Abstract
We profiled the expression of the 48 human nuclear receptors (NRs) by quantitative RT-PCR in 51 human cancer cell lines of the NCI60 collection derived from nine different tissues. NR mRNA expression accurately classified melanoma, colon, and renal cancers, whereas lung, breast, prostate, central nervous system, and leukemia cell lines exhibited heterogeneous receptor expression. Importantly, receptor mRNA levels faithfully predicted the growth-inhibitory qualities of receptor ligands in nonendocrine tumors. Correlation analysis using NR expression profiles and drug response information across the cell line panel uncovered a number of new potential receptor-drug interactions, suggesting that in these cases, individual receptor levels may predict response to chemotherapeutic interventions. Similarly, by cross-comparing receptor levels within our expression dataset and relating these profiles to existing microarray gene expression data, we defined interactions among receptors and between receptors and other genes that can now be mechanistically queried. This work supports the strategy of using NR expression profiling to classify various types of cancer, define NR-drug interactions and receptor-gene networks, predict cancer-drug sensitivity, and identify druggable targets that may be pharmacologically manipulated for potential therapeutic intervention.
This work uses nuclear receptor expression profiling to classify cancers, define nuclear receptor drug and gene interactions, predict cancer-drug sensitivity and identify potential drugable targets.
Nuclear receptors (NRs) are a large superfamily of ligand-inducible transcription factors that play a variety of essential developmental and physiological roles ranging from sexual development to lipid metabolism by modulating the expression of a plethora of target genes. NRs and their target genes direct key cellular events including aspects of cell proliferation, differentiation, and cellular metabolism (1,2,3). Because of their specific proliferative role in certain tissues, some members of this superfamily have been directly implicated in the development or progression of cancer. Examples of this are given by the involvement of ERα and PR in the growth of breast and ovarian cancers, AR in the proliferation of prostate tumors, and TR in the ontogeny of some thyroid tumors (4,5,6,7,8,9,10). Indeed, ERα and AR are considered oncogenes in endocrine tissues because of their proliferative effects.
In contrast, other NRs have been identified as tumor suppressors or differentiation factors and are thus important for the treatment and prevention of cancer. This is illustrated by the functions of VDR in protecting against colon cancer (11), of PPARγ in diminishing the tumorigenic potential of breast, lung, and prostate malignancies (12,13,14,15), and of RARα in reversing premalignant lung lesions and in inducing cancer cell differentiation (16,17). Furthermore, still other NRs regulate drug metabolism, thus controlling tumor sensitivity to chemical interventions. The xenobiotic receptor PXR, for instance, controls genes involved in drug metabolism, protecting the body against toxic substances by inducing their breakdown and similarly inducing the expression of enzymes that metabolize therapeutic drugs (18,19). In addition, interactions between NRs and key tumor suppressors such as p53 have also been described (20). These interactions can affect the function and cellular localization of tumor suppressors, indirectly contributing to malignant phenotypes.
Considering the known roles of NRs in cancer, we reasoned that this transcription factor superfamily may have undescribed associations with cancer development and progression that could be elucidated by querying the expression of the receptors in the major forms of human cancer. To also evaluate correlations between receptor expression and response to drugs, we chose to profile the NR superfamily in the NCI60 collection of human cancer cell lines. This panel of cell lines is derived from the nine most prevalent human cancers including leukemias, melanomas, and malignancies of the lung, colon, ovary, kidney, prostate, breast, and central nervous system (CNS). This collection of cell lines is of particular interest because it has been extensively studied (http://dtp.nci.nih.gov), has been evaluated at the National Cancer Institute (NCI) for sensitivity to over 40,000 compounds in dose-response viability assays and has been profiled for gene expression by microarray analysis. In addition, a number of molecular targets have been measured at the protein level in these cells, and in the case of some proteins, their activity has also been evaluated. These features of the NCI60 panel allowed us to perform a thorough analysis of the implications of NR expression across cancers. We found that NR mRNA expression profiles can accurately classify some cancer types and that the expression of specific druggable NRs in every cell line suggests the use of NRs as therapeutic targets for individualized medicine. We also identified novel correlations between receptor expression patterns and between receptors and other genes that give insights into gene networks that can be assessed mechanistically. Furthermore, our NR expression profile dataset can be used to predict drug sensitivity and explore new NR-drug functional interactions. The informative power offered by mRNA expression derived from the high-resolution, sensitive, high-throughput quantitative real-time PCR (qRT-PCR) method used here, demonstrates the usefulness of this approach while protein tools for the full NR family are developed.
Results
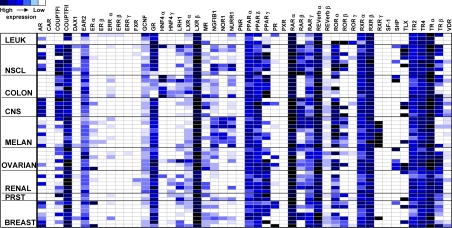
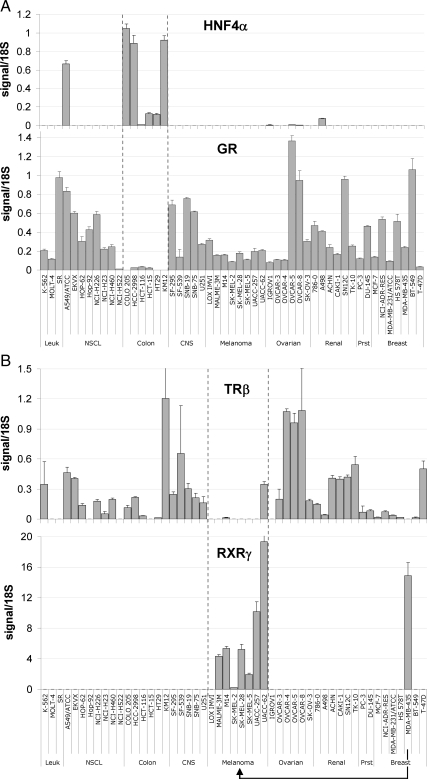
High-throughput qRT-PCR was used as previously described (1) to interrogate the expression of the human NR superfamily in 51 cancer cell lines from the NCI60 collection. We chose qRT-PCR because of its high sensitivity, resolution, and dynamic range that greatly enhance cross-sample comparisons. Figure 1 summarizes the NR profiles in the nine different cancer cell types of the NCI60 panel as a heat map. A number of unique patterns of expression were readily evident. For example, COUPTFII, LXRβ, RARα, REVERBα, and TRα were highly expressed across all cancer types, and EAR2, PPARα, PPARδ, RXRα, RXRβ, TR2, and TR4 were also widely expressed, although at more moderate levels. In contrast, CAR, ERβ, ERRβ and -γ, FXR, PNR, PXR, and SF-1 were expressed at low to undetectable levels in all tissues. A number of NRs showed a marked association with specific types of cancer. HNF4α was almost exclusively found in colon cancer, whereas GR's expression was lowest in this tumor type (Fig. 2A). Similarly, RXRγ was seen only in melanomas, and TRβ was generally excluded from melanomas (Fig. 2B), whereas the expression of other receptors was sporadic across the cell panel (e.g. ERα, PR, MR, TLX, and VDR).
Figure 1.
Heat map representation of mRNA expression levels of the 48 human NRs in a panel of human cancer cell lines (NCI60) as measured by qRT-PCR. Cell lines are grouped according to tissue of origin/cancer type as labeled on rows. Leuk, Leukemia cell lines; Melan, melanomas; NSCL, non-small-cell lung cancer; Prst, prostate cancer lines. The names of the receptors are given across the columns. Dark blue rectangles represent the highest levels of expression, and white rectangles represent low/undetectable expression (cycle time >35). Standard curves were used to quantify signals. The data were normalized to 18S and processed as described in Materials and Methods. Common names and abbreviations for each NR are given in Supplemental Table 3.
Figure 2.
Patterns of mRNA expression across the NCI60 panel for select receptors. A, The HNF4α profile (top) shows almost exclusive expression in colon cancer cell lines as measured by qRT-PCR. In contrast, GR (bottom) is excluded from colon cancers but is otherwise ubiquitously expressed throughout other cancer types. B, The TRβ expression profile (top) shows this receptor to be largely excluded from melanomas. In contrast, the RXRγ (bottom) profile shows exclusive expression in melanomas and reclassifies MDA-MB-435 as a melanoma cell line. The abbreviations used for tissues are as in Fig. 1.
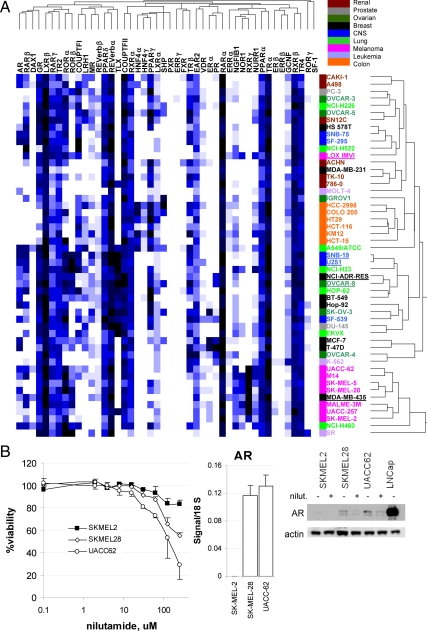
To evaluate whether NR expression profiles could predict cancer type, we performed nonsupervised hierarchical clustering analysis based on centered Pearson correlation coefficients (PCCs) (Fig. 3A). This analysis linked melanomas into a unique group driven mainly by relatively high expression of RXRγ and NOR1 and by relatively low expression of TRβ and COUPTF-I. Similarly, colon cancer formed an independent arm in the clustering tree, characterized by expression of ERRα, HNF4γ, and HNF4α and low levels of GR and RARβ. Renal cancers clustered together in a wider arm, interspersed with other cancer types. In contrast, lung and ovarian cancers were scattered throughout the relational schema. Quite striking was a cluster of tumors moderately expressing TLX, including not only CNS lines but also lung, breast, prostate, and ovarian cancers, which may suggest a stem cell-like or primitive character for these cells (21,22). Thus, NR expression levels can predict cancer type in a subset of cases. Indeed, for melanomas, and probably also for colon cancers, NR expression profiles can even correct past misclassification of cells. This is the case for the MDA-MB-435 cell line, for example, which was classified for decades as a breast cancer and has now been undisputedly shown through genome-wide gene expression analysis, single-nucleotide polymorphism (SNP) studies and biological behavior to actually be a melanoma line (23,24,25). In our expression data of 48 NR, this cell line was clearly predicted to be a melanoma (Fig. 3A). Additionally, NCI-ADR-RES, which recently has been demonstrated to be ovarian and not breast in origin through SNP and karyotypic studies (26), segregates with OVCAR8 in our analysis. NR expression profiles also show tight clustering of CNS lines SNB19 and U251, which have been discovered using genome-wide SNP analysis to be derived from the same patient (27). Taken together, these data define NRs as classifiers of cancer tissue origin for a subgroup of malignancies.
Figure 3.
Receptor-specific patterns of expression help reclassify cancer cells and target cancer growth pharmacologically. A, Hierarchical clustering analysis of NR expression. Using the Matrix 1.2 software, NR expression data across all cell lines studied were subjected to centered, PCC-driven, average linkage analysis. Hierarchical tree schema on the right show cell line clusters, whereas tree schema on the top show receptor clusters. Note color coding of cell lines by cancer of origin. Reclassified or recoded lines by NR expression discussed in the text are underlined. Please refer to the text for details. B, Inhibition of cell viability in AR-positive melanomas by an AR antagonist. Melanomas show heterogeneous expression for AR. SKMEL2, which is AR negative, fails to respond to AR antagonist nilutamide, whereas AR-positive lines SKMEL28 and UCC62 are growth inhibited by nilutamide (left). Representative results of four independent experiments are shown. Error bars represent sd from eight replicate wells. Relative receptor expression levels as measured by qRT-PCR are shown in the middle panel, and protein levels analyzed by Western blotting are shown on the right panel. Note the decrease in AR levels in the AR+ lines after nilutamide treatment.
Our dataset showed some NRs to be sporadically expressed across cancer types and/or within a cancer type, such as AR in melanomas and in lung cancers. To test whether pharmacological manipulation of the activity of heterogeneously expressed receptors can result in differential changes in cell growth depending on the cancer's receptor status, we evaluated the effects of the antiandrogen nilutamide on AR+ vs. AR− melanoma cell growth. SKMEL28 and UACC62 melanomas, which express both AR message and AR protein as seen in the middle and right panels, respectively, of Fig. 3B, were growth inhibited by the AR antagonist, whereas SKMEL2 cells, which do not express AR protein or message, were insensitive to the drug (left panel of Fig. 3B). Nilutamide down-regulated AR protein levels, as has been shown for other AR antagonists (28).
We next examined correlations between NR expression levels and drug sensitivity across the NCI60 cell panel to identify potential interactions between NRs and anticancer compounds. The expression of each receptor across the cell line panel was compared with drug sensitivity data for these lines. This analysis resulted in a number of high-correlation hits for each receptor. Positive correlations, showing associations between high receptor expression level and sensitivity to a drug, and negative correlations, representing associations between high receptor levels and drug resistance, are shown in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). For example, and as expected, we found that ERα expression correlated well with tamoxifen sensitivity (PCC of +0.6). Similarly, sensitivity to 13-cis-retinoic acid and to the steroid difluoroprednisolone showed positive association with ERα levels, as did several other compounds. The connection between ERα and 13-cis-retinoic acid is particularly interesting, because this retinoid has been shown to have greater potency against ERα+ breast cancer cells that maintain ERα levels in response to drug treatment compared with ERα− cells or cells that down-regulate ER (29,30,31).
Through this correlation analysis of receptor levels to drug sensitivity, we also identified receptors whose expression predicts cancer cell response to known anticancer drugs in clinical use/trials (summarized in Table 1). Of particular note is the negative relationship we observed between the levels of PPARα in cancer cells and their response to DNA intercalators such as adriamycin tetrahydropyranyl and daunomycinone (PCC of −0.55). This result is in agreement with the known protective effect of PPARα against doxorubicin-induced apoptosis and cardiac toxicity (32,33,34), providing functional validation for our analysis. Analogously, we found that cells expressing low levels of EAR2, COUPTFII, LRH1, or PR showed significantly higher sensitivity to various microtubule-targeting drugs than cells that expressed higher levels of these receptors. For instance, low levels of COUPTFII were predictive of cancer cell sensitivity to derivatives of vinblastine, colchicines, and taxol (PCCs between −0.6 and −0.5).
Table 1.
Correlation between NR expression and cancer cell sensitivity to clinical anticancer drugs
| Receptor | Anticancer drug | PCC | P value |
|---|---|---|---|
| CoupTFII | 15-tert-butyl-20-deoxyvinblastine | −0.58 | 7.80 × 10−6 |
| Vinblastine | −0.55 | 4.70 × 10−5 | |
| 2,3-Didemethyl colchicine | −0.54 | 4.30 × 10−5 | |
| 3-Dimethyl-3-butyl-thiocolchicine | −0.52 | 1.50 × 10−4 | |
| 7-Epi-10-deacetyltaxol | −0.50 | 2.05 × 10−4 | |
| Bisantrene | −0.51 | 1.60 × 10−4 | |
| Bruceantin | −0.51 | 1.30 × 10−4 | |
| Olivomycin | −0.50 | 1.70 × 10−4 | |
| EAR2 | Taxol derivative NSC608832 | −0.52 | 1.10 × 10−4 |
| PR | Vincristine | −0.56 | 2.00 × 10−5 |
| TMCA | −0.54 | 4.20 × 10−5 | |
| Allocolchicine | −0.50 | 2.20 × 10−4 | |
| Oncodazole | −0.48 | 3.20 × 10−4 | |
| 13-cis-retinoic acid | +0.68 | 3.00 × 10−8 | |
| LRH-1 | Rhizoxin | −0.62 | 1.30 × 10−6 |
| Colchicine 3-desmethyl | −0.59 | 8.40 × 10−5 | |
| PPARα | Daunomycinone | −0.55 | 3.20 × 10−4 |
| Adriamycin tetrahydropyranyl | −0.54 | 3.60 × 10−4 | |
| GCNF | Ecteinascidin | −0.95 | <1.0 × 10−10 |
| ERRβ | Ecteinascidin | −0.91 | <1.0 × 10−10 |
| LXRα | Xanthocillin | −0.52 | 8.00 × 10−5 |
| HNF4γ | Miltefosine | +0.51 | 4.40 × 10−4 |
| PPARγ | Miltefosine | +0.58 | 9.20 × 10−6 |
| FXR | Bleomycin | +0.45 | 8.20 × 10−4 |
| ERα | Tamoxifen | +0.60 | <1.0 × 10−10 |
| 6-α,9-Difluoroprednisolone | +0.53 | 7.30 × 10−5 | |
| 13-cis-retinoic acid | +0.49 | 2.80 × 10−4 | |
| RORα | DAB389-IL-6 | +0.78 | 1.80 × 10−11 |
| TLX | 9-Fluoroprednisolone | +0.56 | 1.60 × 10−5 |
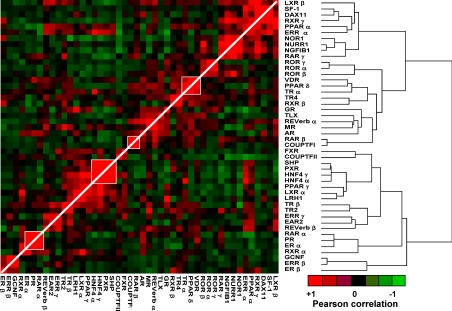
Lastly, we evaluated NR to NR correlations within our qRT-PCR dataset and then correlations between our NR expression data and publicly available microarray gene expression data. A heat map representing receptor-to-receptor correlations derived from the qRT-PCR expression profiles is shown in Fig. 4. Of note was the presence of small clusters of positively associated receptors such as ERα, PR, RARα, and RXRα; SHP, PXR, HNF4γ, and HNF4α; and VDR, PPARα, and TRα. These clusters suggest transcriptional and/or functional interconnections within these receptor subgroups. To examine networks of genes correlated to NRs through coexpression patterns, we also compared the qRT-PCR level of each receptor with available consensus expression data for about 12,000 genes performed across the cell lines on various microarray platforms (see http://dtp.nci.nih.gov/mtargets/release_notes.html for public access). In addition, we analyzed associations between NR levels and the known activity data for some proteins (see the NCI website above). An example of this type of unsupervised analysis using COUPTFII is summarized in Table 2 (the full set of results for all NRs is provided in Supplemental Table 2). There was a strong correlation (PCC = 0.74) between COUPTFII expression in the qRT-PCR and the microarray datasets. Among the top genes whose expression positively correlated with COUPTFII were the corepressor RIP140, the NR coactivator and endothelial differentiation-related factor EDF1, the COUPTFI target gene LAMB1, and the limb developmental gene SHFM1, correlations that appear functionally meaningful given COUPTFII's physiological roles (35,36). Association of COUPTFII with member 6 (FAS) of the TNF receptor superfamily, which correlated by microarray expression (PCC = 0.57) and less strongly by FAS receptor activity measurements (PCC = 0.48) was seen, as well as with other components of TNF signaling such as endothelial TNFAIP1. This potential connection is intriguing, given the reported interactions between COUPTFI and the TNF signaling pathway (37). These observations, along with others shown in Supplemental Table 2, might now be used to generate novel hypotheses and queried directly in functional and mechanistic studies.
Figure 4.
Pairwise PCC analysis of the NRs. Centered, average linkage clustering analysis was performed on a dataset corresponding to pairwise PCC comparisons across receptors and visualized using Eisen Cluster and Tree View software programs. Positive correlations (of 1 for receptor to itself) are depicted in red with the strongest intensities corresponding to higher correlations (0.88 was highest pairwise correlation). Similarly, negative correlations are depicted in green tending toward black as they become less intense. Note that the range of positive correlations (0–0.88) is much wider than that of negative correlations (0–0.3).
Table 2.
Genes and protein activities showing positive correlations with COUPTFII
| PCC | P value | Gene description | Measurement | |
|---|---|---|---|---|
| Gene | ||||
| COUP TF II | 0.74 | 3.7 × 10−10 | Nuclear receptor subfamily 2, group F, member 2 | Microarray |
| WDR40B | 0.65 | 2.4 × 10−7 | WD repeat domain 40B | Microarray |
| KIAA1277 | 0.65 | 2.5 × 10−7 | DENN/MADD domain containing 2A | Microarray |
| RIP140 | 0.62 | 1.0 × 10−6 | Receptor interacting protein 140 | Microarray |
| FAS | 0.57 | 1.0 × 10−5 | Fas (TNF receptor superfamily, member 6) | Microarray |
| LAMB1 | 0.57 | 1.2 × 10−5 | Laminin, β 1 | Microarray |
| LOC387882 | 0.56 | 1.6 × 10−5 | LOC387882 hypothetical protein | Microarray |
| PTHR1 | 0.56 | 1.9 × 10−5 | PTH receptor 1 | Microarray |
| TCF2 | 0.56 | 2.3 × 10−5 | Transcription factor 2, hepatic; LF-B3 | Microarray |
| KIAA0870 | 0.55 | 2.6 × 10−5 | DENN/MADD domain containing 3 | Microarray |
| EDF1 | 0.54 | 4.9 × 10−5 | Endothelial differentiation-related factor 1 | Microarray |
| Protein | ||||
| FAS | 0.48 | 1.2 × 10−3 | Fas (TNF receptor superfamily, member 6) | Activity assay |
Discussion
In this study, we have measured NR expression profiles across 51 human cancer cell lines representing the most prevalent types of malignancies. We demonstrated that melanomas, colon cancers, and to a lesser degree, renal cancers can be identified by their unique NR mRNA expression signature, which may even be used to correct misclassified cell lines. We have also shown that NRs heterogeneously expressed across cancer types may be therapeutic targets for individualized medicine and may have unique roles in the transcriptional deregulation characteristic of tumors. Moreover, we showed the utility of using NR expression profiles to predict cancer drug sensitivity and explore new NR-drug interactions. Finally, the correlations among receptor expression patterns and between NRs and other genes uncovered in this work define novel gene networks that can now be assessed mechanistically. This approach should provide a useful translational tool to mine undefined aspects of NR biology in cancer.
The tight clustering driven by high levels of RXRγ and NOR1 and by relatively low levels of TRβ and COUPTF-I mRNA seen for melanomas cell lines allowed us to confirm that MDA-MB-435, previously thought to be a breast cancer line, is indeed a melanoma cell line. Further inspection of the NR dataset raises the possibility that the LOXIMVI cell line may be misclassified as a melanoma because it fails to cluster within the melanoma arm and expresses relatively high levels of COUPTF-I and no detectable levels of RXRγ (see Fig. 3A). This speculation would need to be validated by an independent method. Our current study established the ability of NR expression measurements to distinguish certain cancers.
Analogous work in lung cancer has recently demonstrated that NR expression can effectively subdivide non-small-cell lung cancers vs. small-cell lung cancers vs. normal bronchial epithelial cells (Jeong, Y., J. D. Minna, and D. J. Mangelsdorf, personal communication), suggesting that NR expression may be generally applicable as a tool to categorize cancers and histological groupings, not seen with random gene sets.
It is of significant interest that several NRs are heterogeneously expressed across cancer types and/or within a cancer. This is the case, for example, for AR in melanomas and lung cancers, for PPARγ in colon and lung cancers, and for RORβ in CNS and other malignancies. These expression profiles may point to a potential role for these receptors in tumorigenic development or progression in which receptor expression is turned on or off in individual cases, reminiscent of ER+ vs. ER− breast tumors. Consistent with this possibility, we found that AR+ but not AR− melanomas were responsive to antiandrogen inhibition, which was accompanied by down-regulation of AR levels, suggesting that AR can play a role in melanoma growth. Similar results have been obtained in an independent study referenced above, in modulating lung cancer growth with AR and PPARγ ligands in cells expressing these receptors. Thus it is reasonable to postulate that NR status could generally lead to individualized therapies even for non-endocrine-derived cancers.
Among the drug-receptor correlations we identified was the one seen between both PPARγ and HNF4α and miltefosine, a known drug used in the treatment of leishmania that has anti-HIV and antineoplastic activity and interferes with CTP:phosphocholine cytidylyl transferase and with phosphatidylinositol 3-kinase signaling (38,39,40,41). Miltefosine is a phospholipid analog, and several reports implicate phospholipids as potential ligands of select NR-binding pockets (42,43,44). Although reporter assays measuring the transcriptional activation of PPARγ showed no direct agonist or antagonist function of miltefosine (data not shown), this drug may engage pathways that overlap with receptor signaling or govern endogenous ligand metabolism. In addition, we found other receptor-cancer drug connections that may have functional significance such as the strong positive correlation between high RORα and RORγ levels (PCC of +0.78 and +0.9, respectively) and sensitivity to DAB389-IL-6, a chimeric toxin containing a fraction of diphtheria toxin upstream of IL-6, known to inhibit the growth of myeloma and sarcoma cells through its interaction with the IL-6 receptor (45,46,47). These novel correlations may have functional significance because the associated compounds may be acting through their correlated NRs, directly as agonists or antagonists or indirectly by modulating pathways that influence or intersect with NR signaling. Overall, these findings demonstrate that NR expression may be used to predict the sensitivity of cancer cells to pharmacological agents, a finding that may have clinical usefulness if validated in tumor samples of patients undergoing therapy.
When analyzing receptor-to-receptor correlations, we observed both receptor clusters, which have also been seen in studies of NR expression in mouse tissues (see for example ERα, PR, and RARα; NOR1, NGF1β, and NURR1; and SHP, PXR, and HNF4α in Ref. 1) as well as unique clusters not previously described. It is possible that the clusters in common with the tissue studies may reflect normal physiology (i.e. the normal regulation of one receptor's transcription by another receptor or the complementary functions of receptor groups within a tissue), whereas the clusters that were not seen in normal mouse tissues but that do come up across the NCI60 panel may represent cancer-derived pathological receptor associations. These latter ones may define cancer-specific receptor networks potentially targetable by combinations of NR ligands, a paradigm that could open up new therapeutic opportunities.
Materials and Methods
Cell lines and RNA extractions
Cancer cells were grown at the NCI under standard, uniform conditions (see http://dtp.nci.nih.gov/branches/btb/ivclsp.html for details). Fifty-one frozen cell pellets corresponding to the available cell lines from the NCI60 panel were received from the NCI under the Molecular Target Measurement program. Cell pellets were then processed for RNA extractions using the QIAGEN (Valencia, CA) RNeasy kit, according to the manufacturer's protocol. Extracted RNA was quantified, aliquoted, and stored at −80 C and used to make the corresponding cDNA with Invitrogen's (Carlsbad, CA) First Strand kit. NCI60 cell lines are routinely tested and authenticated at the NCI cell line repository.
qRT-PCR and data analysis
Analysis of mRNA NR expression was performed in triplicate using the TaqMan-based efficiency-corrected cycle threshold method with 12.5 ng cDNA per reaction for 50 cycles in an ABI 7900HT sequence detection system (Applied Biosystems, Foster City, CA) as described previously (48). NR mRNAs with cycle times of 35 or more were determined to be below detection. Primer concentrations were 75 nm for 18S rRNA and 300 nm for NR primers; probes were added at 250 nm. The sequences of the validated primer/probe sets for the 48 human NRs are available at www.nursa.org under the rapid release tab. Universal cDNA standards generated from human adult RNA (BD Clontech, Palo Alto, CA) were used for analysis of all receptors except CAR, FXRβ, PXR, SHP, DAX-1, ERβ, LRH-1, PNR, SF1, and TLX, which were too limited in expression to use the universal RNA set. For these receptors, commercially available tissue-specific total RNA standards derived from cell lines or adult organ donors were used from liver, ovary, eye, adrenal, and brain, as appropriate. qRT-PCR data were analyzed using ABI instrument software SDS2.1. Baseline values of amplification plots were set automatically, and threshold values were kept constant to obtain normalized cycle times and linear regression data. Because PCR efficiencies for each receptor primer set vary, individual receptor PCR efficiencies were determined to permit receptor-to-receptor comparisons. PCR efficiencies were calculated from the slope of the resulting standard curves as reported previously (1). Normalized mRNA levels are expressed as arbitrary units and were obtained by dividing the averaged, efficiency-corrected values for NR mRNA expression by that for 18S RNA expression for each sample.
Hierarchical clustering
For color coding and heat map generation, the average values resulting from the triplicate RT-PCR data analysis were multiplied by 105 for graphical representation and plotted using Matrix 1.2 software developed by Luc Girard (unpublished) in-house. Two-dimensional unsupervised hierarchical clustering was performed using centered PCCs, and average linkage for receptors that showed detectable expression. The resulting correlation trees were derived and diagrammed using Matrix 1.2 software.
Statistical correlation analysis
PCC-based analysis of the NR expression dataset vs. itself, vs. drug sensitivity data (using 50% growth inhibition values), vs. publicly available microarray expression data [using average consensus data derived from Affymetrix (Santa Clara, CA) U133, U95, and HUM6000 platforms], or vs. molecular targets measured at the DNA, RNA, or protein level under the Molecular Targets Program at NCI (all available at http://dtp.nci.nih.gov/docs/dtp_search.html) were calculated using the COMPARE algorithm (http://dtp.nci.nih.gov/compare). The pairwise cross-receptor comparisons were visualized as heat maps using the Eisen Cluster and Tree View software. Receptor-drug correlation results were sorted using correlation coefficients of 0.5 as the lowest threshold and filtered for statistical significance by P value. Only correlations based on drug sensitivity data for multiple experiments with P values <0.05 are reported. In addition, correlation results were subjected to a filtering schema to distill the most prominent and receptor-specific associations. Finally, manual inspection of drug sensitivity profiles was undertaken to ensure that correlations were not being driven by single outliers.
Viability assays
Cancer cells, growing in RPMI medium supplemented with 10% fetal bovine serum, were split into 96-well plates at 2000 cells per well and allowed to attach overnight. Cells were then treated in eight replicate wells with increasing concentrations of NR ligands and assayed 4 d later for viability using standard MTS reagents from Promega (Madison, WI). ED50 and IC50 values were calculated using the Divisa software, a gift of Dr. Luc Girard. Experiments with charcoal-stripped serum gave the same results. Experiments were carried out a total of four independent times.
Western blots
Western analysis was done as previously described (49) with the following changes: one million cells were plated on 10-cm dishes, treated the next day with 50 μm nilutamide or vehicle, and harvested 24 h later. Twenty micrograms of protein were loaded on a 4–12% sodium dodecyl sulfate gel. An anti-androgen receptor antibody (06-680; Millipore, Bedford, MA) was used to detect androgen receptor in melanoma cell lysates and in untreated LNCap control lysates. An anti-actin antibody was used as a control for loading (Santa Cruz 1616; Santa Cruz Biotechnology, Santa Cruz, CA).
Dataset deposits
Note that the NR expression dataset across the NCI60 panel has been deposited at the NCI Molecular target program site at http://dtp.nci.nih.gov/mtargets/release_notes.html and at the NURSA site at www.nursa.org/10.1621/datasets.04001. Individual receptor expression bar graphs and additional correlation files are available upon request.
Supplementary Material
Acknowledgments
We thank Zimo Zheng, Badri Modi, and Kenneth Spence for technical assistance in processing correlation data. We are grateful to Dr. Monica Spinola for helpful suggestions during manuscript preparation. We are deeply indebted to Dr. Luc Girard for the gift of the Matrix and DIVISA softwares.
Footnotes
This work was supported by the Nuclear Receptor Signaling Atlas (U19DK062434 to D.J.M.), Howard Hughes Medical Institute (to D.J.M.), the Robert A. Welch Foundation (grant I-275 to D.J.M.), the Doctors Cancer Foundation (to E.M.), and the National Cancer Institute (5K22CA118717 to E.M.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 7, 2010
Abbreviations: CNS, Central nervous system; FAS, TNF receptor superfamily member 6; NR, nuclear receptor; PCC, Pearson correlation coefficient; qRT-PCR, quantitative real-time PCR; SNP, single-nucleotide polymorphism.
References
- Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ 2006 Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B 2008 The Year in Basic Science: nuclear receptors and coregulators. Mol Endocrinol 22:2751–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda J, Pei L, Evans RM 2008 Nuclear receptors: decoding metabolic disease. FEBS Lett 582:2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk SP, Knudsen KE 2008 AR, the cell cycle, and prostate cancer. Nucl Recept Signal 6:e001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnetta L, Granata OM, Cocciadiferro L, Saetta A, Polito L, Bronte G, Rizzo S, Campisi I, Agostara B, Carruba G 2004 Sex steroids, carcinogenesis, and cancer progression. Ann NY Acad Sci 1028:233–246 [DOI] [PubMed] [Google Scholar]
- Crescenzi A, Graziano MF, Carosa E, Papini E, Rucci N, Nardi F, Trimboli P, Calvanese A, Jannini EA, D'Armiento M 2003 Localization and expression of thyroid hormone receptors normal and neoplastic human thyroid. J Endocrinol Invest 26:1008–1012 [DOI] [PubMed] [Google Scholar]
- Hartman J, Ström A, Gustafsson JA 2009 Estrogen receptor beta in breast cancer: diagnostic and therapeutic implications. Steroids 74:635–641 [DOI] [PubMed] [Google Scholar]
- Kato Y, Ying H, Willingham MC, Cheng SY 2004 A tumor suppressor role for thyroid hormone β receptor in a mouse model of thyroid carcinogenesis. Endocrinology 145:4430–4438 [DOI] [PubMed] [Google Scholar]
- Persson I 2000 Estrogens in the causation of breast, endometrial and ovarian cancers: evidence and hypotheses from epidemiological findings. J Steroid Biochem Mol Biol 74:357–364 [DOI] [PubMed] [Google Scholar]
- Shao W, Brown M 2004 Advances in estrogen receptor biology: prospects for improvements in targeted breast cancer therapy. Breast Cancer Res 6:39–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross HS, Kállay E, Khorchide M, Lechner D 2003 Regulation of extrarenal synthesis of 1,25-dihydroxyvitamin D3–relevance for colonic cancer prevention and therapy. Mol Aspects Med 24:459–465 [DOI] [PubMed] [Google Scholar]
- Girnun GD, Naseri E, Vafai SB, Qu L, Szwaya JD, Bronson R, Alberta JA, Spiegelman BM 2007 Synergy between PPARγ ligands and platinum-based drugs in cancer. Cancer Cell 11:395–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller E, Sarraf P, Tontonoz P, Evans RM, Martin KJ, Zhang M, Fletcher C, Singer S, Spiegelman BM 1998 Terminal differentiation of human breast cancer through PPARγ. Mol Cell 1:465–470 [DOI] [PubMed] [Google Scholar]
- Mueller E, Smith M, Sarraf P, Kroll T, Aiyer A, Kaufman DS, Oh W, Demetri G, Figg WD, Zhou XP, Eng C, Spiegelman BM, Kantoff PW 2000 Effects of ligand activation of peroxisome proliferator-activated receptor gamma in human prostate cancer. Proc Natl Acad Sci USA 97:10990–10995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwankwo JO, Robbins ME 2001 Peroxisome proliferator-activated receptor- gamma expression in human malignant and normal brain, breast and prostate-derived cells. Prostaglandins Leukot Essent Fatty Acids 64:241–245 [DOI] [PubMed] [Google Scholar]
- Virmani AK, Rathi A, Zöchbauer-Müller S, Sacchi N, Fukuyama Y, Bryant D, Maitra A, Heda S, Fong KM, Thunnissen F, Minna JD, Gazdar AF 2000 Promoter methylation and silencing of the retinoic acid receptor-β gene in lung carcinomas. J Natl Cancer Inst 92:1303–1307 [DOI] [PubMed] [Google Scholar]
- Xu XC, Sozzi G, Lee JS, Lee JJ, Pastorino U, Pilotti S, Kurie JM, Hong WK, Lotan R 1997 Suppression of retinoic acid receptor β in non-small-cell lung cancer in vivo: implications for lung cancer development. J Natl Cancer Inst 89:624–629 [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Goodwin B, Willson TM 2002 The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev 23:687–702 [DOI] [PubMed] [Google Scholar]
- You L 2004 Steroid hormone biotransformation and xenobiotic induction of hepatic steroid metabolizing enzymes. Chem Biol Interact 147:233–246 [DOI] [PubMed] [Google Scholar]
- Sengupta S, Wasylyk B 2004 Physiological and pathological consequences of the interactions of the p53 tumor suppressor with the glucocorticoid, androgen, and estrogen receptors. Ann NY Acad Sci 1024:54–71 [DOI] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM 2008 A role for adult TLX-positive neural stem cells in learning and behaviour. Nature 451:1004–1007 [DOI] [PubMed] [Google Scholar]
- Shi Y, Chichung Lie D, Taupin P, Nakashima K, Ray J, Yu RT, Gage FH, Evans RM 2004 Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature 427:78–83 [DOI] [PubMed] [Google Scholar]
- Ellison G, Klinowska T, Westwood RF, Docter E, French T, Fox JC 2002 Further evidence to support the melanocytic origin of MDA-MB-435. Mol Pathol 55:294–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD 2007 MDA-MB-435 cells are derived from M14 melanoma cells: a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res Treat 104:13–19 [DOI] [PubMed] [Google Scholar]
- Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, Jeffrey SS, Van de Rijn M, Waltham M, Pergamenschikov A, Lee JC, Lashkari D, Shalon D, Myers TG, Weinstein JN, Botstein D, Brown PO 2000 Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet 24:227–235 [DOI] [PubMed] [Google Scholar]
- Liscovitch M, Ravid D 2007 A case study in misidentification of cancer cell lines: MCF-7/AdrR cells (re-designated NCI/ADR-RES) are derived from OVCAR-8 human ovarian carcinoma cells. Cancer Lett 245:350–352 [DOI] [PubMed] [Google Scholar]
- Garraway LA, Widlund HR, Rubin MA, Getz G, Berger AJ, Ramaswamy S, Beroukhim R, Milner DA, Granter SR, Du J, Lee C, Wagner SN, Li C, Golub TR, Rimm DL, Meyerson ML, Fisher DE, Sellers WR 2005 Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 436:117–122 [DOI] [PubMed] [Google Scholar]
- List HJ, Smith CL, Martinez E, Harris VK, Danielsen M, Riegel AT 2000 Effects of antiandrogens on chromatin remodeling and transcription of the integrated mouse mammary tumor virus promoter. Exp Cell Res 260:160–165 [DOI] [PubMed] [Google Scholar]
- Prakash P, Russell RM, Krinsky NI 2001 In vitro inhibition of proliferation of estrogen-dependent and estrogen-independent human breast cancer cells treated with carotenoids or retinoids. J Nutr 131:1574–1580 [DOI] [PubMed] [Google Scholar]
- Toma S, Isnardi L, Raffo P, Dastoli G, De Francisci E, Riccardi L, Palumbo R, Bollag W 1997 Effects of all-trans-retinoic acid and 13-cis-retinoic acid on breast-cancer cell lines: growth inhibition and apoptosis induction. Int J Cancer 70:619–627 [DOI] [PubMed] [Google Scholar]
- Hua S, Kittler R, White KP 2009 Genomic antagonism between retinoic acid and estrogen signaling in breast cancer. Cell 137:1259–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HH, Liu JC, Hong HJ, Lin JW, Chen CH, Cheng TH 23 June 2009 l-Carnitine reduces doxorubicin-induced apoptosis through a prostacyclin-mediated pathway in neonatal rat cardiomyocytes. Int J Cardiol 10.1016/j.i.card.2009.06.010 [DOI] [PubMed] [Google Scholar]
- Lin H, Hou CC, Cheng CF, Chiu TH, Hsu YH, Sue YM, Chen TH, Hou HH, Chao YC, Cheng TH, Chen CH 2007 Peroxisomal proliferator-activated receptor-α protects renal tubular cells from doxorubicin-induced apoptosis. Mol Pharmacol 72:1238–1245 [DOI] [PubMed] [Google Scholar]
- Mitra MS, Donthamsetty S, White B, Latendresse JR, Mehendale HM 2007 Mechanism of protection of moderately diet restricted rats against doxorubicin-induced acute cardiotoxicity. Toxicol Appl Pharmacol 225:90–101 [DOI] [PubMed] [Google Scholar]
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY 2005 Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435:98–104 [DOI] [PubMed] [Google Scholar]
- Lee CT, Li L, Takamoto N, Martin JF, Demayo FJ, Tsai MJ, Tsai SY 2004 The nuclear orphan receptor COUP-TFII is required for limb and skeletal muscle development. Mol Cell Biol 24:10835–10843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LJ, Liu X, Gafken PR, Kioussi C, Leid M 2009 A chicken ovalbumin upstream promoter transcription factor I (COUP-TFI) complex represses expression of the gene encoding tumor necrosis factor α-induced protein 8 (TNFAIP8). J Biol Chem 284:6156–6168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzouz S, Maache M, Garcia RG, Osuna A 2005 Leishmanicidal activity of edelfosine, miltefosine and ilmofosine. Basic Clin Pharmacol Toxicol 96:60–65 [DOI] [PubMed] [Google Scholar]
- Barratt G, Saint-Pierre-Chazalet M, Loiseau PM 2009 Cellular transport and lipid interactions of miltefosine. Curr Drug Metab 10:247–255 [DOI] [PubMed] [Google Scholar]
- Croft SL, Engel J 2006 Miltefosine–discovery of the antileishmanial activity of phospholipid derivatives. Trans R Soc Trop Med Hyg 100(Suppl 1):S4–S8 [DOI] [PubMed] [Google Scholar]
- Santa-Rita RM, Henriques-Pons A, Barbosa HS, de Castro SL 2004 Effect of the lysophospholipid analogues edelfosine, ilmofosine and miltefosine against Leishmania amazonensis. J Antimicrob Chemother 54:704–710 [DOI] [PubMed] [Google Scholar]
- Li Y, Choi M, Cavey G, Daugherty J, Suino K, Kovach A, Bingham NC, Kliewer SA, Xu HE 2005 Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor steroidogenic factor-1. Mol Cell 17:491–502 [DOI] [PubMed] [Google Scholar]
- Ortlund EA, Lee Y, Solomon IH, Hager JM, Safi R, Choi Y, Guan Z, Tripathy A, Raetz CR, McDonnell DP, Moore DD, Redinbo MR 2005 Modulation of human nuclear receptor LRH-1 activity by phospholipids and SHP. Nat Struct Mol Biol 12:357–363 [DOI] [PubMed] [Google Scholar]
- Sablin EP, Blind RD, Krylova IN, Ingraham JG, Cai F, Williams JD, Fletterick RJ, Ingraham HA 2009 Structure of SF-1 bound by different phospholipids: evidence for regulatory ligands. Mol Endocrinol 23:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick DE, Jean LF, Jamal N, Messner HA, Murphy JR, Minden MD 1993 Differential sensitivity of human myeloma cell lines and normal bone marrow colony forming cells to a recombinant diphtheria toxin-interleukin 6 fusion protein. Br J Haematol 85:25–36 [DOI] [PubMed] [Google Scholar]
- Kreitman RJ 2003 Recombinant toxins for the treatment of cancer. Curr Opin Mol Ther 5:44–51 [PubMed] [Google Scholar]
- Masood R, Lunardi-Iskandar Y, Jean LF, Murphy JR, Waters C, Gallo RC, Gill P 1994 Inhibition of AIDS-associated Kaposi's sarcoma cell growth by DAB389-interleukin 6. AIDS Res Hum Retroviruses 10:969–975 [DOI] [PubMed] [Google Scholar]
- Bookout AL, Cummins CL, Mangelsdorf DJ, Pesola JM, Kramer MF 2006 High-throughput real-time quantitative reverse transcription PCR. Curr Protoc Mol Biol Chapter 15:Unit 15.8 [DOI] [PubMed] [Google Scholar]
- Martinez ED, Danielsen M 2002 Loss of androgen receptor transcriptional activity at the G1/S transition. J Biol Chem 277:29719–29729 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.