MT, Norman DP 2004 Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov 3:27–41 [DOI] [PubMed] [Google Scholar]
- Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW 2004 The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1α,25(OH)2-vitamin D3in vivo and in vitro. Mol Endocrinol 18:2660–2671 [DOI] [PubMed] [Google Scholar]
- Boland R, De Boland AR, Buitrago C, Morelli S, Santillán G, Vazquez G, Capiati D, Baldi C 2002 Non-genomic stimulation of tyrosine phosphorylation cascades by 1,25(OH)2D3 by VDR-dependent and -independent mechanisms in muscle cells. Steroids 67:477–482 [DOI] [PubMed] [Google Scholar]
- Buitrago C, Boland R, de Boland AR 2001 The tyrosine kinase c-Src is required for 1,25(OH)2-vitamin D3 signalling to the nucleus in muscle cells. Biochim Biophys Acta 1541:179–187 [DOI] [PubMed] [Google Scholar]
- Boland R, Buitrago C, De Boland AR 2005 Modulation of tyrosine phosphorylation signalling pathways by 1α,25(OH)2-vitamin D3. Trends Endocrinol Metab 16:280–287 [DOI] [PubMed] [Google Scholar]
- Adachi R, Honma Y, Masuno H, Kawana K, Shimomura I, Yamada S, Makishima M 2005 Selective activation of vitamin D receptor by lithocholic acid acetate, a bile acid derivative. J Lipid Res 46:46–57 [DOI] [PubMed] [Google Scholar]
- Buitrago C, Vazquez G, De Boland AR, Boland RL 2000 Activation of Src kinase in skeletal muscle cells by 1, 1,25-(OH)2-vitamin D3 correlates with tyrosine phosphorylation of the vitamin D receptor (VDR) and VDR-Src interaction. J Cell Biochem 79:274–281 [DOI] [PubMed] [Google Scholar]
- Chappel J, Ross FP, Abu-Amer Y, Shaw A, Teitelbaum SL 1997 1,25-Dihydroxyvitamin D3 regulates pp60c-src activity and expression of a pp60c-src activating phosphatase. J Cell Biochem 67:432–438 [DOI] [PubMed] [Google Scholar]
- Lechner D, Kállay E, Cross HS 2007 1α,25-Dihydroxyvitamin D3 downregulates CYP27B1 and induces CYP24A1 in colon cells. Mol Cell Endocrinol 263:55–64 [DOI] [PubMed] [Google Scholar]
- Chiang JY 2009 Hepatocyte nuclear factor 4α regulation of bile acid and drug metabolism. Expert Opin Drug Metab Toxicol 5:137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizwicki MT, Norman AW 2009 The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci Signal 2:re4 [DOI] [PubMed] [Google Scholar]
- Bjorge JD, Jakymiw A, Fujita DJ 2000 Selected glimpses into the activation and function of Src kinase. Oncogene 19:5620–5635 [DOI] [PubMed] [Google Scholar]
- Ronda AC, Buitrago C, Colicheo A, de Boland AR, Roldán E, Boland R 2007 Activation of MAPKs by 1α,25(OH)2-vitamin D3 and 17β-estradiol in skeletal muscle cells leads to phosphorylation of Elk-1 and CREB transcription factors. J Steroid Biochem Mol Biol 103:462–466 [DOI] [PubMed] [Google Scholar]
- Wu W, Zhang X, Zanello LP 2007 1α,25-Dihydroxyvitamin D3 antiproliferative actions involve vitamin D receptor-mediated activation of MAPK pathways and AP-1/p21(waf1) upregulation in human osteosarcoma. Cancer Lett 254:75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toell A, Polly P, Carlberg C 2000 All natural DR3-type vitamin D response elements show a similar functionality in vitro. Biochem J 352:301–309 [PMC free article] [PubMed] [Google Scholar]
- Väisänen S, Dunlop TW, Sinkkonen L, Frank C, Carlberg C 2005 Spatio-temporal activation of chromatin on the human CYP24 gene promoter in the presence of 1α,25-dihydroxyvitamin D3. J Mol Biol 350:65–77 [DOI] [PubMed] [Google Scholar]
- Polly P, Herdick M, Moehren U, Baniahmad A, Heinzel T, Carlberg C 2000 VDR-Alien: a novel, DNA-selective vitamin D3 receptor-corepressor partnership. FASEB J 14:1455–1463 [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Sisk JM, Jurutka PW, Haussler CA, Slater SA, Haussler MR, Thompson CC 2003 Physical and functional interaction between the vitamin D receptor and hairless corepressor, two proteins required for hair cycling. J Biol Chem 278:38665–38674 [DOI] [PubMed] [Google Scholar]
- Kim JY, Son YL, Lee YC 2009 Involvement of SMRT corepressor in transcriptional repression by the vitamin D receptor. Mol Endocrinol 23:251–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Martinez R, Zambrano A, Castillo AI, Aranda A 2008 Vitamin D-dependent recruitment of corepressors to vitamin D/retinoid X receptor heterodimers. Mol Cell Biol 28:3817–3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Fujiki R, Murayama A, Kitagawa H, Yamaoka K, Yamamoto Y, Mihara M, Takeyama K, Kato S 2007 1α,25(OH)2D3-induced transrepression by vitamin D receptor through E-box-type elements in the human parathyroid hormone gene promoter. Mol Endocrinol 21:334–342 [DOI] [PubMed] [Google Scholar]
- Ewing AK, Attner M, Chakravarti D 2007 Novel regulatory role for human Acf1 in transcriptional repression of vitamin D3 receptor-regulated genes. Mol Endocrinol 21:1791–1806 [DOI] [PubMed] [Google Scholar]
- Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K 2002 Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun 298:714–719 [DOI] [PubMed] [Google Scholar]
- Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M 2003 A G protein-coupled receptor responsive to bile acids. J Biol Chem 278:9435–9440 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J 2006 Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439:484–489 [DOI] [PubMed] [Google Scholar]
- Ishizawa M, Matsunawa M, Adachi R, Uno S, Ikeda K, Masuno H, Shimizu M, Iwasaki K, Yamada S, Makishima M 2008 Lithocholic acid derivatives act as selective vitamin D receptor modulators without inducing hypercalcemia. J Lipid Res 49:763–772 [DOI] [PubMed] [Google Scholar]
- Crestani M, Stroup D, Chiang JY 1995 Hormonal regulation of the cholesterol 7α-hydroxylase gene (CYP7). J Lipid Res 36:2419–2432 [PubMed] [Google Scholar]
- Wang DP, Stroup D, Marrapodi M, Crestani M, Galli G, Chiang JY 1996 Transcriptional regulation of the human cholesterol 7α-hydroxylase gene (CYP7A) in HepG2 cells. J Lipid Res 37:1831–1841 [PubMed] [Google Scholar]
- Chen W, Chiang JY 2003 Regulation of human sterol 27-hydroxylase gene (CYP27A1) by bile acids and hepatocyte nuclear factor 4α (HNF4α). Gene 313:71–82 [DOI] [PubMed] [Google Scholar]
- Li T, Chiang JY 2005 Mechanism of rifampicin and pregnane X receptor inhibition of human cholesterol 7α-hydroxylase gene transcription. Am J Physiol Gastrointest Liver Physiol 288:G74–G84 [DOI] [PubMed] [Google Scholar]
- Li T, Chiang JY 2007 A Novel Role of Transforming Growth Factor β1 in transcriptional repression of human cholesterol 7α-hydroxylase gene. Gastroenterology 133:1660–1669 [DOI] [PubMed] [Google Scholar]