Abstract
Endothelins were first identified as potent vasoactive peptides; however, diversity in the biological function of these hormones is now evident. We have identified a novel role for endothelins: a requirement for these peptides within the oviduct during fertilization and/or early embryo development. In vivo, treatment after ovulation with a dual endothelin receptor antagonist (tezosentan) decreased the number of two-cell embryos that could be collected from within the oviducts. In vitro fertilization experiments showed that gamete viability and their ability to fertilize were not affected by treatment with this antagonist, suggesting that the effect observed in vivo was mediated by the oviduct itself. Expression of mRNA for all three isoforms of the endothelins and both receptor subtypes was detectable within the oviduct. Expression of mRNA for endothelin-3 was regulated by gonadotropins in epithelial cells of the oviduct and increased specifically within the isthmus of this structure. Immunostaining revealed localization of both endothelin receptors A and B to the columnar epithelial cells within the oviduct, suggestive of a local role for endothelins in the regulation of epithelial function and ultimately oviductal secretions. A microarray analysis revealed three likely endothelin-regulated protein networks for future analysis: the TGFβ, IL-10, and CCAAT/enhancer-binding protein superfamilies. Overall, these results suggest a novel and requisite role for endothelins within the oviduct during fertilization and/or early embryo development.
Endothelin-3 is regulated by gonadotropins in the epithelial cells of the oviduct and appears to be required for normal fertility.
The oviduct plays a vital role in the establishment of a pregnancy, providing the microenvironment for fertilization and development of the very early embryo. Briefly, ovulated cumulus-oocyte complexes (COCs) enter the oviduct via the infundibulum and are fertilized within the ampulla, the upper section of the oviduct. The developing embryo stays several days within this tubal structure, traversing the lower and muscular isthmus before entering the uterus in preparation for implantation approximately 4 d after fertilization (1). Endocrine, autocrine, and paracrine factors regulate oviductal function, including control of the secretion of essential embryotrophic factors, together facilitating this tissue's role in the establishment of a new pregnancy (2,3,4). However, the detailed regulation of oviductal function during the first few days after ovulation is still not well understood.
Considering the essential nature of the oviduct in the establishment of a pregnancy, it could be expected that oviductal dysfunction is a primary contributor to female-based infertility and, hence, the need for women to use assisted reproductive technologies to establish a pregnancy (5). With approximately 140,000 assisted reproductive technology procedures reported to the Centers for Disease Control and Prevention in a recent annual report (5), the significance of understanding oviductal function as a means of improving women's health should be easily recognized. Inclusive to this would be an understanding in the etiology of diseases causing tubal blockage that are ultimately responsible for a high proportion of cases of female infertility (6,7). However, irregularities in the precisely controlled secretion of oviductal fluids cannot be overlooked as another cause of poor fertility. Tubal secretions are known to affect both the ability of the gametes to fertilize (4) and of the embryo to subsequently develop (3).
In this paper, we report a novel requirement for endothelins in the regulation of oviductal function and fertility. Endothelins were first identified as potent vasoactive molecules (8); however, a role in female reproductive function has also proven itself to be significant (9,10,11). The endothelin family contains three 21-amino-acid peptide ligands [endothelin-1 (EDN1), EDN2, and EDN3] (8,12,13,14), and EDN1 is the most well-characterized isoform with functions identified in a multitude of biological systems (15,16). Studies on EDN2 include roles in the kidney, intestine, ovary, placenta, and uterus (10,13,17,18,19), whereas reports on EDN3 predominantly indicate a neural action for this isoform (20,21). Endothelins exert their biological response by binding to one of two G protein-coupled receptor subtypes, endothelin receptor A (EDNRA) and EDNRB (22). Although the binding affinity of EDNRB to the three ligand isoforms is similar, the affinity of EDNRA strongly favors EDN1 and EDN2 over EDN3 (23).
Smooth muscle contractility of the oviduct is well documented (24,25), as is the contractile action of endothelins (8,26). It is therefore not surprising that a contractile effect of endothelins on this tubal structure has also been revealed (10,27,28,29). However, endothelins have a diverse portfolio of actions including acting as effectors of proliferation (30), steroidogenesis (30,31,32), and apoptosis (33,34,35), and their precise function within an individual tissue must be evaluated with this divergence in mind. Hence, our primary objective was to test the hypothesis that endothelins are required by the oviduct to facilitate fertilization and/or early embryonic development. To meet this objective, experiments were designed to determine whether endothelins are required for fertilization of the male and female gametes themselves or whether endothelin-regulated fertility was mediated by the oviduct. A thorough analysis of the components of the endothelin system within the oviduct was performed to allow accurate extrapolation of any results. Finally, a microarray approach was used to identify novel biological pathways that were affected by endothelins within this structure. Together, the results of these experiments reveal what appears to be a unique role for endothelins within the oviduct, regulating epithelial cell function and fertility.
Materials and Methods
Animals
Mice were purchased from Charles River [Wilmington, MA; for in vitro fertilization (IVF)], Dae Han Biolink Co. (Eumsung, South Korea; for immunohistochemistry), and Harlan Inc. (Harlan, IN; all others). Animal procedures involved in these studies were approved by the Sungshin University, Hanyang University, and the University of Kentucky Animal Care and Use Committees, respectively, according to National Institutes of Health guidelines for the ethical use of animals in research.
Immunohistochemistry
Immature female CD1 mice were injected ip with 5 IU pregnant mare's serum gonadotropin (PMSG) to induce follicular development and, 48 h later, with 5 IU human chorionic gonadotropin (hCG) to induce ovulation. Mice were killed 12, 18, and 24 h later. Ovaries with oviducts attached were retrieved at the time of killing and fixed for 4 h in Bouin's fixative and then embedded in paraffin. Sections were cut to 5 μm, mounted on poly l-lysine-coated glass slides, deparaffinized, and rehydrated. Antigen retrieval was performed by autoclaving for 20 min in 10 mm citrate buffer (pH 6.0). The slides were then incubated for 10 min in the dark in 3.5% H2O2 in methanol to remove endogenous peroxidase activity, blocked for 20 min in 1.5% normal goat serum, and incubated overnight at 4 C with a primary antibody against either EDNRA (rabbit polyclonal, catalog no. IMG71259; IMGENEX, San Diego, CA) or EDNRB (rabbit polyclonal, catalog no. ab65972; Abcam, Cambridge, UK) at a dilution of 1:1000. After washing in PBS, the slides were then incubated for 30 min at room temperature in a 1:200 dilution of biotinylated goat antirabbit IgG (Vector Laboratories, Burlingame, CA) in blocking solution. Slides were rinsed again, and an avidin-biotin peroxidase complex solution was applied for 30 min at room temperature. After additional rinses in PBS, the coloring reaction was conducted with ImmPACT diaminobenzidine peroxidase substrate for 10 min at room temperature, and nuclei were stained with 20% Harris hematoxylin solution. Sections were rinsed again in PBS and then dehydrated in a graded ethanol series, dipped in xylenes, and permanently mounted. Images were obtained using a digital imaging system (DFC320; Leica Microsystems, Wetzlar, Germany), as described previously (36).
Real-time PCR analysis
Immature female CD1 mice were treated with PMSG and hCG, as described above. Mice were killed for tissue collection at 0 h (before treatment with gonadotropins), PMSG +48 h, hCG +12 h, hCG +18 h, and hCG +24 h. Whole oviducts (at all time points) or separated sections of the ampulla and isthmus (at hCG +12, +18, and +24 h) were collected and pooled from at least three mice per time point, with a total of three independent replicates collected for each tissue type and time. Epithelial cells (at all time points) were collected and pooled from the whole oviducts of four mice per time point, with a total of three independent replicates collected. When present, COCs were removed from within the oviduct. Total RNA was isolated from all tissues using Trizol, and cDNA reverse transcribed from each sample of RNA. The concentration and integrity of each sample of RNA was assessed by UV spectroscopy using an Eppendorf Biophotometer-plus (Eppendorf, Hauppauge, NY) as well as by visual distinction of 18S and 28S rRNA bands after ethidium bromide staining in an agarose gel. Real-time PCR was then performed to determine expression levels of mRNA for EDN1, EDN2, EDN3, endothelin converting enzyme-1 (ECE1), ECE2, EDNRA, and/or EDNRB using SYBR Green PCR Master Mix (Bio-Rad, Hercules, CA) and gene-specific primer pairs (Table 1) on a Bio-Rad IQ5 system under similar cycling conditions, as described previously (10). Real-time PCR was performed with a total volume of 25 μl per reaction, with each reaction containing 5 μl cDNA, 1 μl of a 10 μm stock of each primer (forward and reverse), 12.5 μl 2× SYBR Green PCR Master Mix, and 5.5 μl diethylpyrocarbonate-treated water. All primers were designed to amplify a product with a size of 195–205 bases, and the specificity of each primer set was confirmed by running the PCR products on a 1.5% agarose gel. Protocol conditions consisted of denaturation at 95 C for 30 sec, followed by 40 cycles at 94 C for 30 sec, 55 C for 30 sec, and 72 C for 45 sec with a final dissociation (melting) curve analysis. The relative level of expression of each mRNA was standardized against ribosomal protein L19 as a housekeeping gene and analyzed by the 2−ΔΔCT method (37).
Table 1.
Primer sequences employed for real-time PCR measurements
| Name | Primer sequence (5′–3′) |
|---|---|
| EDN1 | |
| Forward | TTC CCG TGA TCT TCT CTC TGC T |
| Reverse | TCT GCT TGG CAG AAA TTC CA |
| EDN2 | |
| Forward | GTC GAT GCT CCT GCA ACT C |
| Reverse | TGG CCT TTC TTG TCA CCT CT |
| EDN3 | |
| Forward | CTG TGT GCT TGA GAC CTG GA |
| Reverse | TCC CCA AGG ATC CAC ATT TA |
| EDNRA | |
| Forward | CAT AGG ACC TGC ATG CTC AA |
| Reverse | GCC AGG TTA ATG CCG ATG TA |
| EDNRB | |
| Forward | CCT GAT GAC CTG CGA AAT G |
| Reverse | TGC TTC TCC TCC AAG GAC TG |
| ECE1 | |
| Forward | ACC CTC GGG GAG AAC ATT |
| Reverse | GCA GCT CCT TCC CTT TTT CT |
| ECE2 | |
| Forward | TCC CTG ATT TCA TCC TGG AG |
| Reverse | GAC GCA GAT TCC CTT CTT TG |
Embryo collection analysis
Immature female CD1 mice were treated with PMSG and hCG, as described above. At the time of hCG administration, females were allocated to pairs, and each pair of females was introduced into the cage of a male of confirmed fertility. Two females were placed with each male to minimize any individual effect of male fertility on the results. At hCG +14 h (after ovulation), one female of each pair was injected with 20 mg/kg body weight (ip) of the dual endothelin receptor antagonist tezosentan (Actelion Pharmaceuticals, Switzerland) and the other with an equal volume of vehicle as a control (PBS, 30 μl). Tezosentan was administered at hCG +14 h because earlier treatment with this antagonist affects ovulation (38) and therefore the number of oocytes that can be fertilized. At hCG +24 h, males were removed and copulation plugs confirmed, and each female received a second dose of the same treatment. The dose of tezosentan was based upon our previous use (10,38) with the second treatment administered to ensure maintained blockade of endothelin receptors. At hCG +48 h, the female mice were killed, the oviducts were flushed, and the number of two-cell embryos within the oviducts of each mouse was counted. The results reflect the mean ± sem of four replicated pairs.
IVF analysis
Six-week-old female CD1 mice were treated with PMSG and hCG, as described above. Females were killed at hCG +9 h (before ovulation), and COCs were collected by follicular puncture of the whole ovaries. Male mice were killed at 8.5 h after the females had been injected with hCG, and the cauda epididymis and vas deferens were isolated for sperm collection. To determine whether endothelin receptor activation is required for fertilization of the female gamete, COCs were maintained for 1 h in medium with or without tezosentan (0, 10, or 100 μg/ml). COCs were then washed several times in plain medium, and the rate of IVF was determined following a well-established protocol (39). Similarly, a requirement for endothelin receptor activation on fertility of the male gamete was evaluated by additionally maintaining sperm in medium containing tezosentan (0, 10, or 100 μg/ml) before IVF. In each case, sperm were incubated for 1.5 h at 37 C in Biggers-Whitten-Whittingham medium to allow capacitation (with or without tezosentan), and then the rate of IVF was determined by the addition of sperm (1–2 × 106/ml) to individual drops of medium containing COCs. Oocytes were then transferred to fresh medium after 4 h, and the rate of IVF was determined by classification under an inverted microscope (Olympus ×70) after 48 h culture. The results reflect the average of seven biological replicates for each treatment group.
Microarray analysis
Immature female CD1 mice were treated with PMSG and hCG, as described above. At hCG +9 h, mice were treated with tezosentan (20 mg/kg body weight) or PBS as a vehicle control. Tezosentan was administered at hCG +9 h to ensure blockade of endothelin receptors before the increase in ovarian EDN2 that occurs at the time of ovulation (38). Mice were killed for tissue collection 9 h later (hCG +18 h). Whole oviducts were collected and pooled from at least four mice per treatment, with a total of two independent replicates (microarray chips) collected for each treatment. When present, COCs were removed from within the oviducts before processing. Total RNA was extracted from all tissues using Trizol and purified with RNeasy (QIAGEN, Valencia, CA) according to the manufacturer's directions. The integrity of total RNA was verified by visualizing the intact and distinct 28S and 18S rRNA bands on a 1.5% agarose gel. Microarray hybridization was then performed using the Affymetrix Mouse 430-2.0 whole genome arrays (Affymetrix; DNA Microarray Core Facility, University of Kentucky, Lexington, KY), as described previously (40). The signal intensity on each chip was normalized using a quantile normalization algorithm following the Affymetrix GeneChip protocol. The signal for each gene (probe set ID) was normalized to the median signal value for that gene across all the chips among treatment groups. The dataset was then analyzed to identify endothelin-regulated biological pathways. Briefly, the level of expression of each gene was compared between the two treatment groups (PBS vs. tezosentan), and differentially expressed genes were extracted. However, marginally expressed genes had a large impact on the differential expression classifications, so to eliminate this potential problem, we excluded all marginally expressed genes with an intensity of less than 200 from the analysis. Differentially expressed genes were then selected by fold change, with only genes with at least a 1.5-fold treatment effect carried further in the analysis. Overall, 942 genes were selected with an intensity of more than 200 and a fold change of more than 1.5 between the two treatments (PBS vs. tezosentan). Selected genes were then subjected to an enrichment analysis using Pathways Studio 6 Software (Ariadne, MD) to indicate potential protein networks affected by treatment. The pattern of gene expression for a random selection of genes that were identified as being differentially expressed by the microarray analysis was then confirmed by real-time PCR analysis, as described above. The selected genes, expression levels, and primer pairs are described in Supplemental Figs. 1–3 and Supplemental Tables 1–4 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Statistical analysis
Datasets were first tested for normality and homogeneity of variance. When appropriate, data were transformed before statistical analysis. Nontransformed data are depicted in all the figures. One-way ANOVA using SigmaStat 3.5 (Systat Software, Inc., Point Richmond, CA) was used to determine differences in expression of mRNA levels and the effect of treatment on rate of IVF. If differences were detected, Tukey's test was used to determine which means differed. The Student's t test was used to determine the effect of treatment in the embryo collection analysis.
Results
Expressions of EDNRA and EDNRB in the oviduct
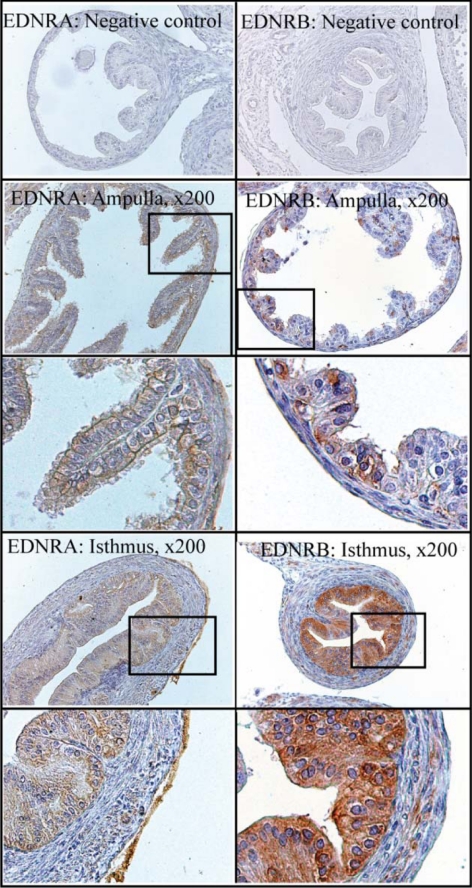
The oviduct is the site of fertilization and very early embryonic development. Studies suggest the involvement of EDNRA and/or EDNRB in mediating the action of endothelins within this structure (10,28), and we hypothesized a requirement for these peptides in fertility. However, no clear analysis of the spatial and temporal expression of both receptor subtypes within this tissue around the time of ovulation and fertilization is available. Hence, real-time PCR and immunohistochemistry were used to determine the relative level of expression and location of endothelin receptors (mRNA and protein) within the oviduct. Expression of mRNA for both EDNRA and EDNRB was detectable in oviducts at all times examined (Fig. 1) and was not affected by treatment with tezosentan in the microarray analysis. Expression of mRNA for EDNRA in whole oviducts was not affected by treatment with gonadotropins (P > 0.05; Fig. 1A). In contrast, expression of mRNA for EDNRB in whole oviducts was increased by gonadotropins (P < 0.05), with maximal levels observed at 12 h after hCG was injected to induce ovulation. Consistent with expression of mRNA in whole oviducts, no differences in levels of mRNA for either subtype were observed in the ampulla at 12–24 h after hCG (P > 0.05; Fig. 1B), when both receptors were shown to be localized to the epithelial cells adjacent to the ampullary lumen (Fig. 2). Interestingly, expression of EDNRA appeared relatively homogeneous among the epithelial cells of the ampulla, whereas some heterogeneity was observed in the expression of EDNRB. Within the isthmus of the oviduct, expression of mRNA for EDNRA was increased at 24 h after hCG, in contrast to the expression of mRNA for EDNRB, which showed a concurrent decrease in expression at this same time of collection (P < 0.05). Immunohistochemistry revealed that both subtypes were strongly expressed within the columnar epithelial cells of the isthmus; however, minor smooth muscle or stromal expression of both receptors can be observed (especially for EDNRB), consistent with reports of endothelin-induced oviductal contraction (10,27,28,29). Because no dramatic differences in the temporal expression or location of endothelin receptors were revealed by immunohistochemical analysis of sections from oviducts collected at hCG +12, +18, or +24 h, immunohistochemical expression at hCG +18 h alone is presented. Note that the intensity of staining cannot be used to compare levels of protein between the two receptor subtypes.
Figure 1.
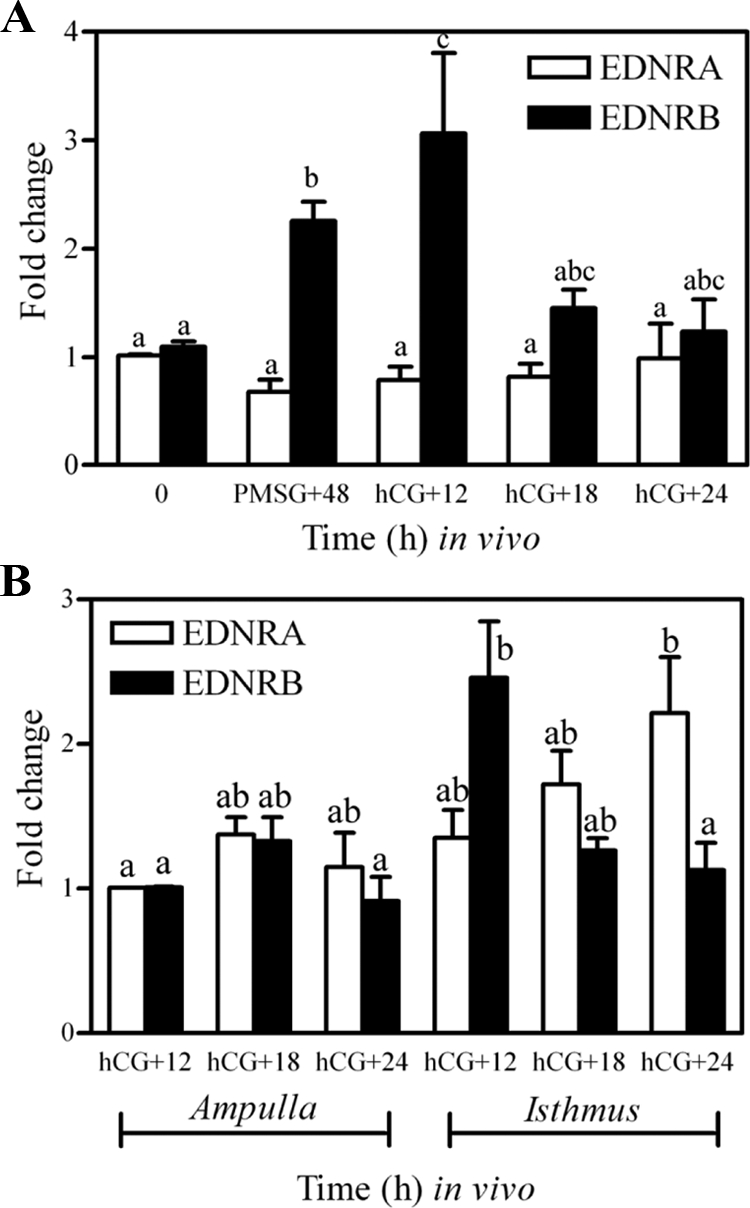
Expression of mRNA for EDNRA and EDNRB in whole oviducts (A) and isolated sections of ampulla and isthmus (B) collected from gonadotropin-primed mice. Immature mice were treated with 5 IU PMSG to induce follicular development and with 5 IU hCG 48 h later to synchronize ovulation. Tissues were collected at the times described in each panel. Data are the means ± sem of triplicate replicates from each of three samples per time point. Levels of mRNA were obtained by real-time PCR and are expressed as fold changes. For each mRNA within a panel, values with different superscript letters differ (P < 0.05).
Figure 2.
Immunohistochemical localization of EDNRA and EDNRB in the ampulla and isthmus of the mouse oviduct. Immature mice were treated with 5 IU PMSG to induce follicular development and with 5 IU hCG 48 h later to synchronize ovulation. Tissues were collected at hCG +18 h, fixed in Bouin's fixative, and embedded in paraffin. A higher magnification of the boxed section is presented for each receptor subtype and section of the oviduct.
Requirement for endothelins in early embryonic development, in vivo
Real-time PCR and immunohistochemistry revealed abundant expression of both receptor subtypes in the oviduct, especially within the secretory epithelial cells lining the oviductal lumen. We hypothesized that endothelin receptor-mediated actions would be required to fulfill the oviduct's physiological goal of facilitating fertilization and early embryonic development and used an in vivo fertility analysis to test this hypothesis. Mice were primed with gonadotropins, bred, and treated with the dual endothelin receptor antagonist tezosentan or PBS as a vehicle control. Treatment of mice with tezosentan had a dramatic effect on the number of two-cell embryos that were retrieved from within the oviducts (Fig. 3). Tezosentan induced a decrease in the number of two-cell embryos to only 21% of vehicle-treated controls (17.8 ± 4.1 vs. 3.8 ± 2.3, P < 0.05), suggesting that endothelins play a vital role in the establishment of a new pregnancy.
Figure 3.
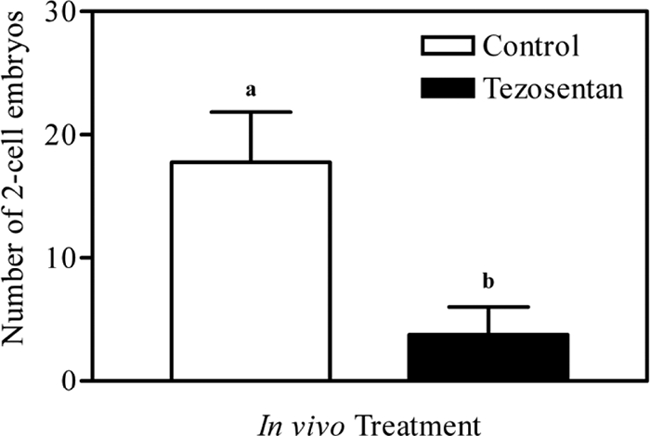
Number of two-cell embryos collected from the oviducts of mice treated with vehicle (PBS, control) or tezosentan. Immature mice were treated with 5 IU PMSG to induce follicular development and with 5 IU hCG 48 h later to synchronize ovulation. At the time of hCG treatment, gonadotropin-primed females were bred with fertile males. Females were treated with PBS or tezosentan at hCG +14 h and hCG +24 h. Mice were killed at hCG +48 h and the number of two-cell embryos counted after flushing the oviducts. Treatment with tezosentan decreased the number of two-cell embryos that were retrieved from within the oviducts at the time of killing (a vs. b, P < 0.05).
Requirement for endothelins in fertilization, in vitro
To determine whether the dramatic reduction in the number of two-cell embryos collected after treatment with tezosentan in vivo was due to a direct inhibitory or potentially toxic effect of that antagonist on the male or female gamete, the same receptor antagonist was used in a series of experiments using IVF, thus removing the oviduct as a site of endothelin-mediated action. Treatment of COCs with tezosentan had no effect on the rate of IVF (P > 0.05; Fig. 4A), nor did treatment of sperm (P > 0.05; Fig. 4B). These results indicate that the effect observed after treatment of the antagonist in vivo is not due to a direct inhibitory or toxic effect on the viability and ability of gametes to fertilize but rather an indirect effect mediated at the level of the oviduct. It is important to note that the higher dose of tezosentan used in vitro (100 μg/ml) is quite comparable to the dose used in vivo. Twenty milligrams per kilogram body weight (in vivo) reflects a total dose of 400 μg injected into a 20-g mouse (vs. 100 μg/ml culture medium). Overall, these results suggest that the requirement for endothelins in the processes of fertilization and/or early embryonic development is mediated by the oviduct itself.
Figure 4.
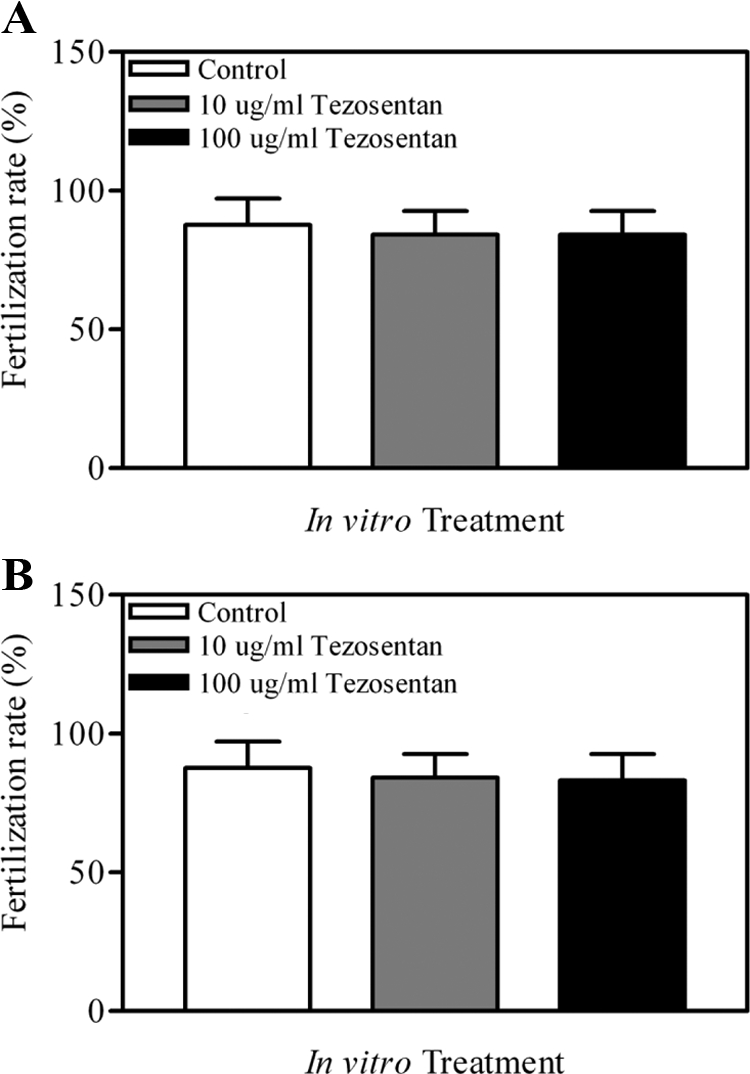
Rate of IVF after treatment of incubation media containing COCs (A) or COCs plus sperm (B) with vehicle (PBS) or 10 or 100 μg/ml tezosentan. COCs were collected from 6-wk-old gonadotropin-primed females; sperm was collected from males of proven fertility. No effect of treatment with tezosentan on the rate of IVF was detected (P > 0.05).
Expression of mRNA for ECE1, ECE2, EDN1, EDN2, and EDN3 in the oviduct
The results above indicated a requirement of endothelin receptor activation for fertilization and/or early embryonic development at the level of the oviduct. Both receptor subtypes are predominantly expressed in the secretory luminal epithelial cells of the oviduct; however, the endothelin isoform mediating this effect is unknown. To begin to elucidate the mechanism whereby endothelins are regulating fertilization and/or early embryonic development, identification of the converting enzymes and isoforms present within the oviduct and their spatial and temporal distribution was needed. Hence, real-time PCR was used to determine the expression of mRNA for ECE1, ECE2, EDN1, EDN2, and EDN3 in whole oviducts as well as sections of isolated ampulla and isthmus. In whole oviducts, no difference was detected in the temporal expression of mRNA for ECE1 (P > 0.05); however, levels of mRNA for ECE2 were lower at hCG +18 h when compared with 0 h and PMSG +48 h (P < 0.05). Consistent with these findings, no differences were detected in expression of mRNA for ECE1 and ECE2 in isolated sections of ampulla and isthmus collected at hCG +12, +18, and +24 h (P > 0.05). Unexpectedly, gonadotropin-induced expression of mRNA for EDN3 was the most readily detected endothelin subtype mRNA observed in the whole oviducts (Fig. 5). Expression of mRNA for EDN3 was increased by treatment with gonadotropins, with maximal expression found in oviducts collected at hCG +12 h (P < 0.05; Fig. 5A). Levels of mRNA for this isoform decreased by hCG +18 h (P < 0.05) and remained low thereafter. Further analysis of mRNA for EDN3 in 1) spatially distinct sections of the oviduct (Fig. 5B) and 2) isolated epithelial cells collected from whole oviducts (Fig. 6) suggested that the increase in mRNA observed at hCG +12 h was specific to epithelial cells of the isthmus. In contrast to the temporal and spatial regulation of mRNA for EDN3, expression of mRNA for EDN1 and EDN2 remained relatively stable with only minor changes in expression of these mRNA observed. Overall, determination that mRNA for EDN3 was abundant and increased within the epithelial cells around the time of ovulation (hCG +12 h) is a very unique finding. Given the expression of EDNRA and EDNRB within the luminal epithelium of the oviduct, with spatial and temporal differences in expression of mRNA for the three endothelin isoforms, it appears that multiple isoform/receptor-specific pathways are involved in affecting oviductal function and fertility.
Figure 5.
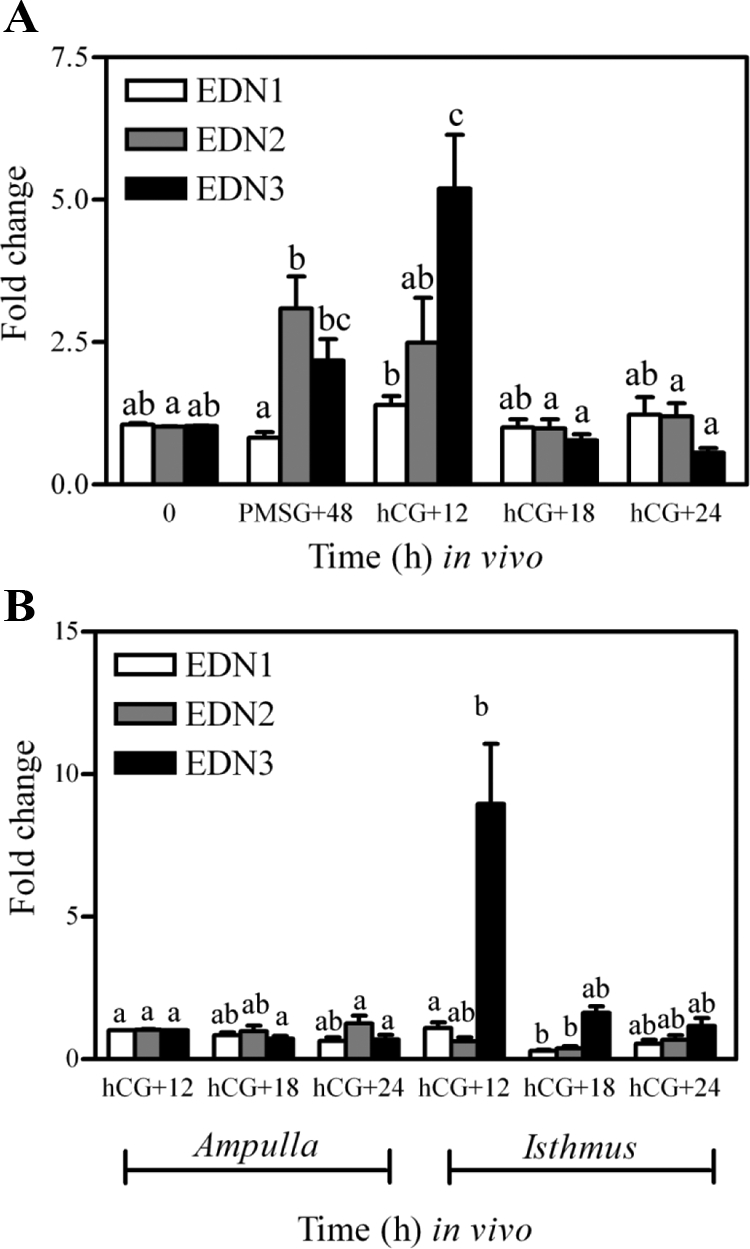
Expression of mRNA for EDN1, EDN2, and EDN3 in whole oviducts (A) and isolated sections of ampulla and isthmus (B) collected from gonadotropin-primed mice. Immature mice were treated with 5 IU PMSG to induce follicular development and with 5 IU hCG 48 h later to synchronize ovulation. Tissues were collected at the times described in each panel. Data are the means ± sem of triplicate replicates from each of three samples per time point. Levels of mRNA were obtained by real-time PCR and are expressed as fold changes. For each mRNA within a panel, values with different superscript letters differ (P < 0.05).
Figure 6.
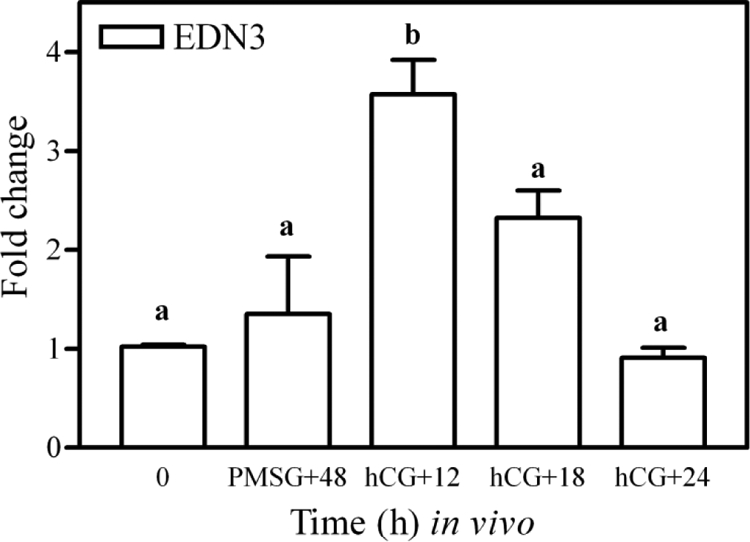
Expression of mRNA for EDN3 in isolated epithelial cells collected from the whole oviducts of gonadotropin-primed mice. Immature mice were treated with 5 IU PMSG to induce follicular development and with 5 IU hCG 48 h later to synchronize ovulation. Tissues were collected at the times indicated and epithelial cells pooled from four mice for each sample. Data are the means ± sem of duplicate replicates from each of three samples per time point. Levels of mRNA were obtained by real-time PCR and are expressed as fold changes. Values with different superscript letters differ (P < 0.05).
Analysis of endothelin-regulated pathways in the oviduct
Endothelin receptor antagonism resulted in a decrease in the number of developing two-cell embryos, and mRNAs encoding all three of the ligands were expressed in the oviduct. To elucidate the biological pathways governed by these peptides, a microarray analysis was performed using a similar experimental paradigm as that used for the in vivo fertility analysis, and genes were extracted and sorted to build a database of endothelin-regulated biological pathways. Using the extraction guidelines described in Materials and Methods, treatment with tezosentan resulted in the regulation of 14 major protein networks (Table 2). Three of these 14 protein networks were suggested by 26–28 reference genes that are shown in Supplemental Figs. 1–3. Overall, the pathway analysis indicated that the TGFβ, IL-10, and CCAAT/enhancer-binding protein (C/EBP) families are under the regulation of endothelins in the oviduct.
Table 2.
Endothelin-regulated pathways identified by microarray analysis
| Name | Number of regulated genes | P value |
|---|---|---|
| TGF family | 28 | 0.025 |
| IL10 | 28 | 0.027 |
| CEBPA | 26 | 0.043 |
| Histone | 13 | 0.039 |
| PRKCA | 10 | 0.038 |
| ACTB | 8 | 0.040 |
| IL3 | 7 | 0.047 |
| MYOD1 | 7 | 0.033 |
| Notch | 7 | 0.029 |
| LEF | 7 | 0.017 |
| RUNX1T1 | 7 | 0.001 |
| NPPA | 6 | 0.037 |
| TERT | 5 | 0.049 |
| SMAD6 | 5 | 0.037 |
Discussion
The oviduct provides the microenvironment for fertilization and subsequent development of the early embryo. We now provide evidence that endothelins are required by the oviduct to facilitate the initiation of a pregnancy. Treatment of mice with an endothelin receptor antagonist in vivo resulted in a dramatic decrease in the number of two-cell embryos that could be collected from within the animals' oviducts. Subsequent in vitro experiments using the same receptor antagonist as well as immunolocalization studies were consistent in their indication that the effect observed was mediated by the oviduct and not the gametes themselves. Furthermore, both endothelin receptor subtypes (EDNRA and EDNRB) were highly expressed in the luminal epithelium of the oviduct, suggestive of requisite endothelin-regulated function of the epithelial cells. Regulated expression of mRNA for EDN3 within the epithelial cells of the oviduct (likely the epithelial cells of the isthmus) indicated a novel and local role for the least understood of the endothelin isoforms; however, with all three isoforms and both receptors expressed within this structure, multiple ligand- and receptor-specific biological pathways are expected to be involved in endothelin-regulated function in the oviduct.
Considering that many proteins such as growth factors, enzymes, protease inhibitors, and cytokines have been identified within oviductal fluid (41), it was not unexpected that receptor localization indicated potential endothelin-regulated epithelial cell functions. This is especially pertinent when considering that epithelial cell secretions have proven crucial for fertilization and early embryonic development (42,43,44). Our observation that mRNA for EDN3 was highest in epithelial cells around the time of ovulation is also consistent with the cyclic changes in the secretory activity of the oviduct as a whole. Maximal numbers of the secretory cells are observed around the time of ovulation (45,46), and endothelins are known mediators of cell secretion in other tissues (47). However, it should be emphasized that a role for EDN3 in the regulation of epithelial cell secretion would be distinct from the known contractile role of these peptides (10,27,28,29), which is likely mediated through stromal expression of the two endothelin receptor subtypes. Previous reports have localized EDN3 to the pituitary gland, brain, and intestinal epithelial cells (48,49,50,51,52), and the literature suggests that this isoform functions in roles other than the typical endothelin-induced contractility (20,48,50,53). EDN3 is required for the development of neurons and melanocytes (50) with both the EDN3 and EDNRB knockouts sharing a similar Hirschsprung's disease-like phenotype (50). Determination of a gonadotropin-induced increase in mRNA for EDN3 in epithelial cells and the isthmus of the oviduct was therefore an exciting finding; however, the mechanism involved in endothelins as facilitators of fertility is far from understood, especially when considering potential differences in the affinity of each receptor subtype for its available ligands (23).
Results of the isoform and receptor analysis suggest the likelihood that multiple endothelin-regulated pathways will be unraveled within the oviduct. With that in mind, it appeared that a microarray analysis was the most appropriate approach to take with the objective of determining the mechanisms whereby endothelins are regulating oviductal function and fertility. Overall, the TGFβ, IL-10, and C/EBP families appeared to be key candidate pathways for further study. However, caution in interpretation is warranted because these biological pathways are drawn from microarray studies and computer-generated literature searches. Of these three candidate pathways, endothelin regulation of the TGFβ superfamily was not a surprising finding given that TGFβ is a known regulator of reproductive functions, including of those within the oviduct (54,55,56,57,58,59). In fact, TGFβ1, TGFβ2, and TGFβ receptors are expressed in the oocyte, embryo, and oviduct of the mouse (59,60,61), and analysis of human oviductal fluid has suggested a role for the TGFβ family in gamete maturation and early embryo development (60,61). Given that the expression level of TGFβ2 in the oviduct is most abundant before fertilization, declining rapidly thereafter (61) (59), it appears likely that infertility due to treatment with tezosentan in the studies described herein is mediated in part through this network.
The gene pathway analysis also indicated that the IL-10 and C/EBP families were regulated by tezosentan. IL-10 has several reported immunosuppressive effects, including suppression in production of proinflammatory cytokines such as IL-1, IL-6, IL-8, TNFα (62,63), and prostaglandins (62). This is suggestive of an endothelin-regulated immune response within the oviduct in which cytokines like IL-10, secreted from epithelial cells of the oviduct, may change the microenvironment faced by the early developing embryo, i.e. regulate immunological factors involved in early embryonic development. Less specific than the regulation of TGFβ and IL-10 is endothelin regulation of C/EBPs. These transcription factors are known regulators of cell differentiation, proliferation, apoptosis, and gene expression in several organs (64,65,66,67). In reproductive function, C/EBPβ is a downstream mediator of ERK1/2, which is activated by gonadotropins and plays an important role in fertilization, embryo development (68), and implantation (69,70).
In conclusion, antagonism of endothelin receptors decreased the number of two-cell embryos developing within the oviduct. The effect was not mediated directly by the gametes but by the oviduct itself. Gonadotropin-induced expression for mRNA for EDN3 within the epithelial cells of the isthmus of the oviduct suggests a specific role for this isoform during the processes of fertilization and/or early embryonic development. However, multiple receptor- and ligand-specific pathways were likely disrupted by the antagonist. Overall, the results indicate that endothelins play an important role within the oviduct in the initiation of a pregnancy.
Supplementary Material
Footnotes
This work was supported by start-up funds from the University of Kentucky (to P.J.B.) and K12 DA014040 (to P.J.B.), P20 RR15592 (to P.J.B. and C.K.), and RO1HD052694 (to C.K.) from the National Institutes of Health.
Disclosure Summary: M.J., S.L., H.H., Y.C, Y.K.J., M.C.G., C.K., and P.J.B. have nothing to disclose. M.I. is employed by Actelion Pharmaceuticals Ltd.
First Published Online March 31, 2010
Abbreviations: C/EBP, CCAAT/enhancer-binding protein; COC, cumulus-oocyte complex; ECE1, endothelin converting enzyme-1; EDN1, endothelin-1; EDNRA, endothelin receptor A; hCG, human chorionic gonadotropin; IVF, in vitro fertilization; PMSG, pregnant mare serum gonadotropin.
References
- Buhi WC, Alvarez IM, Kouba AJ 1997 Oviductal regulation of fertilization and early embryonic development. J Reprod Fertil Suppl 52:285–300 [PubMed] [Google Scholar]
- Jansen RP 1984 Endocrine response in the fallopian tube. Endocr Rev 5:525–551 [DOI] [PubMed] [Google Scholar]
- Kito S, Hayao T, Noguchi-Kawasaki Y, Ohta Y, Hideki U, Tateno S 2004 Improved in vitro fertilization and development by use of modified human tubal fluid and applicability of pronucleate embryos for cryopreservation by rapid freezing in inbred mice. Comp Med 54:564–570 [PubMed] [Google Scholar]
- Munuce MJ, Serravalle A, Caille AM, Zumoffen C, Botti G, Cabada M, Ghersevich S 2009 Human tubal secretion can modify the affinity of human spermatozoa for the zona pellucida. Fertil Steril 91:407–413 [DOI] [PubMed] [Google Scholar]
- Wright VC, Chang J, Jeng G, Chen M, Macaluso M 2007 Assisted reproductive technology surveillance: United States, 2004. MMWR Surveill Summ 56:1–22 [PubMed] [Google Scholar]
- Serafini P, Batzofin J 1989 Diagnosis of female infertility. A comprehensive approach. J Reprod Med 34:29–40 [PubMed] [Google Scholar]
- Mastroianni Jr L 1999 The fallopian tube and reproductive health. J Pediatr Adolesc Gynecol 12:121–126 [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T 1988 A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332:411–415 [DOI] [PubMed] [Google Scholar]
- Rosselli M, Imthurn B, Macas E, Keller PJ 1994 Endothelin production by bovine oviduct epithelial cells. J Reprod Fertil 101:27–30 [DOI] [PubMed] [Google Scholar]
- Al-Alem L, Bridges PJ, Su W, Gong MC, Iglarz M, Ko C 2007 Endothelin-2 induces oviductal contraction via endothelin receptor subtype A in rats. J Endocrinol 193:383–391 [DOI] [PubMed] [Google Scholar]
- Na G, Bridges PJ, Koo Y, Ko C 2008 Role of hypoxia in the regulation of periovulatory EDN2 expression in the mouse. Can J Physiol Pharmacol 86:310–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Naruse M, Naruse K, Demura H, Uemura H 1990 Immunocytochemical localization of endothelin in cultured bovine endothelial cells. Histochemistry 94:475–477 [DOI] [PubMed] [Google Scholar]
- Levin ER 1995 Endothelins. N Engl J Med 333:356–363 [DOI] [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T 1989 The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci USA 86:2863–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson MS 1993 Endothelins: multifunctional renal peptides. Physiol Rev 73:375–411 [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Polokoff MA 1994 Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev 46:325–415 [PubMed] [Google Scholar]
- Fujimori Y, Uchide T, Saida K, Temma K, Sasaki T, Akera T 2003 Cloning of full-length preproendothelin-2 cDNA and its expression in dog. J Vet Med Sci 65:1217–1225 [DOI] [PubMed] [Google Scholar]
- Grimshaw MJ, Naylor S, Balkwill FR 2002 Endothelin-2 is a hypoxia-induced autocrine survival factor for breast tumor cells. Mol Cancer Ther 1:1273–1281 [PubMed] [Google Scholar]
- Smollich M, Wülfing P 2007 The endothelin axis: a novel target for pharmacotherapy of female malignancies. Curr Vasc Pharmacol 5:239–248 [DOI] [PubMed] [Google Scholar]
- Shinmi O, Kimura S, Sawamura T, Sugita Y, Yoshizawa T, Uchiyama Y, Yanagisawa M, Goto K, Masaki T, Kanazawa I 1989 Endothelin-3 is a novel neuropeptide: isolation and sequence determination of endothelin-1 and endothelin-3 in porcine brain. Biochem Biophys Res Commun 164:587–593 [DOI] [PubMed] [Google Scholar]
- Kanyicska B, Lerant A, Freeman ME 1998 Endothelin is an autocrine regulator of prolactin secretion. Endocrinology 139:5164–5173 [DOI] [PubMed] [Google Scholar]
- Masaki T, Ninomiya H, Sakamoto A, Okamoto Y 1999 Structural basis of the function of endothelin receptor. Mol Cell Biochem 190:153–156 [PubMed] [Google Scholar]
- Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S 1990 Cloning and expression of a cDNA encoding an endothelin receptor. Nature 348:730–732 [DOI] [PubMed] [Google Scholar]
- Maia H, Lopes AC, Coutinho EM 1974 Contractility of the human fallopian tube. Basic Life Sci 4:139–145 [DOI] [PubMed] [Google Scholar]
- Coutinho EM 1974 Ovarian contractility and ovulation. Res Reprod 6:3–4 [PubMed] [Google Scholar]
- Cameron IT, Bacon CR, Collett GP, Davenport AP 1995 Endothelin expression in the uterus. J Steroid Biochem Mol Biol 53:209–214 [DOI] [PubMed] [Google Scholar]
- Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, Yanagisawa M 1994 Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell 79:1267–1276 [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Sakamoto S, Kubota T, Aso T, Azuma H 2001 Localization and role of endothelin-1 and endothelin receptors in the human Fallopian tube. Mol Hum Reprod 7:1057–1063 [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M 1998 Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development 125:813–824 [DOI] [PubMed] [Google Scholar]
- Denkova R, Bourneva V, Yaneva E, Baleva K, Nikolov B, Ivanov I, Simeonov K, Timeva T 2002 Potential role of nitric oxide in endothelin-1 provoked inhibition of progesterone secretion by isolated ovarian granulosa cells. Endocr Regul 36:19–22 [PubMed] [Google Scholar]
- Tedeschi C, Lohman C, Hazum E, Ittoop O, Ben-Shlomo I, Resnick CE, Payne DW, Adashi EY 1994 Rat ovarian granulosa cell as a site of endothelin reception and action: attenuation of gonadotropin-stimulated steroidogenesis via perturbation of the A-kinase signaling pathway. Biol Reprod 51:1058–1065 [DOI] [PubMed] [Google Scholar]
- Flores JA, Garmey JC, Lahav M, Veldhuis JD 1999 Mechanisms underlying endothelin's inhibition of FSH-stimulated progesterone production by ovarian granulosa cells. Mol Cell Endocrinol 156:169–178 [DOI] [PubMed] [Google Scholar]
- Vacca F, Bagnato A, Catt KJ, Tecce R 2000 Transactivation of the epidermal growth factor receptor in endothelin-1-induced mitogenic signaling in human ovarian carcinoma cells. Cancer Res 60:5310–5317 [PubMed] [Google Scholar]
- Nelson JB, Udan MS, Guruli G, Pflug BR 2005 Endothelin-1 inhibits apoptosis in prostate cancer. Neoplasia 7:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflug BR, Zheng H, Udan MS, D'Antonio JM, Marshall FF, Brooks JD, Nelson JB 2007 Endothelin-1 promotes cell survival in renal cell carcinoma through the ET(A) receptor. Cancer Lett 246:139–148 [DOI] [PubMed] [Google Scholar]
- Shin I, Kim HJ, Lee JE, Gye MC 2006 Aquaporin7 expression during perinatal development of mouse brain. Neurosci Lett 409:106–111 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Ko C, Gieske MC, Al-Alem L, Hahn Y, Su W, Gong MC, Iglarz M, Koo Y 2006 Endothelin-2 in ovarian follicle rupture. Endocrinology 147:1770–1779 [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Constantini F, Lacy E 1994 Manipulating the mouse embryo: a laboratory manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 146–150 [Google Scholar]
- Jo M, Gieske MC, Payne CE, Wheeler-Price SE, Gieske JB, Ignatius IV, Curry Jr TE, Ko C 2004 Development and application of a rat ovarian gene expression database. Endocrinology 145:5384–5396 [DOI] [PubMed] [Google Scholar]
- Buhi WC, Alvarez IM, Kouba AJ 2000 Secreted proteins of the oviduct. Cells Tissues Organs 166:165–179 [DOI] [PubMed] [Google Scholar]
- Giudice LC 1994 Growth factors and growth modulators in human uterine endometrium: their potential relevance to reproductive medicine. Fertil Steril 61:1–17 [DOI] [PubMed] [Google Scholar]
- Ghosh D, Sengupta J 1998 Recent developments in endocrinology and paracrinology of blastocyst implantation in the primate. Hum Reprod Update 4:153–168 [DOI] [PubMed] [Google Scholar]
- Bongso A, Fong CY, Ng SC, Ratnam S 1994 Human embryonic behavior in a sequential human oviduct-endometrial coculture system. Fertil Steril 61:976–978 [DOI] [PubMed] [Google Scholar]
- Bareither ML, Verhage HG 1981 Control of the secretory cell cycle in cat oviduct by estradiol and progesterone. Am J Anat 162:107–118 [DOI] [PubMed] [Google Scholar]
- Shirley B, Reeder RL 1996 Cyclic changes in the ampulla of the rat oviduct. J Exp Zool 276:164–173 [DOI] [PubMed] [Google Scholar]
- Nelson J, Bagnato A, Battistini B, Nisen P 2003 The endothelin axis: emerging role in cancer. Nat Rev Cancer 3:110–116 [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Suzuki N, Onda H, Fujino M 1989 Abundance of endothelin-3 in rat intestine, pituitary gland and brain. Biochem Biophys Res Commun 164:74–80 [DOI] [PubMed] [Google Scholar]
- Burris TP, Kanyicska B, Freeman ME 1991 Inhibition of prolactin secretion by endothelin-3 is pertussis toxin-sensitive. Eur J Pharmacol 198:223–225 [DOI] [PubMed] [Google Scholar]
- Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M 1994 Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell 79:1277–1285 [DOI] [PubMed] [Google Scholar]
- Kanyicska B, Burris TP, Freeman ME 1991 Endothelin-3 inhibits prolactin and stimulates LH, FSH and TSH secretion from pituitary cell culture. Biochem Biophys Res Commun 174:338–343 [DOI] [PubMed] [Google Scholar]
- Kanyicska B, Freeman ME 1993 Characterization of endothelin receptors in the anterior pituitary gland. Am J Physiol 265:E601–E608 [DOI] [PubMed] [Google Scholar]
- Montani D, Souza R, Binkert C, Fischli W, Simonneau G, Clozel M, Humbert M 2007 Endothelin-1/endothelin-3 ratio: a potential prognostic factor of pulmonary arterial hypertension. Chest 131:101–108 [DOI] [PubMed] [Google Scholar]
- Selick CE, Horowitz GM, Gratch M, Scott Jr RT, Navot D, Hofmann GE 1994 Immunohistochemical localization of transforming growth factor-β in human implantation sites. J Clin Endocrinol Metab 78:592–596 [DOI] [PubMed] [Google Scholar]
- Kane MT, Morgan PM, Coonan C 1997 Peptide growth factors and preimplantation development. Hum Reprod Update 3:137–157 [DOI] [PubMed] [Google Scholar]
- Ge MX, Li H, Xing FQ, Kong LH, Chen SL 2003 [Transforming growth factor-β1, estradiol, progesterone and lutropin levels in follicular fluid after ovarian stimulation]. Di Yi Jun Yi Da Xue Xue Bao 23:463–465 (Chinese) [PubMed] [Google Scholar]
- Knight PG, Glister C 2003 Local roles of TGF-β superfamily members in the control of ovarian follicle development. Anim Reprod Sci 78:165–183 [DOI] [PubMed] [Google Scholar]
- Chow JF, Lee KF, Chan ST, Yeung WS 2001 Quantification of transforming growth factor β1 (TGFβ1) mRNA expression in mouse preimplantation embryos and determination of TGFβ receptor (type I and type II) expression in mouse embryos and reproductive tract. Mol Hum Reprod 7:1047–1056 [DOI] [PubMed] [Google Scholar]
- Kubota K, Omori Y, Ikeda S, Minegishi T 2009 Expression and cyclic change of betaglycan in the rat oviduct. J Reprod Dev 55:200–205 [DOI] [PubMed] [Google Scholar]
- Schmid P, Cox D, van der Putten H, McMaster GK, Bilbe G 1994 Expression of TGF-βs and TGF-β type II receptor mRNAs in mouse folliculogenesis: stored maternal TGF-β2 message in oocytes. Biochem Biophys Res Commun 201:649–656 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Chegini N, Flanders KC 1994 Human fallopian tube expresses transforming growth factor (TGFβ) isoforms, TGFβ type I-III receptor messenger ribonucleic acid and protein, and contains [125I]TGFβ-binding sites. J Clin Endocrinol Metab 79:1177–1184 [DOI] [PubMed] [Google Scholar]
- Barsig J, Küsters S, Vogt K, Volk HD, Tiegs G, Wendel A 1995 Lipopolysaccharide-induced interleukin-10 in mice: role of endogenous tumor necrosis factor-α. Eur J Immunol 25:2888–2893 [DOI] [PubMed] [Google Scholar]
- Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, Padbury J, Sharma S 2000 Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immunol 164:5721–5728 [DOI] [PubMed] [Google Scholar]
- Freytag SO, Geddes TJ 1992 Reciprocal regulation of adipogenesis by Myc and C/EBPα. Science 256:379–382 [DOI] [PubMed] [Google Scholar]
- Burgess-Beusse BL, Timchenko NA, Darlington GJ 1999 CCAAT/enhancer binding protein α (C/EBPα) is an important mediator of mouse C/EBPβ protein isoform production. Hepatology 29:597–601 [DOI] [PubMed] [Google Scholar]
- Timchenko NA, Harris TE, Wilde M, Bilyeu TA, Burgess-Beusse BL, Finegold MJ, Darlington GJ 1997 CCAAT/enhancer binding protein α regulates p21 protein and hepatocyte proliferation in newborn mice. Mol Cell Biol 17:7353–7361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timchenko NA, Wilde M, Nakanishi M, Smith JR, Darlington GJ 1996 CCAAT/enhancer-binding protein α (C/EBPα) inhibits cell proliferation through the p21 (WAF-1/CIP-1/SDI-1) protein. Genes Dev 10:804–815 [DOI] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS 2009 MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 324:938–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantena SR, Kannan A, Cheon YP, Li Q, Johnson PF, Bagchi IC, Bagchi MK 2006 C/EBPβ is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proc Natl Acad Sci USA 103:1870–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante BJ, Kannan A, Bagchi MK, Yuan L, Young SL 2009 Cyclic regulation of transcription factor C/EBPβ in human endometrium. Reprod Biol Endocrinol 7:15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.