Abstract
Uterine leiomyomata (ULs) represent the most common tumor in women and can cause abnormal uterine bleeding, large pelvic masses, and recurrent pregnancy loss. Although the dependency of UL growth on ovarian steroids is well established, the relative contributions of 17β-estradiol and progesterone are yet to be clarified. Conventionally, estradiol has been considered the primary stimulus for UL growth, and studies with cell culture and animal models support this concept. In contrast, no research model has clearly demonstrated a requirement of progesterone in UL growth despite accumulating clinical evidence for the essential role of progesterone in this tumor. To elucidate the functions of ovarian steroids in UL, we established a xenograft model reflecting characteristics of these tumors by grafting human UL tissue beneath the renal capsule of immunodeficient mice. Leiomyoma xenografts increased in size in response to estradiol plus progesterone through cell proliferation and volume increase in cellular and extracellular components. The xenograft growth induced by estradiol plus progesterone was blocked by the antiprogestin RU486. Furthermore, the volume of established UL xenografts decreased significantly after progesterone withdrawal. Surprisingly, treatment with estradiol alone neither increased nor maintained the tumor size. Although not mitogenic by itself, estradiol induced expression of progesterone receptor and supported progesterone action on leiomyoma xenografts. Taken together, our findings define that volume maintenance and growth of human UL are progesterone dependent.
Progesterone is the primary hormone that stimulates growth of uterine leiomyoma, whereas estrogens by themselves are not mitogenic but essential for the action of progesterone.
Uterine leiomyomata (ULs) or fibroids are benign smooth muscle tumors originating from the myometrium. The estimated cumulative incidence of tumors by age 50 yr is greater than 80% for African American women and nearly 70% for Caucasian women (1). Patients with UL can experience symptoms such as pelvic pressure, abnormal uterine bleeding, and recurrent pregnancy loss that require medical intervention (2). Surgical removal is the primary treatment option for symptomatic ULs, and more than 1 million hysterectomies were performed for the treatment of UL between 2001 and 2005 in the United States alone (3). With the high number of hysterectomies for this tumor, management of ULs has placed an enormous burden on the health care system. Therefore, development of efficacious preventative and therapeutic modalities that will allow avoidance of surgery not only improves quality of life but also carries significant public health implications.
Importance of 17β-estradiol (E2) and progesterone (P4) in the etiology of UL is well established. However, the relative contributions of estrogen vs. progestin and their functions in the pathogenesis of UL are still controversial. Conventionally, estrogen has been considered to be the primary growth promoter of ULs, and experimental evidence in both animal and cell culture models support this concept (4,5,6,7,8,9,10,11,12,13,14). In contrast, the effect of P4 on UL has not been well elucidated in these research models. For example, growth of spontaneous UL was stimulated by E2 but not P4 in the Eker rat model (7). Within a guinea pig model, UL formation induced by E2 treatment was inhibited by coadministration of P4 (15). In vitro, progestin can show similar growth-promoting effects as estrogen on primary culture of human UL cells by inhibiting apoptosis and stimulating proliferation (10,16,17). However, progestin can also inhibit UL growth under certain culture conditions (18,19).
In contrast to these research models, clinical studies have suggested that P4 plays an equally important role as E2 in the growth of ULs. Proliferation markers such as Ki67 and proliferating cell nuclear antigen (PCNA) are highest in UL in the luteal/secretory phase (20,21,22). Moreover, quantitative proliferation indices of ULs in postmenopausal women increased significantly with combined estrogen plus progestin replacement but not with estrogen replacement alone (20). These authors have even suggested that P4 may be the primary hormone driving the growth of UL. However, systemic hormone-levels in patients cannot be manipulated experimentally, and thus, the effects of estrogen vs. progestin on UL growth cannot be delineated solely by analysis of clinical specimens.
In total, there remains significant conflict regarding the role of P4 on human UL. Within human subjects, manipulation of systemic hormone levels beyond routine administration of estrogen/progesterone-containing medications is unethical, and thus, study findings are hindered by factors such as dosing and patient compliance. Furthermore, most UL-related procedures are scheduled within the first half of the menstrual cycle to avoid the possibility of pregnancy, thereby limiting the number of UL specimens obtained within the luteal/secretory phase. Thus, to advance research on UL, development of a novel model system that incorporates characteristics of original human tumors is essential.
In human ULs, cells are embedded in extracellular matrix (ECM), which is largely composed of collagens and glycosaminoglycans. ECM is known to influence several critical cellular functions, including apoptosis and cell proliferation (23,24). Indeed, myometrial and UL cells alter their gene expression pattern significantly in the organotypic three-dimensional environment in vivo vs. an artificial, two-dimensional culture within plastic dishes (25,26). In addition, accumulation of ECM contributes significantly to the growth of the tumor (27). These observations raise additional questions regarding the correlation between in vitro studies to human in situ tumors.
We report a method of xenografting and growing human ULs in immunodeficient mice. Xenografting into an immunodeficient mouse host is a standard approach in studying human tissues in vivo. Previously UL tissues with ECM were implanted under the skin of severe combined immunodeficiency mice (28). However, the UL xenografts were maintained only when their original phenotypes were altered by transduction of proangiogenic genes, PTGS2 (COX2) and VEGFA (28). These findings imply that the limiting factor for UL xenograft survival within subcutaneous (sc) tissue with low intrinsic vascular density was angiogenesis. Thus, we chose to use a subrenal capsule graft site because of its superior blood supply (29). On the kidney of nonobese diabetic (NOD)-scid IL2Rγnull mice (30), the survival rate of UL and normal myometrial tissue xenografts was nearly 100%, and the xenografts retained the histological characteristics of original tissue. Using this model, the objective of this study was to address the effects of E2 vs. P4 on UL growth.
Materials and Methods
Subrenal grafting of myometrial and UL tissues
All procedures involving animals in this study were approved by Northwestern University's Animal Care and Use Committee. Protocol for the acquisition of surgical specimens was approved by Northwestern University's Institutional Review Board. Surgically removed UL and normal myometrial tissue from the same patient were cut into small pieces (∼1 × 2 × 2 mm) and grafted onto opposing kidneys of adult female nonobese diabetic-scid IL2Rγnull mouse hosts (Jackson Laboratory, Bar Harbor, ME). The tissue pieces were high in water content, and thus, they became smaller under the pressure of subrenal capsule. The estimated starting volume of tissue grafts under the renal capsule was approximately 1 mm3. The growth of UL in intact female hosts without hormone treatment was minimum and inconsistent. Therefore, all hosts were ovariectomized (OVX) and supplemented with sc implantation of 50 mg P4 plus 50 μg E2 60-d slow-release pellets (Innovative Research of America Inc., Sarasota, Fl). Stable dosages of E2 (50 μg per 60 d) and P4 (50 mg per 60 d) were used throughout the study. These dosages were chosen because previous studies demonstrated that they were able to sustain systemic E2 and P4 levels within cycling women (31,32,33,34,35). Additionally, if required by the experimental design of the specific study, RU486, a progesterone receptor (PR) antagonist, was also given as sc implant (slow release pellet containing 25 mg per 60 d; Innovative Research of America). The effects of ovariectomy and hormone treatments were then confirmed by gross appearance and histology of host female reproductive tracts. The presence of hormone pellets was also confirmed at the time of termination of the host.
Preparation of cell graft
Fresh surgical specimens of human myometrial/UL tissues were digested into single cells by type I collagenase (Sigma, St. Louis, MO) and cultured for 2–3 d as previously described (36). Cells were collected from the culture plates by trypsin digestion and suspended into rat-tail collagen (type I) solution (BD Bioscience, San Jose, CA) at 106 cells per 10 μl. The method has been successfully used to study hormonal response of human endometrial tissue in our previous report (37). The low-density collagen gel consisted mostly of water, and thus, the pellet volume (10 μl) did not reflect the starting volume of tumor. When cell pellets were incubated at 37 C overnight as floating-culture, they became smaller than 1 mm in diameter by contraction of collagen by UL cells. Therefore, the estimated starting volume of cell graft was smaller than 0.6 mm3.
Data analysis
The tumor volume was estimated using the formula: volume (cubic millimeters) = 0.52 (derived from π/6) × length × width × height (millimeters) (38). When tumor volume of the E2+P4 group was less than 1.0 mm3 at 8 wk after grafting, the UL was categorized as growth negative. In the statistical analysis, average value (e.g. tumor volume, cell density, labeling index) of three to six xenografts per group in each experiment was considered as a single measurement. Only the experiments with the complete set of hormone treatment groups (e.g. OVX, E2, P4, E2+P4, and E2+P4+RU486) were included in the analyses of tumor volume, Ki67 labeling, cell size index, and cell density.
Histological analysis
The following histological analyses were performed routinely for all xenografts and the original tissues: hematoxylin and eosin (H&E) staining, immunohistochemistry (IHC) for estrogen receptor (ER)-α (LabVision, Fremont, CA), PR (Dako, Carpinteria, CA), Ki67 (proliferation marker; Novacastra Laboratories, Burlingame, CA) and active caspase-3 (apoptosis marker; Cell Signaling, Danvers, MA) (37,39). Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay was performed using Apo-Brd UTM DNA fragmentation assay kit (BioVision, Mountain View, CA). The cellular component was detected by IHC for β-actin (Abcam, Cambridge, MA). The cell density was determined on H&E sections by counting the number of nuclei per optical field with a ×20 objective lens. Morphometric analysis of immunohistochemical staining has been described previously (39). Images of β-actin-stained tissues were captured with a Leica microscope imaging system (Leica Microsystems Inc., Bannockburn, IL). Total and β-actin-positive tissue areas were measured with ImageJ (National Institutes of Health, http://rsbweb.nih.gov/ij/index.html). The area with OD higher than 180 [from 255 (white) to 0 (black)] in blue channel of RGB mode was considered negative. Relative cell size was calculated by β-actin-positive area per cell number and expressed as relative value to that of 2 wk after grafting.
Results
Growth of UL tissue in mouse hosts
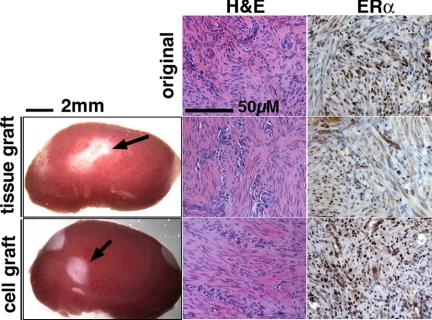
All myometrial grafts (74 grafts) survived for 8 wk but did not show detectable growth with any hormone treatments (Fig. 1), indicating the presence of an intrinsic growth-suppressive mechanism within normal adult myometrium. Due to small tissue size, further analysis was difficult for myometrial xenografts. Thus, we focused on UL in the following analyses. In contrast, UL xenografts increased their size in response to E2+P4 but not to either E2 or P4 alone. Among a total of 29 UL cases, xenografts of 14 cases (48.2%) increased in size in response to E2+P4 treatment (growth-positive UL), whereas there was no detectable growth (tumor size <1 mm3) in the other 15 cases (growth-negative UL). The most noticeable difference between growth-positive and -negative ULs was the cell density in the original patient tissue. Specifically, growth-positive xenografts arose from patient tissue in which the original cell density was significantly higher (831 ± 113 cells/area, n = 12), whereas growth-negative UL cases demonstrated fewer cells within the original patient tissue (425 ± 155 cells/area, n = 12) (Student's t test, P < 0.05). Thus, the number of cells/xenograft significantly affected growth of UL xenografts. This conclusion was further confirmed by cell-grafting experiments in the following section. Because the absence of growth was simply due to the quality of the original tissue, we excluded the growth-negative UL cases from the following analyses.
Figure 1.
Gross appearance and histology of myometrial xenografts. Gross appearance of myometrial tissue and cell xenografts (indicated by an arrow) on the host kidney (treated with E2+P4) at 8 wk after grafting. Although the myometrial xenografts did not increase in size with any hormone treatment, both tissue and cell xenografts (with E2 treatment) showed typical histology of myometrium comparable with the original tissue (top panels) in H&E and ERα-IHC staining.
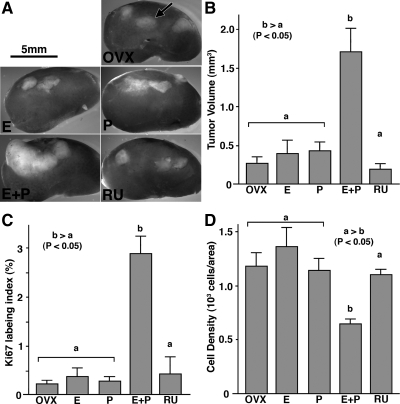
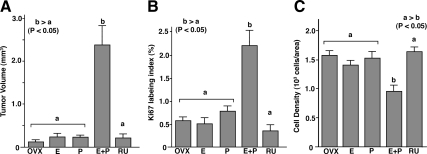
Tumor volume was significantly higher in the E2+P4 group than the other four groups (Fig. 2, A and B). The growth-promoting effect of E2+P4 was completely blocked by coadministration of RU486, a PR antagonist (Fig. 2). Therefore, P4 induced tumor growth via PR. There was no significant difference in the tumor volume (P < 0.05) between E2+P4+RU486, E2 alone, P4 alone, and untreated control groups, confirming the requirement of both E2 and P4 for human UL growth. In fact, E2 alone failed to increase tumor size, even when a supraphysiological dose was administered (∼10 μg/d) (n = 4).
Figure 2.
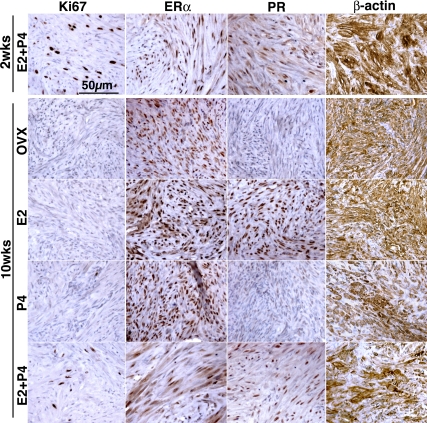
Effect of E2 and P4 on the growth of UL xenografts. OVX, OVX control (no hormone); E, E2 treated; P, P4 treated; E+P, E2+P4 treated; RU, E2+P4+RU486 treated groups. UL tissues were grown as subrenal grafts in OVX mouse host treated with E2 (E), P4 (P), E2+P4 (E+P), or E2+P4+RU486 (RU) for 8 wk. Gross appearance of xenografts on host kidney (A; graft is indicated by an arrow), tumor volume (B), Ki67 labeling index (C), and cell density (D). The bar indicates average value ± sem. Statistical differences were assessed by using the Kruskal-Wallis test (n = 6, P < 0.05). UL tissues enlarged in response to E2 plus P4 treatment (A and B). E2+P4 also increased ki67 labeling index (C) and decreased cell density (D). This growth-promoting effect of E2 plus P4 treatment was blocked by RU486. E2 and P4 alone had no effect on the growth of UL tissues.
The Ki67 index was significantly higher (Fig. 2C) in the E2+P4 group than the other four groups, indicating that E2+P4-stimulated tumor growth via proliferation of UL cells. At the same time, cell density was significantly lower in the E2+P4-treated group (Fig. 2D), suggesting that an increase in ECM volume and/or cell size also contributed to the enlargement of tumor. This issue was further assessed in the following section.
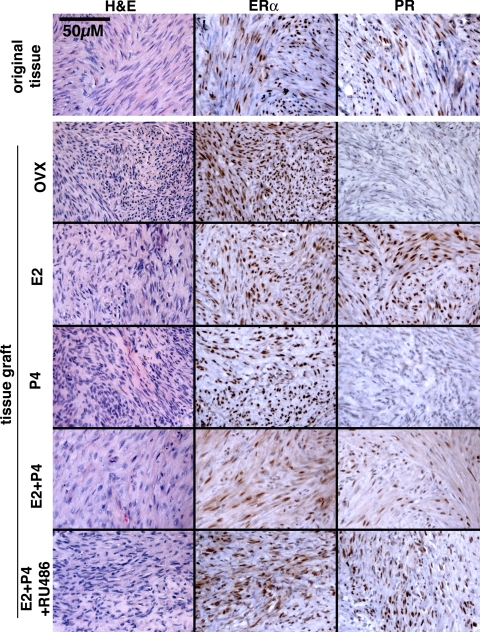
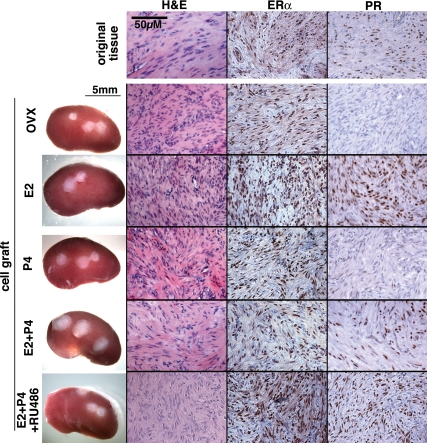
As assessed by IHC, ERα was expressed in the xenografts of all five groups (Fig. 3). In contrast, expression of PR in UL xenografts was totally dependent on E2. The level of PR was very low to undetectable in the untreated-control (OVX) and P4 groups but high in the E2, E2+P4 and E2+P4+RU486 groups (Fig. 3). These results indicate that E2 is essential for P4 to act on ULs in vivo.
Figure 3.
Histology of UL xenografts and original tumor. OVX, No hormone control. UL xenografts survived for 8 wk and retained histology typical for leiomyoma in all hormone treatment groups. Whereas ERα was expressed in all xenografts irrespective of hormone treatment, expression of PR was dependent on E2. Thus, PR was undetectable in OVX and P4 groups. All histology images were taken under a ×20 objective lens and in the same magnification.
Growth of established tumors
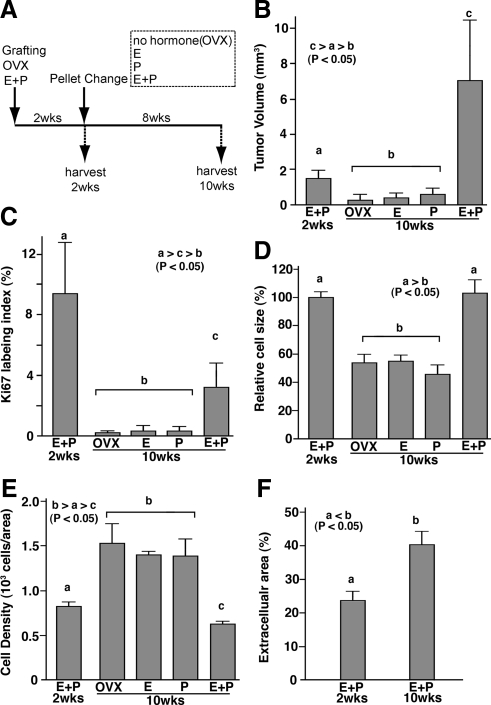
In our xenograft model, tissue grafts must first establish a blood vessel network to survive and subsequently grow. Although the kidney is highly vascular and thus capable of supporting survival of tissues grafted onto it, efficiency in blood vessel recruitment can be a limiting factor for subsequent growth of xenograft (29). Therefore, lack of growth within xenografts treated with E2 alone may be explained by a requirement of P4 for UL xenografts to sufficiently establish an adequate vascular network. Thus, E2 may still be a mitogen for established ULs. To examine this possibility, xenografts were established first in the hosts with the optimum hormone treatment (E2+P4) and then subjected to different hormone treatments. All hosts were ovariectomized and supplemented with E2 and P4 at the time of grafting. Two weeks later, to allow establishment of vascularization, the hosts were divided into four groups, and hormone pellets were changed as indicated (Fig. 4A). After the hormone pellet change, established UL xenografts increased their size further only with E2+P4 treatment (Fig. 4B). In contrast, E2 or P4 alone failed to maintain tumor size. All xenografts of groups in which E2 and/or P4 was withdrawn exhibited significant reduction in tumor volume and Ki67 labeling index (Figs. 4 and 5). These results confirm the essential role of E2 and P4 in growth and maintenance of ULs. All xenografts were negative for apoptotic cells as assessed by active caspase-3 IHC and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay. Absence of apoptotic cells at 8 wk after hormone withdrawal (data not shown) does not necessarily mean that UL cells do not die via apoptosis during regression of tumor. Nevertheless, UL cells appeared healthy and showed no signs of tissue degradation as shown in Fig. 5. Thus, loss of cells via apoptosis does not appear to play a major role in tumor regression. Instead, UL cells reduced their size significantly after withdrawal of E2 and/or P4 (Fig. 4D). Accordingly, cell density increased significantly in these groups (Figs. 4E and 5). This result indicates that cell size reduction is one of the mechanisms via which UL shrinks.
Figure 4.
Effect of hormone withdrawal on UL xenograft. A, Time line. UL xenografts were grown for 2 wk with E2+P4 (E+P 2wks), and then hormone pellets were replaced with E2+P4 (E+P), E2 (E), P4 (P), or no hormone (OVX). Eight weeks after pellet replacement (10 wk after grafting), xenografts were harvested and analyzed. Tumor volume (B), Ki67 labeling index (C), relative cell size (D; relative value to average cell size at 2 wk after grafting), cell density (E), and extracellular area (β-actin negative area; F). Bars indicate average value ± sem. Statistical differences were assessed by using the Kruskal-Wallis test (n = 6, P < 0.05). Tumor volume increased only with E2+P4 treatment (B). In response to withdrawal of E2 and/or P4, tumor volume (B), Ki67 labeling index (C), and cell size (D) significantly declined. Accordingly, cell density increased in OVX, E, and P groups (E). In the E+P group, cell size remained the same; however, the cell density decreased significantly (E) with increase of extracellular component (F) from 2 to 10 wk after grafting, indicating tumor volume increased by both cell number and ECM volume.
Figure 5.
Histology of UL xenografts subjected to hormone withdrawal. Proliferation activity significantly declined in response to withdrawal of E2 and/or P4 as assessed by Ki67 expression. PR was also down-regulated in response to E2 withdrawal. However, UL xenografts did not show sign of tissue degradation or cell death, and expression of ERα and β-actin was maintained without E2 and/or P4, even after 8 wk. All histology images were taken under a ×20 objective lens and in the same magnification.
In the E2+P4-treated group, cell size remained the same (Fig. 4D), but the cell density significantly decreased from 2 to 10 wk after grafting (Fig. 4E), suggesting an enlargement of ECM. Indeed, β-actin-negative areas significantly increased from 2 to 10 wk (Fig. 4F). Therefore, E2 and P4 stimulate UL growth via increase in cell number and ECM volume. Ki67 was coexpressed with ERα and PR (Supplemental Fig. 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org), suggesting that Ki67-positive cells were the direct target of E2 and P4. As tumor volume increased, the Ki67 labeling index decreased significantly from 9.4 ± 3.4% at 2 wk to 3.1 ± 1.6% at 10 wk (mean ± sd) (Fig. 4C). This result indicates that the peak for cell proliferation is an early event in the UL growth, whereas increase of ECM appears to occur at a constant rate.
Although it was not statistically significant, cell size appeared smaller (Fig. 4D) and ECM volume appeared higher (Fig. 4E) in the P4-alone group than control and E2-alone groups (Fig. 5). It suggests that P4 may have some effects on UL cell, even in the absence of E2.
Reconstruction of UL tissues from cultured UL cells
Because of the significant heterogeneity that exists within human tissue, comparison between subjects is difficult. Factors including cell density of the original surgical specimen and the distribution of UL cells within the sampled portion of the tumor can affect growth of the xenograft. To address this issue, tumor tissue was digested into single cells, and UL tissue was reconstructed by suspending the isolated UL cells in type I collagen. After 8 wk of in vivo growth with E2+P4 treatment, UL cell grafts containing 106 cells formed uniform tissues with histology comparable with the original tumor (Fig. 6). For efficient tumor growth, a concentration of 106 cells/graft was the optimum. With lower cell concentrations (5.0 × 105 cell/graft and lower), growth of xenografts was inconsistent. With higher cell concentration (5.0 × 106 UL cells/graft), the collagen gel became too soft to graft. To test whether normal myometrium was able to grow as cell grafts, the same cell-graft protocol was applied to the normal human myometrium. Xenografts of reconstructed myometrium (106 cells/graft) formed a tissue with typical histology of myometrium, but graft volume did not increase with E2, P4, or E2+P4 treatment for 8 wk (Fig. 1, n = 12).
Figure 6.
Gross appearance and the histology of UL cell xenografts. Xenografts constructed with 106 dissociated leiomyoma cells formed tumors with typical histology of UL. Expression of ERα and PR was also comparable with that of tissue xenografts (Fig. 3). All histology images were taken under a ×20 objective lens and in the same magnification.
Previous studies demonstrated that UL cells alter gene expression profile in vitro and lose expression of ERα and PR rapidly (26). Therefore, expression of these receptors in cell grafts was assessed by IHC. As in the tissue xenografts, ERα was expressed in all xenografts of UL cells, whereas PR was expressed only in the presence of E2 (Fig. 6). The growth response of UL cell grafts to E2 and P4 was identical with that of tissue grafts. The growth of UL cell grafts was stimulated by E2+P4 and inhibited by RU486 (Fig. 7). Moreover, E2+P4 treatment increased Ki67 labeling index (Fig. 7B) and reduced cell density significantly (Fig. 7C).
Figure 7.
Growth of UL cell xenografts. OVX, OVX control (no hormone); E, E2 treated; P, P4 treated; E+P, E2+P4 treated; RU, E2+P4+RU486 treated groups. UL cell xenografts were grown in OVX mouse host treated with E2 (E), P4 (P), E2+P4 (E+P), or E2+P4+RU486 (RU) for 8 wk. As assessed by tumor volume (A), Ki67 labeling index (B), and cell density (C), the response of UL cell xenografts to E2 and P4 was essentially identical with that of tissue xenografts (Fig. 2). Xenografts increased their size in response to E2 plus P4 treatment (A). E2+P4 also increased ki67 labeling index (B) and decreased cell density (C). This growth-promoting effect of E2 plus P4 treatment was blocked by RU486. E2, P4 alone had no effect on the growth of UL cell grafts. The bars indicate average value ± sem. Statistical differences were assessed by using the Kruskal-Wallis test (n = 6, P < 0.05).
The efficacy of the cell graft method was experimentally tested by comparing growth of the UL cell graft vs. that of the UL tissue xenograft in tissue/cells obtained from the same subject. Among three UL cases, two cases with relatively low cell density (cases 128 and 135) did not show detectable growth in response to E2+P4 as tissue xenografts (Table 1). However, when cell grafts were generated from these same UL tissues, the volume of all three cases increased significantly in response to E2+P4 treatment (Table 1 and Fig. 7). The overall rate of growth-positive tumors in the entire experiment improved from 48.2% (14 of 29) in tissue grafts to 76.4% (13 of 17) in cell grafts. This result further confirmed that the absence of growth as tissue xenograft was mostly due to the low cell number and/or thick ECM layer in the original tissues from which the tissue xenografts were generated. The growth-positive and -negative ULs do not represent two different entities with different hormone requirement. Our study suggests that all human ULs require E2 and P4 for growth and volume maintenance.
Table 1.
Efficacy comparison between tissue and cell grafts
| Case no. | Donor
|
Original UL
|
Xenograft size (mm3)
|
|||
|---|---|---|---|---|---|---|
| Age (yr) | Phase | Nodule size (cm) | Cell density | Tissue graft | Cell graft | |
| 128 | 31 | Follicular | 12 × 12 × 10 | 513 | Growth negative (<0.5) | 2.4 |
| 130 | 34 | Follicular | 8 × 6 × 6 | 901 | 4.3 | 9.6 |
| 135 | 37 | Luteal | 8 × 8 × 6 | 373 | Growth negative (<0.5) | 1.5 |
Discussion
There has been conflicting evidence for the role of P4 in the regulation of UL growth. This is the first study to definitively demonstrate that both growth and maintenance of human UL in vivo are dependent on P4 action via PR. Our conclusion agrees with the results of recent clinical trials of selective progesterone receptor modulators for ULs (40,41,42,43,44). This study also defined for the first time that estrogen by itself is not a mitogen for human UL in vivo. Nonetheless, our findings do not discount the importance of estrogen in UL. PR is widely recognized as a marker for estrogen action, and regulation of the PR gene (PGR) by estrogen/ER has been well defined (45). Because the PR protein level is thought to be a critical determinant of sensitivity to P4, almost all hormone treatment protocols designed to elicit effects of P4 involve priming with estrogen. This was also true for human UL in vivo, and the expression of PR was dependent on E2 in UL xenografts. Based on the results of the current study, we propose the following model of UL growth control. P4 action via PR increases tumor volume through cell proliferation and ECM accumulation. E2 is not a mitogen but is required for the growth and maintenance of ULs to sensitize cells to P4 by inducing PR. This model is currently being tested by whether PR overexpression replaces E2 action in P4-dependent UL growth.
Because coadministration of E2 with P4 was essential for growth and maintenance, inhibition of ER should also be an effective treatment for UL. However, efficacy of selective ER modulators in clinical trials for UL has been inconsistent (46). This may be due to the technical difficulty in blockade of ER signaling in vivo (47). In addition, our study suggests that E2 is required only for up-regulation of PR, which should be achieved with a relatively low level of E2. In reality, effective blockade of E2/ER signaling is difficult to achieve and should cause severe adverse side effects such as hot flashes and osteoporosis. Hence, the systemic inhibition of E2/ER by antiestrogens may not be a practical option for treatment of symptomatic ULs.
Previously clinical observations associated with pregnancy have raised questions about the growth-promoting effect of P4 on human UL. The major criticism is a lack of conclusive evidence for the enlargement of UL during pregnancy despite the elevated systemic P4 level (48,49,50). Furthermore, epidemiological evidence indicates that parity is protective, rather than promoting, for UL development (51,52). However, there are several reasons these findings may not necessarily reflect the role of P4 on ULs. First, the majority of longitudinal studies examining the natural history of UL in pregnancy enrolled patients that were found to have UL on their obstetrical ultrasounds after pregnancy was well established. It is possible that early exposure to elevated P4 levels induced UL growth that was not detected given the timing of enrollment of the subjects. This is supported by the fact that when ULs do increase in size during pregnancy, the majority of growth occurs by the 10th week of gestation (49). Furthermore, pregnancy-associated stretching and hypertrophy of myometrium may affect tumor volume. Lastly, pregnancy physiology affects multiple systems including immunological and vascular systems within the uterus (53). In total, multiple factors likely contribute to UL size in pregnancy, and the phenotype of UL in pregnancy is not necessarily representative of increased systemic E2 and P4. Indeed, data on miscarriage or induced abortion suggest that the protective effects of parity for UL may be associated with an event at delivery or during the postpartum process (52,54,55,56). Therefore, the absence of detectable growth in ULs during pregnancy does not necessarily contradict with our findings of UL P4 dependency.
Our study also clearly demonstrated that E2 was not mitogenic for human ULs in vivo. In contrast, studies with cell culture and animal models repeatedly demonstrated mitogenic effects of estrogens on UL cells (4,5,6,7,8,9,10,11,12,13,14). Therefore, estrogen/ER signal transduction in cell culture and animal models should significantly differ from that in human UL in vivo. In the case of UL cell culture, the reduced levels of ERα and PR may be the reason for the aberrant actions of E2 and P4 in vitro (26). The difference in hormone responsiveness between human and rodent leiomyoma may reflect differences in the intrinsic growth control mechanism of human and rodent myometrial cells. Previously we demonstrated fundamental differences of human vs. mouse endometrial epithelial cells in the estrogen-regulated proliferation by xenograft experiments (37). Considering fundamental differences in cellular kinetics of menstrual vs. estrous cycles and the duration of cycles, growth control of uterine cells should be significantly different in human vs. rodent. Rodent UL models may have some limitation in the studies of growth control of human UL.
Within the clinical realm, tumor growth can be assessed only by gross volume change. However, the volume of UL reflects multiple factors such as cell number/size, ECM, and water content. Therefore, tumor growth is not necessarily due to the proliferation of UL cells. To envision therapeutic strategies, it is essential to understand how ovarian steroids regulate the tumor volume. For example, if tumors grow solely by accumulation of ECM, inhibition of cell proliferation should not be an effective treatment. The xenograft model of human UL serves as an ideal tool to dissect the mechanism of tumor growth. Our results indicate that the enlargement of UL xenografts involved cell proliferation, hypertrophy of UL cells, and volume increase in extracellular component, all of which were stimulated by E2+P4. Whereas Ki67 labeling index of UL xenografts significantly declined, the rate of tumor growth by volume appeared to increase as the tumor grew. It is quite possible that efficiency in the exchange of oxygen, nutrients, and waste products between UL cells and blood reduces as the ECM thickens, and in turn ECM negatively affects proliferation activity of UL cells. In such a case, inhibition of ECM accumulation may stimulate proliferation of UL cells by improving the molecular exchange efficiency between UL cells and blood. Parallel to the growth, shrinkage of UL also appeared to be primarily via the reduction in the cell size and extracellular components but not the loss of leiomyoma cells. Therefore, inhibition of PR is only a temporary suppression of tumor and not a cure for UL. It agrees with the clinical observation that regrowth of shrunken UL occurs slowly after cessation of RU486 treatment for premenopausal woman (57).
To improve the efficiency of UL xenograft growth, we developed the cell graft system in which UL tissues were reconstructed with dissociated single UL cells. The phenotype and hormone responsiveness of cell grafts were identical with those of tissue xenografts. The advantages of cell graft over tissue graft were reproducibility of cell number in each xenograft and improved success rates in xenograft growth. In addition, transgenic human ULs can be generated from UL cells transduced with genes of interest. In total, the cell graft model is a novel and relevant tool for basic research on UL growth. The knowledge gained from these studies is critical if effective treatments are to be discovered.
Supplementary Material
Acknowledgments
The authors thank Dr. Emily Su (Northwestern University) for critical reading of the paper.
Footnotes
This work was supported by Friends of Prentice and The National Institutes of Health Grant HD057877.
Disclosure Summary: H.I., K.I., V.A.S., R.K., and T.K. have nothing to declare. S.E.B. has served on the advisory board for Repros Inc., Meditrina Inc., and Novartis Inc.
First Published Online April 7, 2010
Abbreviations: E2, 17β-Estradiol; ECM, extracellular matrix; ER, estrogen receptor; H&E, hematoxylin and eosin; IHC, immunohistochemistry; OVX, ovariectomy; P4, progesterone; PR, progesterone receptor; SCID, severe combined immunodeficiency; UL, uterine leiomyoma.
References
- Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM 2003 High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 188:100–107 [DOI] [PubMed] [Google Scholar]
- Parker WH 2007 Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 87:725–736 [DOI] [PubMed] [Google Scholar]
- Merrill RM 2008 Hysterectomy surveillance in the United States, 1997 through 2005. Med Sci Monit 14:CR24–CR31 [PubMed] [Google Scholar]
- Shimomura Y, Matsuo H, Samoto T, Maruo T 1998 Up-regulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J Clin Endocrinol Metab 83:2192–2198 [DOI] [PubMed] [Google Scholar]
- Park SH, Ramachandran S, Kwon SH, Cha SD, Seo EW, Bae I, Cho C, Song DK 2008 Upregulation of ATP-sensitive potassium channels for estrogen-mediated cell proliferation in human uterine leiomyoma cells. Gynecol Endocrinol 24:250–256 [DOI] [PubMed] [Google Scholar]
- Porter KB, Tsibris JC, Nicosia SV, Murphy JM, O'Brien WF, Rao PS, Spellacy WN 1995 Estrogen-induced guinea pig model for uterine leiomyomas: do the ovaries protect? Biol Reprod 52:824–832 [DOI] [PubMed] [Google Scholar]
- Burroughs KD, Fuchs-Young R, Davis B, Walker CL 2000 Altered hormonal responsiveness of proliferation and apoptosis during myometrial maturation and the development of uterine leiomyomas in the rat. Biol Reprod 63:1322–1330 [DOI] [PubMed] [Google Scholar]
- Howe SR, Gottardis MM, Everitt JI, Walker C 1995 Estrogen stimulation and tamoxifen inhibition of leiomyoma cell growth in vitro and in vivo. Endocrinology 136:4996–5003 [DOI] [PubMed] [Google Scholar]
- Blin C, L'Horset F, Romagnolo B, Colnot S, Lambert M, Thomasset M, Kahn A, Vandewalle A, Perret C 1996 Functional and growth properties of a myometrial cell line derived from transgenic mice: effects of estradiol and antiestrogens. Endocrinology 137:2246– 2253 [DOI] [PubMed] [Google Scholar]
- Barbarisi A, Petillo O, Di Lieto A, Melone MA, Margarucci S, Cannas M, Peluso G 2001 17β-Estradiol elicits an autocrine leiomyoma cell proliferation: evidence for a stimulation of protein kinase-dependent pathway. J Cell Physiol 186:414–424 [DOI] [PubMed] [Google Scholar]
- Cramer SF, Robertson Jr AL, Ziats NP, Pearson OH 1985 Growth potential of human uterine leiomyomas: some in vitro observations and their implications. Obstet Gynecol 66:36–41 [PubMed] [Google Scholar]
- Sumitani H, Shozu M, Segawa T, Murakami K, Yang HJ, Shimada K, Inoue M 2000 In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology 141:3852–3861 [DOI] [PubMed] [Google Scholar]
- Andersen J, DyReyes VM, Barbieri RL, Coachman DM, Miksicek RJ 1995 Leiomyoma primary cultures have elevated transcriptional response to estrogen compared with autologous myometrial cultures. J Soc Gynecol Investig 2:542–551 [DOI] [PubMed] [Google Scholar]
- Di X, Yu L, Moore AB, Castro L, Zheng X, Hermon T, Dixon D 2008 A low concentration of genistein induces estrogen receptor-α and insulin-like growth factor-I receptor interactions and proliferation in uterine leiomyoma cells. Hum Reprod 23:1873–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipschütz A, Vargas Jr L 1941 Structure and origin of uterine and extragenital fibroids induced experimentally in the guinea pig by prolonged administration of estrogens. Cancer Res 1:236–249 [Google Scholar]
- Matsuo H, Maruo T, Samoto T 1997 Increased expression of Bcl-2 protein in human uterine leiomyoma and its up-regulation by progesterone. J Clin Endocrinol Metab 82:293–299 [DOI] [PubMed] [Google Scholar]
- Chen W, Ohara N, Wang J, Xu Q, Liu J, Morikawa A, Sasaki H, Yoshida S, Demanno DA, Chwalisz K, Maruo T 2006 A novel selective progesterone receptor modulator asoprisnil (J867) inhibits proliferation and induces apoptosis in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells. J Clin Endocrinol Metab 91:1296–1304 [DOI] [PubMed] [Google Scholar]
- Maruo T, Ohara N, Wang J, Matsuo H 2004 Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum Reprod Update 10:207–220 [DOI] [PubMed] [Google Scholar]
- Yamada T, Nakago S, Kurachi O, Wang J, Takekida S, Matsuo H, Maruo T 2004 Progesterone down-regulates insulin-like growth factor-I expression in cultured human uterine leiomyoma cells. Hum Reprod 19:815–821 [DOI] [PubMed] [Google Scholar]
- Lamminen S, Rantala I, Helin H, Rorarius M, Tuimala R 1992 Proliferative activity of human uterine leiomyoma cells as measured by automatic image analysis. Gynecol Obstet Invest 34:111–114 [DOI] [PubMed] [Google Scholar]
- Kawaguchi K, Fujii S, Konishi I, Nanbu Y, Nonogaki H, Mori T 1989 Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol 160:637–641 [DOI] [PubMed] [Google Scholar]
- Tiltman AJ 1985 The effect of progestins on the mitotic activity of uterine fibromyomas. Int J Gynecol Pathol 4:89–96 [DOI] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM 2001 Taking cell-matrix adhesions to the third dimension. Science 294:1708–1712 [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM 2001 Transmembrane cross talk between the extracellular matrix-cytoskeleton cross talk. Nat Rev Mol Cell Biol 2:793–805 [DOI] [PubMed] [Google Scholar]
- Zaitseva M, Vollenhoven BJ, Rogers PA 2006 In vitro culture significantly alters gene expression profiles and reduces differences between myometrial and fibroid smooth muscle cells. Mol Hum Reprod 12:187–207 [DOI] [PubMed] [Google Scholar]
- Severino MF, Murray MJ, Brandon DD, Clinton GM, Burry KA, Novy MJ 1996 Rapid loss of oestrogen and progesterone receptors in human leiomyoma and myometrial explant cultures. Mol Hum Reprod 2:823–828 [DOI] [PubMed] [Google Scholar]
- Stewart EA, Friedman AJ, Peck K, Nowak RA 1994 Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab 79:900–906 [DOI] [PubMed] [Google Scholar]
- Hassan MH, Eyzaguirre E, Arafa HM, Hamada FM, Salama SA, Al-Hendy A 2008 Memy I: a novel murine model for uterine leiomyoma using adenovirus-enhanced human fibroid explants in severe combined immune deficiency mice. Am J Obstet Gynecol 199:156.e1–156.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xue H, Cutz JC, Bayani J, Mawji NR, Chen WG, Goetz LJ, Hayward SW, Sadar MD, Gilks CB, Gout PW, Squire JA, Cunha GR, Wang YZ 2005 An orthotopic metastatic prostate cancer model in SCID mice via grafting of a transplantable human prostate tumor line. Lab Invest 85:1392–1404 [DOI] [PubMed] [Google Scholar]
- Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R 2005 Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R γ null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 174:6477–6489 [DOI] [PubMed] [Google Scholar]
- Bebo Jr BF, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H 2001 Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol 166:2080–2089 [DOI] [PubMed] [Google Scholar]
- McDonnel AC, Van Kirk EA, Isaak DD, Murdoch WJ 2003 Inhibitory effects of progesterone on plasma membrane fluidity and tumorigenic potential of ovarian epithelial cancer cells. Exp Biol Med (Maywood) 228:308–314 [DOI] [PubMed] [Google Scholar]
- McDonnel AC, Van Kirk EA, Isaak DD, Murdoch WJ 2005 Effects of progesterone on ovarian tumorigenesis in xenografted mice. Cancer Lett 221:49–53 [DOI] [PubMed] [Google Scholar]
- Gross G, Imamura T, Vogt SK, Wozniak DF, Nelson DM, Sadovsky Y, Muglia LJ 2000 Inhibition of cyclooxygenase-2 prevents inflammation-mediated preterm labor in the mouse. Am J Physiol Regul Integr Comp Physiol 278:R1415–R1423 [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Suzuki T, Suzuki S, Niikawa H, Lu L, Miki Y, Moriya T, Hayashi S, Handa M, Kondo T, Sasano H 2005 Progesterone receptor in non-small cell lung cancer—a potent prognostic factor and possible target for endocrine therapy. Cancer Res 65:6450–6458 [DOI] [PubMed] [Google Scholar]
- Yin P, Lin Z, Cheng YH, Marsh EE, Utsunomiya H, Ishikawa H, Xue Q, Reierstad S, Innes J, Thung S, Kim JJ, Xu E, Bulun SE 2007 Progesterone receptor regulates Bcl-2 gene expression through direct binding to its promoter region in uterine leiomyoma cells. J Clin Endocrinol Metab 92:4459–4466 [DOI] [PubMed] [Google Scholar]
- Kurita T, Medina R, Schabel AB, Young P, Gama P, Parekh TV, Brody J, Cunha GR, Osteen KG, Bruner-Tran KL, Gold LI 2005 The activation function-1 domain of estrogen receptor α in uterine stromal cells is required for mouse but not human uterine epithelial response to estrogen. Differentiation 73:313–322 [DOI] [PubMed] [Google Scholar]
- Hann HW, Stahlhut MW, Rubin R, Maddrey WC 1992 Antitumor effect of deferoxamine on human hepatocellular carcinoma growing in athymic nude mice. Cancer 70:2051–2056 [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee K, Saunders PT, Cooke PS, Taylor JA, Lubahn DB, Zhao C, Mäkelä S, Gustafsson JÅ, Dahiya R, Cunha GR 2001 Regulation of progesterone receptors and decidualization in uterine stroma of the estrogen receptor-α knockout mouse. Biol Reprod 64:272–283 [DOI] [PubMed] [Google Scholar]
- Carbonell Esteve JL, Acosta R, Heredia B, Pérez Y, Castañeda MC, Hernández AV 2008 Mifepristone for the treatment of uterine leiomyomas: a randomized controlled trial. Obstet Gynecol 112:1029–1036 [DOI] [PubMed] [Google Scholar]
- Fiscella K, Eisinger SH, Meldrum S, Feng C, Fisher SG, Guzick DS 2006 Effect of mifepristone for symptomatic leiomyomata on quality of life and uterine size: a randomized controlled trial. Obstet Gynecol 108:1381–1387 [DOI] [PubMed] [Google Scholar]
- Engman M, Granberg S, Williams AR, Meng CX, Lalitkumar PG, Gemzell-Danielsson K 2009 Mifepristone for treatment of uterine leiomyoma. A prospective randomized placebo controlled trial. Hum Reprod 24:1870–1879 [DOI] [PubMed] [Google Scholar]
- Williams AR, Critchley HO, Osei J, Ingamells S, Cameron IT, Han C, Chwalisz K 2007 The effects of the selective progesterone receptor modulator asoprisnil on the morphology of uterine tissues after 3 months treatment in patients with symptomatic uterine leiomyomata. Hum Reprod 22:1696–1704 [DOI] [PubMed] [Google Scholar]
- Wilkens J, Chwalisz K, Han C, Walker J, Cameron IT, Ingamells S, Lawrence AC, Lumsden MA, Hapangama D, Williams AR, Critchley HO 2008 Effects of the selective progesterone receptor modulator asoprisnil on uterine artery blood flow, ovarian activity, and clinical symptoms in patients with uterine leiomyomata scheduled for hysterectomy. J Clin Endocrinol Metab 93:4664–4671 [DOI] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P 1990 Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Chen X, Xie L 2007 Selective estrogen receptor modulators (SERMs) for uterine leiomyomas. Cochrane Database Syst Rev CD005287 [DOI] [PubMed] [Google Scholar]
- Donnez J, Hervais Vivancos B, Kudela M, Audebert A, Jadoul P 2003 A randomized, placebo-controlled, dose-ranging trial comparing fulvestrant with goserelin in premenopausal patients with uterine fibroids awaiting hysterectomy. Fertil Steril 79:1380–1389 [DOI] [PubMed] [Google Scholar]
- Neiger R, Sonek JD, Croom CS, Ventolini G 2006 Pregnancy-related changes in the size of uterine leiomyomas. J Reprod Med 51:671–674 [PubMed] [Google Scholar]
- Rosati P, Exacoustòs C, Mancuso S 1992 Longitudinal evaluation of uterine myoma growth during pregnancy. A sonographic study. J Ultrasound Med 11:511–515 [DOI] [PubMed] [Google Scholar]
- Hammoud AO, Asaad R, Berman J, Treadwell MC, Blackwell S, Diamond MP 2006 Volume change of uterine myomas during pregnancy: do myomas really grow? J Minim Invasive Gynecol 13:386–390 [DOI] [PubMed] [Google Scholar]
- Marshall LM, Spiegelman D, Goldman MB, Manson JE, Colditz GA, Barbieri RL, Stampfer MJ, Hunter DJ 1998 A prospective study of reproductive factors and oral contraceptive use in relation to the risk of uterine leiomyomata. Fertil Steril 70:432–439 [DOI] [PubMed] [Google Scholar]
- Baird DD, Dunson DB 2003 Why is parity protective for uterine fibroids? Epidemiology 14:247–250 [DOI] [PubMed] [Google Scholar]
- Schaaps JP, Tsatsaris V, Goffin F, Brichant JF, Delbecque K, Tebache M, Collignon L, Retz MC, Foidart JM 2005 Shunting the intervillous space: new concepts in human uteroplacental vascularization. Am J Obstet Gynecol 192:323–332 [DOI] [PubMed] [Google Scholar]
- Parazzini F, Negri E, La Vecchia C, Chatenoud L, Ricci E, Guarnerio P 1996 Reproductive factors and risk of uterine fibroids. Epidemiology 7:440–442 [DOI] [PubMed] [Google Scholar]
- Chen CR, Buck GM, Courey NG, Perez KM, Wactawski-Wende J 2001 Risk factors for uterine fibroids among women undergoing tubal sterilization. Am J Epidemiol 153:20–26 [DOI] [PubMed] [Google Scholar]
- Walker CL, Cesen-Cummings K, Houle C, Baird D, Barrett JC, Davis B 2001 Protective effect of pregnancy for development of uterine leiomyoma. Carcinogenesis 22:2049–2052 [DOI] [PubMed] [Google Scholar]
- Eisinger SH, Bonfiglio T, Fiscella K, Meldrum S, Guzick DS 2005 Twelve-month safety and efficacy of low-dose mifepristone for uterine myomas. J Minim Invasive Gynecol 12:227–233 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.