Abstract
Depletion of estrogens and progesterone at menopause has been linked to an increased risk for the development of Alzheimer's disease (AD) in women. A currently controversial literature indicates that although treatment of postmenopausal women with hormone therapy (HT) may reduce the risk of AD, several parameters of HT may limit its potential efficacy and perhaps, even exacerbate AD risk. One such parameter is continuous vs. cyclic delivery of the progestogen component of HT. Recent experimental evidence suggests that continuous progesterone can attenuate neural actions of estradiol (E2). In the present study, we compared the effects of continuous and cyclic progesterone treatment in the presence and absence of E2 in ovariectomized 3×Tg-AD mice, a transgenic mouse model of AD. We found that ovariectomy-induced hormone depletion increases AD-like pathology in female 3×Tg-AD mice, including accumulation of β-amyloid, tau hyperphosphorylation, and impaired hippocampal-dependent behavior. E2 treatment alone prevents the increases in pathology. Continuous progesterone did not affect β-amyloid levels when delivered alone but blocked the Aβ-lowering action of E2. In contrast, cyclic progesterone significantly reduced β-amyloid levels by itself and enhanced rather than inhibited the E2 effects. These results provide new insight into the neural interactions between E2 and progesterone that may prove valuable in optimizing HT regimens in postmenopausal women.
Delivery of progesterone in a cyclic rather than a continuous manner, both in the presence and absence of estradiol treatment, provides better protection against pathology in a mouse model of Alzheimer's disease.
Women have a higher incidence of Alzheimer's disease (AD) than men (1,2,3,4), suggesting that depletion of ovarian hormones at menopause may increase vulnerability to AD (5,6). Consistent with this possibility, several case-control (7,8) and prospective studies (9,10,11,12) have found that the use of estrogen-based hormone therapy (HT) in postmenopausal women is associated with a reduced risk of AD. However, the Women's Health Initiative Memory Study, a large well-controlled clinical trial, found that HT comprised of conjugated equine estrogens (CEE) alone benefited neither cognitive function nor dementia risk (13,14). In fact, risk of dementia was significantly higher in women receiving HT consisting of both CEE and the progestogen medroxyprogesterone acetate (MPA) (15). These findings raised serious concerns about the potential efficacy of HT for prevention of age-related cognitive decline.
The disconnection between the Women's Health Initiative Memory Study and the broader HT and sex steroid literatures has prompted much discussion about whether discordant findings indicate flaws in the design and use of HT and/or limitations in its therapeutic benefits (16,17,18,19). One potential problem with HT is the delivery of the progestogen component in a continuous manner (20,21,22). Both the estrogen estradiol (E2) (23,24,25) and progesterone (P4) (26,27) have a range of well-established protective neural actions predicted to increase the plasticity and resiliency of the brain. However, the interactions between E2 and P4 in brain are less well understood. Recent experimental evidence indicates that protective neural actions of E2 can be attenuated by cotreatment with continuous P4 (28,29,30,31). For example, in a transgenic mouse model of AD, we recently reported that continuous P4 blocks E2-mediated reductions in β-amyloid (32), a protein strongly implicated in AD pathogenesis (33). Together, these observations show that interactions between E2 and P4 can affect the established protective actions of each hormone, which in turn may impact neural efficacy of HT.
Inclusion of a progestogen component in HT is necessary to counteract the risk of endometrial cancer associated with use of unopposed estrogens (34,35). Although progestogens are delivered continuously in most HT regimens, P4 levels fluctuate across the natural ovarian cycle. Thus, one strategy that may improve HT is the use of cyclic rather than continuous progestogen (36,37). The effects of continuous vs. cyclic P4 on development of AD have not been assessed in either clinical or experimental models. In this study, we compared the effects of cyclic vs. continuous P4 in the presence and absence of an E2, on indices of pathology in the 3×Tg-AD triple transgenic mouse model of AD.
Materials and Methods
Animals
Colonies of 3×Tg-AD (38) and background strain, wild-type (WT) (C57BL6/129S; Jackson Laboratory, Bar Harbor, ME) mice were bred and maintained in our vivarium. Female mice used in these studies were housed individually on 12-h light, 12-h dark cycles and provided ad libitum access to food and water. Experiments were conducted in accordance with National Institutes of Health guidelines in the care and use of laboratory animals and under an approved institutional animal protocol.
Experimental design and treatments
At 3 months of age, both WT and 3×Tg-AD mice were divided into the following groups (n = 7/group): sham-ovariectomized (Sham), bilaterally ovariectomized (OVX), OVX with continuous E2 (OVX+E2), OVX with continuous P4 (OVX+Pco), OVX with cyclic P4 (OVX+Pcy), OVX with continuous E2 and continuous P4 (OVX+E2+Pco), and OVX with continuous E2 and cyclic P4 (OVX+E2+Pcy). Mice were OVX or sham-OVX and immediately replaced with sc, slow-release pellet (Innovative Research of America, Sarasota, FL) designed to deliver hormones over the 90-d treatment period. The Sham and OVX groups received placebo pellets. E2-treated groups received a 90-d pellet containing 0.025 mg E2. Pco groups received a 90-d pellet with 25 mg P4. The Pcy groups were treated on the 20th day of each of three consecutive 30-d cycles (i.e. on d 20, 50, and 80) with a 10-d pellet containing 2.8 mg P4 (the same P4 dose on a per day basis as the Pco groups). The used doses of 90-d E2 and P4 hormone pellets were successfully used in prior studies with 3×Tg-AD mice (32,39) and have been shown to produce serum levels of E2 and P4 in the physiological range (39,40,41). These regimens of hormone exposure were chosen not to mimic the 4- to 5-d mouse estrus cycle but rather to model ongoing clinical trials of HT in postmenopausal women that involve continuous E2 with cyclic delivery of P4 for 10–12 d per month (40,41,42). To verify efficacy and duration of the 10-d P4 pellets used in the Pcy groups, we conducted an additional, short-term, time-course experiment. WT female mice aged 4–6 months were randomly assigned to one of four conditions (n = 5/group): sham OVX, OVX, OVX+Pco, and OVX+Pcy. Importantly, the OVX+Pcy group was repeated four times (total n = 20 mice). All mice in the OVX+Pcy group were implanted with a 10-d P4 pellet immediately after OVX on d 0. A subset of OVX+Pcy mice (n = 5/group) were killed at each of four different time points after hormone treatment: d 5, 10, 20, and 30. Immediately before the animals were killed, all animals were assessed on P4-sensitive behaviors, as described below.
All WT and 3×Tg-AD mice in the primary study were killed 90 d after OVX at 6 months of age. On the day the animals were killed, mice were deeply anesthetized (100 mg/kg sodium pentobarbital), transcardially perfused with cold PBS, and killed by decapitation. One hemibrain was immersion fixed in 4% paraformaldehyde for 48 h and then stored at 4 C in PBS per 1% sodium azide until use. Also, uteri were dissected, blotted, and weighed.
Immunohistochemistry
Hemibrains were cut into blocks containing frontal cortex and subiculum plus amygdala. Tissue blocks were sectioned exhaustively (40 μm sections) with a vibratome, the frontal cortex block in the coronal plane, and the subiculum/amygdala block in the horizontal plane. Free-floating sections were immunostained using standard avidin-biotin immunohistochemistry with ABC Elite and diaminobenzidine kits (Vector, Burlingame, CA), as previously described (32,39). In brief, every eighth section was immunostained using primary antibodies directed against either β-amyloid (Aβ no. 71-5800; Zymed, San Francisco CA; 1:300 dilution) or hyperphosphorylated tau (AT8; Pierce, Rockford, IL; 1:1000 dilution). Before Aβ immunostaining, sections were pretreated for 5 min with 95% formic acid for antigen unmasking. To minimize interexperiment variability, immunostaining was conducted in three large batches that were balanced according to treatment groups.
Immunoreactivity quantification
Aβ immunoreactivity was quantified by determining immunohistochemical load using a previously described protocol (32,39). Briefly, in each brain three to five adjacent, high-magnification fields from immunolabeled sections (12 sections for subiculum, six sections for frontal cortex and amygdala) were collected and digitized using a video capture system (B/W charge-coupled device camera coupled to a BX40 upright microscope; Olympus, Tokyo, Japan). Images were processed using Image software 1.61 (National Institutes of Health, Bethesda, MD) with a predetermined, constant threshold value such that each pixel was recognized as either positively or negatively immunoreactive. The percentage of pixels positive for Aβ immunoreactivity is termed Aβ load.
To determine levels of tangle-like cells with tau hyperphosphorylation, we counted the total number of AT8 immunoreactive cells from every 12th section of the subiculum/amygdala tissue block (such labeling is rarely observed in frontal cortex), as previously described (32). To be included in the counts, cells were required to exhibit strong AT8 immunoreactivity covering more than 50% of the cell body and positive staining in processes extending from the cell body.
Spontaneous alternation behavior
WT and 3×Tg-AD mice were tested for spontaneous alternation behavior (SAB) in a Y-maze, a hippocampal-dependent task of working memory and visual attention, as previously described (32,39). Briefly, within 5 d of when the animals were killed, each animal was placed in a Y-maze and allowed to explore freely for 8 min. Arm entry was defined as both forepaws and hind paws fully entering arm. The total number and sequence of arm entries was scored. SAB score was calculated as the proportion of alternations (an arm choice differing from the previous two choices) to the total number of alternation opportunities (total arm entries − 2).
Elevated plus maze (EPM)
All mice were tested on the EPM as a behavioral assay for P4 action based on a previously described protocol (43,44). Within 5 d of the animals being killed (or on specified days after P4 treatment in the Pcy validation experiment), mice were placed into the center of a plus shaped-maze (26 in. × 2 in.) with two open arms and two arms protected by 6-in. walls (modeled after model 7001-0225; San Diego Instruments, San Diego, CA), which was elevated 15 in. from a padded floor. During a 5-min testing period, both the time spent in open arms and the number of entries into the open and closed arms (an arm entry was defined as all four paws in an arm) were recorded.
Forced swim test (FST)
To behaviorally assess neural efficacy of E2 treatment, mice were tested on the FST as previously described (43,44). Within 5 d of the animals being killed, animals were placed for 5 min into a 20-cm-diameter cylinder filled to about 13 cm with 24 ± 1.0 C water. FST scores were calculated as the proportion of freezing incidents (activity that is motionless except for respiration) to the total incidents observed (39).
Statistical analyses
Raw data from all experiments were first analyzed by ANOVA and, as appropriate, subjected to between-group comparisons using the Fisher least squares difference test. Significance was determined as P < 0.05.
Results
Efficacy of hormone treatments
The efficacy of treatment conditions was confirmed using several assays of hormone action. First, we observed a statistically significant effect of treatment condition on uterine weights [F (6,34) = 17.0, P < 0.0001]. In comparison with the Sham group of 3×Tg-AD mice (77.9 ± 10.8 mg), uterine weight in the sex steroid-depleted OVX condition was significantly lower (15.7 ± 5.2 mg), whereas the OVX+E2 group was significantly higher (147.9 ± 14.3 mg). The OVX+E2+Pco (108.0 ± 14.3 mg) and OVX+E2+Pcy (157.7 ± 14.5 mg) groups exhibited uterine weights significantly higher than the OVX mice.
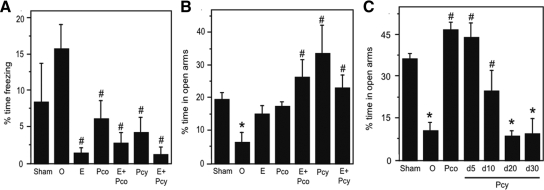
Neural efficacies of hormone treatments were also assessed behaviorally, using the FST and EPM as measures of E2 and P4 actions, respectively. OVX-induced depletion of sex steroid hormones is associated with evidence of depressive and anxious behaviors as evidenced by increased immobility in the FST and reduced time spent in the open arms of the EPM, effects that are reversed by E2 and/or P4 treatments (45,46). Consistent with these established findings, we observed significant group differences [F (6,34) = 4.15, P = 0.003] in freezing behavior in the FST (Fig. 1A). Relative to the approximately 2-fold increase observed in the OVX group, freezing behavior was significantly reduced in all three groups receiving E2 treatment: OVX+E2, OVX+E2+Pco, and OVX+E2+Pcy. Treatment with P4 was also associated with decreased freezing behavior (Fig. 1A). In parallel, results in the EPM (Fig. 1B) demonstrated a significant effect of treatment on anxiety-related behavior [F (6,34) = 5.5, P < 0.001]. In comparison with the Sham group, only the OVX group showed significant decreases in the proportion of time spent in open arms of the maze (Fig. 1B). All P4-treated groups exhibited significantly greater amounts of time spent in open arms than the OVX group. This effect of P4 was the same in WT mice under the same hormonal manipulations and tested in the same experimental paradigm (data not shown).
Figure 1.
Behavioral confirmation of hormone manipulations. Hormone-treated 3×Tg-AD mice were tested on the FST as a bioassay of E2 action and on the EPM as a bioassay of P4 action. A, On the FST, significant group differences were observed (F = 4.15, P = 0.003), and all groups with E2 treatments showed significant reductions in anxiety-related behavior. B, On the EPM, significant group differences were also observed (F = 5.5, P = 0.0006). The Pco group demonstrated reductions in anxiety-related behavior (C), implying a significant P4 effect similar to the Pcy group tested after 5 and 10 d but not 20 or 30 d after initiation of the10-d Pcy treatment. Sham, Sham-OVX; O, OVX; E, OVX+E2; Pco, OVX+constant P4; E+Pco, OVX+E2+constant P4; Pcy, OVX+cyclic P4; E+Pcy, OVX+E2+cyclic P4. *, P < 0.05 relative to sham-OVX group; #, P < 0.05 relative to OVX.
Because constrained cyclic delivery of P4 is a key component of our experimental design, we used EPM performance to confirm that cyclic P4 treatment resulted in P4 action limited to about 10 d. As expected, WT mice in the OVX group showed significantly lower proportion of time spent in the open arm in comparison with the Sham group, an effect that was reversed in the OVX+Pco group (Fig. 1C) [F (6,27) = 13.5, P < 0.001]. To evaluate the effect of cyclic P4, we examined EPM performance in four separate groups of mice in the OVX+Pcy condition that were behaviorally assessed at d 5, 10, 20, and 30 after administration of the 10-d P4 pellet. Importantly, 10-d cyclic P4 was associated with significantly increased EPM performance at d 5, which was moderately diminished by d 10 (Fig. 1C). At d 20 and 30 after initiation of cyclic P4, no improvement was apparent in EPM performance (Fig. 1C), demonstrating neural efficacy of the cyclic P4 pellet over an approximately 10-d period but not thereafter.
Differential effects of continuous vs. cyclic progesterone on Aβ accumulation
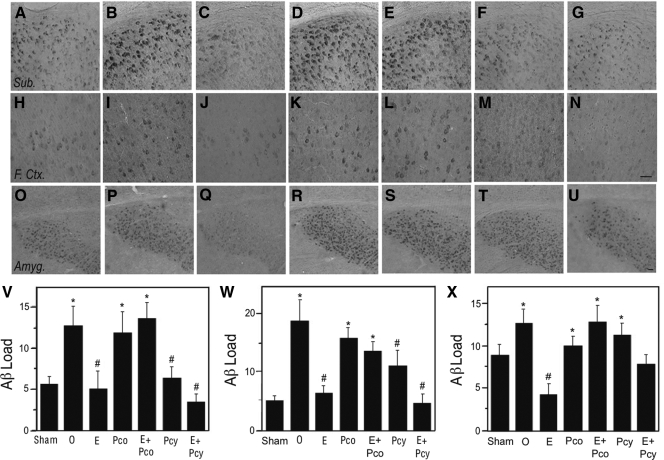
In prior research, we found that OVX female 3×Tg-AD mice exhibit significantly increased Aβ levels, which are reduced by continuous E2 but not continuous P4 (32). In agreement with these observations, we found that in comparison with the Sham group, OVX 3×Tg-AD mice showed significantly increased Aβ levels in the subiculum (Fig. 2, A, B, and V), frontal cortex (Fig. 2, H, I, and W), and amygdala (Fig. 2, O, P, and X). The OVX-induced increases were completely attenuated in the OVX+E2 group (Fig. 2, C, J, Q, and V-X) but not significantly reduced by continuous P4 treatment in the OVX+Pco group (Fig. 2, D, K, R, and V-X). Interestingly, the OVX+Pcy group with cyclic P4 treatment exhibited Aβ levels that were significantly lower than the OVX group in the subiculum and frontal cortex but not the amygdala (Fig. 2, F, M, T, and V-X). Continuous and cyclic P4 treatment also exhibited qualitatively different effects when delivered in combination with continuous E2. Continuous treatment with both hormones in the OVX+E2+Pco group resulted in high Aβ levels that were significantly higher than the Sham and OVX+E2 groups but indistinguishable from the OVX group (Fig. 2, E, L, S, and V-X). In contrast, the OVX+E2+Pcy group that combines continuous E2 with cyclic P4 exhibited significantly lower Aβ levels than OVX group in the subiculum and frontal cortex (Fig. 2, G, N, V, and W); the amygdala showed intermediate Aβ levels that were significantly different from neither the Sham nor OVX groups (Fig. 2, U and X).
Figure 2.
Continuous and cyclic P4 differentially affect E2 reduction of Aβ accumulation. Aβ immunoreactivity was visualized in female 3×Tg-AD mice at age 6 months across treatment groups in the subiculum (A–G), frontal cortex (H–N), and amygdala (O–U), as shown in representative images. Scale bar, 100 μm. Immunoreactivity load data demonstrate that mean Aβ levels (±sem) differed significantly across treatment groups in the subiculum (F = 4.23, P = 0.003) (V), frontal cortex (F = 6.38, P < 0.0001) (W), and the amygdala (F = 4.17, P = 0.003) (X). In both the subiculum and frontal cortex but not the amygdala, continuous P4 (Pco) attenuated E2 action, whereas cyclic P4 (Pcy) did not. Sham, Sham-OVX; O, OVX; E, OVX+E2; Pco, OVX+constant P4; E+Pco, OVX+E2+ constant P4; Pcy, OVX+cyclic P4; E+Pcy, OVX+E2+cyclic P4. *, P < 0.05 relative to sham-OVX group; #, P < 0.05 relative to OVX.
Both continuous and cyclic P4 lower tau hyperphosphorylation
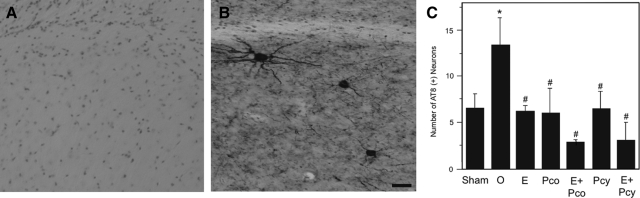
To investigate P4 regulation of AD-like neuropathology, we also determined the effects of continuous and cyclic P4 treatments on levels of tau hyperphosphorylation. In contrast to WT mice, 3×Tg-AD mice exhibit progressive tau pathology including the formation of tangle-like neurons that are strongly immunoreactive with phosphorylation-specific tau antibodies, such as AT8, and are predominantly found in the subiculum (Fig. 3, A and B). We found that the depletion of endogenous E2 and P4 in the OVX groups was associated with a significant increase in the number of AT8-immunoreactive neurons (Fig. 3C). The individual hormone treatment groups OVX+E2, OVX+Pco, and OVX+Pcy all significantly reduced numbers of AT8-immunoreactive cells (Fig. 3C). The combination of E2 with either continuous P4 (OVX+E2+Pco) or cyclic P4 (OVX+E2+Pcy) yielded the lowest levels of tau phosphorylation showing a nonsignificant trend toward an additive hormone effect (Fig. 3C).
Figure 3.
Both continuous and cyclic progesterone lower tau hyperphosphorylation. Representative images demonstrate that compared with WT female mice (A), 3×Tg-AD females exhibit intense immunoreactivity for AT8, an antibody that recognizes phosphorylation of tau at Ser202 (B). Scale bar, 40 μm. Quantification of intensely AT8-immunoreactive (AT8+) cells (±sem) across groups show that all hormone treatments significantly decreased tau hyperphosphorylation in comparison with the OVX group (F = 2.71, P = 0.028) (C). Sham, Sham-OVX; O, OVX; E, OVX+E2; Pco, OVX+constant P4; E+Pco, OVX+E2+constant P4; Pcy, OVX+cyclic P4; E+Pcy, OVX+E2+cyclic P4. *, P < 0.05 relative to sham-OVX group; #, P < 0.05 relative to OVX.
Differential effects of continuous vs. cyclic P4 on hippocampal-dependent working memory and visual attention
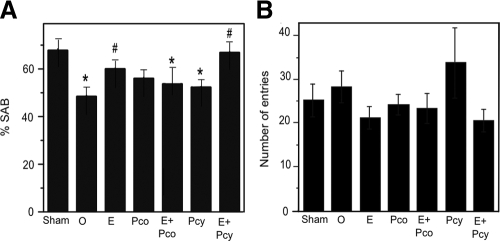
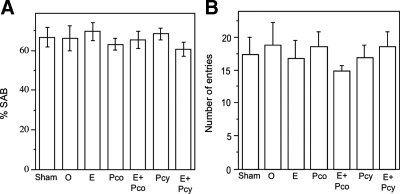
To further investigate P4 regulation of AD-related neuropathology, we assessed SAB, a hippocampal-dependent test of working memory and visual attention. We observed that SAB performance was significantly altered across hormone status (Fig. 4A). The OVX group showed a significant decline in SAB performance compared with the Sham group. The decrease in the hormone-depleted OVX group was partially and significantly attenuated in the OVX+E2 group but not significantly improved in the OVX+Pco and OVX+Pcy groups. The improved performance in the OVX+E2 group was attenuated by combination with continuous P4 because the OVX+E2+Pco group exhibited SAB values significantly lower than Sham but not significantly improved relative to OVX (Fig. 4A). In contrast, the highest SAB values among the hormone-treated groups were observed in the OVX+E2+Pcy group. SAB performance was not associated with the number of arm entries across treatment groups (Fig. 4B). Furthermore, to ensure that the effects of hormone manipulations on SAB reflected underlying neuropathology rather than direct hormone effects on SAB, we also evaluated the effect of all hormone manipulations on SAB in age-matched WT female mice. There was no statistically significant effect of treatment group in WT mice on either SAB performance (Fig. 5A) or number of arm entries (Fig. 5B).
Figure 4.
Continuous but not cyclic progesterone attenuates E2's beneficial effect on working memory. A, SAB in the Y-maze significantly differed across treatment groups of 6-month-old female 3×Tg-AD mice (F = 3.05, P = 0.016). Relative to the sham OVX (Sham) group, SAB was significantly lower in the OVX (O), an effect that was rescued by E2. The SAB improving action of E2 was attenuated by continuous P4 (Pco) but not cyclic P4 (Pcy). B, There was no main effect of condition on the number of arm entries (F = 1.2, P = 0.32). *, P < 0.05 relative to Sham; #, P < 0.05 relative to OVX.
Figure 5.
Hormone status does not affect SAB in nontransgenic mice. WT female mice treated under the same hormone regimen as 3×Tg-AD mice were tested for SAB in the Y-maze at 6 months of age. There were no significant main effects of treatment on either SAB performance (F = 1.2, P = 0.32) (A) or the number of arm entries (F = 0.29, P = 0.93) (B). Sham, Sham-OVX; O, OVX; E, OVX+E2; Pco, OVX+constant P4; E+Pco, OVX+E2+constant P4; Pcy, OVX+cyclic P4; E+Pcy, OVX+E2+cyclic P4. *, P < 0.05 relative to sham-OVX group; #, P < 0.05 relative to OVX.
Discussion
The goal of this study was to compare the effects of continuous vs. cyclic P4 on E2 regulation of indices of AD-like neuropathology in female 3×Tg-AD mice. We replicated our previous findings that continuous P4 treatment in OVX mice did not affect Aβ accumulation and, when combined with continuous E2, blocked the established Aβ-lowering action of E2 (32). The new findings in this study indicate that a cyclic regimen of P4, designed to more closely mimic the natural P4 fluctuations that occur across women's normal ovarian cycle, yields significant beneficial effects on AD-related pathology both alone and in combination with E2. We observed that cyclic P4 delivered alone significantly reduced the increase in Aβ resulting from OVX, and exhibited a trend of increasing rather than attenuating the Aβ-lowering effect of E2. Furthermore, the significant improvement in hippocampal-dependent working memory and visual attention afforded by E2 treatment was mildly enhanced by cyclic P4 but attenuated by continuous P4. All hormone treatments reduced levels of tau hyperphosphorylation. Taken together, these results demonstrate that in contrast to continuous P4, a cyclic P4 treatment schedule in combination with continuous E2 replacement is beneficial in lowering indices of AD-like neuropathology in female 3×Tg-AD mice.
P4 exhibited some evidence of independently reducing AD-related pathology. When delivered alone to OVX 3×Tg-AD mice, both cyclic and continuous P4 treatments were associated with decreased levels of tau hyperphosphorylation. These observations are consistent with evidence of P4-mediated neuroprotection actions in several in vivo paradigms, including animal models of traumatic brain injury (47,48), spinal cord injury (49), ischemia (50,51), demyelinating diseases (52), excitotoxicity (53), and seizure (54) (reviewed in Refs. 26 and 27). Interestingly, we observed that Aβ accumulation was differentially affected by P4 treatments in OVX 3×Tg-AD mice, with cyclic P4 significantly reducing Aβ levels but continuous P4 having no effect. The mechanism(s) underlying this effect is unclear, although our latest results suggest that cyclic and continuous P4 differentially regulate expression of Aβ-degrading enzymes (Jayaraman, A., and C. J. Pike, unpublished observations). Behaviorally, both cyclic and continuous P4 reduced anxiety and depressive behaviors in OVX 3×Tg-AD mice as indicated by the EPM and FST, respectively. However, despite some evidence of reduced pathology, neither P4 treatment improved performance on spontaneous alternation, a hippocampal-dependent measure of working memory and visual attention.
In prior studies with the 3×Tg-AD mouse, we observed a strong predictive relationship between increased Aβ load and worsening SAB performance (32,39,55). However, in this study we found that the OXV+Pcyc group showed no significant improvement in SAB performance relative to the OVX group despite significant reductions in Aβ load and tau hyperphosphorylation. The reasons for this discrepancy between pathology and behavior is unclear but may include the need for a more rigorous behavioral testing battery, the relatively young age of the mice and corresponding modest level of pathology, and the oversimplified assumption that Aβ accumulation and tau hyperphosphorylation are the only causes for the observed SAB impairment. For example, SAB can be affected by cholinergic function, which is regulated by E2 alone (28,56,57) and E2+P4 (58). Interestingly, our findings are consistent with those of Frye and Walf, who reported that a similar regimen of continuous P4 treatment in OVX female APPSWE+PSEN1ΔΔε9, a transgenic mouse model of AD, did not improve performance in two hippocampal-dependent tasks, object placement, and Morris water maze (59), but did reduce anxiety and depressive behaviors (60). Taken together, these studies indicate that in the absence of E2, P4 treatment can reduce some but not all indices of AD-related pathology and does not improve SAB performance.
Aside from independent actions of P4, our data indicate significant interactions between P4 and E2 in regulating AD-like neuropathology. An important finding from this study is that continuous P4 antagonizes some of the pathology-lowering effects of E2. Specifically, the significant decrease in Aβ accumulation and improvement in spontaneous alternation behavior observed in the E2-alone group were not observed in the group in which E2 was combined with continuous P4. This finding is consistent with a growing literature that indicates some neuroprotective actions of E2 are attenuated by cotreatment with continuous P4 by various mechanisms (21). For example, progestogens antagonize E2 protection against excitotoxicity in female rats (30,55) and organotypic culture (56); E2 up-regulation of Bcl-2 (57) and the neurotrophic factors brain-derived neurotrophic factor, nerve growth factor, and neurotrophin 3 (29,56); E2-mediated increase in hippocampal spine density (58); E2 enhancement of spatial memory performance (28,59); and cholinergic activity in cynomolgus monkeys (61). The modulatory effects of P4 on E2-mediated enhancement of basal forebrain cholinergic function (61) may be of particular interest, given the role of α7-nicotinic acetylcholine receptor in affecting Aβ production and accumulation (reviewed in Ref. 62). The mechanism(s) underlying the P4 attenuation of E2 neural actions is not known but may involve regulation of estrogen receptor (ER) expression. Recent cell culture data from our laboratory demonstrated that P4 strongly reduces neuronal expression of both ERα and ERβ (63). This effect is paralleled in a time-dependent manner by decreases in E2-mediated genomic action (63) and neuroprotection (63,64). If such mechanisms operate in vivo, continuous P4 would be expected to down-regulate ER expression and, as a consequence, dampen at least some E2 actions.
In contrast to the largely inhibitory effects of continuous P4 on E2 actions, our data demonstrate that cyclic P4 complements rather than antagonizes E2 effects. Specifically, the combination of cyclic P4 with E2 resulted in statistically nonsignificant trends toward lower Aβ levels and improved performance in the spontaneous alternation task. In agreement with some (65,66) but not all (67,68) prior reports, we found that hormone levels in WT female mice were not associated with significant differences in SAB performance, suggesting that the observed effects of hormone treatments on SAB in 3×Tg-AD mice reflect changes in underlying pathology. Our finding of qualitative differences in the interactions of cyclic vs. continuous P4 with E2 in 3×Tg-AD mice is similar to prior results by Gibbs (28), who found that cholinergic function in OVX female rats was significantly improved by cyclic P4 and E2 treatment but not continuous P4 and E2 treatment. Similarly, acute P4 treatment enhances but prolonged P4 treatment reduces E2-mediated increases in hippocampal spine density (69). Thus, evidence from experimental comparisons of cyclic and continuous P4 in animal models suggest that neural benefits of E2 may be maximized by combination with repeated cycles of P4 and minimized by continuous combined treatment.
There are some limitations to this study. Technically, the use of slow-release hormone delivery pellets can be problematic. Although these pellets reduce the level of animal intervention associated with other delivery methods [e.g. SILASTIC brand capsules (Dow Corning, Midland, MI), daily injections] and offer extended hormone treatment that is advantageous in long-term experiments such as the present study, they also have significant disadvantages. In particular, similar slow-release E2 pellets have been shown to produce an initial spike of hormone release, resulting in supraphysiological E2 levels that may last for 1 or more weeks before achieving physiological levels (70,71,72). Some have argued that such a supraphysiological spike in E2 levels can have deleterious effects (70,72). Although E2 treatment was associated with beneficial rather than detrimental effects in this study, we cannot exclude the possibility that some of the observed hormone effects were related in part to supraphysiological levels associated with the pellet delivery method.
Conceptually this study is limited in the use of a rodent paradigm to study issues relevant to human clinical applications. Our hormone regimens resulted in E2 and P4 exposure patterns that significantly differ from the natural 4- to 5-d estrus cycle of mice, and thus, it is uncertain how the results reflect interactions between endogenous estrogens and progestogens in rodents. Although a 30-d hormone cycle is not physiologically relevant in mice, it may provide valuable basic science insight into similar regimens of E2 and cyclic P4 currently under evaluation in two ongoing human clinical trials, the Early vs. Late Intervention Trial with Estradiol (41) and Kronos Early Estrogen Prevention Study (KEEPS) (40,41,42).
Another limitation of our study with respect to its potential clinical implications is that we used the sex steroid hormones E2 and P4, whereas the most common corresponding clinical drugs are CEE and MPA. CEE consists of numerous bioactive estrogens of which E2 is a relatively small component (73). The synthetic progestogen MPA mimics some but not all P4 actions and may have deleterious effects (26,27). Furthermore, clinical administration of HT components is typically oral, whereas our hormone delivery was transdermal. It is noteworthy that the KEEPS trial is using P4 rather than MPA and is comparing efficacies of transdermal E2 vs. oral CEE (42).
Our results demonstrate significant interactive effects of P4 and E2 treatments on regulation of pathology in a mouse model of AD. These findings provide novel experimental insight into the controversial issue of clinical HT use in postmenopausal women as a strategy to combat age-related cognitive decline and development of AD. The most common HT regimen is continuous combined treatment with an estrogen (CEE) and a progestogen (MPA) despite the fact that naturally fluctuating P4 levels are elevated for only a portion of the ovarian cycle. In terms of reducing AD-related neuropathology and improving associated behavioral deficits, our data indicate significantly improved efficacy with hormone treatments that involve delivery of P4 in a cyclic rather than continuous manner and thus support the use of a cyclic progestogen component in future HT paradigms. Although the relative efficacy of HT with cyclic vs. continuous progestogen for neural endpoints remains to be determined, the two ongoing studies Early vs. Late Intervention Trial with Estradiol and KEEPS use cyclic P4 regimens and promise to yield valuable information on the neural benefits of HT in women at midlife (41).
Footnotes
This work was supported by National Institutes of Health Grants AG026572 [to R. Brinton (USC) and C.J.P.], NS059174 (to J.C.C.), and NS52143 (to E.R.R.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 21, 2010
Abbreviations: AD, Alzheimer's disease; CEE, conjugated equine estrogen; E2, estradiol; EPM, elevated plus maze; ER, estrogen receptor; FST, forced swim test; HT, hormone therapy; KEEPS, Kronos Early Estrogen Prevention Study; MPA, medroxyprogesterone acetate; OVX, ovariectomized; P4, progesterone; SAB, spontaneous alternation behavior; WT, wild type.
References
- Ruitenberg A, Ott A, van Swieten JC, Hofman A, Breteler MMB 2001 Incidence of dementia: does gender make a difference? Neurobiol Aging 22:575–580 [DOI] [PubMed] [Google Scholar]
- Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A 1999 Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. Neurology 53:1992–1997 [DOI] [PubMed] [Google Scholar]
- Bachman DL, Wolf PA, Linn R, Knoefel JE, Cobb J, Belanger A, D'Agostino RB, White LR 1992 Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology 42:115–119 [DOI] [PubMed] [Google Scholar]
- Jorm AF, Korten AE, Henderson AS 1987 The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand 76:465–479 [DOI] [PubMed] [Google Scholar]
- Henderson VW 2008 Cognitive changes after menopause: influence of estrogen. Clin Obstet Gynecol 51:618–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton JV, Henderson VW 2005 Estrogen and cognition, with a focus on Alzheimer's disease. Semin Reprod Med 23:172–179 [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Henderson VW 1996 Estrogen replacement therapy and risk of Alzheimer disease. Arch Intern Med 156:2213–2217 [PubMed] [Google Scholar]
- Henderson VW, Paganini-Hill A, Emanuel CK, Dunn ME, Buckwalter JG 1994 Estrogen replacement therapy in older women. Comparisons between Alzheimer's disease cases and nondemented control subjects. Arch Neurol 51:896–900 [DOI] [PubMed] [Google Scholar]
- Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E 1997 A prospective study of estrogen replacement therapy and the risk of developing Alzheimer's disease: The Baltimore Longitudinal Study of Aging. Neurology 48:1517–1521 [DOI] [PubMed] [Google Scholar]
- Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R 1996 Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet 348:429–432 [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JCS 2002 Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA 288:2123–2129 [DOI] [PubMed] [Google Scholar]
- Waring SC, Rocca WA, Petersen RC, O'Brien PC, Tangalos EG, Kokmen E 1999 Postmenopausal estrogen replacement therapy and risk of AD: a population-based study. Neurology 52:965–970 [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH 2004 Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA 291:2947–2958 [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J 2004 Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA 291:2959–2968 [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones 3rd BN, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J 2003 Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 289:2651–2662 [DOI] [PubMed] [Google Scholar]
- Resnick SM, Henderson VW 2002 Hormone therapy and risk of Alzheimer disease: a critical time. JAMA 288:2170–2172 [DOI] [PubMed] [Google Scholar]
- Couzin J 2003 Estrogen research. The great estrogen conundrum. Science 302:1136–1138 [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R 2003 Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res 74:637–643 [DOI] [PubMed] [Google Scholar]
- Brinton RD 2004 Impact of estrogen therapy on Alzheimer's disease: a fork in the road? CNS Drugs 18:405–422 [DOI] [PubMed] [Google Scholar]
- Craig MC, Maki PM, Murphy DG 2005 The Women's Health Initiative Memory Study: findings and implications for treatment. Lancet Neurol 4:190–194 [DOI] [PubMed] [Google Scholar]
- Pike CJ, Carroll JC, Rosario ER, Barron AM 2009 Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol 30:239–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD 2006 Hormone in the hot seat. Nat Med 12:379–380 (Reply) [DOI] [PubMed] [Google Scholar]
- Lee SJ, McEwen BS 2001 Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annu Rev Pharmacol Toxicol 41:569–591 [DOI] [PubMed] [Google Scholar]
- Green PS, Simpkins JW 2000 Neuroprotective effects of estrogens: potential mechanisms of action. Int J Dev Neurosci 18:347–358 [DOI] [PubMed] [Google Scholar]
- Wise PM 2002 Estrogens and neuroprotection. Trends Endocrinol Metab 13:229–230 [DOI] [PubMed] [Google Scholar]
- Schumacher M, Sitruk-Ware R, De Nicola AF 2008 Progesterone and progestins: neuroprotection and myelin repair. Curr Opin Pharmacol 8:740–746 [DOI] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J 2008 Progesterone receptors: form and function in brain. Front Neuroendocrinol 29:313–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB 2000 Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience 101:931–938 [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC 2006 Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci 24:229–242 [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Nelson ME, Granholm AC 2004 Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport 15:2659–2663 [DOI] [PubMed] [Google Scholar]
- Rosario ER, Ramsden M, Pike CJ 2006 Progestins inhibit the neuroprotective effects of estrogen in rat hippocampus. Brain Res 1099:206–210 [DOI] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ 2007 Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3×Tg-AD mice. J Neurosci 27:13357–13365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ 2002 The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297:353–356 [DOI] [PubMed] [Google Scholar]
- Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D 1995 Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol 85:304–313 [DOI] [PubMed] [Google Scholar]
- Whitehead MI, Hillard TC, Crook D 1990 The role and use of progestogens. Obstet Gynecol 75:59S–76S; discussion 81S–83S [PubMed] [Google Scholar]
- PEPI Writing Group 1995 Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA 273:199–208 [PubMed] [Google Scholar]
- Castelo-Branco C, Figueras F, Sanjuan A, Pons F, Vicente JJ, Vanrell JA 1999 Long-term postmenopausal hormone replacement therapy effects on bone mass: differences between surgical and spontaneous patients. Eur J Obstet Gynecol Reprod Biol 83:207–211 [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM 2003 Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron 39:409–421 [DOI] [PubMed] [Google Scholar]
- Carroll JC, Pike CJ 2008 Selective estrogen receptor modulators differentially regulate Alzheimer-like changes in female 3×Tg-AD mice. Endocrinology 149:2607–2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon RL, Sugarbaker PH 2005 Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol 2:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW 2009 Estrogens, episodic memory, and Alzheimer's disease: a critical update. Sem Reprod Med 27:283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VM, Black DM, Brinton EA, Budoff MJ, Cedars MI, Hodis HN, Lobo RA, Manson JE, Merriam GR, Naftolin F, Santoro N, Taylor HS, Harman SM 2009 Using basic science to design a clinical trial: baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). J Cardiovasc Trans Res 2:228–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Boyce-Rustay JM, Millstein R, Yang R, Wiedholz LM, Murphy DL, Holmes A 2007 Effects of mild early life stress on abnormal emotion-related behaviors in 5-HTT knockout mice. Behav Genet 37:214–222 [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A 2006 Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacology 31:2405–2414 [DOI] [PubMed] [Google Scholar]
- Frye CA, Sumida K, Dudek BC, Harney JP, Lydon JP, O'Malley BW, Pfaff DW, Rhodes ME 2006 Progesterone's effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology (Berl) 186:312–322 [DOI] [PubMed] [Google Scholar]
- Frye CA, Wawrzycki J 2003 Effect of prenatal stress and gonadal hormone condition on depressive behaviors of female and male rats. Horm Behav 44:319–326 [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Braswell L, Stein DG 1994 Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol 129:64–69 [DOI] [PubMed] [Google Scholar]
- Stein DG, Hoffman SW 2003 Estrogen and progesterone as neuroprotective agents in the treatment of acute brain injuries. Pediatr Rehabil 6:13–22 [DOI] [PubMed] [Google Scholar]
- Labombarda F, Gonzalez SL, Deniselle MC, Vinson GP, Schumacher M, De Nicola AF, Guennoun R 2003 Effects of injury and progesterone treatment on progesterone receptor and progesterone binding protein 25-Dx expression in the rat spinal cord. J Neurochem 87:902–913 [DOI] [PubMed] [Google Scholar]
- Cervantes M, González-Vidal MD, Ruelas R, Escobar A, Moralí G 2002 Neuroprotective effects of progesterone on damage elicited by acute global cerebral ischemia in neurons of the caudate nucleus. Arch Med Res 33:6–14 [DOI] [PubMed] [Google Scholar]
- Gibson CL, Constantin D, Prior MJ, Bath PM, Murphy SP 2005 Progesterone suppresses the inflammatory response and nitric oxide synthase-2 expression following cerebral ischemia. Exp Neurol 193:522–530 [DOI] [PubMed] [Google Scholar]
- Garay L, Deniselle MC, Meyer M, Costa JJ, Lima A, Roig P, De nicola AF 2009 Protective effects of progesterone administration on axonal pathology in mice with experimental autoimmune encephalomyelitis. Brain Res 1283:177–185 [DOI] [PubMed] [Google Scholar]
- Ciriza I, Carrero P, Frye CA, Garcia-Segura LM 2006 Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J Neurobiol 66:916–928 [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA 2004 Progestins in the hippocampus of female rats have antiseizure effects in a pentylenetetrazole seizure model. Epilepsia 45:1531–1538 [DOI] [PubMed] [Google Scholar]
- Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ 2006 Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer's disease. J Neurosci 26:13384–13389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC 2002 Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience 115:547–558 [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Hashash A, Johnson DA 1997 Effects of estrogen on potassium-stimulated acetylcholine release in the hippocampus and overlying cortex of adult rats. Brain Res 749:143–146 [DOI] [PubMed] [Google Scholar]
- Gibbs RB 1996 Fluctuations in relative levels of choline acetyltransferase mRNA in different regions of the rat basal forebrain across the estrous cycle: effects of estrogen and progesterone. J Neurosci 16:1049–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA 2008 Effects of progesterone administration and APPswe+PSEN1Δe9 mutation for cognitive performance of mid-aged mice. Neurobiol Learn Mem 89:17–26 [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA 2009 Progesterone reduces depression-like behavior in a murine model of Alzheimer's Disease. Age (Dordr) 31:143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Nelson D, Anthony MS, Clarkson TB 2002 Effects of long-term hormone replacement and of tibolone on choline acetyltransferase and acetylcholinesterase activities in the brains of ovariectomized, cynomolgus monkeys. Neuroscience 113:907–914 [DOI] [PubMed] [Google Scholar]
- D'Andrea MR, Nagele RG 2006 Targeting the α7 nicotinic acetylcholine receptor to reduce amyloid accumulation in Alzheimer's disease pyramidal neurons. Curr Pharm Des 12:677–684 [DOI] [PubMed] [Google Scholar]
- Jayaraman A, Pike CJ 2009 Progesterone attenuates oestrogen neuroprotection via downregulation of oestrogen receptor expression in cultured neurones. J Neuroendocrinol 21:77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre CC, Baudry M 2009 Progesterone reverses 17β-estradiol-mediated neuroprotection and BDNF induction in cultured hippocampal slices. Eur J Neurosci 29:447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Tanaka T, Zou LB, Senzaki K, Yano K, Osada T, Ana O, Ren X, Kameyama T, Nabeshima T 1999 Long-term deprivation of oestrogens by ovariectomy potentiates β-amyloid-induced working memory deficits in rats. Br J Pharmacol 128:419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD 2008 Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: evidence that the duration of hormone deprivation after ovariectomy compromises 17β-estradiol effectiveness in altering CA1 spines. Horm Behav 54:386–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MM, Hyder SM, Assayag R, Panarella SR, Tousignant P, Franklin KB 1999 Estrogen modulates spontaneous alternation and the cholinergic phenotype in the basal forebrain. Neuroscience 91:1143–1153 [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce C, Manley K, Frye CA 2009 Proestrous compared to diestrous wildtype, but not estrogen receptor β knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res 196:254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS 1993 Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol 336:293–306 [DOI] [PubMed] [Google Scholar]
- Strom JO, Theodorsson A, Theodorsson E 2009 Dose-related neuroprotective versus neurodamaging effects of estrogens in rat cerebral ischemia: a systematic analysis. J Cereb Blood Flow Metab 29:1359–1372 [DOI] [PubMed] [Google Scholar]
- Ström JO, Theodorsson E, Theodorsson A 2008 Order of magnitude differences between methods for maintaining physiological 17β-oestradiol concentrations in ovariectomized rats. Scand J Clin Lab Invest 68:814–822 [DOI] [PubMed] [Google Scholar]
- Theodorsson A, Hilke S, Rugarn O, Linghammar D, Theodorsson E 2005 Serum concentrations of 17β-estradiol in ovariectomized rats during two times six weeks crossover treatment by daily injections in comparison with slow-release pellets. Scand J Clin Lab Invest 65:699–705 [DOI] [PubMed] [Google Scholar]
- Rozovsky I, Hoving S, Anderson CP, O'Callaghan J, Finch CE 2002 Equine estrogens induce apolipoprotein E and glial fibrillary acidic protein in mixed glial cultures. Neurosci Lett 323:191–194 [DOI] [PubMed] [Google Scholar]