Abstract
LH triggers the maturation of the cumulus-oocyte complex (COC), which is followed by ovulation. These ovarian follicular responses to LH are mediated by epidermal growth factor (EGF)-like growth factors produced by granulosa cells and require the participation of oocyte-derived paracrine factors. However, it is not clear how oocytes coordinate with the EGF receptor (EGFR) signaling to achieve COC maturation. The aim of the present study was to test the hypothesis that oocytes promote the expression of EGFR by cumulus cells, thus enabling them to respond to the LH-induced EGF-like peptides. Egfr mRNA and protein expression were dramatically reduced in cumulus cells of mutant mice deficient in the production of the oocyte-derived paracrine factors growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15). Moreover, microsurgical removal of oocytes from wild-type COCs dramatically reduced expression of Egfr mRNA and protein, and these levels were restored by either coculture with oocytes or treatment with recombinant GDF9 or GDF9 plus recombinant BMP15. Blocking Sma- and Mad-related protein (SMAD)2/3 phosphorylation in vitro inhibited Egfr expression in wild-type COCs and in GDF9-treated wild-type cumulus cells, and conditional deletion of Smad2 and Smad3 genes in granulosa cells in vivo resulted in the reduction of Egfr mRNA in cumulus cells. These results indicate that oocytes promote expression of Egfr in cumulus cells, and a SMAD2/3-dependent pathway is involved in this process. At least two oocyte-derived growth factors, GDF9 and BMP15, are required for EGFR expression by cumulus cells.
Oocyte-derived GDF9 and BMP15 promote expression of EGFR by cumulus cells thus enabling maturation in response to EGF-like peptides induced by the preovulatory LH surge.
In healthy Graafian follicles of mammalian ovaries, oocytes are maintained at immature germinal vesicle stage and form a gap junction-mediated syncytium-like structure with surrounding layers of compact cumulus cells, which is termed the “cumulus-oocyte complex” (COC). COCs persist at the immature stage until the preovulatory surge of LH induces them to mature. During COC maturation, oocytes undergo germinal vesicle breakdown and resume meiosis, and its surrounding cumulus oophorus undergoes a process called “expansion.” Expansion involves cumulus cell production of a sticky mucinous extracellular matrix essential for ovulation, fertilization, and the subsequent embryonic development. Failure to undergo these maturational processes causes female infertility (1,2,3,4).
Expression of LH/choriogonadotropin receptors is restricted to theca and mural granulosa cells that line the follicular wall, and murine cumulus cells or oocytes express no detectable LH/choriogonadotropin receptors (5,6,7). Therefore, the mechanism by which LH induces maturation of COCs in intact follicles was a longstanding puzzle. Recently, however, compelling evidence from studies in mice showed that a locally produced epidermal growth factor (EGF) receptor (EGFR) network within the follicle mediates LH-induced COC maturation (8,9). LH induces a rapid and transient expression of three members of the EGF family of growth factors, i.e. amphiregulin (AREG), epiregulin (EREG), and betacellulin (BTC) in mural granulosa cells, which then bind to the common receptor (EGFR) for members of the EGF family in cumulus cells and induce COC maturation (10,11,12). Several lines of evidence indicate that the expression of EGFR in cumulus cells is essential for transducing the EGFR signaling propagated from mural granulosa cells. For example, EGFR ligands, AREG, EREG, BTC, and EGF, stimulated oocyte maturation in cultured COCs but not in denuded oocytes without the surrounding cumulus cells (11,13). Furthermore, in a genetic mouse model carrying a hypomorphic allele of Egfr, Egfrwa2 (waved 2), COC maturation was significantly compromised (10). The defect in COC maturation was further potentiated when a null allele of Areg was placed onto the Egfrwa2 background, creating Areg−/− Egfrwa2/wa2 compound mutants (10). Because AREG binds exclusively to EGFR, the severely impaired maturation of COCs in Areg−/− Egfrwa2/wa2 mice indicates that a functional EGFR network in the follicle, especially the expression of Egfr in cumulus cells, is indispensable for the induction of COC maturation and ovulation in vivo. The same EGFR network appears functioning in other mammalian species studied and plays an indispensable role in mediating LH-induced COC maturation and ovulation (9,14). The key downstream effectors of EGFR signaling in cumulus cells after ligand binding are MAPK3/1 (also commonly known as ERK1/2), and their phosphorylation is essential for COC maturation (15,16,17).
In addition to the requirement of a functional EGFR network in the follicle, the process of cumulus expansion also requires paracrine signals from the oocyte. Removing oocytes from COCs by microsurgery abrogates EGF-induced cumulus expansion, whereas coculture of the resulting oocytectomized (OOX) cumulus cells with fully grown oocytes restores normal cumulus expansion, thus indicating that mouse oocyte-secreted factors enable EGFR-dependent induction of cumulus expansion (18,19). Within the repertoire of mouse oocyte-secreted factors, growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15), two members of the TGFβ superfamily, were found to play crucial roles in the regulation of granulosa development and function including cumulus expansion (1,20). In Gdf9+/−Bmp15−/− (hereafter referred to as double mutant, or DM) mice that are deficient in both GDF9 and BMP15 production, cumulus cells cannot undergo normal expansion in vitro and, when ovulated in vivo, the DM cumuli oophori are very fragile and unstable (21,22). This suggests that normal cumulus expansion requires the full complement of oocyte-derived GDF9 and BMP15. Studies in vitro using recombinant proteins also demonstrated that both GDF9 and BMP15 are crucial for cumulus expansion (17,19,23,24,25,26). The cumulus expansion enabling effect of mouse oocytes and GDF9 appears to be mediated by a Sma- and Mad-related protein (SMAD; MAD homolog, Drosophila) 2/3-dependent pathway (19,27,28).
The requirement of both EGFR signaling generated by mural granulosa cells and paracrine signals emanating from oocytes for LH to induce COC maturation suggests that these two signals, originating from different sources within the follicle, must cooperate. However, it is unclear how and where this cooperation occurs. Previous studies have shown that mouse oocytes enable the downstream response of the cumulus to EGF-like peptide signals communicated by EGFR (18,19,20,27,29). The present study was designed to investigate whether the effect of mouse oocytes to enable the response of the cumulus to EGF-like peptide signals can occur in cumulus cells even before the LH surge. Our data indicate that mouse oocytes program cumulus cells in preparation for receiving the maturation-inducing EGF-like signals propagated by the peripheral follicular compartments after the LH surge. Key to this preparation is GDF9/BMP15 elevation of Egfr mRNA and protein levels in cumulus cells.
Results
Egfr expression in cumulus cells from Bmp15−/− and DM mice
In freshly isolated cumulus cells from equine chorionic gonadotropin (eCG)-primed mice, expression of steady-state levels of Egfr mRNA and protein were significantly lower in both Bmp15−/−and DM mice, as compared with wild-type (WT) mice. As shown in Fig. 1A, Egfr mRNA levels in Bmp15−/− cumulus cells were about 55% less than WT, and a more dramatic difference was observed in DM cells, i.e. about 75% less. EGFR protein levels were similarly less in mutant cumulus cells compared with WT (Fig. 1B). Associated with lower levels of Egfr mRNA and protein in the mutant cumulus cells, levels of phosphorylated SMAD2, a downstream effector of the activated type I receptors (activin receptor-like kinase-4, -5, or -7) for a group of specific TGFβ superfamily ligands (e.g. activins, TGFBs, and GDF9), were also significantly lower in both mutant cumulus cells (Fig. 1B).
Figure 1.
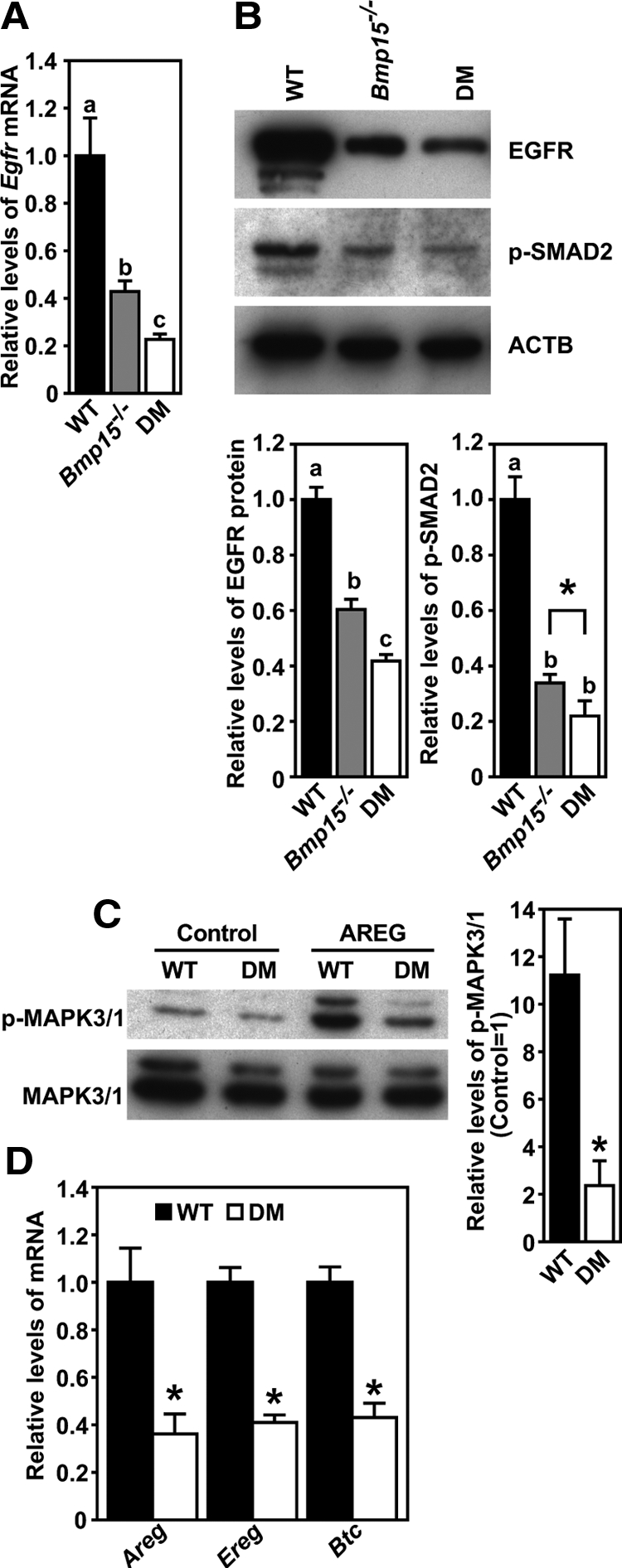
Impaired EGFR signaling in cumulus cells freshly isolated from eCG-primed Bmp15−/− and DM mice. A, Real-time RT-PCR quantification of Egfr mRNA expressed in WT-, Bmp15−/−- and DM cumulus cells. B, Western blot analysis of EGFR, phosphorylated SMAD2 (p-SMAD2), and β-actin (ACTB) in WT-, Bmp15−/−-, and DM cumulus cells. *, P < 0.05, t test. C, EGFR-dependent acute response of WT- and DM-cumulus cells. Freshly isolated WT- and DM-COCs were treated with or without 250 ng/ml AREG for 30 min, indicated as “AREG” and “Control” groups, respectively, and the levels of p-MAPK3/1 and total MAPK3/1 were then assayed. D, Quantification of Areg, Ereg, and Btc mRNA in WT- and DM-cumulus cells 4 h after hCG injection in vivo. Levels of each transcript are presented as relative to WT. In all panels, groups indicated with different letters are significantly different, P < 0.05. In C and D, groups denoted with asterisks are significantly different from the corresponding WT groups, P < 0.05, t test. Note that each Western blot image shown is of the same membrane sequentially immunoblotted for the proteins indicated. This applies to all the following figures.
Coincident with lower levels of Egfr mRNA and protein in DM cumulus cells, the acute response of these cumulus cells to the stimulation of EGFR ligand, AREG, was also dramatically impaired. As shown in Fig. 1C, AREG treatment for 30 min induced about 11-fold up-regulation of the levels of phosphorylated MAPK3/1 (ERK1/2) in WT cumulus cells, whereas in DM cumulus cells, the increase was only about 2-fold. Another biochemical response in cumulus cells upon the activation of EGFR is the up-regulation of the expression of three genes encoding EGF-like growth factors, Areg, Ereg, and Btc (30). The impaired expression of Egfr in mutant cumulus cells suggests that expression of these genes may be also impaired in vivo. To test this hypothesis, we measured the expression levels of Areg, Ereg, and Btc mRNA in cumulus cells of WT and DM after administration of human (h)CG in vivo. Because the most robust up-regulation of these three transcripts in cumulus cells occurred 4 h after hCG injection, which is comparable to the peak levels expressed in mural granulosa cells (see Supplemental Fig. 1 published on The Endocrine Society's Journals web site at http://mend.endojournals.org), expression of these transcripts in cumulus cells was assessed 4 h after hCG treatment. As shown in Fig. 1D, expression levels of Areg, Ereg, and Btc mRNA in DM cumulus cells 4 h after administration of hCG were significantly lower, i.e. only about 40% of that in WT cumulus cells.
Oocytectomy (OOX) reduced Egfr expression in normal WT-COCs
Culture of OOX cumulus cells results in a time-dependent reduction of Egfr mRNA observed as early as 2 h after OOX and most dramatically at 20 h (Fig. 2A). OOX also dramatically reduced levels of EGFR protein (Fig. 2B). Levels of EGFR protein in OOX cumulus cells were reduced about 80% compared with cumulus cells of intact COCs at 20 h culture. Contemporaneous with the reduction in Egfr mRNA and protein levels, OOX also reduced the levels of phosphorylated SMAD2 by more than 80% (Fig. 2B).
Figure 2.
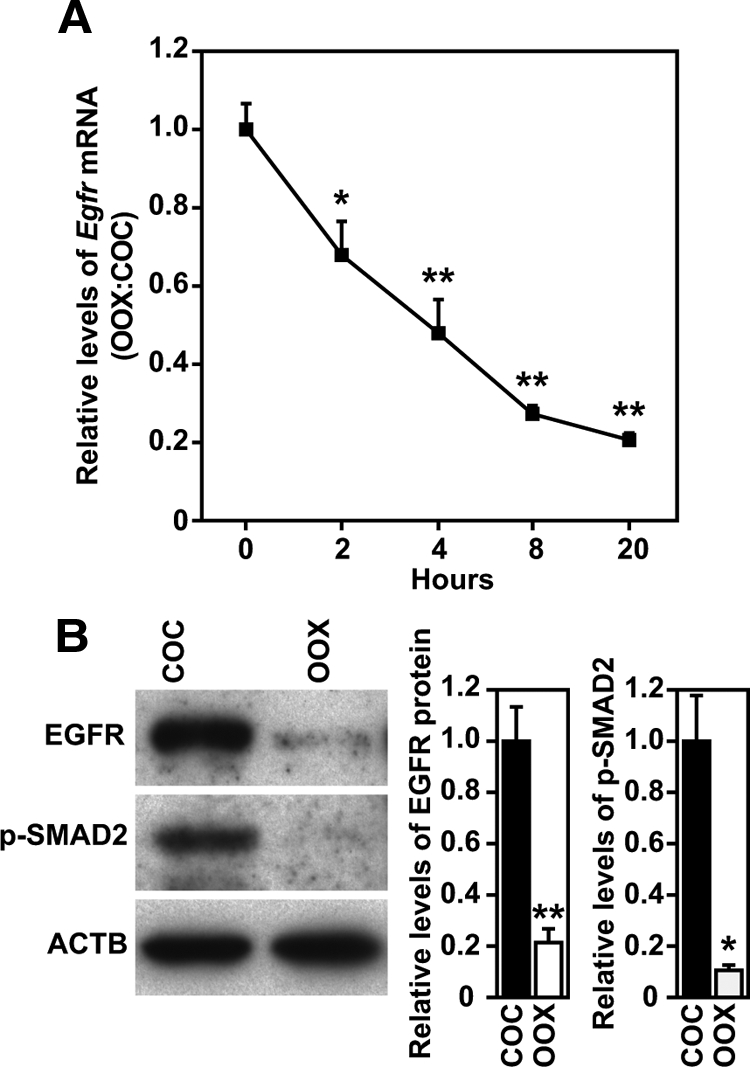
Reduction of Egfr expression and levels of p-SMAD2 in WT OOX-cumulus cells. A, Changes of Egfr mRNA expressed in WT COCs and OOX cumulus cells during 20-h culture period. Levels of Egfr mRNA are presented as the ratio of OOX cumulus cells to COCs. *, P < 0.05; **, P < 0.01, compared with 0-h group by t test. B, Western blot analysis of EGFR, p-SMAD2, and β-actin (ACTB) in WT COCs and OOX cumulus cells 20 h after culture. *, P < 0.05; **, P < 0.01, compared with COC group by t test.
WT, but not DM, oocytes prevented the reduction of Egfr expression in OOX cumulus cells
Coculture of WT-OOX cumulus cells with WT oocytes prevented the reduction of Egfr mRNA (Fig. 3A) and protein (Fig. 3B) but coculture with DM oocytes did not (Fig. 3, A and B). Likewise WT, but not DM, oocytes prevented the decrease in the levels of phosphorylated SMAD2 (Fig. 3B) in WT-OOX cumulus cells. Coincident with the reduction in the levels of both Egfr mRNA and protein in cultured OOX cumulus cells, the acute response of these cultured OOX cumulus cells to AREG treatment was also attenuated. As shown in Fig. 3C, high levels of phosphorylated MAPK3/1 were detected in COCs but not in OOX cumulus cells that were initially cultured for 20 h and then treated with AREG for 30 min. Coculture of OOX cumulus cells with WT- but not DM-oocytes for 20 h significantly improved the levels of phosphorylated MAPK3/1 induced by AREG treatment.
Figure 3.
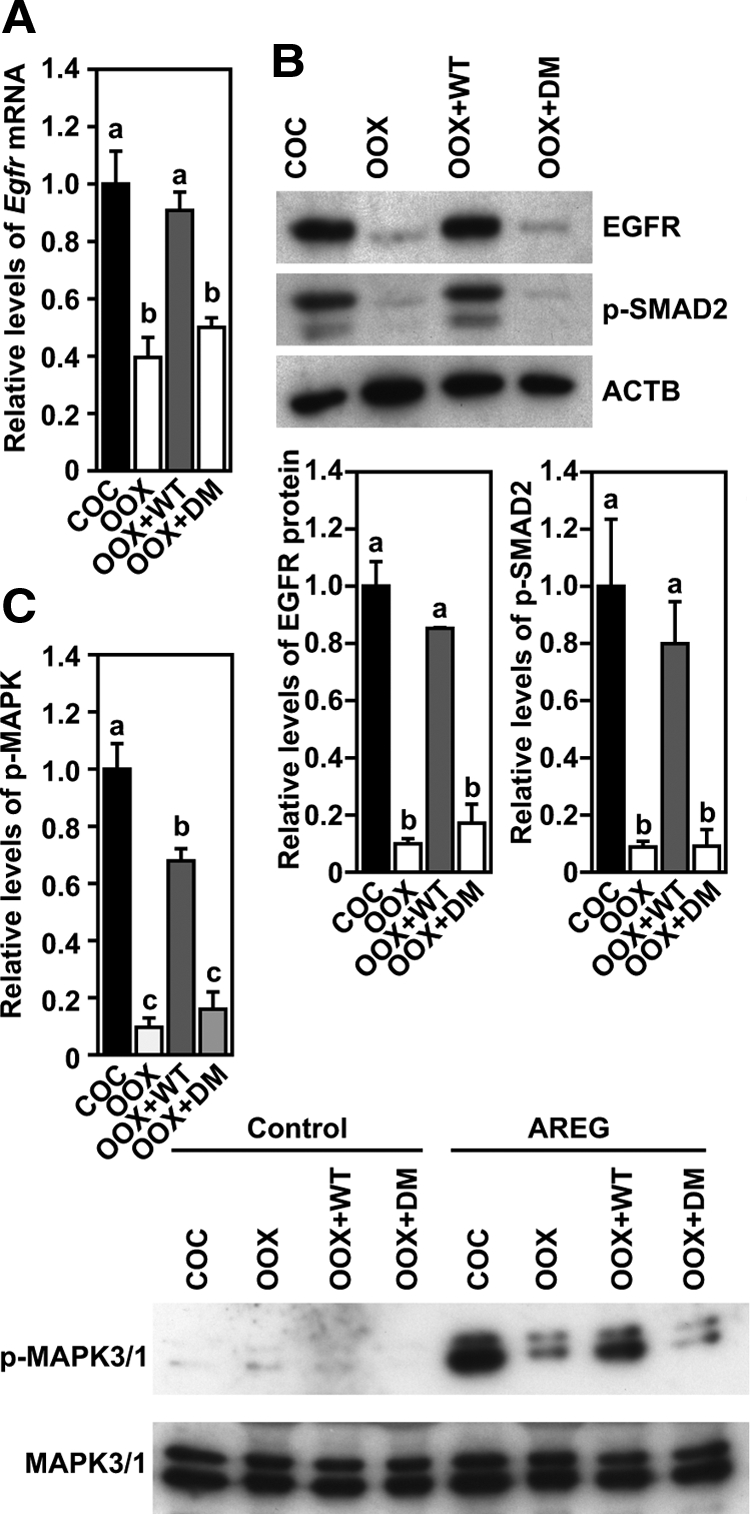
Coculture with WT- but not DM-oocytes promotes EGFR signaling in WT OOX cumulus cells. A, Levels of Egfr mRNA expressed in WT, COCs, OOX cumulus cells (OOX), OOX cumulus cell cocultured with WT Oocytes (OOX+WT), or DM oocytes (OOX+DM), 20 h after culture. B, Western blot analysis of EGFR, p-SMAD2, and β-actin (ACTB) in the same experimental groups as stated in panel A 20 h after culture. C, Changes of the levels of p-MAPK3/1 in the same experimental groups as stated in panel A after the initial 20-h culture and then treated with or without (control) AREG for 30 min. Groups indicated with different letters are significantly different, P < 0.05.
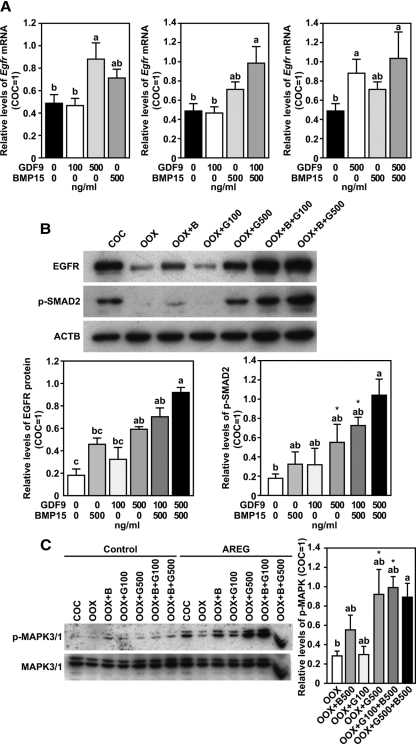
Recombinant GDF9 and BMP15 promoted Egfr expression in OOX cumulus cells
When used alone, 500 ng/ml (32.05 nm) but not 100 ng/ml (6.41 nm) recombinant GDF9 promoted the expression of both Egfr mRNA and protein in cultured OOX cumulus cells (Fig. 4, A and B). Recombinant BMP15, even at a dose of 500 ng/ml (35.97 nm), did not significantly promote the expression of either Egfr mRNA or protein (Fig. 4, A and B). However, 500 ng/ml BMP15, when used together with 100 ng/ml GDF9, did promote the expression of both Egfr mRNA and protein in cultured OOX cumulus cells (Fig. 4, A and B). BMP15 (500 ng/ml), when used together with 500 ng/ml GDF9, did not enhance the expression of Egfr mRNA or protein above that induced by 500 ng/ml GDF9 alone (Fig. 4, A and B). A similar effect of GDF9 and/or BMP15 on the phosphorylation of SMAD2 was observed in cultured OOX cumulus cells (Fig. 4B). Similar changes in the levels of AREG-induced phosphorylation of MAPK3/1 were observed in OOX cumulus cells that were initially treated with GDF9 and/or BMP15 for 20 h (Fig. 4C).
Figure 4.
Recombinant GDF9 and/or BMP15 promote EGFR signaling in WT OOX cumulus cells. A, Egfr mRNA expression in OOX cumulus cells after being treated with GDF9 and/or BMP15 for 20 h. Levels of Egfr mRNA are presented as relative to COCs (COC = 1). B, Western blot analysis of EGFR, p-SMAD2, and β-actin (ACTB) in the same experimental groups as stated in panel A 20 h after culture. C, Changes of the levels of p-MAPK3/1 in the same experimental groups as stated in panel A after the initial 20-h culture and then treated with or without (control) AREG for 30 min. Groups indicated with different letters are significantly different, P < 0.05. *, P < 0.05, compared with OOX group by t test.
Activation of SMAD2/3, but not SMAD3 alone, is required for Egfr expression in cumulus cells
To test whether a SMAD2- and/or 3-dependent pathway participates in the process of oocyte promotion of Egfr expression in cumulus cells, we first examined the effect of specific inhibitors for phosphorylation of SMAD2/3 or SMAD3 on the expression of Egfr in cumulus cells of intact WT-COCs. As shown in Fig. 5, A and B, treatment with 10 μm SMAD2/3 inhibitor SB431542 reduced Egfr mRNA and protein expression in COCs, which was correlated with the diminishment of SMAD2 phosphorylation. However, 20 μm specific inhibitor of SMAD3 (SIS) did not affect expression of Egfr mRNA or protein or levels of phosphorylated SMAD2 in COCs. In contrast to the robust induction of MAPK3/1 activation in COCs initially treated with dimethylsulfoxide (DMSO) or SIS for 20 h, AREG treatment induced barely detectable levels of phosphorylated MAPK3/1 in COCs that were initially treated with 10 μm SB431542 for 20 h (Fig. 5C).
Figure 5.
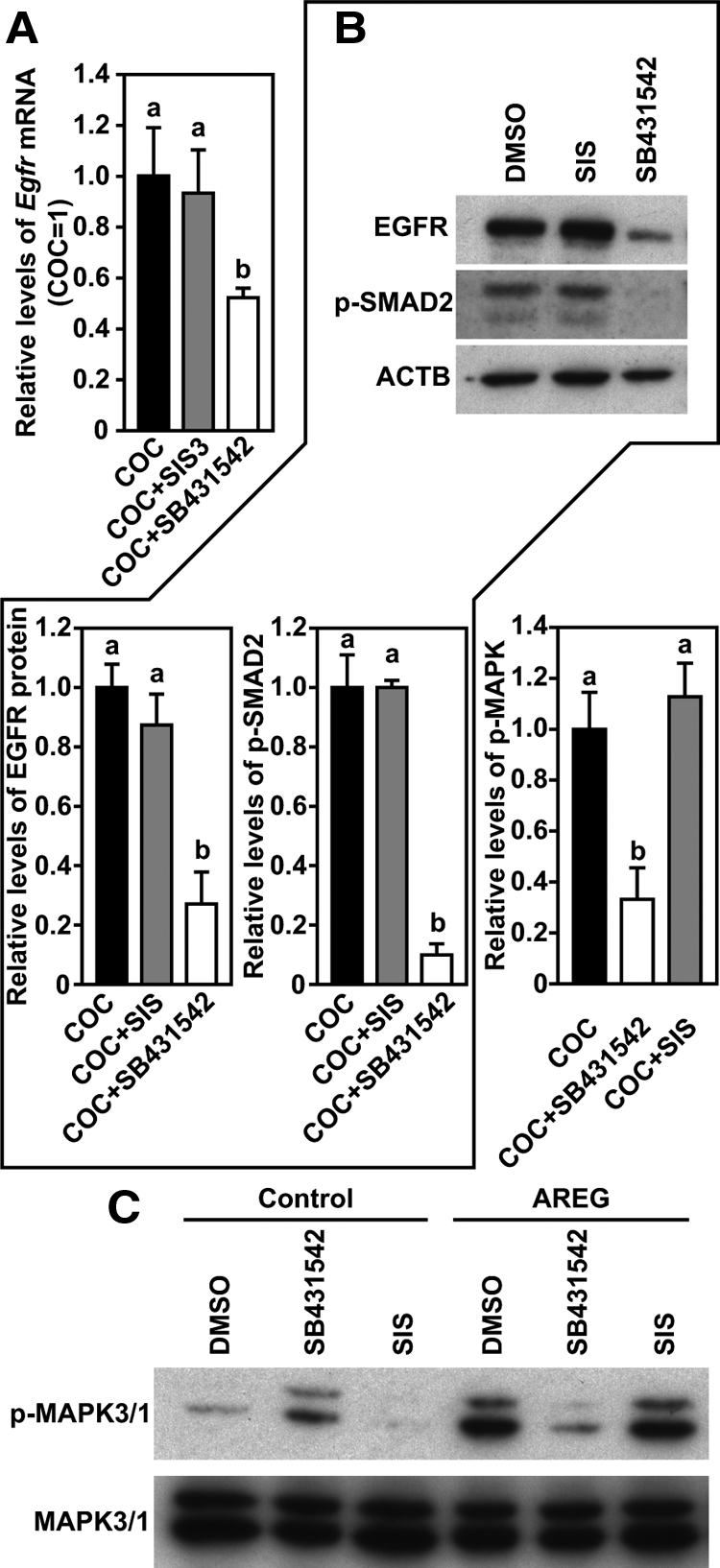
SB431542, but not SIS, inhibited EGFR signaling in WT COCs. A, Egfr mRNA expression in COCs treated with DMSO, SB431542, or SIS for 20 h. B, Western blot analysis of EGFR, p-SMAD2, and β-actin (ACTB) in the same experimental groups as stated in panel A 20 h after culture. C, Changes of the levels of p-MAPK3/1 in the same experimental groups as stated in panel A after the initial 20-h culture and then treated with or without (control) AREG for 30 min. Groups indicated with different letters are significantly different, P < 0.05.
We then studied the effect of the same inhibitors on GDF9-induced Egfr expression in WT OOX cumulus cells. SB431542 (10 μm), but not 20 μm SIS, inhibited GDF9 (500 ng/ml)-induced expression of Egfr mRNA and protein and levels of phosphorylated SMAD2 in OOX cumulus cells (Fig. 6, A and B). Associated with inhibition of GDF9-induced Egfr expression and SMAD2 phosphorylation by 10 μm SB431542 in OOX cumulus cells, the response of OOX cumulus cells to AREG treatment was also impaired. Lower levels of phosphorylated MAPK3/1 were detected in OOX cumulus cells initially treated with SB431542+GDF9 for 20 h and then treated with AREG, as compared with OOX cumulus cells treated with GDF9+DMSO or GDF9+SIS (Fig. 6C).
Figure 6.
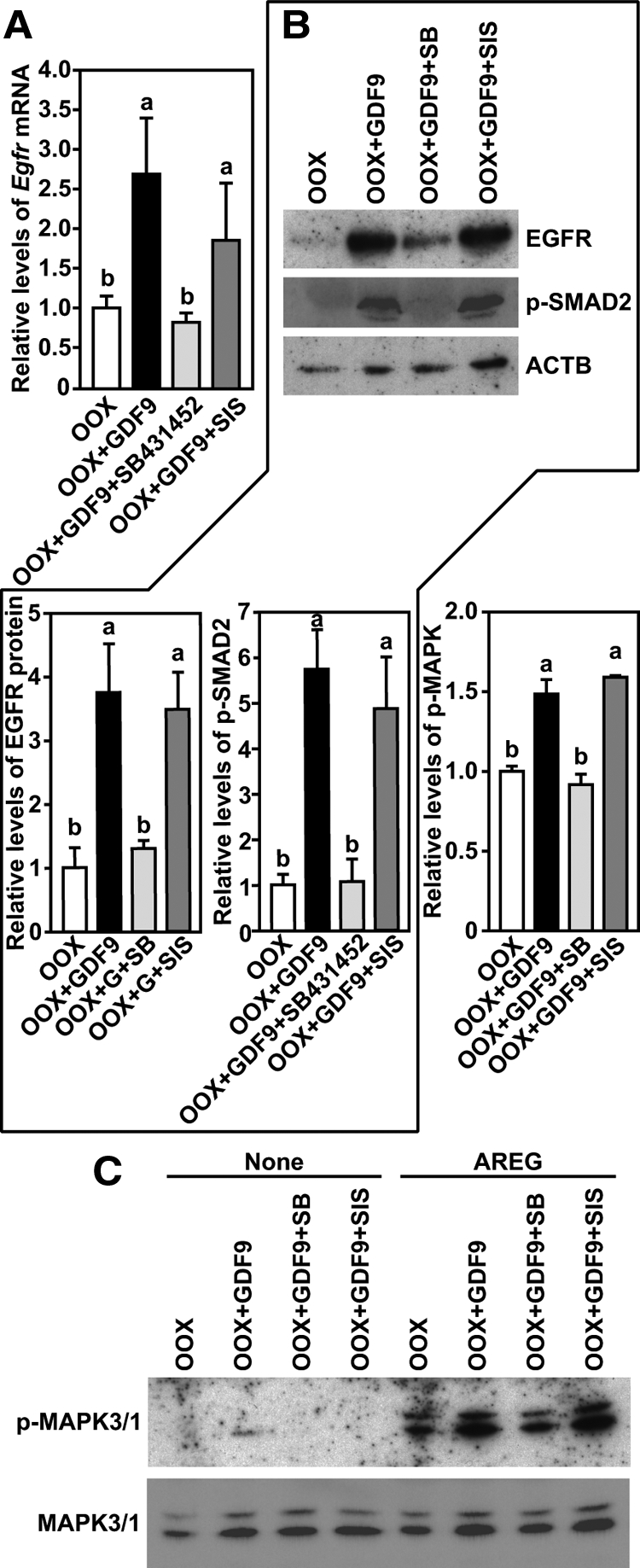
SB431542, but not SIS, inhibited GDF9-induced EGFR signaling in WT OOX cumulus cells. A, Quantification of Egfr mRNA expressed in OOX cumulus cells (OOX), OOX cumulus cells treated with GDF9 (OOX+GDF9), GDF9 and SB431542 (OOX+GDF9+SB), or SIS (OOX+GDF9+SIS) for 20 h. B, Western blot analysis of EGFR, p-SMAD2, and β-actin (ACTB) in the same experimental groups as stated in panel A 20 h after culture. C, Changes of the levels of p-MAPK3/1 in the same experimental groups as stated in panel A after the initial 20-h culture and then treated with or without (control) AREG for 30 min. Groups indicated with different letters are significantly different, P < 0.05.
Finally, we employed the Smad2/3 granulosa cell-conditioned knockout mice to test the role of SMAD2/3 in regulation of Egfr expression in cumulus cells. As indicated in Fig. 7, the steady-state levels of Egfr mRNA in freshly isolated cumulus cells of Smad2/3flox/−Amhr2Cre/+ mice were significantly lower than in WT cumulus cells, with only about 55% of the level in WT cumulus cells.
Figure 7.
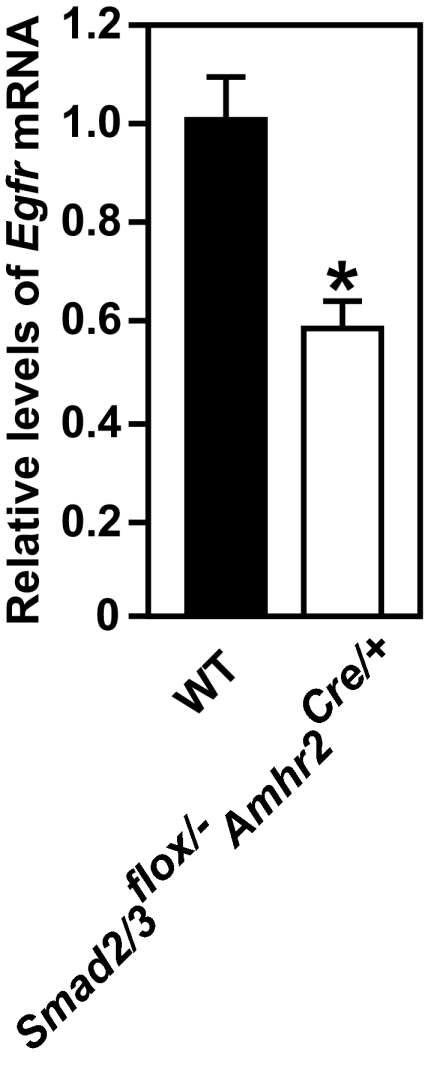
Reduction of Egfr mRNA in freshly isolated cumulus cells from eCG-primed Smad2/3flox/−Amhr2Cre/+ mice. *, P < 0.05, compared with WT group by t test.
Discussion
EGFR expression in cumulus cells is essential for propagation of peripheral LH signaling to COCs and for induction of COC maturation (10,11,12). Moreover, oocyte-derived paracrine factors GDF9 and BMP15 enable the cumulus expansion response to activation of EGFR (18,19). Here we demonstrate that oocytes promote expression of Egfr by cumulus in preparation for receiving EGF-like peptide signals generated by the peripheral mural granulosa cells in response to preovulatory surge of LH. Thus there are two roles for oocytes in enabling the expansion (maturation) of the cumulus oophorus. First, as shown here, oocytes promote the expression of cumulus cell EGFR and, second, oocytes also enable the responses of cumulus cells to EGF-like peptide signals that are downstream of EGFR activation (18,19,20,27,29). Oocyte-derived GDF9 and BMP15 are crucial for both roles.
That microsurgical removal of oocytes from normally developed WT COCs dramatically reduced levels of both Egfr mRNA and protein in cumulus cells indicates that oocytes promote the expression of Egfr in cumulus cells. Coculture of these OOX cumulus cells with fully grown WT oocytes almost completely prevented the decrease of Egfr mRNA and protein in OOX cumulus cells, suggesting that the stimulatory effect of oocytes on cumulus cell Egfr expression is mediated by oocyte-derived paracrine factors. Expression of both Egfr mRNA and protein was significantly reduced in Bmp15−/− and DM cumulus cells, thus indicating that BMP15 and GDF9 are oocyte-secreted paracrine factors participating in the induction of Egfr expression in cumulus cells. Egfr expression was reduced more dramatically in DM cumulus cells than in Bmp15−/− cumulus cells, suggesting that these two growth factors cooperate in the process of regulating Egfr expression in cumulus cells. Because both GDF9 and BMP15 affect the development and function of granulosa cells as early as the preantral stage of follicular development, as evidenced by the defective early follicular development in Gdf9−/− mice and transgenetic mice with oocyte-specific overexpression of the mature form of BMP15 (31,32), it is possible that impaired expression of Egfr in Bmp15−/− and DM cumulus cells could be due to the improper differentiation of cumulus caused by the long-term deficiency in GDF9 and BMP15 production during follicular development. However, we found that recombinant GDF9 alone or GDF9 and BMP15 promoted Egfr expression by WT OOX cumulus cells. In fact, a low dose of GDF9 and BMP15 had a greater effect than either alone, further supporting the genetic data that suggest the cooperation of these two ligands. High-dose GDF9, but not BMP15 alone, promoted expression of Egfr in WT cumulus cells and is probably the major component of oocyte-secreted factors that promote Egfr expression. BMP15, on the other hand, affects cumulus expression of Egfr through cooperation with GDF9. Cooperation between BMP15 and GDF9 has also been reported in the regulation of other activities of granulosa cells, such as proliferation and gonadotropin-induced differentiation, in a variety of mammalian species (33,34,35,36). How BMP15 cooperates with GDF9 is not clear. The cooperative actions of these two growth factors may be modulated through the combination of BMP receptor II and one or more of the type I receptors, namely activin receptor-like kinase 5/7 (33,34). In addition, the proregion of mouse BMP15 (34), as well as the phosphorylation status of both GDF9 and BMP15 (37), may also regulate the cooperative interactions of GDF9 and BMP15.
The SMAD2/3-dependent pathway is one of the two distinct signaling cascades for the TGFβ superfamily. This pathway has been shown to be essential for the proper development and certain types of functions of cumulus cells. For example, conditional knockout of both Smad2 and 3, but not Smad3 or Smad2 alone, in granulosa cells results in the defective expansion of cumulus and the subsequent subfertility (28). In vitro, inhibiting phosphorylation of SMAD2 and 3, but not just SMAD3, prevents mouse oocytes from promoting the expression of cumulus cell marker transcripts (27). Inhibition of SMAD2/3 phosphorylation also abolishes the ability of mouse oocytes and recombinant GDF9 to enable EGF-induced cumulus expansion (19,27). SB431542 (10 μm) completely inhibits phosphorylation of both SMAD2 and -3 in intact mouse COCs and OOX cumulus cells cocultured with oocytes (27). Here, using the same dose of this inhibitor, inhibition of SMAD2/3 phosphorylation dramatically reduced the levels of Egfr mRNA and protein in intact mouse COCs and blocked GDF9-induced Egfr expression in WT OOX cumulus cells. Thus a SMAD2/3-dependent pathway is required for oocytes and GDF9 to promote the expression of Egfr in cumulus cells. In contrast, there was no effect of 20 μm SIS on Egfr expression although this dose of SIS has been shown to effectively inhibit SMAD3 phosphorylation (27). Therefore, activation of SMAD2 is indispensable for promoting the expression of Egfr in cumulus cells and is consistent with studies showing redundant roles of these two SMADs in the regulation of cumulus cell development and function (28). Conditional knockout of Smad2/3 in cumulus resulted in only about a 50% reduction of the normal Egfr mRNA steady-state level. This is probably due to an incomplete depletion of Smad2/3 mRNA expression in cumulus cells (28). The resulting low levels of SMAD2/3 produced in the mutant cumulus cells could be sufficient to sustain the expression of about half of Egfr mRNA. Nevertheless, lower levels of Egfr mRNA in Smad2/3 mutant cumulus cells provide genetic evidence for the participation of the SMAD2/3-dependent pathway in promoting expression of Egfr.
BMP15, as well as other members of the BMP family, such as BMP6, can activate the SMAD1/5/8 pathway (20,38). Nevertheless, the level of phosphorylated SMAD2 was reduced in cumulus cells from Bmp15−/− mice. Given that BMP15 cooperates with GDF9 to elicit some of its effects on granulosa cells (Refs. 33,34,35,36 and present study), and that the cooperation between BMP15 and GDF9 can be abolished by inhibitors of SMAD2/3 phosphorylation (34), it is possible that the reduction of SMAD2 phosphorylation in Bmp15−/− cumulus cells could be due to the loss of cooperativity between BMP15 and GDF9.
We conclude that oocyte-derived paracrine factors, particularly GDF9 and BMP15, program cumulus cells to receive LH-induced EGF-like peptide signals from peripheral mural granulosa cells by promoting Egfr expression in cumulus cells before the LH surge.
Materials and Methods
Mice
Female mice (22 d of age) B6SJLF1, Bmp15−/−, DM, Smad2/3flox/−Amhr2Cre/+, and wild type (WT) on the B6/129 genetic background, were used in experiments. The Administrative Panels on Laboratory Animal Care approved all animal protocols, and all experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Cell isolation and culture
Female mice were primed with 5 IU eCG (EMD Biosciences, Inc., San Diego, CA; Calbiochem, La Jolla, CA) for 48 h to stimulate follicular development. COCs, cumulus cells, and oocytes were then isolated as described previously (25). Microsurgical removal of oocytes from COCs (OOX) was carried out as described previously (18). COCs, OOX cumulus cells, and OOX cumulus cells + oocytes (two oocytes/μl of medium) were cultured in a drop of medium covered with washed mineral oil at a density of 1 COC or OOX cumulus cells/μl of medium in a four-well multi-dish (Nuclon, Rochester, NY). Media and culture conditions were as described previously (25). Cells were cultured for various periods of time according to each experimental design and then collected for RNA or protein extraction.
Cell treatments
SB431542 (Sigma-Aldrich, St. Louis, MO) and SIS (EMD Biosciences, Inc.; Calbiochem) were dissolved in DMSO at stock concentration of 10 and 20 mm, respectively. Treatment of COCs or OOXs with 10 μm SB431542 or 20 μm SIS was achieved by diluting the stock in medium with the final concentration of DMSO was 0.1%. In a separate group, the same concentration of DMSO was added to serve as control. These doses were based on our previous studies (27). Our in-house purified recombinant mouse GDF9 and human BMP15 (39,40) were initially used to test their effect on Egfr expression in cumulus cells. While this project was ongoing, these recombinant proteins became available commercially by R&D System (Minneapolis, MN). Because both our preparations and those of R&D (GDF9: catalog no. 739-G9-010, BMP15: catalog no. 5096-BM) showed the same bioactivity in promoting Egfr expression, and the commercially available products are more convenient to obtain, GDF9 and BMP15 from R&D were used in all experiments reported here. To test the effect of these recombinant proteins on Egfr expression in cumulus cells, OOX cumulus cells were treated with 100, or 500 ng/ml of GDF9 and/or 500 ng/ml BMP15 and cultured for 20 h. To test the effect of inhibitors for SMAD2 and/or -3 on GDF9-induced Egfr expression, OOX cumulus cells were first treated with 10 μm SB431542 or 20 μm SIS for 1 h, and then GDF9 was added to medium at a final concentration of 500 ng/ml. OOX cumulus cells in this experiment were cultured for a total of 20 h.
Real-time RT-PCR analysis
RNA extraction, in vitro transcription, and real-time PCR analyses were carried out as described previously (25) to quantify the steady-state mRNA levels of Egfr, Areg, Ereg, Btc, and housekeeping gene Rpl19 (internal control). The primers used were Egfr-forward: 5′-GTGGAGGGACATCGTCCAAA-3′; Egfr-reverse: 5′-ATTGGGACAGCTTGGATCACAT-3′; Rpl19-forward: 5′-CCGCTGCGGGAAAAAGAAG-3′; and Rpl19-reverse: 5′-CAGCCCATCCTTGATCAGCTT-3′. Primers for Areg, Ereg, and Btc were the same as we have used in a previous study (41). Calculation of the relative fold change of Egfr, Areg, Ereg, and Btc was done using the method of 2-ΔΔCt as described previously (42). In each experiment, levels of Egfr, Areg, Ereg, or Btc mRNA were presented as relative changes to a specific group (control) in which its expression level was set at 1.
Western blot analysis
Protein samples were prepared as described previously (43). Protein content in the sample was determined by Bradford Analysis using the Pierce 660 nm Protein Assay Regent (Fisher Scientific, Pittsburgh, PA). About 1 μg total protein from each sample was loaded onto SDS-PAGE gels. For detection of EGFR and MAPK3/1, 7.5% and 10% gels were used, respectively, for protein resolution. Electrophoresis and transfer and subsequent immunodetection procedures were carried out as described previously (43). The following primary and secondary antibodies were used for protein detection: EGFR (pan) rabbit monoclonal antibody (Epitomics, Burlingame, CA), Monoclonal anti-β-actin (Sigma-Aldrich), rabbit antiphospho-Smad2 (Ser465/Ser467) (Zymed Laboratories, Inc., South San Francisco, CA; Invitrogen), monoclonal antidiphosphorylated ERK-1 and 2 antibody (Sigma-Aldrich), polyclonal anti-MAPK antibody (Sigma-Aldrich), stabilized peroxidase-conjugated goat antimouse IgG (Thermo Fisher Scientific, Rockford, IL), and stabilized peroxidase conjugated goat antirabbit IgG (Thermo Fisher Scientific, Rockford, IL). After membranes were incubated with appropriate primary and secondary antibodies, respectively, they were incubated in SuperSignal West Femto Maximum Sensitivity substrate (Thermo Scientific) for 10 min and then scanned using Fujifilm LAS-3000 Imaging System (Fuji, Edison, NJ). The intensity of each specific band was then quantified using Image Gauge version 3.46 software. Restore Plus Western Blot Stripping Buffer (Thermo Scientific) was used to remove antibodies initially bound to membranes before subsequently reprobing membranes with another antibody. To quantify the relative changes of the levels of EGFR and p-SMAD2, the pixel intensity of EGFR and p-SMAD2 in each sample was first normalized to that of β-actin in the same sample, and the normalized pixel intensity of EGFR and p-SMAD2 in each sample was then divided by that in a specific group (control) in which its expression was set at 1. For quantification of the levels of p-MAPK3/1, the pixel intensity of p-MAPK3/1 in each sample was first normalized to that of total MAPK3/1 in the same sample, after which the changes of the levels of p-MAPK3/1 caused by AREG treatment were then calculated by dividing the normalized pixel intensity of p-MAPK3/1 in AREG-treated samples with that in the corresponding control group that was not treated with AREG. The levels of p-MAPK3/1 were presented as relative changes to a specific group in which its expression was set at 1. For all the Western blot experiments, a representative Western blot image of three independent experiments was presented, and the quantification data of three independent experiments were listed beside each group of representative images.
Statistical analysis
All experiments were performed independently at least three times, and data are presented as mean ± sem. Differences between groups were analyzed by one-way ANOVA followed by Tukey's Honesty Significant Difference (HSD) test using JMP software (SAS Institute, Cary, NC). In some experiments, differences between two groups were analyzed by t test using Microsoft Excel software and are indicated in the figure legends. P < 0.05 was considered significantly different.
Supplementary Material
Acknowledgments
We thank Drs. Mary Ann Handel and Sophie LaSalle (The Jackson Laboratory, Bar Harbor, ME) for comments and suggestions on this manuscript; we thank Jesse Hammer at Multimedia Services (supported by grant CA34196 from the National Cancer Institute) of The Jackson Laboratory for helping to configure the figures.
Footnotes
This work was supported by grants HD23839 (to Y.-Q.S., K.S., J.J.E.); HD21970 (to K.S., K.W., J.J.E.); and HD32067 and HD33438 (to Q.L., M.M.M.) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Disclosure Summary: Y.-Q.S., K.S., Q.L., K.W., M.M.M., and J.J.E. have nothing to disclose.
First Published Online April 9, 2010
Abbreviations: AREG, Amphiregulin; BMP, bone morphogenetic protein; BTC, betacellulin; CG, chorionic gonadotropin; COC, cumulus-oocyte complex; DM, double mutant; DMSO, dimethylsulfoxide; EGF, epidermal growth factor; EGFR, EGF receptor; EREG, epiregulin; GDF, growth differentiation factor; OOX, oocytectomized/oocytectomy; SIS, specific inhibitor of SMAD3; SMAD, Sma- and Mad-related protein; WT, wild type.
References
- Eppig JJ 2001 Oocyte control of ovarian follicular development and function in mammals. Reproduction 122:829–838 [DOI] [PubMed] [Google Scholar]
- Russell DL, Salustri A 2006 Extracellular matrix of the cumulus-oocyte complex. Semin Reprod Med 24:217–227 [DOI] [PubMed] [Google Scholar]
- Richards JS 2005 Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol 234:75–79 [DOI] [PubMed] [Google Scholar]
- Richards JS 2007 Genetics of ovulation. Semin Reprod Med 25:235–242 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Pendola F, Hirao Y 1997 Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod 56:976–984 [DOI] [PubMed] [Google Scholar]
- Lawrence TS, Dekel N, Beers WH 1980 Binding of human chorionic gonadotropin by rat cumuli oophori and granulosa cells: a comparative study. Endocrinology 106:1114–1118 [DOI] [PubMed] [Google Scholar]
- Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T 1991 Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology 129:3200–3207 [DOI] [PubMed] [Google Scholar]
- Conti M, Hsieh M, Park JY, Su YQ 2006 Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol 20:715–723 [DOI] [PubMed] [Google Scholar]
- Hsieh M, Zamah AM, Conti M 2009 Epidermal growth factor-like growth factors in the follicular fluid: role in oocyte development and maturation. Semin Reprod Med 27:52–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M 2007 Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 27:1914–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M 2004 EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303:682–684 [DOI] [PubMed] [Google Scholar]
- Panigone S, Hsieh M, Fu M, Persani L, Conti M 2008 Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol 22:924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SM, Daniel SA, Eppig JJ 1988 Induction of maturation in cumulus cell-enclosed mouse oocytes by follicle-stimulating hormone and epidermal growth factor: evidence for a positive stimulus of somatic cell origin. J Exp Zool 245:86–96 [DOI] [PubMed] [Google Scholar]
- Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A 2005 Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology 146:77–84 [DOI] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS 2009 Mapk3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 324:938–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela-Abramovich S, Chorev E, Galiani D, Dekel N 2005 Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology 146:1236–1244 [DOI] [PubMed] [Google Scholar]
- Su YQ, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ 2002 Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology 143:2221–2232 [DOI] [PubMed] [Google Scholar]
- Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ 1990 FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev Biol 138:16–25 [DOI] [PubMed] [Google Scholar]
- Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Thompson JG, Armstrong DT, Gilchrist RB 2007 Oocyte-secreted factor activation of smad 2/3 signaling enables initiation of mouse cumulus cell expansion. Biol Reprod 76:848–857 [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Lane M, Thompson JG 2008 Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update 14:159–177 [DOI] [PubMed] [Google Scholar]
- Su YQ, Wu X, O'Brien MJ, Pendola FL, Denegre JN, Matzuk MM, Eppig JJ 2004 Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop. Dev Biol 276:64–73 [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM 2001 Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol 15:854–866 [DOI] [PubMed] [Google Scholar]
- Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Armstrong DT, Gilchrist RB 2005 Role of oocyte-secreted growth differentiation factor 9 in the regulation of mouse cumulus expansion. Endocrinology 146:2798–2806 [DOI] [PubMed] [Google Scholar]
- Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM 1999 Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol 13:1035–1048 [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Wigglesworth K, O'Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ 2008 Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 135:111–121 [DOI] [PubMed] [Google Scholar]
- Yoshino O, McMahon HE, Sharma S, Shimasaki S 2006 A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse. Proc Natl Acad Sci USA 103:10678–10683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz FJ, Wigglesworth K, Eppig JJ 2007 Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci 120:1330–1340 [DOI] [PubMed] [Google Scholar]
- Li Q, Pangas SA, Jorgez CJ, Graff JM, Weinstein M, Matzuk MM 2008 Redundant roles of SMAD2 and SMAD3 in ovarian granulosa cells in vivo. Mol Cell Biol 28:7001–7011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz FJ, O'Brien MJ, Wigglesworth K, Eppig JJ 2006 The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion. Dev Biol 299:91–104 [DOI] [PubMed] [Google Scholar]
- Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS 2006 Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol 20:1352–1365 [DOI] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM 1996 Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 383:531–535 [DOI] [PubMed] [Google Scholar]
- McMahon HE, Hashimoto O, Mellon PL, Shimasaki S 2008 Oocyte-specific overexpression of mouse bone morphogenetic protein-15 leads to accelerated folliculogenesis and an early onset of acyclicity in transgenic mice. Endocrinology 149:2807–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SJ, Reader KL, Lun S, Western A, Lawrence S, McNatty KP, Juengel JL 2008 The cooperative effect of growth and differentiation factor-9 and bone morphogenetic protein (BMP)-15 on granulosa cell function is modulated primarily through BMP receptor II. Endocrinology 149:1026–1030 [DOI] [PubMed] [Google Scholar]
- McIntosh CJ, Lun S, Lawrence S, Western AH, McNatty KP, Juengel JL 2008 The proregion of mouse BMP15 regulates the cooperative interactions of BMP15 and GDF9. Biol Reprod 79:889–896 [DOI] [PubMed] [Google Scholar]
- McNatty KP, Juengel JL, Reader KL, Lun S, Myllymaa S, Lawrence SB, Western A, Meerasahib MF, Mottershead DG, Groome NP, Ritvos O, Laitinen MP 2005 Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function in ruminants. Reproduction 129:481–487 [DOI] [PubMed] [Google Scholar]
- McNatty KP, Juengel JL, Reader KL, Lun S, Myllymaa S, Lawrence SB, Western A, Meerasahib MF, Mottershead DG, Groome NP, Ritvos O, Laitinen MP 2005 Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function. Reproduction 129:473–480 [DOI] [PubMed] [Google Scholar]
- McMahon HE, Sharma S, Shimasaki S 2008 Phosphorylation of bone morphogenetic protein-15 and growth and differentiation factor-9 plays a critical role in determining agonistic or antagonistic functions. Endocrinology 149:812–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimasaki S, Moore RK, Otsuka F, Erickson GF 2004 The bone morphogenetic protein system in mammalian reproduction. Endocr Rev 25:72–101 [DOI] [PubMed] [Google Scholar]
- Li Q, Rajanahally S, Edson MA, Matzuk MM 2009 Stable expression and characterization of N-terminal tagged recombinant human bone morphogenetic protein 15. Mol Hum Reprod 15:779–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ 2009 Fibroblast growth factors and epidermal growth factor cooperate with oocyte-derived members of the TGFβ superfamily to regulate Spry2 mRNA levels in mouse cumulus cells. Biol Reprod 81:833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Eppig JJ 2009 Targeted suppression of Has2 mRNA in mouse cumulus cell-oocyte complexes by adenovirus-mediated short-hairpin RNA expression. Mol Reprod Dev 76:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Woo Y, Wigglesworth K, Kamdar S, Affourtit J, Eppig JJ 2007 Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev Biol 302:104–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YQ, Rubinstein S, Luria A, Lax Y, Breitbart H 2001 Involvement of MEK-mitogen-activated protein kinase pathway in follicle-stimulating hormone-induced but not spontaneous meiotic resumption of mouse oocytes. Biol Reprod 65:358–365 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.