Abstract
Vascular smooth muscle cell (VSMC) proliferation contributes to vascular remodeling in atherosclerosis and hypertension. Calcium-dependent signaling through calcium/calmodulin-dependent kinase II (CaMKII) and ERK1/2 activation plays an important role in the regulation of VSMC proliferation by agents such as α-adrenergic receptor agonists. Nevertheless, how the CaMKII and ERK pathways interact in VSMCs has yet to be characterized. The aim of the present study was to clarify this interaction in response to α1-adrenergic receptor-mediated VSMC proliferation. We discovered that phenylephrine stimulation resulted in complex formation between CaMKII and ERK in a manner that facilitated phosphorylation of both protein kinases. To assess the effects of CaMKII/ERK association on VSMC proliferation, we inhibited endogenous CaMKII either pharmacologically or by adenoviral-mediated gene transfer of a kinase-inactive CaMKII mutant. Inhibition of CaMKII activation but not CaMKII autonomous activity significantly decreased formation of the CaMKII/ERK complex. On the contrary, the expression of constitutively active CaMKII enhanced VSMC growth and CaMKII/ERK association. In addressing the mechanism of this effect, we found that CaMKII could not directly phosphorylate ERK but instead enhanced Raf1 activation. By contrast, ERK interaction with CaMKII facilitated CaMKII phosphorylation and promoted its nuclear localization. Our results reveal a critical role for CaMKII in VSMC proliferation and imply that CaMKII facilitates assembly of the Raf/MEK/ERK complex and that ERK enhances CaMKII activation and influences its subcellular localization.
Phenylephrine-induced assembly and nuclear translocation of a CaMKII-ERK macromolecular complex regulates VSMC proliferation.
Vascular smooth muscle cell (VSMC) accumulation and proliferation characterize neointimal hyperplasia and lumen narrowing and contribute to the development of atherosclerosis and hypertension (1,2,3). The regulation of VSMC proliferation can be initiated by diverse stimuli (4,5,6), but all signaling pathways converge on the activation of the ERK1/2 (7,8,9). In general, the signaling cascade leading to ERK activation is reported to require the sequential activation of Ras, Raf1, and MAPK/ERK kinase (MEK) (10,11). For example, catecholamine-mediated α1-adrenergic receptor (α1AR) activation leads to an increase of intracellular Ca2+, which initiates signaling cascades involved in VSMC proliferation (7,12). Indeed, changes in intracellular calcium dynamics control several important cell functions (12,13) that are mediated by the multifunctional calcium/calmodulin-dependent protein kinases (CaMKs), a family of serine/threonine protein kinases including CaMKII (14,15,16). CaMKII is a multimeric enzyme and its activity is regulated by the binding of Ca2+/calmodulin (CaM), which activates its protein kinase activity and promotes intrasubunit autophosphorylation (14). The autophosphorylated enzyme retains its kinase activity after the release of Ca2+/CaM, a phenomenon called autonomous activity (14).
Connections between Ca2+ signaling and the ERK pathway have been documented in many cellular systems (4,10): ERK is activated by CaMKII and Raf-dependent mechanism (17,18), and CaMKII facilitates adhesion-dependent activation of ERK in VSMCs (19). Furthermore, pretreatment with a CaM antagonist or a CaMKII inhibitor attenuates ERK activation in response to several stimuli (18,20), and coexpression of CaMKII or a CaMKII inactive mutant in CHO cells down-regulates Ca2+-induced ERK activation (4). These data suggest that CaMKII and ERK are essential mediators of cell proliferation, but so far the mechanism by which these two kinases conspire to regulate proliferation has not been fully elucidated.
In the present study, we explored the roles of CaMKII and ERK in α1AR-mediated VSMC proliferation. Our results suggest a model in which CaMKII binds ERK after α1AR stimulation in a manner that is independent of the generation of CaMKII autonomous activity. Moreover, the inhibition of endogenous CaMKII function by pharmacological antagonists or adenoviral-mediated gene transfer of kinase-inactive dominant negative CaMKII (CaMKII-DN) significantly reduced VSMC proliferation as well as the formation of the CaMKII/ERK complex. Intriguingly, ERK potentiated CaMKII activation and regulated its nuclear localization. We propose that CaMKII plays a regulatory role in VSMC proliferation by contributing to the Ca2+-dependent assembly of the ERK cascade components and to the subcellular localization of itself and ERK. Understanding the molecular elements of the CaMKII-ERK interaction and their functional significance offers a novel therapeutic approach to limit pathological intimal hyperplasia.
Materials and Methods
For extended and more detailed methods, please refer to Supplemental Materials and Methods published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Cell culture, infection, and agonist/antagonists treatment
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by U.S. National Institutes of Health (publication 85-23, revised 1996) and approved by the Ethics Committee of the Federico II University. Primary cultures of VSMCs were obtained from thoracic aortas of WKY rats as described previously (20). VSMCs were incubated with an adenoviral construct encoding either CaMKII-DN constitutively active (CaMKII-CA), or wild-type (CaMKII-WT) CaMKII or the empty virus as a negative control (see Supplemental Materials and Methods for detailed description of the mutants). The cells were used for experiments 48 h after infection.
All stimulations were performed with 100 nmol/liter phenylephrine (PE; Sigma, St. Louis, MO) after 12 h serum starvation or, where indicated, with 2 μmol/liter ionomycin (Sigma).
Doxazosin (Doxa; 100 nmol/liter) was kindly provided by Pfizer (New York, NY).
CaMKII pharmacological inhibition was obtained with the CaMK inhibitor KN93 (5 μmol/liter; Seikagaku, Tokyo, Japan) or, alternatively, 10 nmol/liter of the CaMKII selective inhibitor, AntCaNtide (13,21,22). ERK inhibition was obtained by adding the MEK inhibitor UO126 (10 μmol/liter; Promega, Madison, WI).
Cell transfections
HEK293A cells were transfected with PRK5, full-length WT rat (r) CaMKIIα (CaMKII-WT) or PRK5/rCaMKIIα, full length, T286V (T286V rCaMKIIα), or empty PRK5 vector and Lipofectamine 2000 reagent (Invitrogen, Grand Island, NY) according to the manufacturer's recommendations. After 24 h the cells were stimulated with Ionomycin for 15 min and lysed in the presence or absence of 2 mmol/liter EGTA and 2 mmol/liter EDTA.
Nuclear extract preparation
Nuclei were obtained by cell fractionation: VSMCs were pelleted by centrifugation at 12,000 rpm for 30 min in hypotonic buffer. Nuclei were resuspended in hypertonic buffer for 30 min, centrifuged at 14,000 rpm for 5 min, and the supernatant (nuclear extract) was saved. Nuclear extracts were confirmed by immunoblot analysis using antiactin and histone 3 antibodies.
Immunoblot and immunoprecipitation analysis
VSMCs were stimulated with PE as indicated. Alternatively, the cells were pretreated with the appropriate inhibitor (Doxa, KN-93, and AntCaNtide) for 30 min before stimulation with PE.
Endogenous CaMKII was immunoprecipitated with 5 μl of anti-CaMKII antibody and 25 μl of protein G plus/protein A agarose beads per milligram total cell extract (Santa Cruz Biotechnology, Santa Cruz, CA). The immunoprecipitated kinases were either used to assay activity or resolved on SDS-PAGE to visualize the associated proteins by immunoblot. Commercial antibodies were diluted 1:1000 and purchased as follows: total MEK1/2, Raf1, ERK1/2, CaMKII, actin, histone 3, and phospho-p44/p42 ERK1/2 (Santa Cruz); total retinoblastoma, and phosphoretinoblastoma (Cell Signaling, Danvers, MA); phosphothreonine-286-CaMKII (Promega).
Cell proliferation
VSMCs were plated in 12-well plates (20,000 cells/well) and serum starved overnight. Cells were pretreated with KN93 or AntCaNtide and then stimulated with PE. Six, 12, and 24 h after stimulation with PE, the medium was removed, cells detached from the plates, and counted.
[3H]thymidine incorporation
Cells were plated in 12-well plates (20,000 cells/well). VSMCs were pretreated with KN93 or AntCaNtide and then incubated with PE and 0.5 μCi of [3H]thymidine (Amersham, Buckinghamshire, UK). Twenty-four hours after stimulation, VSMCs were processed.
Kinase assays
CaMKII activity assays
In all CaMKII assays, active recombinant full-length CaMKII (Signal Chem, La Jolla, CA) or immunoprecipitated CaMKII, where indicated, were incubated for 30 min at 30 C with either 1 mmol/liter CaCl2 and 1 μmol/liter CaM or 1 mmol/liter EGTA (negative control) in 50 μl of a reaction mixture (50 mmol/liter HEPES, pH 7.5; 10 mmol/liter MgCl2; 0.5 mmol/liter dithiothreitol; 100 nmol/liter microcystin; 0.1 mmol/liter nonradiolabeled ATP). Then aliquots from these reactions were incubated with the different substrates and [γ32P]ATP (Amersham) (see Supplemental Materials and Methods); EGTA was added to quantify CaMKII autonomous activity.
Raf-1 activity assay
Raf-1 activity was evaluated using a commercial Raf1 kinase activity assay (Upstate Biotechnology, Lake Placid, NY).
Confocal microscopy
For immunocytochemistry, cells were fixed in 0.04 g/liter paraformaldehyde for 30 min at 4 C and permeabilized with 0.01 g/liter Triton X-100 for 30 min at 4 C. Incubation with anti-CaMKII (BD Biosciences, San Diego, CA) and anti-ERK1/2 (Santa Cruz) antibodies was performed in PBS and 3% BSA overnight at 4 C. Cells were then washed and stained with Alexa Fluor 594 goat antirabbit IgG (0.5 μg/ml; Molecular Probes, Eugene, OR) and fluorescein isothiocyanate conjugated goat-antimouse IgG (BD Biosciences) for 30 min and counterstained with Hoechst 33342 (Vector, Burlingame, CA). Images were acquired with a LSM510 inverted confocal microscope (Zeiss, Oberkochen, Germany) using a ×63 oil objective and processed using LSM software (Zeiss).
Statistical analysis
One-way ANOVA was performed to compare different groups using a Bonferroni post hoc analysis. Two-way ANOVA was applied to analyze different parameters among the different groups. A significance level of P < 0.05 was assumed for all statistical evaluations. Statistics were computed with GraphPad Prism software (San Diego, CA). Each value in the figures is presented as mean ± sem of at least three independent experiments.
Results
α1-AR stimulation by PE induces ERK and CaMKII activation in VSMCs
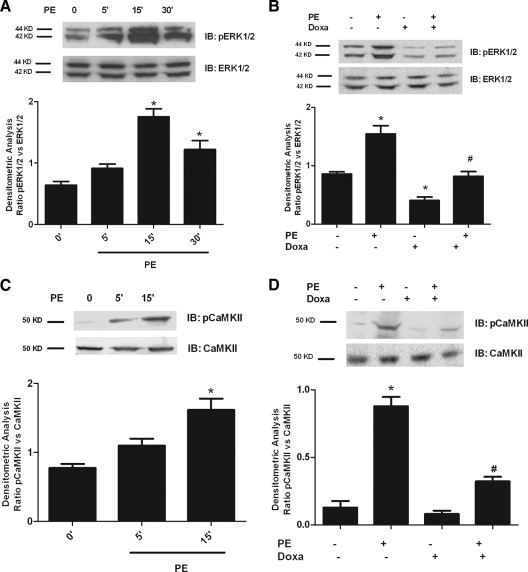
Figure 1A shows that PE stimulation of VSMCs resulted in time-dependent ERK phosphorylation (Fig. 1A). PE stimulation of ERK was achieved through activation of the α1AR, because Doxa, a specific α1AR antagonist, completely abrogated PE-induced ERK activation (Fig. 1B). We also demonstrated that PE induced maximal CaMKII phosphorylation 15 min after the stimulation (Fig. 1C), and Doxa significantly attenuated CaMKII activation (Fig. 1D).
Figure 1.
Specific α1AR stimulation by phenylephrine induces ERK and CaMKII activation in VSMCs. A, VSMCs were stimulated with PE at the indicated time points. Total cell lysates were analyzed by Western blotting (WB) for phosphotyrosine ERK1/2 (pERK1/2) and total ERK1/2 with specific antibodies. Immunoblots (IB) were quantified by densitometric analysis (DA). The densitometry of pERK1/2 were averaged and normalized to averaged ERK1/2 densitometry (F = 109.2, P < 0.0001, ANOVA). *, P < 0.01 vs. 0′. B, VSMCs were stimulated with PE for 15 min in presence or absence of Doxa pretreatment. pERK1/2 and ERK1/2 levels were evaluated by WB. Immunoblots were quantified by DA. pERK1/2 levels were normalized to ERK1/2 densitometry as in A (F = 29.89, P < 0.0001, ANOVA). *, P < 0.01 vs. control; #, P < 0.01 vs. PE. C, VSMCs were stimulated with PE at the indicated time points. Whole lysates were analyzed by WB. Total CaMKII was visualized by a specific antibody (CaMKII) and phosphorylated CaMKII by antiphosphothreonine-286-CaMKII antibody (pCaMKII). Immunoblots were quantified by DA. pCaMKII levels were normalized to CaMKII densitometry (F = 32.23, P < 0.0001, ANOVA). *, P < 0.05 vs. 0′. D, VSMCs were exposed to Doxa for 30 min and stimulated with PE for 15 min. Total cellular extracts were analyzed by WB for pCaMKII and total CaMKII. Immunoblots were quantified by DA. pCaMKII levels were normalized to CaMKII densitometry (F = 168.5, P < 0.0001, ANOVA). *, P < 0.001 vs. control; #, P < 0.05 vs. PE.
CaMKII regulates PE-induced ERK phosphorylation
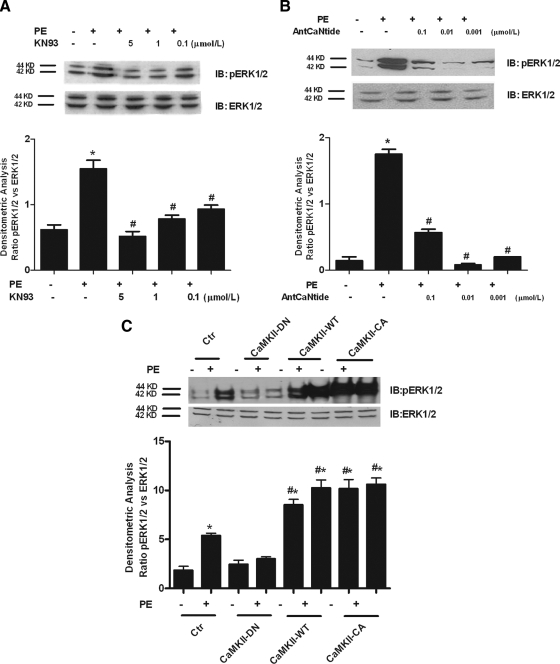
To investigate cross talk between CaMKII and ERK in VSMCs, we stimulated the cells with PE in the presence or absence of CaMK inhibitors and evaluated ERK phosphorylation. The CaMK inhibitor, KN93, dose-dependently inhibited ERK phosphorylation (Fig. 2A). In addition, CaMKII-specific inhibition was achieved by a cell-permeant peptide, AntCaNtide (Fig. 2B), or CaMKII-DN (Fig. 2C), and both treatments resulted in inhibition of ERK phosphorylation. Conversely, the overexpression of CaMKII-WT or CaMKII-CA leads to increased basal and α1AR-mediated ERK phosphorylation (Fig. 2C). These results demonstrate CaMKII-dependent ERK activation after PE stimulation.
Figure 2.
CaMKII regulates PE-induced ERK phosphorylation. A, VSMCs were pretreated with KN93 for 30 min at indicated micromoles per liter concentrations and then stimulated with PE for 15 min. Whole extracts were analyzed by Western blotting (WB) with anti-pERK1/2 or anti-total ERK1/2 antibodies. Immunoblots (IB) were quantified by densitometric analysis (DA). The densitometry of pERK1/2 were averaged and normalized to averaged ERK1/2 densitometry (F = 32.58, P < 0.0001, ANOVA). *, P < 0.05 vs. control; #, P < 0.05 vs. PE. B, VSMCs were pretreated with AntCaNtide for 30 min at indicated micromoles per liter concentrations and then stimulated with PE for 15 min, and ERK1/2 and pERK1/2 levels were evaluated by WB. Immunoblots were quantified by DA. pERK1/2 levels were normalized to ERK1/2 densitometry as in A (F = 243.6, P < 0.001, ANOVA). *, P < 0.05 vs. control; #, P < 0.05 vs. PE. C, VSMCs were infected with adenovirus-expressing CaMKII-WT, CaMKII-CA, CaMKII-DN, or the empty virus [control (Ctr)] as a negative control. After 48 h, the cells were serum starved overnight and then stimulated with PE for 15 min. Total extracts were analyzed by WB with antiphospho-ERK1/2 or antitotal ERK1/2 antibodies. Densitometry was analyzed and normalized as in A (F = 38.92, P < 0.0001, ANOVA). *, P < 0.05 vs. control; #, P < 0.05 vs. PE.
CaMKII is required for PE activation of VSMC proliferation
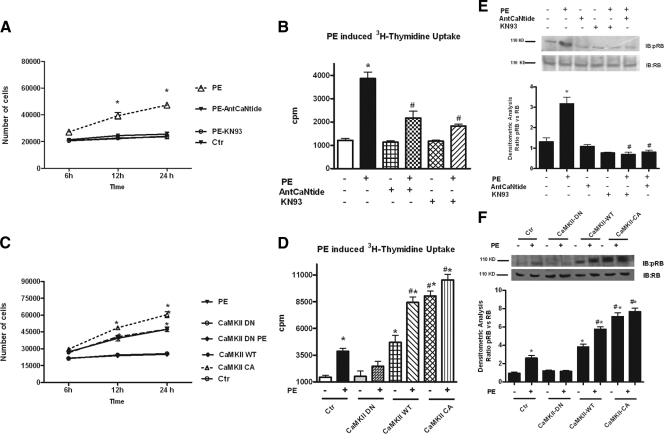
To investigate the role of CaMKII on VSMC growth, we assessed the effect of CaMKII inhibition on PE-induced cell proliferation and 3H-thymidine uptake. Pretreatment with KN93 or AntCaNtide caused a significant reduction in cell proliferation (Fig. 3A) and 3H-thymidine uptake (Fig. 3B) 24 h after PE. Furthermore, the adenoviral-mediated overexpression of WT- or CA-CaMKII but not DN-CaMKII resulted in increased VSMC proliferation (Fig. 3C) and 3H-thymidine uptake (Fig. 3D). To confirm these results, we evaluated the phosphorylation status of retinoblastoma (Rb) protein, which is indicative of cell cycle progression. The decrease in CaMKII activity due to inhibition by either KN93 or AntCaNtide (Fig 3E) as well as the adenoviral gene transfer (Fig. 3F) of DN-CaMKII inhibited PE-induced Rb phosphorylation. On the contrary, expression of WT- or CA-CaMKII stimulated Rb phosphorylation in the absence of PE. These results demonstrate that CaMKII activity is an essential component of the VSMC proliferative response.
Figure 3.
CaMKII is required for PE activation of VSMC proliferation. A, 2 × 104 VSMCs were plated in 12-well plates and serum starved overnight. After pretreatment with KN93 (PE-KN93) or AntCaNtide (PE-AntCaNtide) for 30 min, VSMCs were stimulated with PE. Then 6-, 12-, and 24-h cells were harvested and counted by hemocytometer (F=26.51 P < 0.0001 ANOVA). *, P < 0.0001 vs. PE-AntCaNtide and PE-KN93. B, 2 × 104 VSMCs were plated in 12-well plates and serum starved overnight. Cells were pretreated with KN93 or AntCaNtide for 30 min and cultured in the presence or absence of PE and 0.5 μCi/ml [3H]thymidine. After 24 h, the rate of [3H]thymidine incorporation was determined by β-counter. Values of [3H]thymidine incorporation are presented as total incorporation (counts per minute) (F= 18.03, P < 0.0001, ANOVA). *, P < 0.05 vs. control (Ctr). C, VSMCs were infected with adenovirus expressing CaMKII-WT, CaMKII-CA, CaMKII-DN, or the empty virus (Ctr) as described in Materials and Methods. Forty-eight hours after the infection, 2 × 104 cells were plated in 12-well plates and serum starved overnight. After 30 min of pretreatment with KN93 or AntCaNtide, the cells were stimulated with PE. After 6, 12, and 24 h, the cells were harvested and counted by hemocytometer (F = 19.55, P < 0.0001, ANOVA). *, P < 0.001 vs. CaMKII-DN and CaMKII-DN-PE. D, VSMCs were infected with adenovirus expressing CaMKII-WT, CaMKII-CA, CaMKII-DN, or the empty virus (Ctr) as described in Materials and Methods. Forty-eight hours after the infection, 2 × 104 cells were plated in 12-well plates and serum starved overnight. Subsequently VSMCs were pretreated with KN93 or AntCaNtide and cultured with PE, and 0.5 μCi/ml [3H]thymidine. Twenty-four hours later, the rate of [3H]thymidine incorporation was determined by β-counter. Values of [3H]thymidine incorporation are presented as total incorporation (counts per minute) (F = 58.64, P < 0.0001, ANOVA). *, P < 0.05 vs. Ctr; #, P < 0.05 vs. PE. E, VSMCs were exposed to KN93 or AntCaNtide and then stimulated with PE for 15 min. Total cellular extracts were analyzed by Western blotting (WB) for Rb protein phosphorylation (pRb) with a specific antibody. Data from immunoblots were quantified by densitometric analysis. pRb levels were normalized to total Rb protein densitometry (F = 105.1, P < 0.0001, ANOVA). *, P < 0.05 vs. Ctr; #, P < 0.05 vs. PE. F, VSMCs were infected with adenoviruses expressing CaMKII-WT, CaMKII-CA, CaMKII-DN, or the empty virus (Ctr) as described in Materials and Methods. Forty-eight hours after the infection, the cell were serum starved overnight and then stimulated with PE for 15 min. Total cellular extracts were analyzed by WB for Rb protein phosphorylation. Densitometry was analyzed and normalized as indicated above (F = 27.42, P < 0.0001, ANOVA). *, P < 0.05 vs. Ctr; #, P < 0.05 vs. PE.
PE-induced CaMKII-ERK interaction is regulated by Ca2+/CaM-dependent CaMKII activation
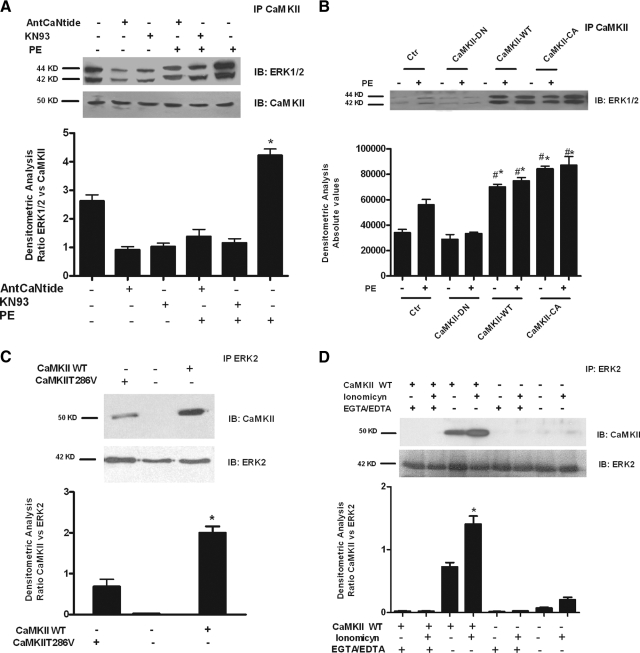
To study whether the CaMKII effect on PE-induced proliferation was mediated by the interaction with ERK, we performed coimmunoprecipitation experiments. As shown in Fig. 4A, ERK is bound to CaMKII in resting cells. The extent of this interaction is increased after PE stimulation but prevented by CaMKII inhibition with KN93 or AntCaNtide (Fig. 4A). A CaMKII/ERK interaction was confirmed by adenoviral gene transfer in that DN-CaMKII did not associate with ERK, even after PE stimulation, whereas WT-CaMKII and CA-CaMKII associated with ERK, even in the absence of PE stimulation (Fig. 4B). These data confirm that CaMKII activation promotes its interaction with ERK.
Figure 4.
PE-induced CaMKII-ERK interaction is regulated by Ca2+/CaM-dependent CaMKII activation. A, VSMCs were pretreated with KN93 or AntCaNtide for 30 min and stimulated with PE. Cell lysates were immunoprecipitated (IP) using anti-CaMKII antibody, and the samples were analyzed by Western blotting (WB) for ERK1/2 to evaluate the association with CaMKII. Immunoblots (IB) were quantified by densitometric analysis (DA). The densitometry of ERK1/2 was averaged and normalized to CaMKII densitometry (F = 48.18, P < 0.0001, ANOVA). *, P < 0.05 vs. control (Ctr). B, VSMCs were infected with adenovirus expressing CaMKII-WT, CaMKII-CA, CaMKII-DN, or the empty virus (Ctr) as described in Materials and Methods. Forty-eight hours after the infection, cells were stimulated as above with PE after pretreatment with KN93 or AntCaNtide. Total cell lysates were immunoprecipitated using anti-CaMKII antibody. The samples underwent WB to visualize ERK1/2, and evaluate its association with CaMKII. Immunoblots were quantified by DA. ERK1/2 levels were averaged and expressed as absolute values (F = 130.7, P < 0.0001, ANOVA). *, P < 0.05 vs. Ctr; #, P < 0.05 vs. PE. C, CaMKII WT, CaMKII T286V, and control plasmids were transfected in HEK293A cells. After 24 h, total cell lysates were immunoprecipitated using anti-ERK2 antibody and probed for CaMKII and ERK2. Immunoblots were quantified by DA and CaMKII levels were normalized to ERK2 densitometry (F = 78.56, P < 0.0001, ANOVA). *, P < 0.05 vs. CaMKII-WT. D, Cell lysates were immunoprecipitated for ERK2 in presence or absence of EGTA/EDTA from HEK293A transfected with CaMKII-WT or control plasmid and stimulated or not with 2 μmol/liter ionomycin for 15 min. The precipitates were subjected to WB for CaMKII and ERK2. Immunoblots were quantified by DA. CaMKII levels were normalized to ERK2 densitometry (F = 126.9, P < 0.0001, ANOVA). *, P < 0.05 vs. CaMKII WT.
Next, to test whether CaMKII kinase activity and/or autonomous activity were required for the association with ERK, HEK293A cells were transfected with CaMKII-WT or the CaMKII-T286V point mutant that is unable to undergo intrasubunit autophosphorylation in response to Ca2+/CaM binding and, as such, lacks autonomous activity. Both CaMKII and its T286V mutant coimmunoprecipitated with ERK2, suggesting that their interaction is not dependent on CaMKII autonomous activity (Fig. 4C). Next, to test whether CaMKII activation was required for the interaction with ERK, we transfected the cells with CaMKII-WT; stimulated them with the Ca2+ ionophore, ionomycin; and immunoprecipitated CaMKII in the presence or absence of the Ca2+ chelators EGTA and EDTA. Basal association between CaMKII and ERK2 was increased by ionomycin, whereas EDTA/EGTA completely disrupted the interaction, suggesting that Ca2+/CaM-dependent activation of CaMKII is required for the formation of the CaMKII/ERK complex (Fig. 4D).
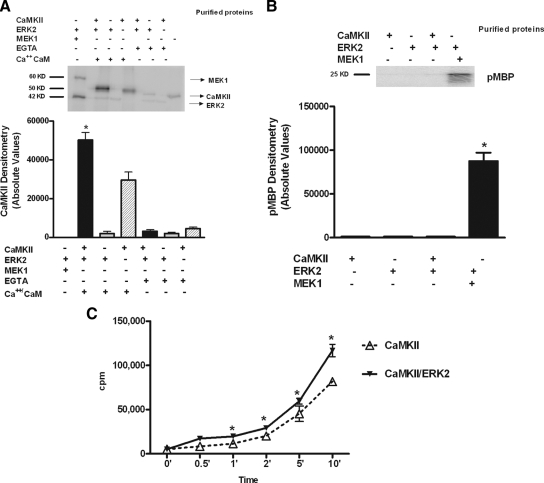
CaMKII does not phosphorylate ERK in vitro
To investigate whether CaMKII/ERK association results in ERK phosphorylation, we performed an in vitro 32P kinase assay with purified proteins. Figure 5A shows that active CaMKII cannot directly phosphorylate ERK. A myelin basic protein (MBP) phosphorylation assay validated this result by confirming that the interaction of ERK with CaMKII was unable to activate ERK to phosphorylate MBP (Fig. 5B). On the other hand, and quite unexpectedly, CaMKII phosphorylation was increased in presence of ERK (Fig. 5A). This result was confirmed by an in vitro time course of autocamtide phosphorylation by CaMKII. As shown in Fig. 5C, the presence of ERK in the reaction mixture enhanced autocamtide phosphorylation and CaMKII activation.
Figure 5.
CaMKII does not phosphorylate ERK in vitro. A, To determine whether CaMKII was able to phosphorylate ERK in vitro, active recombinant CaMKII and recombinant ERK2 were incubated with [γ32P]ATP, CaCl2 and CaM, or EGTA as described in Materials and Methods. Active MEK1 was incubated with inactive ERK2 as positive control. After PAGE, MEK1, CaMKII, and ERK2 were identified by their molecular weights. CaMKII phosphorylation was quantified by phosphorimager and presented as absolute values (F = 67.01, P < 0.0001, ANOVA). *, P < 0.0001 CaMKII in Ca2+/CaM. B, To verify whether CaMKII was able to activate ERK2 in vitro, active recombinant CaMKII and inactive ERK2 were incubated in presence of [γ32P]ATP, MBP, CaCl2, and CaM. Active MEK1 was incubated with inactive ERK2 and MBP as positive control. The reaction mixture was subjected to PAGE and autoradiography, and the band corresponding to phosphorylated MBP (pMBP) was identified by molecular weight and quantified by phosphorimager and presented as absolute values (F = 234.2, P < 0.0001, ANOVA). *, P < 0.001 CaMKII. C, To determine whether ERK2 was able to modify the kinetics of CaMKII activation, active recombinant CaMKII and its substrate autocamtide were incubated in a reaction mixture containing [γ32P]ATP, CaCl2, and CaM in the presence or absence of inactive purified ERK2. Quantification of incorporated radioactivity was performed by liquid scintillation. The results are presented as total incorporation (counts per minute) (F = 15.89, P < 0.0001, ANOVA). *, P < 0.05 vs. CaMKII.
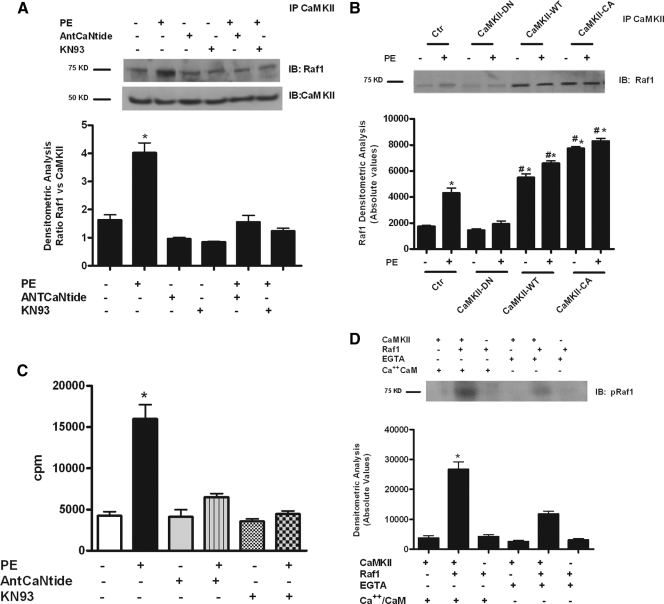
PE induces the association of CaMKII with Raf1 and regulates its activation
Figure 6A shows that in VSMCs CaMKII and Raf1 are associated under basal conditions, and PE stimulation increased the extent of this association. Pharmacological inhibition of CaMKII significantly reduced PE-induced CaMKII/Raf1 interaction (Fig. 6A). As confirmation of the specific effects of CaMKII activity on the interaction with Raf1, we demonstrated that CaMKII-WT and CaMKII-CA but not CaMKII-DN associated with Raf1 after PE stimulation (Fig. 6B). Finally, we discovered that PE stimulation induced a significant increase of Raf1-activity, which was completely abrogated by either KN93 or AntCaNtide (Fig. 6C). The idea that Raf1 might be a substrate of CaMKII suggested by these results was confirmed by the immunoprecipitation of CaMKII and Raf1 from serum-starved VSMCs. Finally, an in vitro kinase assay also showed that the addition of Ca2+/CaM stimulated CaMKII to phosphorylate Raf1 (Fig. 6D).
Figure 6.
PE induces the association of CaMKII with Raf1 and regulates its activation. A, VSMCs were stimulated with PE after pretreatment with KN93 or AntCaNtide. Cell lysates were immunoprecipitated (IP) using anti-CaMKII antibody. The protein samples underwent Western blotting (WB) procedure to visualize Raf1 and evaluate the association with CaMKII. Immunoblots (IB) were quantified by densitometric analysis (DA). Raf1 levels were normalized to CaMKII densitometry (F = 36.80, P < 0.0001, ANOVA). *, P < 0.05 vs. control (Ctr). B, VSMCs were infected with adenovirus expressing CaMKII-WT, CaMKII-CA, CaMKII-DN, or the empty virus (Ctr) as described in Materials and Methods. Forty-eight hours after the infection, the cells were serum starved overnight and then stimulated with PE. Total cellular extracts were immunoprecipitated (IP) using anti-CaMKII antibody and subjected to WB to evaluate the association of Raf1 with CaMKII. Immunoblots (IB) were quantified by densitometric analysis. Raf1 levels were expressed as absolute values (F = 82.31, P < 0.0001, ANOVA). *, P < 0.05 vs. Ctr; #, P < 0.05 vs. PE. C, VSMCs were stimulated with PE after 30 min pretreatment with KN93 or AntCaNtide. Raf1 was immunoprecipitated from cell extracts and its activity measured by a cascade kinase assay with MEK1, ERK2, [γ32P]ATP, and MBP as a substrate. [γ32P]ATP incorporation in MBP was determined by liquid scintillation. The results are presented as total incorporation (counts per minute) (F = 71.14, P < 0.0001, ANOVA). *, P < 0.05 vs. Ctr; #, P < 0.05 vs. PE. D, Raf1 was immunoprecipitated from cell extracts of serum starved VSMCs and incubated with active recombinant CaMKII in a reaction mixture containing [γ32P]ATP, CaCl2, and CaM or EGTA. After PAGE, phosphorylated Raf1 (pRaf1) was quantified by phosphorimager. Data were normalized total CaMKII assessed by WB on total cell extracts (data not shown) and presented as absolute values (F = 64.09, P < 0.0001, ANOVA). *, P < 0.001 vs. Ctr.
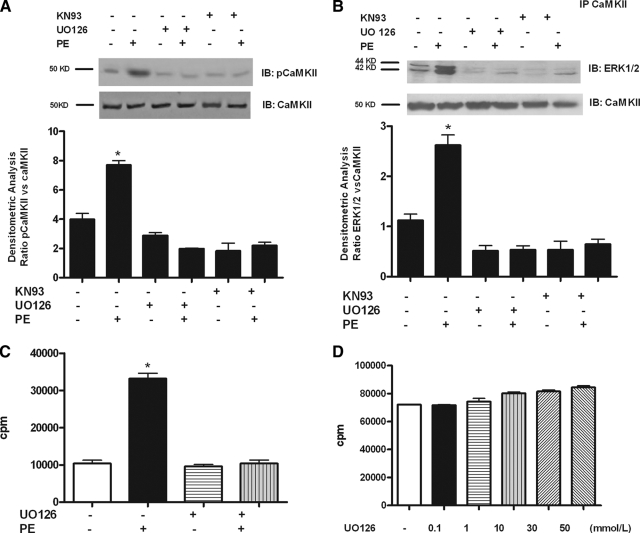
The inhibition of the MEK/ERK pathway down-regulates PE-induced CaMKII activation
Interestingly, the interaction between CaMKII and ERK resulted in a significant increase of autonomous CaMKII activity (Fig. 5). We next explored the role of active ERK on CaMKII phosphorylation and activation. First, we pretreated VSMCs with the MEK/ERK inhibitor UO126 and found that this completely abrogated PE-induced CaMKII phosphorylation (Fig. 7A) and significantly reduced ERK/CaMKII interaction (Fig. 7B). Second, we performed an activity assay with immunoprecipitated CaMKII and showed that UO126 pretreatment significantly inhibited PE-induced CaMKII activation (Fig. 7C). Because UO126 was unable to directly inhibit CaMKII at the concentration used in the present assay (Fig. 7D), we concluded that ERK activity is required to interact with and activate CaMKII.
Figure 7.
The inhibition of the MEK/ERK pathway down-regulates PE-induced CaMKII activation. A, VSMCs were exposed to UO126 for 30 min and then stimulated with PE. Total cell extracts were analyzed by Western blotting (WB) for pCaMKII and quantified by densitometric analysis. pCaMKII levels were normalized to CaMKII densitometry (F = 47.45, P < 0.0001, ANOVA). *, P < 0.05 vs. control (Ctr). IB, Immunoblot. B, VSMCs were stimulated with PE after UO126 pretreatment. CaMKII was immunoprecipitated (IP) from cell lysates and subjected to WB to visualize ERK1/2. Immunoblots were quantified by densitometric analysis. ERK1/2 levels were averaged and normalized to CaMKII densitometry (F = 36.15, P < 0.0001, ANOVA). *, P < 0.05 vs. Ctr. C, VSMCs were stimulated with PE for 15 min after pretreatment with UO126 for 30 min. CaMKII was immunoprecipitated from cell extracts, incubated with autocamtide and [γ32P]ATP and spotted on nitrocellulose. The level of [γ32P]ATP incorporation into autocamtide was determined by liquid scintillation. The results are presented as total incorporation (counts per minute) (F = 132.2, P < 0.0001, ANOVA). *, P < 0.05 vs. Ctr. D, Active recombinant CaMKII was incubated with increasing concentration of UO126 using autocamtide as substrate. The level of [γ32P]ATP incorporation into autocamtide was determined by liquid scintillation and presented as total incorporation (counts per minute) (F = 8.226, P < 0.0001, ANOVA).
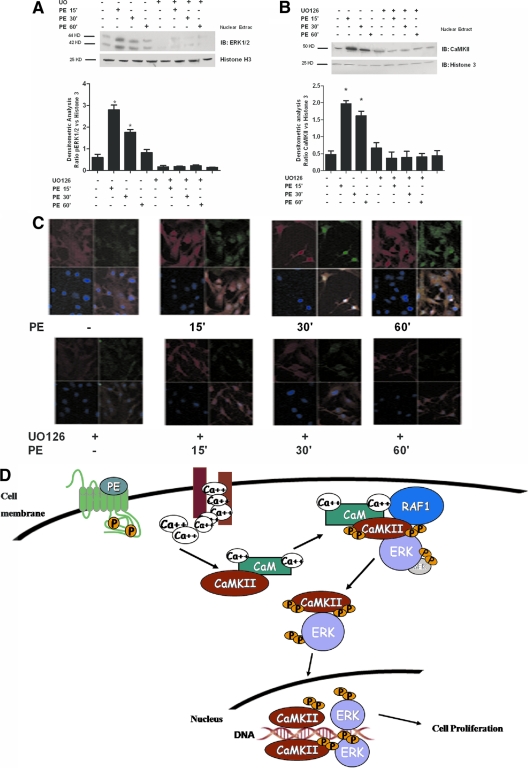
Inhibition of the MEK/ERK pathway affects the subcellular distribution of CaMKII
To investigate whether the potentiation of CaMKII activity might influence the subcellular compartmentalization of one or both kinases, we evaluated the intracellular localization of ERK and CaMKII. As expected, most of the activated ERK translocated into the nucleus 15 min after PE stimulation (Fig. 8A). Similarly, stimulation with PE increased the amount of CaMKII present in the nuclear extracts (Fig. 8A). Moreover, the pharmacological inhibition of the ERK pathway with UO126 abrogated PE-induced nuclear localization of ERK and CaMKII (Fig. 8B). Similar results were obtained by confocal microscopy, which showed that PE induced a time-dependent accumulation of both ERK and CaMKII in the nucleus (Fig. 8C, upper panel). Furthermore and consistent with our biochemical results, UO126 prevented not only ERK but also CaMKII nuclear localization (Fig. 8C, lower panel). These results support the concept that ERK activation facilitates and is required for CaMKII nuclear localization.
Figure 8.
The inhibition of MEK/ERK pathway regulates CaMKII subcellular compartmentalization. A, VSMCs were stimulated with PE for the indicated time points after 30 min pretreatment with UO126. Nuclear extracts were analyzed by Western blotting (WB) for total ERK1/2 with specific antibody. Immunoblots (IB) were quantified by densitometric analysis (DA). ERK1/2 levels were averaged and normalized to histone 3 densitometry (F = 1.885, P < 0.0001, ANOVA). *, P < 0.05 vs. control. B, VSMCs were stimulated with PE for indicated time points after UO126 pretreatment. Nuclear extracts were analyzed by WB for total CaMKII with specific antibody. Immunoblots were quantified by DA. CaMKII levels were normalized to histone 3 densitometry (F = 23.72, P < 0.0001, ANOVA). *, P < 0.05 vs. control; #, P < 0.05 vs. PE. C, Confocal fluorescent immunocytochemistry images of VMSCs stimulated with PE for indicated time points, without (upper panel) or with (lower panel) UO126 for 30 min. For each time point, CaMKII (green), ERK1/2 (red), nuclei (blue), and the overlay of the three colors are shown. D, A new model of CaMKII/ERK1/2 signal transactivation interaction is proposed. After receptor stimulated Ca2+ intracellular influx, a scaffold is created with CaMKII, Raf1, and ERK that facilitates ERK activation. This latter in turn phosphorylates CaMKII, promoting its localization within the nucleus. p, Phosphorous attached.
Discussion
Excessive proliferation of VSMCs is the basis of neointimal hyperplasia and restenosis (23) and contributes to the development of atherosclerosis (1,2,3). The clinical relevance of this phenomenon has lead to the development of strategies for pharmacological inhibition of VSMCs, i.e. with a medicated stent (24,25). Nevertheless, there is still a need to discover novel therapeutic targets to achieve a more effective pharmacological inhibition of VSMC proliferation and migration.
Several intracellular signaling pathways are involved in VSMC proliferation and among these cascades, Ca2+-mediated ones play a major role. CaMKs are important transducers of changes in intracellular Ca2+, and although they are reported to play a role in ERK activation in vascular models (4,26), the mechanisms involved in mediating cross talk between CaMKs and ERK has yet to be fully investigated in VSMCs. Here we report a thorough examination of this issue and make several striking findings that inform the biology of VSMCs. First, we show that PE induces CaMKII association with Raf1, MEK, and ERK and the formation of this macromolecular complex requires CaMKII activation by Ca2+/CaM but is independent of the generation of CaMKII autonomous activity. Second, CaMKII fails to directly activate ERK, whereas, in agreement with our previous results and other published reports (11,17,27), CaMKII regulates Raf1 activation in VSMCs. Together these observations suggest that the interplay between Raf1 and CaMKII regulates ERK activation downstream of a number of different membrane-associated receptors. Third and very intriguingly, our results support a novel role for CaMKII as a scaffold for the components of the ERK pathway and, based on the interaction of ERK with CaMKII-CA, suggest that the kinase domain is sufficient to nucleate the assembly of the CaMKII-Raf1-MEK-ERK signaling complex. Scaffold proteins play a regulatory role by linking the components of the signaling cascade into a multienzyme complex by which the amplitude and duration of ERK-mediated signals are fine-tuned. Our results suggest that CaMKII may serve a similar role by contributing to the assembly of ERK cascade components in response to Ca2+-dependent, Ras-independent stimuli and, by so doing, achieve cytoplasmic spatial selectivity of ERK signals as well by facilitating translocation to the nucleus.
Fourth, we demonstrate that ERK activation participates in α-adrenergic-induced VSMC proliferation in a CaMKII-dependent fashion that does require the kinase activity of CaMKII. Indeed, specific CaMKII inhibition significantly reduces α-adrenergic-dependent ERK activation and cell proliferation. Previous pharmacological studies in VSMCs and transient transfection experiments in COS-7 cells (4,28) suggested that CaMKs play a role in Ca2+-dependent ERK activation, but we extend these results by showing that ERK inhibition can be achieved indirectly through inhibition of CaMKII.
Our fifth original finding is that ERK interaction with CaMKII potentiates CaMKII activation in vitro. PE stimulation activates CaMKII in the cytoplasm (and what little CaMKII resides in the nucleus of VSCM under basal culture conditions) and also promotes the time-dependent accumulation of CaMKII in the nucleus. We find that ERK phosphorylates CaMKII and inhibition of ERK prevents both CaMKII activation and nuclear translocation. Like other multifunctional protein kinases, CaMKII isozymes gain specificity by signaling pathway-dependent targeting to various subcellular locales such as nucleus, plasma membrane, cytoskeleton, and other more specialized structures. Heterooligomerization of CaMKII monomers influences its targeting (29). Indeed, cytosolic CaMKII isoforms can interact with and redirect nuclear-targeted CaMKII isoforms to the cytosol (30). Our results suggest that ERK plays a role in directing the cytoplasmic to nuclear translocation of CaMKII that is initiated by ERK phosphorylation of CaMKII. Although our data are not sufficient to prove the precise mechanism involved, we envision two possible mechanisms: ERK could phosphorylate CaMKII residues present in a potential nuclear translocation sequence in a manner that activates this signal and directs CaMKII to undergo nuclear translocation, or, alternatively, the phosphorylation of CaMKII might allow it to be shuttled into the nucleus and associated with ERK by using the ERK nuclear localization sequence. Further studies are required to clarify this exciting issue.
In conclusion, our results describe a novel mechanism that clarifies the cross talk between CaMKII and ERK. On the one hand, CaMKII, through direct phosphorylation of and scaffolding with Raf1, leads to ERK activation. On the other hand, ERK activation leads to the phosphorylation and nuclear translocation of CaMKII. This unique means of interplay suggests that CaMKII could represent a potential molecular target to consider for therapeutic inhibition of VSMC proliferation.
Supplementary Material
Acknowledgments
We are grateful to Elizabeth MacDougall for editing the manuscript.
Footnotes
This work was supported by a grant as “Progetto Strategico di Ricerca-Regione Puglia 2006-2009-PS_131” and National Institutes of Health Grants DK074701 (to A.R.M.) and R01 NS052644 (to K.U.B).
Disclosure Summary: E.C., S.M., A.S.M., L.V., P.C., L.P., C.F., E.N., V.L., K.U.B., A.R.M., G.R., B.T., G.I., and M.I. have nothing to disclose.
First Published Online April 14, 2010
Abbreviations: α1AR, α1-Adrenergic receptor; CA, constitutively active; CaM, calmodulin; CaMKII, calmodulin-dependent kinase II; DN, kinase-inactive dominant negative; Doxa, doxazosin; MBP, myelin basic protein; MEK, MAPK/ERK kinase; PE, phenylephrine; r, rat; Rb, retinoblastoma; VSMC, vascular smooth muscle cell; WT, wild type.
References
- Ross R 1995 Cell biology of atherosclerosis. Annu Rev Physiol 57:791–804 [DOI] [PubMed] [Google Scholar]
- Ross R 1999 Atherosclerosis—an inflammatory disease. N Engl J Med 340:115–126 [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Majesky MW, Murry CE 1995 The intima: development and monoclonal responses to injury. Atherosclerosis 118(Suppl): S125–S140 [PubMed] [Google Scholar]
- Abraham ST, Benscoter HA, Schworer CM, Singer HA 1997 A role for Ca2+/calmodulin-dependent protein kinase II in the mitogen-activated protein kinase signaling cascade of cultured rat aortic vascular smooth muscle cells. Circ Res 81:575–584 [DOI] [PubMed] [Google Scholar]
- Della Rocca GJ, Maudsley S, Daaka Y, Lefkowitz RJ, Luttrell LM 1999 Pleiotropic coupling of G protein-coupled receptors to the mitogen-activated protein kinase cascade. Role of focal adhesions and receptor tyrosine kinases. J Biol Chem 274:13978–13984 [DOI] [PubMed] [Google Scholar]
- van Biesen T, Hawes BE, Luttrell DK, Krueger KM, Touhara K, Porfiri E, Sakaue M, Luttrell LM, Lefkowitz RJ 1995 Receptor-tyrosine-kinase- and Gβγ-mediated MAP kinase activation by a common signalling pathway. Nature 376:781–784 [DOI] [PubMed] [Google Scholar]
- Hu ZW, Shi XY, Lin RZ, Chen J, Hoffman BB 1999 α1-Adrenergic receptor stimulation of mitogenesis in human vascular smooth muscle cells: role of tyrosine protein kinases and calcium in activation of mitogen-activated protein kinase. J Pharmacol Exp Ther 290:28–37 [PubMed] [Google Scholar]
- Xin X, Yang N, Eckhart AD, Faber JE 1997 α1D-adrenergic receptors and mitogen-activated protein kinase mediate increased protein synthesis by arterial smooth muscle. Mol Pharmacol 51:764–775 [DOI] [PubMed] [Google Scholar]
- Lawrence MC, Jivan A, Shao C, Duan L, Goad D, Zaganjor E, Osborne J, McGlynn K, Stippec S, Earnest S, Chen W, Cobb MH 2008 The roles of MAPKs in disease. Cell Res 18:436–442 [DOI] [PubMed] [Google Scholar]
- Ginnan R, Singer HA 2002 CaM kinase II-dependent activation of tyrosine kinases and ERK1/2 in vascular smooth muscle. Am J Physiol 282:C754–C761 [DOI] [PubMed] [Google Scholar]
- Illario M, Cavallo AL, Bayer KU, Di Matola T, Fenzi G, Rossi G, Vitale M 2003 Calcium/calmodulin-dependent protein kinase II binds to Raf-1 and modulates integrin-stimulated ERK activation. J Biol Chem 278:45101–45108 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL 2003 Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4:517–529 [DOI] [PubMed] [Google Scholar]
- House SJ, Ginnan RG, Armstrong SE, Singer HA 2007 Calcium/calmodulin-dependent protein kinase II-Δ isoform regulation of vascular smooth muscle cell proliferation. Am J Physiol 292:C2276–C2287 [DOI] [PubMed] [Google Scholar]
- Fujisawa H 2001 Regulation of the activities of multifunctional Ca2+/calmodulin-dependent protein kinases. J Biochem 129:193–199 [DOI] [PubMed] [Google Scholar]
- Hook SS, Means AR 2001 Ca2+/CaM-dependent kinases: from activation to function. Annu Rev Pharmacol Toxicol 41:471–505 [DOI] [PubMed] [Google Scholar]
- Soderling TR 1999 The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem Sci 24:232–236 [DOI] [PubMed] [Google Scholar]
- Illario M, Cavallo AL, Monaco S, Di Vito E, Mueller F, Marzano LA, Troncone G, Fenzi G, Rossi G, Vitale M 2005 Fibronectin-induced proliferation in thyroid cells is mediated by αvβ3 integrin through Ras/Raf-1/MEK/ERK and calcium/CaMKII signals. J Clin Endocrinol Metab 90:2865–2873 [DOI] [PubMed] [Google Scholar]
- Tebar F, Lladó A, Enrich C 2002 Role of calmodulin in the modulation of the MAPK signalling pathway and the transactivation of epidermal growth factor receptor mediated by PKC. FEBS Lett 517:206–210 [DOI] [PubMed] [Google Scholar]
- Lu KK, Armstrong SE, Ginnan R, Singer HA 2005 Adhesion-dependent activation of CaMKII and regulation of ERK activation in vascular smooth muscle. Am J Physiol 289:C1343–C1350 [DOI] [PubMed] [Google Scholar]
- Muthalif MM, Benter IF, Uddin MR, Malik KU 1996 Calcium/calmodulin-dependent protein kinase IIα mediates activation of mitogen-activated protein kinase and cytosolic phospholipase A2 in norepinephrine-induced arachidonic acid release in rabbit aortic smooth muscle cells. J Biol Chem 271:30149–30157 [DOI] [PubMed] [Google Scholar]
- Chen L, Xin X, Eckhart AD, Yang N, Faber JE 1995 Regulation of vascular smooth muscle growth by α1-adrenoreceptor subtypes in vitro and in situ. J Biol Chem 270:30980–30988 [DOI] [PubMed] [Google Scholar]
- Chang BH, Mukherji S, Soderling TR 1998 Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci USA 95:10890–10895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indolfi C, Mongiardo A, Curcio A, Torella D 2003 Molecular mechanisms of in-stent restenosis and approach to therapy with eluting stents. Trends Cardiovasc Med 13:142–148 [DOI] [PubMed] [Google Scholar]
- Marks AR 2003 Sirolimus for the prevention of in-stent restenosis in a coronary artery. N Engl J Med 349:1307–1309 [DOI] [PubMed] [Google Scholar]
- Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE 2003 Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 349:1315–1323 [DOI] [PubMed] [Google Scholar]
- Borbiev T, Verin AD, Birukova A, Liu F, Crow MT, Garcia JG 2003 Role of CaM kinase II and ERK activation in thrombin-induced endothelial cell barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 285:L43–L54 [DOI] [PubMed] [Google Scholar]
- Roberson MS, Bliss SP, Xie J, Navratil AM, Farmerie TA, Wolfe MW, Clay CM 2005 Gonadotropin-releasing hormone induction of extracellular-signal regulated kinase is blocked by inhibition of calmodulin. Mol Endocrinol 19:2412–2423 [DOI] [PubMed] [Google Scholar]
- Abraham ST, Benscoter H, Schworer CM, Singer HA 1996 In situ Ca2+ dependence for activation of Ca2+/calmodulin-dependent protein kinase II in vascular smooth muscle cells. J Biol Chem 271:2506–2513 [DOI] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Subramanian K, Meyer T 1998 CaMKIIβ functions as an F-actin targeting module that localizes CaMKIIα/β heterooligomers to dendritic spines. Neuron 21:593–606 [DOI] [PubMed] [Google Scholar]
- Caran N, Johnson LD, Jenkins KJ, Tombes RM 2001 Cytosolic targeting domains of γ and Δ calmodulin-dependent protein kinase II. J Biol Chem 276:42514–42519 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.