Abstract
The androgen receptor (AR) mediates the effect of androgens through its transcriptional function during both normal prostate development and in the emergence and progression of prostate cancer. AR is known to assemble coactivator complexes at target promoters to facilitate transcriptional activation in response to androgens. Here we identify the ATP-dependent chromatin remodeling factor chromodomain helicase DNA-binding protein 8 (CHD8) as a novel coregulator of androgen-responsive transcription. We demonstrate that CHD8 directly associates with AR and that CHD8 and AR simultaneously localize to the TMPRSS2 enhancer after androgen treatment. In the LNCaP cell line, reduction of CHD8 levels by small interfering RNA treatment severely diminishes androgen-dependent activation of the TMPRSS2 gene. We demonstrate that the recruitment of AR to the TMPRSS2 promoter in response to androgen treatment requires CHD8. Finally, CHD8 facilitates androgen-stimulated proliferation of LNCaP cells, emphasizing the physiological importance of CHD8. Taken together, we present evidence of a functional role for CHD8 in AR-mediated transcriptional regulation of target genes.
CHD8 and AR physically interact and colocalize at AREs. CHD8 regulates transcription of androgen-responsive genes by mediating AR recruitment to target AREs upon androgen stimulation.
Prostate cancer is currently the most commonly diagnosed form of cancer and the second leading cause of cancer deaths among males in the United States (1). Androgens, such as testosterone and dihydrotestosterone (DHT), regulate transcription via the androgen receptor (AR) and play a critical role in the normal growth and function of the prostate (2). The dysregulation of AR signaling has also been implicated in the development and progression of prostate cancer. An example includes the aberrant regulation of the ETS family of transcription factors, which are the most frequently overexpressed protooncogenes in prostate cancer (3). Overexpression commonly results from gene fusions between the 5′ untranslated region of the androgen-responsive TMPRSS2 gene to members of the ETS family, placing these protooncogenes under the control of TMPRSS2 androgen-responsive elements (AREs) (4).
Over 80% of prostate cancers are androgen dependent at initial diagnosis, and thus most common therapeutic approaches are directed toward androgen ablation or inhibition of AR (5). Initially, these methods prove to be effective in causing the regression of androgen-dependent tumors, thus highlighting the role of AR activity in early prostate tumorigenesis. However, these treatments often ultimately fail due to progression of the prostate cancer to a hormone-refractory state (6). Some of the proposed mechanisms for this transition to an androgen-independent state include the increased expression of AR or its associated factors, mutations of AR that make it responsive to a broader spectrum of ligands, activation of the receptor through alternate pathways, and the altered function of AR coregulators (7,8,9). Studying the association and interplay of AR with its many coregulators is therefore critical for the development of novel and more effective therapies for prostate cancer.
Several AR coactivators have been implicated in prostate cancer, specifically in AR-mediated control of primary prostate cancer tumorigenesis and progression (10). Alterations in levels or function of AR coactivators have also been proposed to contribute to the emergence of the hormone-refractory disease (11). A recurring function for AR coactivators is the catalysis of site-specific modification events, such as histone acetylation or methylation, at target promoters to modify the chromatin structure (12,13). These events are not just limited to covalent modifications of chromatin; ATP-dependent chromatin remodeling enzymes also play a key role in AR-mediated transcriptional activation (14,15,16,17,18,19,20,21,22).
ATP-dependent chromatin remodeling enzymes use energy derived from ATP hydrolysis to mobilize and modulate nucleosomes (23). A characteristic feature of these enzymes is the presence of a central ATPase component referred to as the Snf2 helicase domain. The Snf2 superfamily is then classified into families based upon the presence of other conserved domains (24). One such family is the chromodomain helicase DNA-binding (CHD) family of enzymes. In mammals, this family can be further divided into three subfamilies: CHD1-CHD2, CHD3-CHD5, and CHD6-CHD9 (25). Although the CHD1-2 and CHD3-5 subfamily proteins have been well studied in the context of their chromatin remodeling activity and their functional role in transcriptional regulation, relatively little is known about the CHD6–9 subfamily.
Previous work from our laboratory has shown that CHD8 is an ATP-dependent chromatin remodeling enzyme involved in transcriptional regulation of β-catenin-responsive genes as well as at the Hox locus (26,52). Other studies have linked CHD8 to CTCF-mediated insulator function (27), to RNA polymerase III transcription in association with hStaf (28), to control of p53-mediated apoptosis (29), and to RNA polymerase II-associated transcription of the cyclin E2 gene (30). Thus, CHD8 exhibits a diverse range of functions in transcriptional regulation. A recent report shows that CHD8 is required for optimal estrogen-responsive induction of cyclin E2 (31), raising the possibility that CHD8 may also be involved in transcriptional regulation by other nuclear receptors.
Here we report that in the androgen-dependent prostate cancer cell line LNCaP, endogenous CHD8 associates with AR as determined by coimmunoprecipitations from nuclear extract. We demonstrate that this association is due to a direct physical interaction by coexpressing recombinant proteins in insect cells followed by coimmunoprecipitation experiments. Using chromatin immunoprecipitation (ChIP) experiments, we demonstrate that CHD8 is present at the TMPRSS2 ARE both before and after induction with DHT. Using re-ChIP experiments, we show AR and CHD8 simultaneously localize to the TMPRSS2 ARE after DHT treatment. In androgen-dependent LNCaP cells, reduction of CHD8 levels by small interfering RNA (siRNA) treatment severely diminishes DHT-dependent activation of the TMPRSS2 gene. However, in several androgen-independent cells, reduction of CHD8 did not alter the expression of the TMPRSS2 gene. Thus, CHD8 is required for optimal AR-mediated transcriptional activation of the TMPRSS2 gene in an androgen-dependent context. To investigate the function of CHD8, we performed ChIP experiments for AR under conditions of CHD8 depletion. Using this approach, we found that the recruitment of AR to the TMPRSS2 promoter in response to DHT treatment requires the presence of CHD8. Finally, by using siRNA against CHD8, we demonstrate that CHD8 facilitates proliferation of LNCaP cells in response to treatment with the synthetic androgen R1881. Taken together, we present evidence of a functional role for CHD8 in AR-mediated transcriptional activation of target genes.
Results
Identification of CHD8 as a novel AR interacting protein
To identify novel chromatin-remodeling enzymes that may function as cofactors for AR in the progression of prostate cancer, publicly available gene expression sets were examined using the ONCOMINE database (www.oncomine.org; search gene CHD8) (32). This investigation revealed that CHD8 was found to be up-regulated in several prostate cancer vs. normal tissue data sets (33,34,35,36,37). We have previously identified and characterized CHD8 as an ATP-dependent chromatin remodeling enzyme involved in the regulation of gene transcription (26). Given the fact that many AR coactivators are up-regulated in prostate cancer (22), the finding that CHD8 is up-regulated in several studies suggests that CHD8 may function in the regulation of AR-mediated transcription.
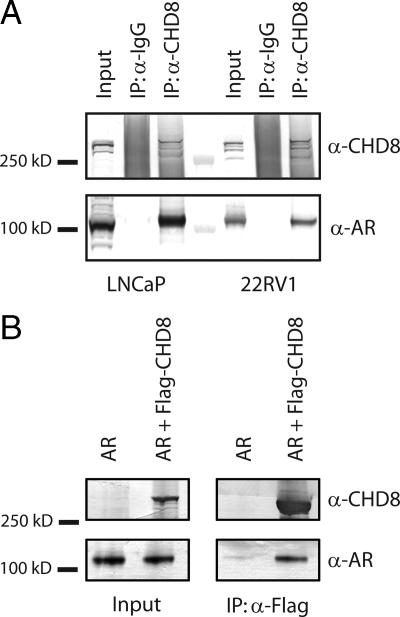
AR coregulators commonly function through a direct interaction between the receptor and the coregulator protein. We therefore examined whether there is a physical association between CHD8 and AR. To test this possibility, coimmunoprecipitations of endogenous proteins were performed from several different prostate cancer cell lines. Nuclear extracts were prepared from the androgen-dependent LNCaP cell line as well as from the androgen-independent cell lines 22RV1, PC-3, and DU-145 (38,39,40,41,42). AR was detected in the LNCaP and 22RV1 nuclear extracts but not in extracts from PC-3 or DU-145 (Supplemental Fig. 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Immunoprecipitations were then performed from the AR-positive extracts using anti-CHD8 polyclonal antibodies. After Western blot analysis with antibodies for CHD8 and AR, it was observed that CHD8 antibodies immunoprecipitate both CHD8 and AR, whereas normal rabbit IgG fails to precipitate any detectable AR or CHD8 (Fig. 1A). These results demonstrate that endogenous AR and CHD8 do indeed interact in these cell lines.
Figure 1.
CHD8 interacts with AR. A, Nuclear extracts were prepared from the indicated cell lines and immunoprecipitated with CHD8 antibodies. After washing, the input and immunoprecipitated samples (IP) were subjected to Western blot analysis using the indicated antibodies. B, Cellular extracts were prepared from SF9 cells after coinfection with the indicated viruses. Immunoprecipitations were performed with anti-Flag antibody-linked M2 agarose beads. Immunoprecipitated samples were subjected to Western blot analysis using the indicated antibodies.
To further investigate the interaction between AR and CHD8, coimmunoprecipitation experiments were performed with recombinant proteins produced in a baculovirus expression system. SF9 cells were infected with either baculovirus encoding AR or coinfected with AR and Flag-CHD8-encoding baculoviruses. After infection, cell extracts were prepared and immunoprecipitation experiments were performed using anti-Flag antibodies. As shown in Fig. 1B, AR was immunoprecipitated with anti-Flag antibodies only in the presence of Flag-CHD8. This result confirms the previously identified interaction of CHD8 and AR and also demonstrates that this association is due to a direct physical interaction between these two proteins.
CHD8 and AR simultaneously bind the TMPRSS2 ARE
TMPRSS2 is a prostate-specific transmembrane serine protease expressed under the control of the AR. The TMPRSS2 locus contains a previously defined enhancer region located approximately 13.5 kb upstream from the start site that contains an ARE that is required for the androgen-responsive expression of this gene (43). In prostate cancer, overexpression of members of the ETS family of protooncogenes commonly results from gene fusions between the 5′ untranslated region of TMPRSS2 to members of the ETS family, placing these protooncogenes under the control of TMPRSS2 AREs (4).
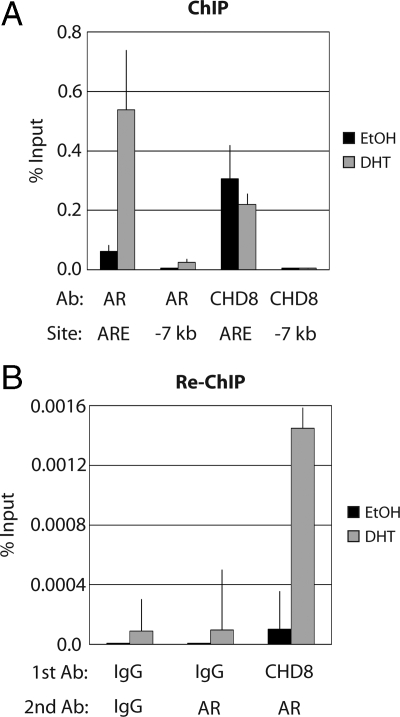
The direct physical interaction between AR and CHD8 suggests that CHD8 may be recruited to endogenous AR target promoters, such as TMPRSS2, to modulate chromatin structure. To examine this hypothesis, ChIP experiments were performed in LNCaP cells because AR is known to regulate androgen-responsive gene expression in these cells. LNCaP cells were either mock treated with ethanol or induced with DHT for 6 h. ChIP experiments were then performed using antibodies against AR, CHD8, or normal rabbit IgG as a control. The immunoprecipitated chromatin was analyzed by quantitative PCR using primers directed against the TMPRSS2 ARE. Upon DHT induction, both AR and CHD8 bound the TMPRSS2 ARE region and not to a control promoter region located 7 kb upstream from the start site (Fig. 2A). As expected, AR was not bound to the target ARE region or the control region without induction by DHT. It was observed that CHD8 localized to the TMPRSS2 ARE region both with and without induction by DHT, indicating that CHD8 may be targeted to the TMPRSS2 ARE in a DHT-independent manner. Similar localization of CHD8 and AR was also observed on the AREs of the prostate-specific antigen (PSA) promoter (Supplemental Fig. 2).
Figure 2.
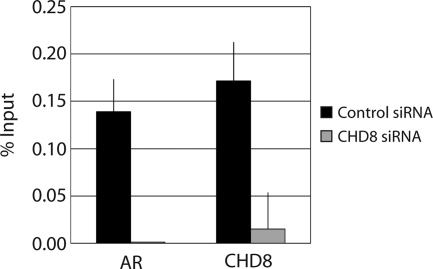
CHD8 and AR colocalize to the TMPRSS2 ARE. A, LNCaP cells were treated with ethanol (EtOH) or 10 nm DHT for 6 h. Chromatin was cross-linked in vivo with formaldehyde. Cells were lysed, and ChIP was performed with the indicated antibodies (Ab). Bound DNA was detected by quantitative PCR using primers to the ARE of TMPRSS2 (ARE) or a control TMPRSS2 promoter region (−7 kb). Control IgG-precipitated samples were less than 0.005% of input and therefore are not shown. Data are representative of multiple experiments. B, LNCaP cells were treated as in A. Re-ChIP experiments were performed by successively immunoprecipitating the cross-linked chromatin with the indicated antibodies. Bound DNA was detected by quantitative PCR using primers to the TMPRSS2 ARE. Data are representative of multiple experiments.
Although these results demonstrate that both CHD8 and AR can bind to the TMPRSS2 ARE in vivo, they do not address whether CHD8 and AR simultaneously occupy this ARE. To test this hypothesis, re-ChIP assays were performed in the LNCaP cell line with and without DHT induction. After cross-linking of the chromatin-protein complexes, a first immunoprecipitation used either CHD8 antibodies or normal rabbit IgG. Upon elution of the bound material, a second immunoprecipitation used either AR antibodies or normal rabbit IgG. Immunoprecipitated chromatin was then analyzed by quantitative PCR using primers directed against the TMPRSS2 ARE. As illustrated in Fig. 2B, AR antibodies precipitated the TMPRSS2 ARE only after prior immunoprecipitation with CHD8 antibodies and only with DHT treatment. This result demonstrates that AR and CHD8 are indeed simultaneously bound to the TMPRSS2 ARE upon DHT induction.
CHD8 activates AR-dependent TMPRSS2 gene expression
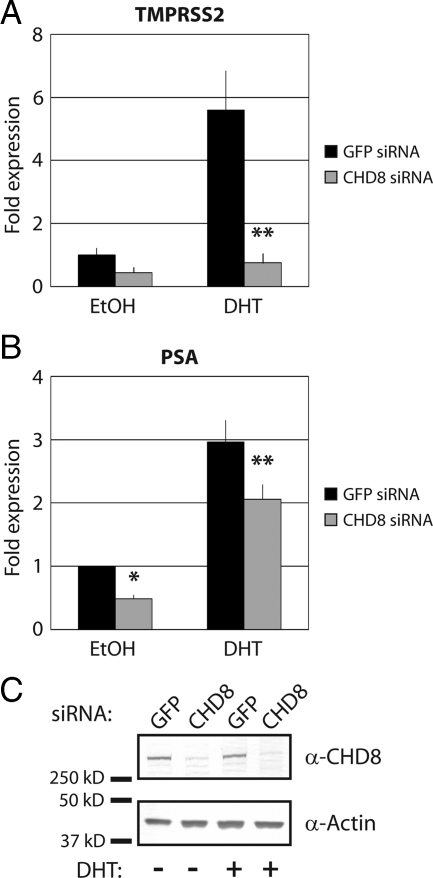
Having established a direct physical interaction between AR and CHD8 and their simultaneous colocalization at the TMPRSS2 ARE, we next examined whether CHD8 has a functional role in the regulation of TMPRSS2 gene expression. This was accomplished by using a vector-based siRNA approach to deplete endogenous CHD8 (44). LNCaP cells were transfected with either a control siRNA construct or with an siRNA construct directed against CHD8. These vectors also coexpress a puromycin resistance marker and therefore allow for the selection of transfected cells. After selection with puromycin for 48 h, the cells were treated with either ethanol or DHT for 6 h, and cDNA was prepared for quantitative PCR analysis of TMPRSS2 expression levels. As expected, in the cells treated with control siRNA, TMPRSS2 expression was induced approximately 6-fold upon DHT treatment (Fig. 3A). In contrast, under conditions of CHD8 depletion, DHT-induced expression of TMPRSS2 was almost completely abrogated (Fig. 3A). CHD8 siRNA treatment resulted in lowered PSA expression in both uninduced and DHT-induced cells. However, PSA expression may still be responsive to hormonal induction (Fig. 3B). The effectiveness of siRNA depletion is shown in Fig. 3C, where we see considerable reduction of CHD8 levels by Western blot analysis. This result demonstrates that CHD8 is required for optimal androgen-induced transcriptional activation of the AR target gene TMPRSS2.
Figure 3.
CHD8 activates AR-mediated transcription of the TMPRSS2 gene. LNCaP cells were transfected with the indicated siRNA constructs. After selection of the transfected cells, cultures were treated with ethanol (EtOH) or 10 nm DHT for 6 h. A and B, Total RNA was isolated, and TMPRSS2 expression (A) or PSA expression (B) was analyzed by quantitative RT-PCR. *, P < 0.05; **, P < 0.01 by Student's t test. C, Efficiency of CHD8 knockdown was determined by Western blot analysis with CHD8 antibodies. Actin is blotted as a loading control.
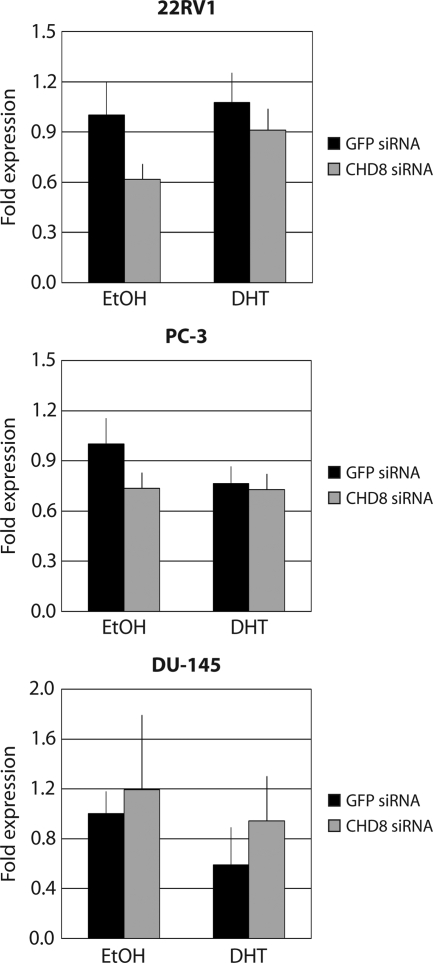
As shown in Fig. 1A, we observed an in vivo interaction between CHD8 and AR not only in the androgen-responsive LNCaP cell line but also in the androgen-independent 22RV1 cell line. This led us to question whether regulation of TMPRSS2 expression by CHD8 is dependent on direct AR interaction and/or in response to androgen stimulation. We tested CHD8 regulation of TMPRSS2 expression in the AR-positive, androgen-independent line 22RV1. We also tested CHD8 regulation of TMPRSS2 expression in the AR-negative, androgen-independent PC-3 and DU-145 cell lines. Expression of TMPRSS2 and PSA is not responsive to androgen treatment in any of these three cell lines either due to the absence of functional AR (PC-3 and DU-145) or due to transition into a hormone-refractory state (22RV1) (45,46,47). As expected in all three of these cell lines, DHT stimulation of TMPRSS2 gene expression was not observed (Fig. 4). Furthermore, upon depletion of CHD8, no significant changes were observed in the pattern of TMPRSS2 gene expression. Similar results were obtained with PSA expression levels in each of these cell lines (Supplemental Fig. 3). Taken together with our previous observation that CHD8 regulates AR-mediated expression in LNCaP cells, these results support the hypothesis that CHD8 plays an important role in androgen-responsive transcriptional activation.
Figure 4.
CHD8 activates TMPRSS2 in an androgen-dependent context. The indicated androgen-independent cell lines were transfected with the specified siRNA constructs. After selection of the transfected cells, cultures were treated with ethanol (EtOH) or 10 nm DHT for 6 h. Total RNA was isolated, and TMPRSS2 expression was analyzed by quantitative RT-PCR.
CHD8 is required for optimal binding of AR to the TMPRSS2 ARE
A possible function for CHD8 in androgen-dependent TMPRSS2 transcriptional activation could be that CHD8 plays a role in modulating the chromatin structure to enhance AR binding to target sites. To test this hypothesis, we examined the effects of CHD8 depletion on the localization of AR at the TMPRSS2 ARE site. As outlined above, endogenous CHD8 was depleted via siRNA. After puromycin selection, cells were treated with DHT for 6 h. ChIP experiments were then performed using antibodies against AR, CHD8, and normal rabbit IgG as a control. Analysis of the immunoprecipitated chromatin by quantitative PCR used primers directed against the ARE of TMPRSS2 (Fig. 5). As expected, depletion of CHD8 results in a significant reduction of CHD8 bound to the TMPRSS2 ARE. In the presence of CHD8, AR was appropriately recruited to the TMPRSS2 ARE. However, upon depletion of CHD8, the binding of AR to the ARE of TMPRSS2 was severely abrogated. This result indicates that CHD8 is required for the proper binding of AR to target sites in the TMPRSS2 ARE. Because CHD8 is present at these target promoters even before DHT-induced AR recruitment, it is possible that the chromatin-remodeling activity of CHD8 may be required to allow AR to bind the TMPRSS2 ARE upon androgen induction.
Figure 5.
CHD8 is required for optimal androgen-responsive binding of AR to the TMPRSS2 ARE. LNCaP cells were transfected with the indicated siRNA constructs. After selection of the transfected cells, cultures were treated with ethanol or 10 nm DHT for 6 h. Chromatin was cross-linked in vivo with formaldehyde. Cells were lysed, and ChIP was performed with the indicated antibodies. Bound DNA was detected by quantitative PCR using primers to the TMPRSS2 ARE. Control IgG-precipitated samples were less than 0.005% of input and therefore are not shown. Shown is a typical result from multiple experiments.
CHD8 is required for androgen-induced cell proliferation
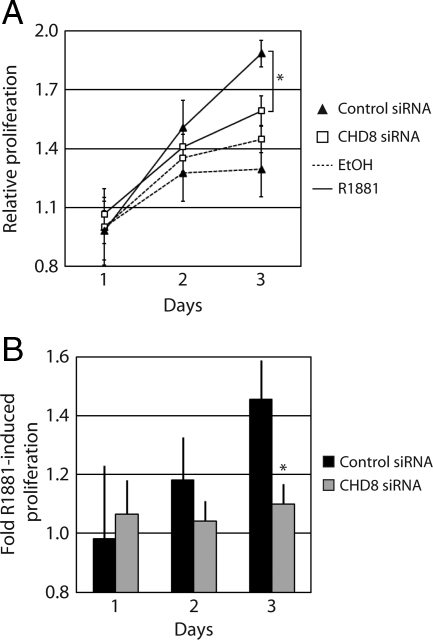
Our results indicate that CHD8 is required for the optimal transcriptional activation of the TMPRSS2 gene upon induction by DHT and that CHD8 is required for the appropriate androgen-responsive recruitment of AR to target promoters. Therefore, it is likely that CHD8 plays an important role in androgen-dependent cell growth of LNCaP cells. Proliferation assays were conducted in LNCaP cells transfected with either control or CHD8-targeting siRNA constructs. After selection with puromycin, cells were either induced with 4 nm of the synthetic androgen R1881 or mock treated with ethanol. Although R1881 treatment of the control LNCaP cells results in a marked increase in proliferation, R1881-induced proliferation is strongly inhibited by CHD8 depletion (Fig. 6). Thus, it appears that CHD8 is indeed required for androgen-induced cell proliferation of LNCaP cells.
Figure 6.
CHD8 regulates androgen-dependent cell proliferation. LNCaP cells were transfected with the indicated siRNA constructs. After selection of the transfected cells, cultures were treated with ethanol (EtOH) or 4 nm R1881. At the indicated time points, proliferation was determined using a luminescent-based assay of metabolically active cells. A, Data are expressed as relative proliferation, which is calculated as the luminescent signal for each condition normalized to the uninduced sample at d 1. B, Data are expressed as fold R1881-induced proliferation, which is calculated as the ratio of the luminescent signals from the induced to the uninduced cells for each siRNA treatment at each indicated time point. *, P < 0.02 by Student's t test.
Discussion
Analysis of publicly available microarray data sets comparing the expression profiles of normal prostate tissue to prostate tumors led to the discovery that CHD8 is up-regulated in prostate cancer. Because several AR coactivators also exhibit similar up-regulation in prostate cancer and many of these coactivators are known chromatin-remodeling enzymes (48), we hypothesized that CHD8 also functions as a coactivator for AR via the modulation of chromatin structure. In support of this hypothesis, we have demonstrated a direct interaction both in vivo and in vitro between CHD8 and AR. Furthermore, we have shown that CHD8 is localized simultaneously with AR to the TMPRSS2 ARE. However, CHD8 is unique from many other AR coactivators in that it is localized to the TMPRSS2 ARE before androgen stimulation. In conjunction with the localization of CHD8 to the TMPRSS2 ARE, we demonstrate that CHD8 is required for optimal DHT-induced transcriptional activation of the TMPRSS2 gene. Mechanistically, it appears CHD8 may be functioning in enhancing or stabilizing the interaction of AR with chromatinized targets because depletion of CHD8 results in loss of AR bound to the TMPRSS2 ARE. Finally, the physiological importance of CHD8 in the regulation of R1881-induced cell proliferation is also demonstrated, because androgen-induced proliferation of LNCaP cells is strongly inhibited by CHD8 depletion.
The role of CHD8 in nuclear hormone signaling is additionally supported by other lines of evidence. CHD8 is a member of the highly related CHD6-9 subfamily of proteins. Several members of this family have been shown to functionally associate with nuclear hormone receptors. CHD9 (CReMM/PRIC320) has been shown to interact with peroxisome proliferator-activated receptor (PPAR)-α, constitutive androstane receptor, estrogen receptor-α, retinoid X receptor, and glucocorticoid receptor and has also been shown to function as a coactivator for PPARα (49,50). CHD7 has also been isolated as a component of a corepressor complex that inhibits PPARγ-mediated transcription (51). Recently, CHD8 has also been reported to be required for the estrogen-mediated up-regulation of the cyclin E2 gene (31). Taken together with our studies on CHD8 and AR, these results suggest that the CHD6-9 family is an important regulator of nuclear hormone signaling.
Deciphering the mechanistic role of CHD8 in transcriptional regulation is complicated by the numerous functions reported for CHD8. Previous work from our group has shown that CHD8 is an ATP-dependent chromatin-remodeling enzyme involved in transcriptional regulation of β-catenin-responsive genes (26). However, CHD8 was found to act in the negative regulation of activated β-catenin-responsive genes, unlike our current report of a role for CHD8 in the activation of TMPRSS2 in response to androgens. This suggests that CHD8 can differentially regulate numerous target genes. Indeed, expression profiling of control and CHD8-depleted cells identified transcripts both positively and negatively regulated by CHD8 (30).
Further insight into the function of CHD8 can be found by examining the reported functions of Kismet, the Drosophila ortholog of CHD8. Kismet was originally identified as an extragenic suppressor of Polycomb and therefore assigned as a member of the trxG of activators (53). Further studies revealed that Kismet assists in an early step in transcriptional elongation (54,55). These reported data are consistent with CHD8 regulating the cyclin E2 gene via interactions with the elongating polymerase (30). AR plays a role in not only transcriptional initiation but also transcriptional elongation. AR has been reported to interact with COBRA1 (NELF-B), a subunit of negative elongation factor (NELF), and depletion of endogenous NELF-B enhances DHT-mediated transcriptional activation (56). AR also interacts with the positive elongation factor P-TEFb, and this interaction serves to enhance transcriptional elongation (57). In addition, AR has been shown to regulate transcriptional initiation as well as elongation via interactions with the general transcription factors TFIIF and TFIIH (58,59). Taken together with our current studies, these reports suggest CHD8 could possibly be regulating AR-mediated transcription by modulating transcriptional elongation.
In this study, we have identified the binding of CHD8 to the TMPRSS2 enhancer region located approximately 13.5 kb upstream from the start site. These data initially seem to be at odds with the model predicted above. However, the investigation of various nuclear receptors binding to DNA at both proximal and distal sites accompanied by reports of RNA polymerase II localization to these sites suggests that enhancer/promoter looping may play an important role in the regulation of nuclear hormone-regulated transcription (60). Indeed, a direct interaction is reported between the TMPRSS2 −13.5-kb enhancer and the promoter region (43). Therefore, the recruitment of CHD8 to the TMPRSS2 distal enhancer does not preclude CHD8 functioning in transcriptional elongation. More experiments need to be performed to determine the precise point of action for CHD8 in the transcriptional cycle.
In summary, the activity of AR is critical for normal prostate development and function but also plays a major role in the development and progression of prostate cancer. Understanding the mechanisms of transcriptional regulation by AR and AR-associated cofactors is critical to the development of new therapies for prostate cancer. Here we report the characterization of a novel AR-associated cofactor required for the proper regulation of the androgen-responsive gene TMPRSS2. Our results present CHD8 as a novel diagnostic, preventative, or therapeutic target in prostate cancer.
Materials and Methods
Cell culture
LNCaP, 22RV1, PC-3, and DU-145 cell lines were obtained from the American Type Culture Collection (Manassas, VA). Cell lines were maintained in RPMI medium 1640 (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS) (Hyclone, Logan, UT) and 1× penicillin-streptomycin-glutamine (Invitrogen) at 37 C in 5% CO2. Twenty-four hours before androgen stimulation, cells were switched to phenol red-free RPMI medium 1640 (Invitrogen) supplemented with 10% dextran/charcoal-stripped FBS (Hyclone) and 1× penicillin-streptomycin-glutamine. DHT was dissolved in ethanol and used at a final concentration of 10 nm. R1881 was dissolved in ethanol and used at a final concentration of 4 nm. SF9 insect cells were cultured at 25 C in 1× Grace's insect medium (Invitrogen) containing 10% FBS and 1× penicillin-streptomycin-glutamine.
Antibodies
Rabbit polyclonal antibodies raised against CHD8 were previously described (26). Rabbit polyclonal (N-20) and mouse monoclonal AR antibodies for Western blotting were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and from BD Pharmingen (San Diego, CA), respectively. Mouse monoclonal (441) AR antibodies for ChIP were obtained from Santa Cruz Biotechnology. Normal rabbit IgG and actin control antibodies were purchased from Sigma Chemical Co. (St. Louis, MO). Antirabbit IgG conjugated to alkaline phosphatase was purchased from Promega (Madison, WI).
Recombinant protein production
Recombinant baculoviruses were used to express AR and CHD8 in SF9 insect cells. The AR baculovirus was a kind gift from J. T. Dalton (Ohio State University, Columbus, OH). The Flag-CHD8 baculovirus was previously described (26). For protein interaction studies, 5 × 106 SF9 cells were coinfected with 1 ml each of AR and Flag-CHD8 baculovirus and incubated at 25 C for 2 d. Cells were then harvested by centrifugation at 500 × g for 2 min, washed with cold PBS, and suspended in 500 μl IP buffer [20 mm Tris-HCl (pH 7.9), 0.2 mm EDTA, 10% glycerol, 0.2 mm phenylmethylsulfonyl fluoride] with 150 mm KCl and 0.1% Nonidet P-40 (NP-40). Cell lysates were cleared by centrifugation at 20,800 × g for 10 min at 4 C and used for protein interaction studies as described below.
Protein interaction studies
For the in vivo interaction studies between endogenous CHD8 and AR, nuclear extracts were prepared from the indicated cell line as described by Dignam et al. (61). Nuclear extracts were incubated overnight at 4 C with the specified antibody bound to protein A-agarose beads (Repligen, Waltham, MA). Beads were then washed sequentially with 150 mm KCl in IP buffer containing 0.2% NP-40, 350 mm KCl in IP buffer, 500 mm KCl in IP buffer, 750 mm KCl in IP buffer (three times), 150 mm KCl in IP buffer containing 0.2% NP-40 (twice), and finally in 150 mm KCl in IP buffer. Samples were eluted in sodium dodecyl sulfate (SDS)-loading buffer [125 mm Tris-HCl (pH 6.8), 0.5% SDS, 10% glycerol, 175 mm β-mercaptoethanol] and subjected to SDS-PAGE followed by Western blot analysis with the indicated antibodies. For the interaction studies between recombinant CHD8 and AR, the cleared SF9 cell lysates described above were incubated overnight at 4 C with anti-Flag M2 agarose beads (Sigma). Beads were washed sequentially with 150 mm KCl in IP buffer, 150 mm KCl in IP buffer with 0.1% NP-40, and 150 mm KCl in IP buffer before eluting as described above. Samples were then subjected to SDS-PAGE and Western blot analysis with the indicated antibodies.
ChIP
ChIP experiments were performed essentially as described in the ChIP assay kit (Upstate Biotechnology, Lake Placid, NY). Briefly, approximately 1 × 106 cells per immunoprecipitation were fixed with 2.5% formaldehyde for 10 min at 37 C. Cells were washed with cold PBS and lysed with ChIP lysis buffer. The chromatin was sheared by sonication (∼200- to 1000-bp fragments) and cleared by centrifugation at 20,000 × g for 10 min at 4 C. Samples were diluted 10-fold in ChIP dilution buffer and then precleared with protein A agarose beads (Repligen) blocked with salmon sperm DNA (Invitrogen). Samples were incubated overnight at 4 C with the indicated antibody. Chromatin-antibody complexes were precipitated by incubation with protein A agarose beads blocked with salmon sperm DNA. Samples were then washed and eluted as described in the instructions, except washes were done for 15 min each. For re-ChIP experiments, the first ChIP was done as described above except the washed chromatin-antibody complexes were eluted in 50 μl Tris-EDTA with 10 mm dithiothreitol. Samples were then diluted 20-fold in re-ChIP buffer [150 mm NaCl, 0.5 mm dithiothreitol, 1% Triton X-100, 20 mm Tris-HCl (pH 8.1), 2 mm EDTA] and immunoprecipitated with the second antibody, washed, and eluted as per the standard ChIP protocol.
RT-PCR and quantitative PCR
cDNA was prepared by extracting total RNA from the indicated cells using the RNeasy kit (QIAGEN, Valencia, CA) following the manufacturer's instructions. Reverse transcription reactions employed total RNA, random decamers (Ambion, Austin, TX), and Superscript II reverse transcriptase (Invitrogen) following the manufacturers' instructions. Real-time quantitative analysis employed the indicated primers, iQ SYBR Green Supermix (Bio-Rad, Hercules, CA), and a MyiQ single-color real-time PCR detection system (Bio-Rad). All real-time PCR were performed in triplicate. For RNA expression analysis, threshold cycle values were normalized to levels of RNA polymerase III-transcribed human H1 RNA. For ChIP experiments, DNA quantities were expressed relative to input levels. The following primers were used for ChIP experiments: TMPRSS2 −13.5 kb, TGGTCCTGGATGATAAAAAAAGTTT and GACATACGCCCCACAACAGA, and TMPRSS2 −7 kb, ACGCCTTCGCTGTCCTACCT and TGCAATGAAGTTCCCTGCAA. The following primers were used for quantitative RT-PCR analysis: TMPRSS2, GGACAGTGTGCACCTCAAAGA and TTGCTGCCCATGAACTTCC, and H1 control, ACTCCACTCCCATGTCCCTTG and CCGTTCTCTGGGAACTCACCT.
RNA interference knockdown experiments
RNA interference experiments in the various prostate cancer cell lines used the UI2-Puro-SIBR siRNA vectors (44). CHD8 was knocked down using two siRNA cassettes cloned into the UI2-Puro-SIBR vector (26). Vectors containing an siRNA cassette targeting GFP were used as a control. Constructs were transfected into cells in either six-well plates (for expression studies) or in 10-cm dishes (for ChIP) using Lipofectamine 2000 (Invitrogen) as described by the manufacturer. Transfections were done in phenol red-free RPMI 1640 medium containing 10% dextran/charcoal-stripped FBS. After 24 h, the cells were selected with 10 μg/ml puromycin and grown for an additional 48 h. Cells were then treated with DHT or ethanol for 6 h. Efficacy of knockdown was verified by Western blot analysis.
Cell proliferation assays
LNCaP cells were transfected and selected as described above. The cells were washed with PBS, trypsinized, and counted. The cells were then plated in 96-well plates at 5 × 103 cells per well and treated with either 4 nm R1881 or vehicle. Cells were harvested at the specified time point, and the cell growth was measured using the CellTiter-Glo luminescent cell viability assay (Promega) following the manufacturer's instructions.
Supplementary Material
Acknowledgments
We thank Drs. J. T. Dalton, J. A. Iniguez-Lluhi, and D. M. Robins for useful reagents and advice. We also thank D. M. Robins and members of the Bochar lab for critical reading of this manuscript.
Footnotes
This work was supported by in part by the National Institutes of Health through the University of Michigan's Cancer Center Support Grant (5 P30 CA46592) and Department of Defense Prostate Cancer Research Program New Investigator Award (PC061052) (to D.A.B.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 22, 2010
Abbreviations: AR, Androgen receptor; ARE, androgen-responsive element; CHD, chromodomain helicase DNA-binding; ChIP, chromatin immunoprecipitation; DHT, dihydrotestosterone; FBS, fetal bovine serum; NP-40, Nonidet P-40; PPAR, peroxisome proliferator-activated receptor; PSA, prostate-specific antigen; SDS, sodium dodecyl sulfate; siRNA, small interfering RNA.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ 2009 Cancer statistics, 2009. CA Cancer J Clin 59:225–249 [DOI] [PubMed] [Google Scholar]
- Dehm SM, Tindall DJ 2006 Molecular regulation of androgen action in prostate cancer. J Cell Biochem 99:333–344 [DOI] [PubMed] [Google Scholar]
- Petrovics G, Liu A, Shaheduzzaman S, Furusato B, Furasato B, Sun C, Chen Y, Nau M, Ravindranath L, Chen Y, Dobi A, Srikantan V, Sesterhenn IA, McLeod DG, Vahey M, Moul JW, Srivastava S 2005 Frequent overexpression of ETS-related gene-1 (ERG1) in prostate cancer transcriptome. Oncogene 24:3847–3852 [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM 2005 Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310:644–648 [DOI] [PubMed] [Google Scholar]
- Denis LJ, Griffiths K 2000 Endocrine treatment in prostate cancer. Semin Surg Oncol 18:52–74 [DOI] [PubMed] [Google Scholar]
- Feldman BJ, Feldman D 2001 The development of androgen-independent prostate cancer. Nat Rev Cancer 1:34–45 [DOI] [PubMed] [Google Scholar]
- Gelmann EP 2002 Molecular biology of the androgen receptor. J Clin Oncol 20:3001–3015 [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C 2004 Androgen receptor in prostate cancer. Endocr Rev 25:276–308 [DOI] [PubMed] [Google Scholar]
- Taplin ME, Balk SP 2004 Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem 91:483–490 [DOI] [PubMed] [Google Scholar]
- Li P, Yu X, Ge K, Melamed J, Roeder RG, Wang Z 2002 Heterogeneous expression and functions of androgen receptor co-factors in primary prostate cancer. Am J Pathol 161:1467–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL 2004 Molecular determinants of resistance to antiandrogen therapy. Nat Med 10:33–39 [DOI] [PubMed] [Google Scholar]
- Xu J, Li Q 2003 Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol 17:1681–1692 [DOI] [PubMed] [Google Scholar]
- Stallcup MR, Kim JH, Teyssier C, Lee YH, Ma H, Chen D 2003 The roles of protein-protein interactions and protein methylation in transcriptional activation by nuclear receptors and their coactivators. J Steroid Biochem Mol Biol 85:139–145 [DOI] [PubMed] [Google Scholar]
- Li J, Fu J, Toumazou C, Yoon HG, Wong J 2006 A role of the amino-terminal (N) and carboxyl-terminal (C) interaction in binding of androgen receptor to chromatin. Mol Endocrinol 20:776–785 [DOI] [PubMed] [Google Scholar]
- Link KA, Burd CJ, Williams E, Marshall T, Rosson G, Henry E, Weissman B, Knudsen KE 2005 BAF57 governs androgen receptor action and androgen-dependent proliferation through SWI/SNF. Mol Cell Biol 25:2200–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZQ, Li J, Sachs LM, Cole PA, Wong J 2003 A role for cofactor-cofactor and cofactor-histone interactions in targeting p300, SWI/SNF and Mediator for transcription. EMBO J 22:2146–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Furukawa T, Giannakopoulos S, Zhou S, King DS, Tanese N 2002 Largest subunits of the human SWI/SNF chromatin-remodeling complex promote transcriptional activation by steroid hormone receptors. J Biol Chem 277:41674–41685 [DOI] [PubMed] [Google Scholar]
- Marshall TW, Link KA, Petre-Draviam CE, Knudsen KE 2003 Differential requirement of SWI/SNF for androgen receptor activity. J Biol Chem 278:30605–30613 [DOI] [PubMed] [Google Scholar]
- Monroy MA, Schott NM, Cox L, Chen JD, Ruh M, Chrivia JC 2003 SNF2-related CBP activator protein (SRCAP) functions as a coactivator of steroid receptor-mediated transcription through synergistic interactions with CARM-1 and GRIP-1. Mol Endocrinol 17:2519–2528 [DOI] [PubMed] [Google Scholar]
- Domanskyi A, Virtanen KT, Palvimo JJ, Jänne OA 2006 Biochemical characterization of androgen receptor-interacting protein 4. Biochem J 393:789–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau N, Domans'kyi A, Reeben M, Moilanen AM, Havas K, Kang Z, Owen-Hughes T, Palvimo JJ, Jänne OA 2002 Novel ATPase of SNF2-like protein family interacts with androgen receptor and modulates androgen-dependent transcription. Mol Biol Cell 13:2106–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitz JH, Tigges M, Baumgärtel K, Khaspekov LG, Lutz B 2004 Dyrk1A potentiates steroid hormone-induced transcription via the chromatin remodeling factor Arip4. Mol Cell Biol 24:5821–5834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker PB, Hörz W 2002 ATP-dependent nucleosome remodeling. Annu Rev Biochem 71:247–273 [DOI] [PubMed] [Google Scholar]
- Smith CL, Peterson CL 2005 ATP-dependent chromatin remodeling. Curr Top Dev Biol 65:115–148 [DOI] [PubMed] [Google Scholar]
- Marfella CG, Imbalzano AN 2007 The Chd family of chromatin remodelers. Mutat Res 618:30–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BA, Tremblay V, Lin G, Bochar DA 2008 CHD8 is an ATP-dependent chromatin remodeling factor that regulates β-catenin target genes. Mol Cell Biol 28:3894–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Oshimura M, Nakao M 2006 CTCF-dependent chromatin insulator is linked to epigenetic remodeling. Mol Cell 23:733–742 [DOI] [PubMed] [Google Scholar]
- Yuan CC, Zhao X, Florens L, Swanson SK, Washburn MP, Hernandez N 2007 CHD8 associates with human Staf and contributes to efficient U6 RNA polymerase III transcription. Mol Cell Biol 27:8729–8738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama M, Oshikawa K, Tsukada Y, Nakagawa T, Iemura S, Natsume T, Fan Y, Kikuchi A, Skoultchi AI, Nakayama KI 2009 CHD8 suppresses p53-mediated apoptosis through histone H1 recruitment during early embryogenesis. Nat Cell Biol 11:172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Paredes M, Ceballos-Chávez M, Esteller M, García-Domínguez M, Reyes JC 2009 The chromatin remodeling factor CHD8 interacts with elongating RNA polymerase II and controls expression of the cyclin E2 gene. Nucleic Acids Res 37:2449–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldon CE, Sergio CM, Schütte J, Boersma MN, Sutherland RL, Carroll JS, Musgrove EA 2009 Estrogen regulation of cyclin E2 requires cyclin D1, but not c-Myc. Mol Cell Biol 29:4623–4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM 2004 ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 6:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaTulippe E, Satagopan J, Smith A, Scher H, Scardino P, Reuter V, Gerald WL 2002 Comprehensive gene expression analysis of prostate cancer reveals distinct transcriptional programs associated with metastatic disease. Cancer Res 62:4499–4506 [PubMed] [Google Scholar]
- Luo JH, Yu YP, Cieply K, Lin F, Deflavia P, Dhir R, Finkelstein S, Michalopoulos G, Becich M 2002 Gene expression analysis of prostate cancers. Mol Carcinog 33:25–35 [DOI] [PubMed] [Google Scholar]
- Wallace TA, Prueitt RL, Yi M, Howe TM, Gillespie JW, Yfantis HG, Stephens RM, Caporaso NE, Loffredo CA, Ambs S 2008 Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res 68:927–936 [DOI] [PubMed] [Google Scholar]
- Welsh JB, Sapinoso LM, Su AI, Kern SG, Wang-Rodriguez J, Moskaluk CA, Frierson Jr HF, Hampton GM 2001 Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res 61:5974–5978 [PubMed] [Google Scholar]
- Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, McDonald C, Thomas R, Dhir R, Finkelstein S, Michalopoulos G, Becich M, Luo JH 2004 Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol 22:2790–2799 [DOI] [PubMed] [Google Scholar]
- Palayoor ST, Youmell MY, Calderwood SK, Coleman CN, Price BD 1999 Constitutive activation of IκB kinase α and NF-κB in prostate cancer cells is inhibited by ibuprofen. Oncogene 18:7389–7394 [DOI] [PubMed] [Google Scholar]
- Kikuchi E, Horiguchi Y, Nakashima J, Kuroda K, Oya M, Ohigashi T, Takahashi N, Shima Y, Umezawa K, Murai M 2003 Suppression of hormone-refractory prostate cancer by a novel nuclear factor κB inhibitor in nude mice. Cancer Res 63:107–110 [PubMed] [Google Scholar]
- Hartel A, Didier A, Pfaffl MW, Meyer HH 2003 Characterisation of gene expression patterns in 22RV1 cells for determination of environmental androgenic/antiandrogenic compounds. J Steroid Biochem Mol Biol 84:231–238 [DOI] [PubMed] [Google Scholar]
- Hartel A, Didier A, Ulbrich SE, Wierer M, Meyer HH 2004 Characterisation of steroid receptor expression in the human prostate carcinoma cell line 22RV1 and quantification of androgen effects on mRNA regulation of prostate-specific genes. J Steroid Biochem Mol Biol 92:187–197 [DOI] [PubMed] [Google Scholar]
- Sramkoski RM, Pretlow 2nd TG, Giaconia JM, Pretlow TP, Schwartz S, Sy MS, Marengo SR, Rhim JS, Zhang D, Jacobberger JW 1999 A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev Biol Anim 35:403–409 [DOI] [PubMed] [Google Scholar]
- Wang Q, Li W, Liu XS, Carroll JS, Jänne OA, Keeton EK, Chinnaiyan AM, Pienta KJ, Brown M 2007 A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell 27:380–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KH, Hart CC, Al-Bassam S, Avery A, Taylor J, Patel PD, Vojtek AB, Turner DL 2006 Polycistronic RNA polymerase II expression vectors for RNA interference based on BIC/miR-155. Nucleic Acids Res 34:e53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JX, Guo L, Revenko AS, Tepper CG, Gemo AT, Kung HJ, Chen HW 2009 Androgen-induced coactivator ANCCA mediates specific androgen receptor signaling in prostate cancer. Cancer Res 69:3339–3346 [DOI] [PubMed] [Google Scholar]
- Agoulnik IU, Vaid A, Bingman 3rd WE, Erdeme H, Frolov A, Smith CL, Ayala G, Ittmann MM, Weigel NL 2005 Role of SRC-1 in the promotion of prostate cancer cell growth and tumor progression. Cancer Res 65:7959–7967 [DOI] [PubMed] [Google Scholar]
- Pignon JC, Koopmansch B, Nolens G, Delacroix L, Waltregny D, Winkler R 2009 Androgen receptor controls EGFR and ERBB2 gene expression at different levels in prostate cancer cell lines. Cancer Res 69:2941–2949 [DOI] [PubMed] [Google Scholar]
- Chmelar R, Buchanan G, Need EF, Tilley W, Greenberg NM 2007 Androgen receptor coregulators and their involvement in the development and progression of prostate cancer. Int J Cancer 120:719–733 [DOI] [PubMed] [Google Scholar]
- Surapureddi S, Viswakarma N, Yu S, Guo D, Rao MS, Reddy JK 2006 PRIC320, a transcription coactivator, isolated from peroxisome proliferator-binding protein complex. Biochem Biophys Res Commun 343:535–543 [DOI] [PubMed] [Google Scholar]
- Marom R, Shur I, Hager GL, Benayahu D 2006 Expression and regulation of CReMM, a chromodomain helicase-DNA-binding (CHD), in marrow stroma derived osteoprogenitors. J Cell Physiol 207:628–635 [DOI] [PubMed] [Google Scholar]
- Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S 2007 A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-γ transactivation. Nat Cell Biol 9:1273–1285 [DOI] [PubMed] [Google Scholar]
- Yates JA, Menon T, Thompson BA, Bochar DA 2010 Regulation of HOXA2 gne expression by the ATP-dependent chromatin remodeling enzyme CHD8. FEBS Lett 584:689–693 [DOI] [PubMed] [Google Scholar]
- Daubresse G, Deuring R, Moore L, Papoulas O, Zakrajsek I, Waldrip WR, Scott MP, Kennison JA, Tamkun JW 1999 The Drosophila kismet gene is related to chromatin-remodeling factors and is required for both segmentation and segment identity. Development 126:1175–1187 [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Armstrong JA, Deuring R, Dahlsveen IK, McNeill H, Tamkun JW 2005 The Drosophila trithorax group protein Kismet facilitates an early step in transcriptional elongation by RNA polymerase II. Development 132:1623–1635 [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Dorighi KM, Tamkun JW 2008 Drosophila Kismet regulates histone H3 lysine 27 methylation and early elongation by RNA polymerase II. PLoS Genet 4:e1000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Blair AL, Aiyar SE, Li R 2007 Cofactor of BRCA1 modulates androgen-dependent transcription and alternative splicing. J Steroid Biochem Mol Biol 107:131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Duan HO, Chang C 2001 Androgen receptor interacts with the positive elongation factor P-TEFb and enhances the efficiency of transcriptional elongation. J Biol Chem 276:9978–9984 [DOI] [PubMed] [Google Scholar]
- Lee DK, Duan HO, Chang C 2000 From androgen receptor to the general transcription factor TFIIH. Identification of cdk activating kinase (CAK) as an androgen receptor NH2-terminal associated coactivator. J Biol Chem 275:9308–9313 [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Ball A, McEwan IJ 2006 The role of the general transcription factor IIF in androgen receptor-dependent transcription. Mol Endocrinol 20:2052–2061 [DOI] [PubMed] [Google Scholar]
- Kininis M, Kraus WL 2008 A global view of transcriptional regulation by nuclear receptors: gene expression, factor localization, and DNA sequence analysis. Nucl Recept Signal 6:e005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG 1983 Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11:1475–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.