Abstract
The nuclear receptor cofactor receptor-interacting protein 140 (RIP140) is essential for cumulus cell-oocyte complex (COC) expansion, follicular rupture, and oocyte release during ovulation. The expression of many genes necessary for COC expansion is impaired in the absence of RIP140, but the studies herein document that their expression can be restored and COC expansion rescued by treatment with the epidermal growth factor (EGF)-like factor amphiregulin (AREG) both in vitro and in vivo. We demonstrate by several approaches that RIP140 is required for the expression of the EGF-like factors in granulosa cells, but the dependence of genes involved in cumulus expansion, including Ptgs2 Has2, Tnfaip6, and Ptx3, is indirect because they are induced by AREG. Treatment of granulosa cells with forskolin to mimic the effects of LH increases AREG promoter activity in a RIP140-dependent manner that 1) requires an intact cAMP response element in the proximal promoter region of the Areg gene and 2) involves its actions as a coactivator for cAMP response element-binding protein/c-Jun transcription factors. Although human chorionic gonadotropin and AREG coadministration is sufficient to restore ovulation fully in RIP140 heterozygous mice in vivo, both follicular rupture and ovulation remain impaired in the RIP140 null mice. Thus, we conclude that although the level of RIP140 expression in the ovary is a crucial factor required for the transient expression of EGF-like factors necessary for cumulus expansion, it also plays a role in other signaling pathways that induce follicular rupture.
Receptor interacting protein-140 (Nrip1) stimulates transcription from the Amphiregulin gene family in the ovary and is therefore necessary for cumulus expansion and ovulation.
Ovulation comprises a precise sequence of events controlled by multiple signaling pathways in response to the LH surge. The process is initiated in mural granulosa cells and leads to cumulus cell expansion, the resumption of meiosis in prophase-arrested oocytes, the rupture of the follicle wall, and luteinization of the cells within the ruptured follicle (1,2,3,4). Among the key mediators of LH are the epidermal growth factor (EGF)-like growth factors, amphiregulin (AREG), epiregulin (EREG), and betacellulin (BTC), which are transiently and sequentially expressed in the ovarian follicle to induce oocyte maturation and stimulate cumulus expansion (5,6,7,8,9,10). Although expression of these EGF-like factors is initially localized in the mural granulosa cells, they are subsequently induced within the cumulus cells as a result of autocrine and paracrine regulation by the factors themselves and by additional signaling pathways, including the action of prostaglandin E2 (PGE2) (11). Moreover, the cumulus cell-oocyte complex (COC) seems to be capable of responding to and coordinating signals not only from mural granulosa cells but also from thecal cells and the oocyte itself (12,13,14,15,16,17,18,19,20,21,22,23).
Numerous genes have been identified that influence one or more steps in ovulation (4,24,25). We have found that receptor-interacting protein 140 (RIP140), a coregulator for nuclear receptors, is essential for ovulation but not for luteinization (26). It is necessary for cumulus expansion (27) and follicular rupture so that RIP140 null mice are infertile with an ovarian phenotype resembling luteinized unruptured follicle syndrome (26). Heterozygous mice exhibit an intermediate phenotype with approximately 50% oocytes released and 50% retained (26). RIP140 is also an important regulator of metabolic activity in adipocytes (28,29), skeletal muscle (30), and liver (31) and of inflammation in macrophages (32). It was initially characterized as a corepressor for nuclear receptors and accordingly has been found to suppress the activity of estrogen-related receptors and peroxisome proliferator-activated receptors (PPARs) (33) and thereby inhibit transcription from a network of genes involved in catabolic pathways (29). Its function as a corepressor depends on the binding of additional corepressor and chromatin remodelling enzymes that may be subject to posttranslational modifications (34,35,36,37). However, RIP140 also seems to have the potential to function as a coactivator as shown by its role in increasing triglyceride synthesis in the liver (31) and in the expression of certain inflammatory cytokines in macrophages (32). However, the function of RIP140 as a coactivator is poorly understood, although it has been shown to form a ternary complex with p65 and cAMP response element (CRE)-binding protein (CREB)-binding protein (32).
Gene expression profiling indicates that RIP140 is required in the ovary for the expression of genes involved in signaling, extracellular matrix formation, cell-cell attachment, and adhesion (27). It is noteworthy that the expression of Areg was markedly reduced in the ovaries of RIP140 null mice (27). We have therefore investigated the hypothesis that RIP140 functions upstream of Areg expression and that it may be possible to rescue the anovulatory phenotype by exogenous administration of one or more of the EGF-like family of growth factors. Studies with wild-type (WT) and RIP140 null mice and with isolated COCs demonstrate that cumulus expansion, but not follicular rupture and oocyte release, can be restored by AREG administration. Reporter gene analysis and knockdown experiments in granulosa cells together with chromatin immunoprecipitation (ChIP) assays demonstrate that RIP140 activates the AREG promoter in response to forskolin by a mechanism dependent on a proximal CRE element and the basic leucine zipper transcription factors, CREB and c-Jun.
Materials and Methods
Animals
Generation of RIP140 null animals has been described previously (26). Mice used in this study were backcrossed six generations to C57BL/6J background. The animals were housed under standard conditions and given food and water ad libitum. Six-week-old animals were used for the in vitro cumulus expansion experiments, whereas immature mice were used for protein samples and in situ hybridization analysis. All animal studies were conducted in accordance with the United Kingdom Home Office guidelines. For superovulation experiments, mice were injected ip with 5 IU Folligon [equine chorionic gonadotropin (eCG); Intervet, Milton Keynes, UK] followed 48 h later with an ovulatory dose of 5 IU Chorulon [human CG (hCG); Intervet] (5). In the experiments where AREG was administered along with hCG, AREG at a concentration of 300 ng/ml was supplemented with hCG (5 IU), and 100 μl were administered to each animal. To determine ovulation, superovulated animals were killed 16 h after hCG treatment, and dissected ovaries were fixed for preparation of wax blocks after carefully separating out the oviduct in culture medium. While the ovulated COCs were taken out from the ampulla of the oviduct using 26-gauge (G) needles (BD Biosciences, San Jose, CA) and treated with hyaluronidase (Sigma Aldrich, St. Louis, MO) to detach the oocyte from the cumulus to count the exact number of oocytes, the prepared wax blocks were serially sectioned throughout the ovary for analysis.
For generating an immortalized granulosa cell line, H-2Kb-tsA58 mice (38) were crossed with C57BL/6J mice to obtain offspring carrying one copy of the Simian Viris 40 large tumor antigen transgene.
Western blotting
Protein extracts (30 μg) were resolved by SDS-PAGE (4–12% gels; Invitrogen, Carlsbad, CA) and transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA). Blocking in 5% milk in Tris-buffered saline with Tween 20 was followed by probing with primary antibodies and horseradish peroxidase-linked antimouse antibody (Dako, Cambridge, UK), diluted 1:5000. After three to four washes in Tris-buffered saline with Tween 20, the bound antibodies were visualized using the enhanced chemiluminescence substrate (Pierce, Rockford, IL). The dilutions used for the primary antibodies were as follows: RIP140 (6D7 generated against residues 301–478), 1:500, and β-actin (Abcam, Cambridge, MA), 1:5000.
In situ hybridization
RIP140 Exon 1b and Areg, Ereg, and Btc sequences were amplified using specific primers (see Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) and cloned into pCRII-TOPO vector (Invitrogen). Digoxigenin-labeled RNA probes were transcribed using the Megascript SP6 or T7 kit (Ambion, Austin, TX) and hybridized to 5-μm paraffin sections of mouse ovary as described previously (39).
COC cultures
Ovaries were collected from animals treated with 5 IU eCG for 48 h. Large antral follicles visible on the surface of the ovaries were punctured with a 26-G needle allowing the unexpanded COCs to be collected in the culture medium [MEM with Earle's salts containing 1% fetal bovine serum (FBS), 25 mm HEPES, 0.25 mm sodium pyruvate, 3 mm l-glutamine, 1 mg/ml BSA, and Pen/Strep; GIBCO, Carlsbad, CA]. COCs were cultured in the presence and absence of 100 ng/ml or 300 ng/ml AREG (R&D Systems, Minneapolis, MN) for 4, 8, and 16 h at 37 C for a time-course study in a humidified incubator (95% air and 5% CO2). The COCs were photographed for scoring expansion. A scoring system (0 to +4) was used for evaluating the degree of cumulus expansion for each COC. Unexpanded COCs received a score of 0. Complexes in which the outer layers of cumulus cells had begun to expand received a score of +1 to +2. A score of +3 was given to COCs in which all the layers except the corona radiata (the cumulus cell layer adjacent to the oocyte) had expanded, and fully expanded COCs were given a score of +4 (9). COCs were then collected for RNA isolation.
RNA isolation and expression analysis
Total RNA was isolated using the RNeasy micro kit (QIAGEN, Valencia, CA), and cDNA was prepared using the Super Script first strand synthesis kit (Invitrogen) according to the manufacturer's instructions. Real-time PCR was performed using Opticon-2 (MJ Research, Inc., Watertown, MA) using the SYBR Green Jumpstart Taq Ready Mix (Sigma Aldrich) and gene specific primers (Supplemental Table 2). Expression levels for all genes were normalized against the expression of ribosomal coding gene RPL7.
Granulosa cell primary cultures
Primary granulosa cells were derived from animals injected with eCG for 48 h. Large preovulatory follicles were punctured and tapped to release the granulosa cells in the culture medium (MEM with Earle's salts containing 1% FBS, 25 mm HEPES, 0.25 mm sodium pyruvate, 3 mm l-glutamine, 1 mg/ml BSA, and Pen/Strep). The COCs released after puncturing these follicles were separated out to be used in COC expansion assays. The medium containing released cells but devoid of oocytes was spun at 1000 rpm for 5 min, and the pelleted cells were resuspended in fresh medium and divided into different experimental groups, i.e. control, 10 μm forskolin (Calbiochem, San Diego, CA), 300 ng/ml AREG, or 500 ng/ml PGE2 (Calbiochem).
Generation of WTF4 cell line
Immortalized granulosa cells were derived by puncturing large follicles from ovaries of mice carrying the thermolabile large T-Ag with 26.5-G needles. Single cell clones from the isolated granulosa cells were grown to develop cell lines. The cells were grown in DMEM/F12 medium containing 10% FBS and Pen/Strep (Sigma, St. Louis, MO) at 33 C in the presence of 2 ng/ml γ-interferon (Chemicon, Temecula, CA) and shifted to 37 C without γ-interferon for being stimulated. The cell lines were characterized and selected for their granulosa cell properties on the basis of cAMP and growth factor responsiveness and expression of genes involved in granulosa cell functions such as steroidogenic acute regulatory protein, Inhibin-α, Areg, Ereg, Btc, nuclear receptor-interacting protein 1, and prostaglandin-endoperoxide synthase 2. For this study, the WTF4 granulosa cells were selected because of their ability to show Areg induction in response to forskolin, AREG, and PGE2 in a manner similar to that observed in primary granulosa cells in culture.
Plasmids and transfection experiments
A −935/+65 fragment of mouse AREG promoter was cloned upstream of the luciferase reporter gene into a pGL3 basic vector (Promega, Madison, WI). Using specific primers (Supplemental Table 2), various truncations of the 5′-flanking region −758/+65, −574/+65, −341/+65, −158/+65 of the −935/+65 fragment were also cloned into pGL3. Using the −158/+65 deletion construct, mutations in a SP1 and CRE element were generated with the QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). The primer sequences used to incorporate the mutation were GGGTTTTTGGAGTAGTAACCTCaataaTCCACCCAGGGCTGGTCCGTGA for SP1 and GCCTCCACCCAGGGCTGGTCCGTagaaTgtCGGGCGGTGGCT for CRE, the mutations are shown in bold lowercase. Mutations generated at the SP1 and CRE sites were verified by automated sequencing at the MRC core DNA facility (Hammersmith Campus, Imperial College London). pCIEF-RIP140 was generated by replacing the cytomegalovirus promoter in the pCI-RIP140 with the elongation factor promoter from a pEF-BOS mammalian expression vector. Reporter assays were carried out in 96-well plates with 40 ng of reporter, 5 ng green fluorescent protein in the presence of 100 ng pCIEF-RIP140 or pCIEF (empty vector) using FuGENE HD (Roche, Indianapolis, IN). After 24 h transfection, cells were stimulated with forskolin (10 μm) alone or along with the protein kinase A (PKA) inhibitor, H89 (10 μm) and/or p38 MAPK inhibitor, SB203580 (20 μm; Calbiochem). Reporter activity was measured 4 h later using the Steady Lite HTS luciferase assay kit (PerkinElmer, Waltham, MA) on a Wallac Victor 1420 multilabel counter (PerkinElmer).
ChIP assays
Cells were treated with forskolin for 1 h and then incubated with 1% formaldehyde in PBS for 10 min at 37 C. The cross-linked cells were then lysed, sonicated, and proteins immunoprecipitated with Dynabeads, protein G (Invitrogen) using the following antibodies: mouse IgG (sc-2025; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and rabbit IgG (sc-2027; Santa Cruz Biotechnology, Inc.), p-CREB (06-519; Upstate Biotechnology, Lake Placid, NY), c-Jun (no. 9165; Cell Signaling, Beverly, MA), and RIP140 (kindly provided by Hongwu Chen, University of California, Davis, CA). Briefly, 5 μg of antibody was prebound for 4 h to protein G dynabeads, washed three times with PBS 0.2% Tween, and then added to diluted chromatin for immunoprecipitation overnight. Beads were then washed thoroughly and protein-DNA complexes eluted. DNA fragments were purified with QIAquick PCR purification kit (QIAGEN), and enrichment relative to input were determined by quantitative PCR (Q-PCR) using primers for the AREG and glyceraldehyde-3-phosphate dehydrogenase promoter (Supplemental Table 2).
RNA interference assays
Control and small interfering RNAs (siRNAs) for depleting CREB and c-Jun were bought from NBS Biologicals (Cambridgeshire, UK) (Supplemental Table 2). The oligos were transfected using Lipofectamine 2000 (Invitrogen) in 96-well plates along with the −935/+65 AREG reporter construct, and reporter activity was analyzed as described above.
Statistics
Data are presented as the mean ± sem and were analyzed by Student's t test. P < 0.05 was considered statistically significant.
Results
Expression patterns of RIP140 (Nrip1) and Areg in the ovary
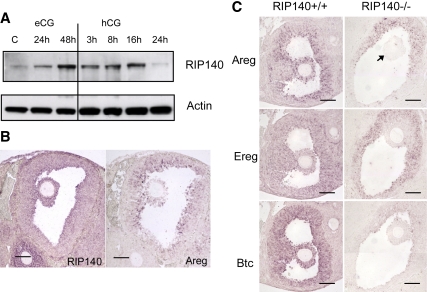
Because the expression of EGF-like factors was reduced in the RIP140 null mice, we first determined whether RIP140 was coexpressed with AREG (Areg) in the mouse ovary in vivo. Whole-cell ovarian extracts obtained from mice injected with eCG and hCG for different time points were first used to analyze the expression of RIP140 protein. Levels of ovarian RIP140 protein increased in response to eCG treatment and were maintained for approximately 16 h after hCG administration when levels declined, indicating that RIP140 is present in the ovary during the ovulation period (Fig. 1A). The cellular localization of RIP140 and EGF-like factor, Areg, was determined by in situ hybridization (Fig. 1B and Supplemental Fig. 1). In untreated and ovaries primed with eCG for 6 h, RIP140 mRNA was detected in secondary and preantral follicles with highest levels of expression in granulosa cells (Supplemental Fig. 1). RIP140 expression was maintained after longer periods of eCG treatment in large follicles, localized primarily to mural granulosa cells, and became apparent in cumulus cells. When eCG-primed mice were treated with hCG, RIP140 expression slightly decreased in mural granulosa cells with expression maintained in the inner layers and in cumulus cells in both unexpanded and expanded COCs (Supplemental Fig. 1). The expression of Areg, Ereg, and Btc was undetectable in mice primed with eCG alone (data not shown), but they were transiently expressed in both mural and cumulus granulosa cells, with pronounced expression after 3-h hCG treatment in WT ovaries (Fig. 1C). In contrast, in situ hybridization analysis of RIP140 null ovaries indicates that the transient expression of Areg, Ereg, and Btc was markedly reduced (Fig. 1C), consistent with previous expression analysis using DNA microarrays (27). Furthermore, their expression was not delayed as judged by an analysis of ovaries after 8-h hCG treatment (data not shown). Importantly, we conclude that although there was some residual expression of EGF-like growth factors in mural cells, there was no detectable expression in cumulus cells from RIP140 null mice.
Figure 1.
Expression of RIP140 and EGF-like factors in the ovary. A, Western blot analysis showing RIP140 protein from extracts obtained from ovaries of 3-wk-old mice, untreated or treated with eCG for 24 and 48 h and eCG for 48 h followed by hCG for 3, 8, 16, and 24 h (n = 2 for each treatment group). β-Actin was used as a loading control. B, In situ hybridization analysis showing expression of both RIP140 and Areg in mural and cumulus granulosa cells in ovary sections obtained from mice treated with eCG for 48 h followed by hCG for 3 h. C, In situ hybridization analysis to detect expression of Areg, Ereg, and Btc in WT and RIP140 knockout (KO) ovary sections obtained from mice treated with eCG for 48 h followed by hCG for 3 h. The RIP140 KO sections show a reduced expression of all three EGF-like factors in the granulosa cells and no expression in the cumulus cells (arrow). Scale bars, 100 μm. Images are representative of experiments done using three animals in each group.
AREG rescues cumulus expansion in RIP140 null COCs
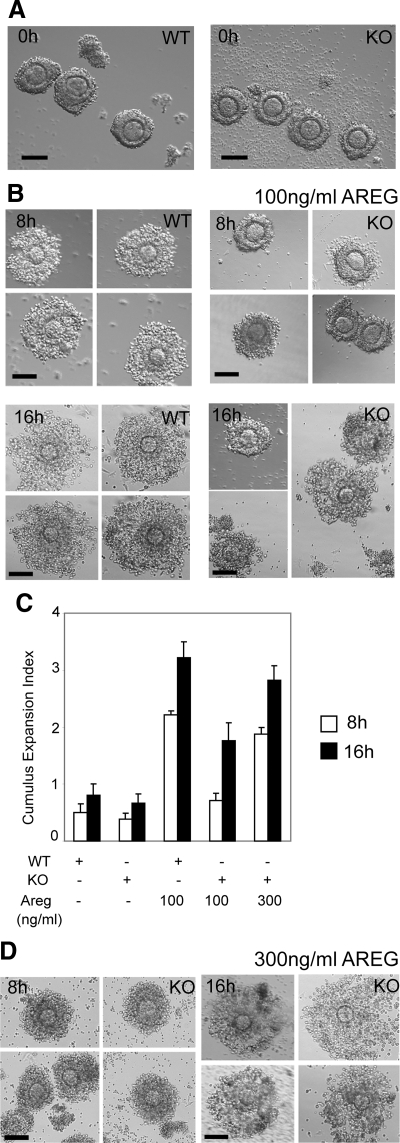
We next investigated the ability of EGF-like growth factors to induce cumulus expansion in cultured COCs isolated from WT and RIP140 null mice. After priming with eCG for 48 h, similar numbers of nonexpanded COCs could be obtained from both genotypes with no apparent morphological defects in the RIP140 null COCs (Fig. 2A). Because cumulus expansion becomes evident around 8 h and increases by 16 h, after stimulation in culture, COCs were photographed at these two time points. Initially, we tested the effect of 100 ng/ml of AREG and found that it was capable of inducing partial expansion of WT COCs at 8 h, which increased further by 16 h after the beginning of the culture (Fig. 2B). Q-PCR analysis confirmed the presence of RIP140 in the COCs and indicated that there was no change in the expression of RIP140 mRNA throughout this period of expansion (Supplemental Fig. 2). The ability of RIP140 null COCs to respond to this concentration of AREG was impaired at 8 h, although by 16 h, more than 50% of the null COCs showed signs of matrix formation (Fig. 2, B and C). We therefore tested the effect of increasing the concentration of AREG to 300 ng/ml and found that this resulted in improved expansion of the RIP140 null COCs, to resemble that of WT COCs at both 8 and 16 h (Fig. 2, C and D). Thus, cumulus expansion could be rescued in COCs devoid of RIP140 by treatment with increased concentrations of AREG. The effect of 100 ng/ml of each of the EGF-like factors on COC expansion in culture was also tested. We observed a significant improvement in cumulus expansion in the null COCs by 16 h, although the degree of expansion was impaired at 8 h when compared with that of WT COCs at the same time (Supplemental Figs. 3 and 4).
Figure 2.
Restoration of cumulus expansion in RIP140 null COCs. A, COCs released from WT and RIP140 knockout (KO) ovaries after 48-h eCG treatment. Scale bar, 100 μm. B, COCs isolated from WT and RIP140 KO ovaries after 48-h eCG treatment and cultured for 8 and 16 h in the presence of 100 ng/ml AREG. Scale bar, 100 μm. Compared with the WT COCs, the KO COCs showed defects in expansion at 8 h (P < 0.0001) with some COCs showing matrix formation by 16 h. C, Cumulus expansion index scored for COCs. D, COCs isolated from RIP140 KO ovaries after 48-h eCG treatment and cultured for 8 and 16 h in the presence of 300 ng/ml AREG showed improvement in cumulus expansion at 8 h and 16 h. Scale bar, 100 μm. Each treatment was performed with a pool of eight to ten COCs isolated from two to three mice, and three to four independent experiments were performed.
RIP140 is a key regulator of a gene network required for COC expansion
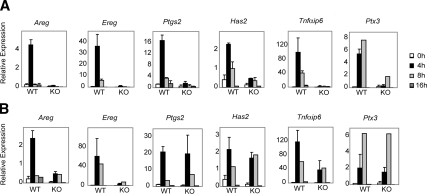
To investigate the role of RIP140 in mediating the LH response, we cultured COCs obtained from WT and RIP140 null mice in the presence of AREG for 4, 8, and 16 h at either 100 ng/ml (Fig. 3A) or 300 ng/ml (Fig. 3B). A 4-h time point was included here to study the induction of EGF-like growth factors that peak in expression around 3–4 h after hCG injection in vivo and in culture, in vitro (5,11). An analysis of markers of expansion demonstrated that AREG at 100ng/ml induced a marked but transient increase in Ptgs2, Has2, Tnfaip6, and Ptx3, as well as Areg and Ereg expression in WT COCs, whereas RIP140 null COCs responded poorly with negligible induction of Areg, Ereg, and Tnfaip6 expression (Fig. 3A). Expression of Ptgs2, Has, Tnfaip6, and Ptx3 was restored in the RIP140 null COCs by AREG treatment at 300 ng/ml (Fig. 3B); however, the expression of Areg and Ereg remained impaired. Thus, it appears that RIP140 is essential for the expression of EGF-like factors, but exogenous treatment with AREG is capable of inducing the expression of Ptgs2 Has2, Tnfaip6, and Ptx3 in the absence of RIP140.
Figure 3.
Time-course expression profile of genes involved in cumulus expansion. COCs were isolated from WT and RIP140 knockout (KO) mice primed with eCG for 48 h and cultured for 4, 8, and 16 h with either 100 ng/ml AREG (A) or 300 ng/ml AREG (B). RNA was isolated from the cultured COCs, and the expression of genes assessed using Q-PCR. Expression of all analyzed genes was induced in WT COCs by 100 ng/ml AREG in a time course. In contrast, 100 ng/ml AREG failed to induce the expression of these genes in the KO COCs. The expression of Ptgs2, Has2, Tnfαip6, and Ptx3 was remarkably induced in the KO COCs cultured with 300 ng/ml AREG with impaired induction of Areg and Ereg. Most treatment groups represent up to four independent experiments containing pools of eight to ten COCs.
AREG administration reverses ovulatory defects in RIP140 heterozygous mice
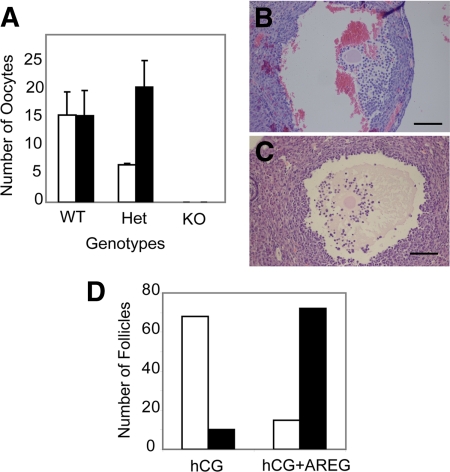
Because expansion could be induced by AREG in RIP140 null COCs in vitro, we investigated whether ovulation could be rescued in RIP140 null mice and in heterozygous mice that exhibit reduced oocyte release. Mice were primed with eCG and were treated with either hCG alone (5 IU) or hCG (5 IU) supplemented with 300 ng/ml AREG, and the number of ovulated COCs in the oviducts were counted after 16 h. As expected, there was no significant difference between the number of COCs released in WT animals treated with hCG alone vs. hCG plus AREG (Fig. 4A). However, the reduced number of COCs ovulated from heterozygous mice in response to hCG alone was markedly increased to a level similar to that found in WT mice when AREG was also administered. In contrast, no oocytes were released from RIP140 null females that had been treated with either hCG alone or hCG plus 300 ng/ml AREG, which induced superovulation in both WT and heterozygous mice (Fig. 4A).
Figure 4.
Effect of AREG on ovulation in vivo. Coadministration of AREG and hCG in vivo rescues ovulation from RIP140 heterozygous mice but not RIP140 null mice. A, COCs recovered from the oviduct were counted after treating WT, RIP140 heterozygous, and RIP140 null mice with hCG for 16 h (open bars) or hCG plus 300 ng/ml AREG (closed bars) for 16 h (n = 3–4 for each group). Heterozygous animals showed an increase in ovulation on administration of AREG (P < 0.04). To analyze in vivo cumulus expansion, serial ovarian sections were analyzed from RIP140 null mice treated with hCG for 16 h (B) or hCG plus 300 ng/ml AREG for 16 h (C). Scale bar, 100 μm. D, Number of unexpanded (open bars) and expanded (closed bars) COCs counted from RIP140 null ovaries treated with hCG or hCG plus 300 ng/ml AREG for 16 h (n = 3 for each group).
Serial sections of the entire ovary for both treatment groups for all three genotypes were analyzed. As expected, superovulated WT ovaries showed the presence of corpora lutea, whereas ovaries from hCG-treated heterozygous females also contained unruptured follicles with trapped oocytes. However, these unruptured follicles were no longer observed after treatment with both hCG and AREG in heterozygous mice. In RIP140 null ovaries after hCG treatment alone, majority of the follicles contained unexpanded COCs attached to the wall of the follicles (Fig. 4, B and D). In contrast, null ovaries with hCG plus AREG treatment had most follicles with expanded COCs detached from the follicle wall as shown by the representative image (Fig. 4, C and D). Therefore, although AREG was unable to overcome the anovulatory defect in RIP140 null mice, COC expansion was improved in agreement with its effect in vitro.
Regulation of Areg by RIP140 in ovarian granulosa cells
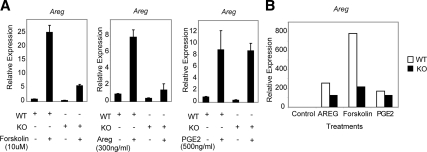
Previous work suggested that the expression of EGF-like growth factors in the ovary is induced not only by LH in mural granulosa cells but also by autocrine augmentation and paracrine control by AREG itself and by PGE2 (11). To investigate whether the ability of each of these signaling pathways to increase Areg is dependent on RIP140, we generated primary cultures of mural granulosa cells from eCG-primed WT and RIP140 null ovaries. To mimic post-LH signals, cells were treated with 10 μm forskolin, 300 ng/ml AREG, or 500 ng/ml PGE2 for 4 h, and Q-PCR was used to determine Areg mRNA levels. As expected, Areg expression in WT granulosa cells was stimulated by all three treatments (Fig. 5A) with forskolin resulting in the highest level of expression. In RIP140 null cells, the response to PGE2 was maintained, but induction by forskolin or by AREG alone was reduced approximately 5-fold. Similarly, the expression of Ereg in response to forskolin and AREG was also impaired in the absence of RIP140 (Supplemental Fig. 5). We also examined Areg expression in COC culture for 8 h and observed similar responses (Fig. 5B). Thus, we conclude that RIP140 contributes to signaling pathways induced by forskolin and by AREG but not PGE2.
Figure 5.
Regulation of Areg in primary granulosa cells. WT and RIP140 null mice were treated with eCG for 48 h and granulosa cells (A) and COCs (B), isolated from large follicles, and cultured for 4 and 8 h, respectively, in the absence or presence of 10 μm forskolin, 300 ng/ml AREG, or 500 ng/ml PGE2. Areg expression relative to the untreated controls was determined by real-time PCR (n = 3 for each treatment; B represents data obtained from COCs pooled from three different animals). Areg expression in the RIP140 knockout (KO) granulosa cells and COCs was reduced in the presence of forskolin and AREG.
RIP140 acts as an activator for the AREG promoter acting through a conserved CRE
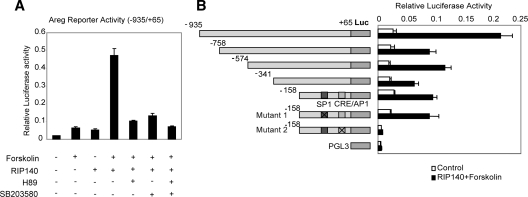
The ability of RIP140 to regulate AREG transcription was investigated by analyzing the activity of reporter genes containing upstream fragments of the AREG promoter in WTF4 granulosa cells, which were generated as described in Materials and Methods (Supplemental Fig. 6). Forskolin treatment stimulated the activity of a reporter gene consisting of −935 to +65 AREG promoter sequence approximately 3-fold but was markedly increased by exogenous expression of RIP140. The increase in activity was suppressed by the kinase inhibitors H89 and SB203580 (Fig. 6A), indicating that RIP140 may be required for the ability of PKA and/or p38 MAPK to stimulate AREG promoter activity.
Figure 6.
RIP140 acts as a coactivator of Areg. A, Reporter activity of a −935/+65 fragment of the Areg promoter fused to a luciferase reporter, transiently transfected in WTF4 granulosa cells together with either pCIEF-RIP140 or pCIEF empty vector. After 24-h transfection, cells were treated in the presence or absence of forskolin with or without PKA inhibitor, H89 (10 μm), or p38 MAPK inhibitor SB203580 (20 μm) for 4 h. In the inhibitor-treated groups, the inhibitors were added 30 min before forskolin stimulation (n = 8). B, Reporter activity of a series of Areg deletion constructs and point mutants fused to a luciferase reporter, transiently transfected in WTF4 granulosa cells with either pCIEF or pCIEF-RIP140 in the presence of forskolin (10 μm). pGL3 basic was used as a control. Schematic representations of the WT and mutated promoter constructs are shown.
We next mapped the region of the AREG promoter that was necessary to mediate the positive effects of RIP140. The response of a series of deletion mutants to RIP140 in the presence of forskolin was slightly reduced, but as shown in Fig. 6B, RIP140 was able to stimulate reporter activity driven by proximal promoter sequences from −158 to +65. This region contains a GC-rich putative binding site for SP1 and a conserved CRE (tgacgtca). Mutagenesis of the CRE abolished both basal activity and RIP140-stimulated reporter activity, whereas mutagenesis of the SP1 binding site had no effect. Thus, we conclude that the stimulatory effect of RIP140 is mediated at least in part by the proximal CRE element in the AREG promoter.
RIP140 is recruited to the CRE of the Areg promoter and acts through CREB and c-Jun
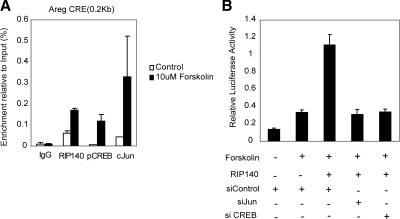
We next examined whether RIP140 itself was recruited to the CRE element together with CREB/c-Jun family members by performing ChIP analysis. WTF4 granulosa cells were treated with forskolin for 1 h, and antibodies for IgG, RIP140, p-CREB, and c-Jun were used to immunoprecipitate DNA protein complexes (Fig. 7A). Using specific primers (Supplemental Table 2), both RIP140 and c-Jun but not p-CREB were found in the vicinity of the CRE element in unstimulated cells. Upon forskolin treatment, recruitment of all three factors increased significantly compared with basal levels and the IgG controls. In contrast, none of the factors was recruited to an upstream region in the Areg promoter or the glyceraldehyde-3-phosphate dehydrogenase promoter in either basal or stimulated conditions (Supplemental Fig. 7).
Figure 7.
RIP140 is directly recruited to the Areg promoter together with CREB and c-Jun. A, ChIP assays were performed in WTF4 granulosa cells in unstimulated and cells stimulated with forskolin (10 μm), using antibodies specific for RIP140, p-CREB, and c-Jun or nonspecific IgG. Precipitated fragments were analyzed by real-time PCR using Areg primers in the proximity of the CRE (n = 2). B, WTF4 granulosa cells were transiently transfected with siRNA oligos for c-Jun, CREB, or control, and after 48 h, cells were cotransfected with the Areg reporter gene (−935/+65), with either pCEIF or pCIEF-RIP140. After 24 h, cells were stimulated with forskolin for 4 h, and reporter activity was determined (n = 6).
Finally, we investigated whether CREB and c-Jun were required for RIP140 stimulated AREG promoter activity by performing knockdown experiments. We found that the stimulatory effect of RIP140 in the presence of forskolin was markedly reduced after reducing the expression of these transcription factors (Fig. 7B), indicating that the stimulatory effect of RIP140 is mediated, at least in part, by CREB/c-Jun family members.
Discussion
RIP140 null mice exhibit normal folliculogenesis but are anovulatory because oocytes remain trapped within nonexpanded COCs and follicles fail to rupture (26). The COC forms a unique microenvironment within the follicle, and recent gene profiling data have established that expansion involves the expression of many matrix, neuronal, and immune cell-related genes (40,41). Defective expression of many of these genes in RIP140 null ovaries and the impaired COC expansion found in RIP140 null mice led us to identify a number of genes that may be targets of RIP140. In particular, the expression of many genes involved in signaling, extracellular matrix formation, cell-cell attachment, and adhesion were aberrantly regulated in the absence of RIP140 and varied according to the hormonal status of the mice (27).
Recent studies have demonstrated that the EGF-like factors, AREG and epiregulin, are important intrafollicular mediators of LH and regulate the expression of Ptgs2, Has2, Tnfaip6, and Ptx3 (2,5,6,9,11,42). In particular, AREG induces the expression of genes involved in COC expansion, steroidogenesis, and immune cell-like functions and also induces its own expression in COCs in a paracrine manner (11). Mice devoid of the genes encoding Areg, Ereg, or Btc are fertile with no overt reproductive phenotype (43,44,45), but a double mutant mouse that was null for Areg and homozygous for a hypomorphic allele of Egfr showed impaired ovulation with defects in cumulus expansion and luteinisation, indicating that EGF family members are crucial for ovulation (9). Moreover, mice in which ERK1/2 genes are disrupted in granulosa cells also fail to ovulate, indicating that these kinases are essential downstream mediators of EGF-like factor action in granulosa cells and cumulus cells (46).
Although LH receptor (Lhcgr) expression in the ovary is normal in the absence of RIP140 (26), the expressions of Areg, Ereg, and Btc (Fig. 1C) (27) are all reduced, suggesting that these genes may be key targets of RIP140 in follicular cells. Therefore, the lack of RIP140 expression in the ovary appears to lead to a general reduction in signaling via the EGF network.
In this paper, we show that absence of RIP140 can be overcome at least in part by exogenous expression of EGF-like growth factors. Notably, AREG treatment was able to rescue the expression of many matrix genes in a concentration-dependent manner in RIP140 null cumulus cells and overcame the defect in the process of COC expansion. However, it is striking that the expression of the EGF-like factors themselves remained impaired, indicating a fundamental link between RIP140 and signaling pathways downstream of LH, leading to Areg expression.
The transient increase in Areg expression that stimulates COC expansion is initiated in response to the LH surge and further potentiated by autocrine- and paracrine-mediated effects of AREG itself and PGE2 (11,47). We were able to confirm the ability of these pathways to stimulate Areg expression in primary cultures of granulosa cells. Although the induction of Areg by PGE2 was maintained in RIP140 null cells, the increase observed in the presence of forskolin and AREG remained impaired. Promoter-reporter assays, ChIP, and siRNA knockdown experiments provide strong evidence that RIP140 acts as a coactivator for CREB/c-Jun family members at the CRE element in the proximal promoter region of the Areg gene. Therefore, we conclude that RIP140 plays a critical coregulatory role in discrete pathways that control Areg production. Accordingly, the combined treatment of RIP140 heterozygous mice with hCG and AREG was able to induce COC expansion in vivo and restore ovulation. Interestingly, although the combination of hCG and AREG was able to improve COC expansion in RIP140 null mice, it did not result in follicle rupture or ovulation, suggesting that additional targets of RIP140 are essential in the control of these processes. Such targets could include nuclear receptors such as PPARγ and progesterone receptor. Conditional knockdown of PPARγ from the granulosa cells leads to an anovulatory phenotype because of a failure of rupture of the follicle wall (48), and knockout mice for progesterone receptor fail to ovulate despite a normal development of the follicles to the ovulatory stage (49,50). RIP140 has been shown to interact with many nuclear receptors and a number of other transcription factors (35,51); however, its function in the processes that lead to the rupture of the follicle remain to be determined.
Our initial metabolic studies indicated that RIP140 functions as a corepressor for a number of nuclear receptors to regulate gene networks involved in catabolic pathways in metabolic tissues (29,30,33,35), but our recent work has demonstrated that RIP140 may also function as a coactivator for the transcription of certain genes in macrophages and hepatocytes (31,32). The ability of RIP140 to potentiate the transcription of a number of inflammatory genes in macrophages seems to depend on the formation of a ternary complex containing nuclear factor κB and CREB-binding protein (32). The mechanisms by which LH and AREG stimulate Areg expression have yet to be elucidated fully, but it appears that RIP140 may also function as a transcriptional coactivator for CREB/c-Jun family members to stimulate transcription from the Areg gene promoter. Thus, although the precise signaling pathways targeted by RIP140 in the complex processes of folliculogenesis and ovulation are diverse, it is evident that RIP140 is an important cofactor in the regulation of Areg expression by LH and AREG itself.
Supplementary Material
Footnotes
This work was supported by the Wellcome Trust Grant 079200/Z/06/Z (to J.N., J.H.S., R.W., and M.G.P.), the Biotechnology and Biological Sciences Research Council Grant BB/C504327/1 (to M.M.R. and M.G.P.), and the Institute of Obstetrics and Gynaecology Trust.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 22, 2010
Abbreviations: AREG, Amphiregulin; BTC, betacellulin; ChIP, chromatin immunoprecipitation; COC, cumulus cell-oocyte complex; CRE, cAMP response element; CREB, CRE-binding protein; eCG, equine chorionic gonadotropin; EGF, epidermal growth factor; EREG, epiregulin; FBS, fetal bovine serum; G, gauge; hCG, human CG; PGE2, prostaglandin E2; PKA, protein kinase A; PPAR, peroxisome proliferator-activated receptors; RIP140, receptor-interacting protein 140; siRNA, small interfering RNA; Q-PCR, quantitative PCR; WT, wild type.
References
- Richards JS 2005 Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol 234:75–79 [DOI] [PubMed] [Google Scholar]
- Richards JS 2007 Genetics of ovulation. Semin Reprod Med 25:235–242 [DOI] [PubMed] [Google Scholar]
- Russell DL, Robker RL 2007 Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update 13:289–312 [DOI] [PubMed] [Google Scholar]
- Edson MA, Nagaraja AK, Matzuk MM 2009 The mammalian ovary from genesis to revelation. Endocr Rev 30:624–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M 2004 EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303:682–684 [DOI] [PubMed] [Google Scholar]
- Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A 2005 Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology 146:77–84 [DOI] [PubMed] [Google Scholar]
- Hsieh M, Zamah AM, Conti M 2009 Epidermal growth factor-like growth factors in the follicular fluid: role in oocyte development and maturation. Semin Reprod Med 27:52–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholt de Prada JK, Lee YS, Latham KE, Chaffin CL, VandeVoort CA 2009 Role for cumulus cell-produced EGF-like ligands during primate oocyte maturation in vitro. Am J Physiol Endocrinol Metab 296:E1049–E1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M 2007 Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 27:1914–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigone S, Hsieh M, Fu M, Persani L, Conti M 2008 Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol 22:924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS 2006 Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol 20:1352–1365 [DOI] [PubMed] [Google Scholar]
- Richard FJ, Fortier MA, Sirard MA 1997 Role of the cyclic adenosine monophosphate-dependent protein kinase in the control of meiotic resumption in bovine oocytes cultured with thecal cell monolayers. Biol Reprod 56:1363–1369 [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Eppig JJ 2009 Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med 27:32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motola S, Popliker M, Tsafriri A 2008 Response of follicle cells to ovulatory stimuli within the follicle and in primary culture. Mol Cell Endocrinol 282:26–31 [DOI] [PubMed] [Google Scholar]
- Li Q, McKenzie LJ, Matzuk MM 2008 Revisiting oocyte-somatic cell interactions: in search of novel intrafollicular predictors and regulators of oocyte developmental competence. Mol Hum Reprod 14:673–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, O'Brien MJ, Matzuk MM, Shimasaki S, Eppig JJ 2007 Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development 134:2593–2603 [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Wigglesworth K, O'Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ 2008 Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 135:111–121 [DOI] [PubMed] [Google Scholar]
- Diaz FJ, Wigglesworth K, Eppig JJ 2007 Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Dev Biol 305:300–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz FJ, O'Brien MJ, Wigglesworth K, Eppig JJ 2006 The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion. Dev Biol 299:91–104 [DOI] [PubMed] [Google Scholar]
- Combelles CM, Carabatsos MJ, Kumar TR, Matzuk MM, Albertini DF 2004 Hormonal control of somatic cell oocyte interactions during ovarian follicle development. Mol Reprod Dev 69:347–355 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ 2002 Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 296:2178–2180 [DOI] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ 2009 Fibroblast growth factors and epidermal growth factor cooperate with oocyte-derived members of the TGFβ superfamily to regulate Spry2 mRNA levels in mouse cumulus cells. Biol Reprod 81:833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz FJ, Wigglesworth K, Eppig JJ 2007 Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci 120:1330–1340 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ 2008 The biology of infertility: research advances and clinical challenges. Nat Med 14:1197–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM 2000 Revelations of ovarian follicle biology from gene knockout mice. Mol Cell Endocrinol 163:61–66 [DOI] [PubMed] [Google Scholar]
- White R, Leonardsson G, Rosewell I, Ann Jacobs M, Milligan S, Parker M 2000 The nuclear receptor co-repressor nrip1 (RIP140) is essential for female fertility. Nat Med 6:1368–1374 [DOI] [PubMed] [Google Scholar]
- Tullet JM, Pocock V, Steel JH, White R, Milligan S, Parker MG 2005 Multiple signaling defects in the absence of RIP140 impair both cumulus expansion and follicle rupture. Endocrinology 146:4127–4137 [DOI] [PubMed] [Google Scholar]
- Leonardsson G, Steel JH, Christian M, Pocock V, Milligan S, Bell J, So PW, Medina-Gomez G, Vidal-Puig A, White R, Parker MG 2004 Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc Natl Acad Sci USA 101:8437–8442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, Kiskinis E, Debevec D, Leonardsson G, White R, Parker MG 2005 RIP140-targeted repression of gene expression in adipocytes. Mol Cell Biol 25:9383–9391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A, Steel JH, Nichol D, Pocock V, Kumaran MK, Fritah A, Mobberley M, Ryder TA, Rowlerson A, Scott J, Poutanen M, White R, Parker M 2007 The transcriptional corepressor RIP140 regulates oxidative metabolism in skeletal muscle. Cell Metab 6:236–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog B, Hallberg M, Seth A, Woods A, White R, Parker MG 2007 The nuclear receptor cofactor, receptor-interacting protein 140, is required for the regulation of hepatic lipid and glucose metabolism by liver X receptor. Mol Endocrinol 21:2687–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschiedrich I, Hardeland U, Krones-Herzig A, Berriel Diaz M, Vegiopoulos A, Müggenburg J, Sombroek D, Hofmann TG, Zawatzky R, Yu X, Gretz N, Christian M, White R, Parker MG, Herzig S 2008 Coactivator function of RIP140 for NFκB/RelA-dependent cytokine gene expression. Blood 112:264–276 [DOI] [PubMed] [Google Scholar]
- Debevec D, Christian M, Morganstein D, Seth A, Herzog B, Parker M, White R 2007 Receptor interacting protein 140 regulates expression of uncoupling protein 1 in adipocytes through specific peroxisome proliferator activated receptor isoforms and estrogen-related receptor α. Mol Endocrinol 21:1581–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskinis E, Hallberg M, Christian M, Olofsson M, Dilworth SM, White R, Parker MG 2007 RIP140 directs histone and DNA methylation to silence Ucp1 expression in white adipocytes. EMBO J 26:4831–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, White R, Parker MG 2006 Metabolic regulation by the nuclear receptor corepressor RIP140. Trends Endocrinol Metab 17:243–250 [DOI] [PubMed] [Google Scholar]
- Gupta P, Huq MD, Khan SA, Tsai NP, Wei LN 2005 Regulation of co-repressive activity of and HDAC recruitment to RIP140 by site-specific phosphorylation. Mol Cell Proteomics 4:1776–1784 [DOI] [PubMed] [Google Scholar]
- Mostaqul Huq MD, Gupta P, Wei LN 2008 Post-translational modifications of nuclear co-repressor RIP140: a therapeutic target for metabolic diseases. Curr Med Chem 15:386–392 [DOI] [PubMed] [Google Scholar]
- Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D 1991 Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA 88:5096–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel JH, Jeffery RE, Longcroft JM, Rogers LA, Poulsom R 1998 Comparison of isotopic and non-isotopic labelling for in situ hybridisation of various mRNA targets with cRNA probes. Eur J Histochem 42:143–150 [PubMed] [Google Scholar]
- Liu Z, Shimada M, Richards JS 2008 The involvement of the Toll-like receptor family in ovulation. J Assist Reprod Genet 25:223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS, Liu Z, Shimada M 2008 Immune-like mechanisms in ovulation. Trends Endocrinol Metab 19:191–196 [DOI] [PubMed] [Google Scholar]
- Conti M, Hsieh M, Park JY, Su YQ 2006 Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol 20:715–723 [DOI] [PubMed] [Google Scholar]
- Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC 1999 Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development 126:2739–2750 [DOI] [PubMed] [Google Scholar]
- Lee D, Pearsall RS, Das S, Dey SK, Godfrey VL, Threadgill DW 2004 Epiregulin is not essential for development of intestinal tumors but is required for protection from intestinal damage. Mol Cell Biol 24:8907–8916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC 2003 Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J 22:2704–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS 2009 MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 324:938–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami I, Freimann S, Armon L, Dantes A, Strassburger D, Friedler S, Raziel A, Seger R, Ron-El R, Amsterdam A 2006 PGE2 up-regulates EGF-like growth factor biosynthesis in human granulosa cells: new insights into the coordination between PGE2 and LH in ovulation. Mol Hum Reprod 12:593–599 [DOI] [PubMed] [Google Scholar]
- Kim J, Sato M, Li Q, Lydon JP, Demayo FJ, Bagchi IC, Bagchi MK 2008 Peroxisome proliferator-activated receptor γ is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol Cell Biol 28:1770–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery Jr CA, Shyamala G, Conneely OM, O'Malley BW 1995 Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS 2000 Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA 97:4689–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Morganstein D, Christian M, Seth A, Herzog B, Parker MG 2008 Role of RIP140 in metabolic tissues: connections to disease. FEBS Lett 582:39–45 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.