Abstract
Signal transducer and activator of transcription (Stat)5a is a critical regulator of mammary gland development. Previous studies have focused on Stat5a's role in the late pregnant and lactating gland, and although active Stat5a is detectable in mammary epithelial cells in virgin mice, little is known about its role during early mammary gland development. In this report, we compare mammary gland morphology in pubertal and adult nulliparous wild-type and Stat5a−/− mice. The Stat5a-null mammary glands exhibited defects in secondary and side branching, providing evidence that Stat5a regulates these processes. In addition, Stat5a−/− mammary glands displayed an attenuated proliferative response to pregnancy levels of estrogen plus progesterone (E+P), suggesting that it plays an important role in early pregnancy. Finally, we examined one potential mediator of Stat5a's effects, receptor activator of nuclear factor-κB ligand (RANKL). Stat5a−/− mammary glands were defective in inducing RANKL in response to E+P treatment. In addition, regulation of several reported RANKL targets, including inhibitor of DNA binding 2 (Id2), cyclin D1, and the cyclin-dependent kinase inhibitor p21Waf1/Cip1, was altered in Stat5a−/− mammary cells, suggesting that one or more of these proteins mediate the effects of Stat5a in E+P-treated mammary epithelial cells.
Stat5a regulates branching and proliferation in the developing mammary gland, potentially via a pathway(s) involving RANKL, Id2, cyclin D1, and/or p21Waf1/Cip1.
Unlike most other tissues, the mammary gland undergoes the majority of its development after birth. This postnatal program of mammogenesis occurs in two distinct stages. The first is initiated by the surge of ovarian hormones at puberty and results in elongation of the ductal tree to the edges of the mammary fat pad, along with arborization by primary, secondary, and side branches. The second major stage of mammary development occurs during pregnancy, when extensive proliferation of the epithelial compartment results in additional side branching and the formation of milk-producing alveoli. Both stages of mammary gland development are driven by ovarian hormones, with ductal outgrowth during puberty being driven primary by estrogen (E), and the expansion and alveologenesis seen during pregnancy requiring high levels of both E and progesterone (P) (reviewed in Refs. 1 and 2). However, ovarian hormones alone are not sufficient for mammary development, and cooperation with several other hormone, growth factor, and integrin signaling pathways is necessary to produce a fully functional mammary gland (1,2).
Signal transducer and activator of transcription (Stat)5 is a critical regulator of normal mammogenesis. The Stat proteins are a family of transcription factors that transduce extracellular cytokine and growth factor signals from cell surface receptors to the nucleus (3,4). Two Stat5 isoforms exist (a and b, collectively referred to as Stat5), which share approximately 96% identity at the amino acid level (5). Stat5a has emerged as the chief isoform involved in mammary development, likely because it is expressed at much higher levels in the mammary gland than Stat5b (6).
One function of Stat5 during pregnancy is to promote proliferation of mammary epithelial cells. Cells in Stat5a−/−, b−/− epithelial outgrowths in wild-type stroma incorporate less 5-bromo-2′-deoxyuridine (BrdU) in response to E+P treatment than wild-type explants (7), demonstrating a proliferative defect that is autonomous to the epithelium. In addition, Stat5a−/−, b−/− mammary glands contain fewer phosphorylated histone H3 (a marker of cell proliferation) positive cells during pregnancy compared with control glands (8). Consistent with a role for Stat5a in proliferation, transgenic animals overexpressing Stat5a in the mammary gland display hyperplasia and a predisposition to develop mammary tumors (9). Stat5 is also reported to play important roles in cell differentiation and survival during pregnancy. Targeted deletion of both Stat5 isoforms in the mammary gland results in a loss of alveolar mammary cell markers and maintenance of ductal cell markers during pregnancy, whereas conditional deletion during late pregnancy results in an increase in apoptotic epithelial cells (8). The importance of the Stat5a isoform during pregnancy is highlighted by the fact that both Stat5a−/−, b−/− and Stat5a−/− mice exhibit reduced alveolar development at parturition and fail to lactate (6,10).
Although the roles of Stat5a in late pregnancy and lactation have been well studied, its potential function(s) during pubertal development and early pregnancy have been largely unexplored. We and others have demonstrated that Stat5a is present and activated in mouse mammary epithelial cells as early as 5 wk of age (11,12), suggesting that it plays a role in the development of the gland before pregnancy. Furthermore, we showed that Stat5a expression in virgin animals requires E+P, suggesting that it may mediate some of the effects of these hormones. Finally, we demonstrated that Stat5a positive luminal epithelial cells rarely proliferate in response to pregnancy levels of E+P, whereas adjacent Stat5a negative cells do proliferate (11). This strongly suggests that a paracrine molecule mediates Stat5a's proliferative effects in early pregnancy.
The phenotype of Stat5a−/− mice should give insights into its importance in early mammary development; however, previous studies of these mice have led to contradictory conclusions. Although one report described incomplete ductal outgrowth and branching 8 wk after Stat5a−/− epithelium was transplanted into wild-type stroma (13), others observed no clear phenotype in outgrowths from transplanted Stat5a−/−, b−/− epithelium (7) or in intact, adult Stat5a−/− mice (6). To gain additional information about the role of Stat5a in the virgin mammary gland, a detailed examination of mammary gland morphology in intact virgin Stat5a−/− mice was conducted. We conclude that Stat5a−/− mice exhibit a defect in both secondary branching and side branching in the virgin gland. In addition, treatment of ovariectomized (OVX) mice with pregnancy levels of E+P revealed that Stat5a is required for a full proliferative response to these hormones. Finally, we identify a defect in the induction of receptor activator of nuclear factor-κB ligand (RANKL) in response to E+P treatment in Stat5a−/− mammary glands. RANKL is essential for the development of side branches (14) and alveologenesis (15) during mammary gland development, and it has been previously identified as a Stat5a target gene in vitro (16). Several downstream targets of RANKL have been identified in the mammary gland, including cyclin D1 (17), inhibitor of DNA binding (Id)2, and p21Waf1/Cip1 (p21) (18). Our results suggest that the expression and/or subcellular localization of all of these molecules are regulated by RANKL signaling, which would provide a mechanism by which it induces proliferation during mammary gland development.
Materials and Methods
Animals
Cryopreserved Stat5a+/− embryos were obtained from James Ihle (6), and recovered animals were maintained in a C57BL/6-129/SvJ mixed background mammary glands were obtained from pubertal (6 wk old) and adult (18 wk old) Stat5a−/−, +/−, and +/+ mice. For hormone treatments, 18-wk-old mice were OVX, treated with E+P or saline for 5 d, and injected with BrdU as described (11). For genotyping, tail DNA was prepared (19), and PCR analysis was performed using previously described primers (6). Genotyping was confirmed by immunohistochemical analysis for Stat5a as described (11). All animal experimentation was conducted in accordance with accepted standards of humane animal care and approved by the All University Committee on Animal Use and Care at Michigan State University.
Whole mounts and immunohistochemistry
For whole-mount analyses, left inguinal mammary glands (gland no. 4) were excised and fixed in 10% phosphate-buffered formalin overnight at 4 C and stained overnight at 4 C in Carmine alum as previously described (20).
For immunohistochemistry, mammary glands were formalin fixed, paraffin embedded, and sectioned at 5 μm. The sections were processed for antigen retrieval as previously described (11). Each incubation step in the staining procedure was followed by two 5-min washes in PBS. Stat5a and cyclin D1 staining was carried out as previously described (11,21). For RANKL, Id2, progesterone receptor A (PRA), and p21 staining, sections were first blocked for 30 min in PBS (pH 7.3) containing 2% BSA (PBSA). Incubation with primary antibodies (goat α-RANKL; R&D Systems, Minneapolis, MN), rabbit α-Id2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit α-progesterone receptor (PR), which detects only PRA (Dako, Carpinteria, CA), or goat α-p21 (Santa Cruz Biotechnology, Inc.) was carried out in 2% PBSA overnight at 4 C. Sections were then incubated with secondary antibodies (rabbit α-goat for RANKL and p21, goat α-rabbit antibody conjugated to Alexa 488 (green) for Id2, or goat α-rabbit conjugated to Alexa 546 (red) for PRA; Invitrogen, Carlsbad, CA) for 30 min in PBS. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma, St. Louis, MO) and images captured using a Nikon Eclipse fluorescent microscope (Nikon, Tokyo, Japan). For Id2 staining, nuclei were counterstained using TO-PRO-3 iodide (Invitrogen) and visualized using a Pascal laser scanning confocal microscope (Zeiss, Oberkochen, Germany).
For BrdU labeling, sections were first blocked with goat α-mouse IgG antigen-binding fragment (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted 1:100 in 1% PBSA for 1 h and with normal goat serum (Vector Laboratories, Burlingame, CA) diluted 1:1 in PBS for 30 min. The tissue was then incubated with mouse α-BrdU antibody (provided as a kit from GE Healthcare, Piscataway, NJ) for 1 h. This was followed by 30 min with a biotinylated goat α-mouse antibody (Dako) (1:400) and ABC reagent (Vector Laboratories). Immunoperoxidase localization of antibody staining was achieved using 3′-3′-diaminobenzidene. The sections were counterstained with hematoxylin and visualized using a Nikon Eclipse 50i microscope.
Branch quantitation and statistical analysis
Primary and secondary branch points were quantitated from mammary gland whole mounts from 18-wk-old mice. Using ImageJ software (National Institutes of Health, Bethesda, MD), a rectangle of identical size corresponding to approximately 25% of the mammary gland epithelium was aligned on photographs of each gland, with its placement determined by the lymph node location. All ducts within the rectangle were traced, and all branch points except those giving rise to side branches were counted. Side branches were identified based on their short length and the fact that they arise at 90-degree angles relative to the duct. The number of branch points was normalized to the epithelial area within the rectangle, calculated as the number of pixels located within the perimeter of duct ends. The final data represent the average number of branch points for three to nine mice per genotype ± sd.
The percentages of BrdU and PRA positive cells were quantitated from captured images. Five mice per treatment were analyzed, and a minimum of 1200 total cells and two independent sections per mouse were analyzed. Results are expressed as mean ± sem. All differences were considered significant at P < 0.05 by using Student's t test or ANOVA where appropriate.
Immunoblot analysis
After the lymph nodes were removed, inguinal mammary glands were homogenized using a Polytron homogenizer in a buffer containing 50 mm Tris HCl (pH 7.2), 6 mm MgCl2, 1 mm EDTA, 10% sucrose (wt/vol) (1 ml/0.2 g tissue) containing complete mini protease inhibitor cocktail tablet (Roche, Indianapolis, IN). Homogenates were centrifuged at 14,000 × g for 30 min, and supernatants were used for immunoblots. Samples (20 μg of protein) were resolved on 12% polyacrylamide gels under denaturing conditions and transferred onto polyvinylidene difluoride membranes (PerkinElmer, Waltham, MA). Membranes were first blocked with 5% milk in PBS with 0.5% Tween 20 before incubation with goat α-RANKL antibody (R&D Systems), then with horseradish peroxidase-labeled rabbit α-goat (Bio-Rad Laboratories, Hercules, CA). Membranes were incubated with Super Signal West Pico chemiluminescent substrate (Pierce Biotechnology, Rockford, IL) and exposed to x-ray film.
Results
Stat5a is required for branching during mammary gland development
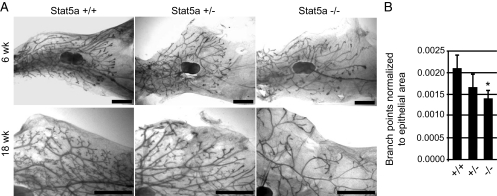
To investigate the effects of Stat5a depletion on early mammary gland development, whole mounts from 6-wk pubertal and 18-wk adult virgin Stat5a−/−, +/−, and +/+ mice were compared. At 6 wk, ductal outgrowth had progressed the same distance beyond the lymph node in all of the genotypes (Fig. 1A), indicating that Stat5a is not required for ductal elongation. No difference was found in the number of terminal end buds between the genotypes in 6-wk pubertal glands. We did observe a slight decrease in ductal branches in the 6-wk Stat5a−/− glands, but it did not meet statistically significance (data not shown). We also observed a general disorganized appearance of the epithelial tree in pubertal Stat5a−/− glands, and this persisted in the 18-wk-old mice. Mammary glands from 18-wk-old Stat5a−/− mice had a clear paucity of side branches (Fig. 1A). Interestingly, the heterozygote glands displayed a morphology intermediate between Stat5a−/− and Stat5a+/+ animals, suggesting a dosage effect of Stat5a on side branching. These findings support a previous report that Stat5a−/− mammary epithelium transplants contained fewer side branches than wild type (8).
Figure 1.
Whole-mount analysis of pubertal and adult virgin mice. The fourth inguinal mammary glands were removed from Stat5a+/+, Stat+/−, and Stat−/− mice and prepared as whole mounts. A, Mammary glands from 6-wk-old pubertal mice and 18-wk-old mature mice. Scale bars, 2 mm. B, Analysis of primary branch points in 18-wk-old Stat5a+/+, Stat5a+/−, and Stat5a−/− mammary glands. Quantitation was carried out as described in Materials and Methods for three to nine animals per genotype and is shown as the average ± sd. *, P < 0.05 that the number of branch points in Stat5a−/− mammary glands were less than in Stat5a+/+ glands.
In addition to a lack of side branches, the 18-wk Stat5a−/− glands appeared to contain fewer primary and secondary ducts. To quantitate this observation, the number of primary and secondary branch points within similar regions of −/−, +/−, and +/+ mammary glands were counted and normalized to epithelial area as described in Materials and Methods. This analysis demonstrated that adult Stat5a−/− glands contain significantly fewer (∼33%) branch points than wild-type glands (Fig. 1B). The Stat5a+/− glands had an intermediate number of branch points, although this number was not statistically different from either the wild-type or knockout glands. Together, the results of morphological analyses establish a specific requirement for the Stat5a isoform in both primary/secondary and side branching during mammary gland development before pregnancy.
Effects of E+P treatment on morphology and proliferation in Stat5a−/− mammary glands
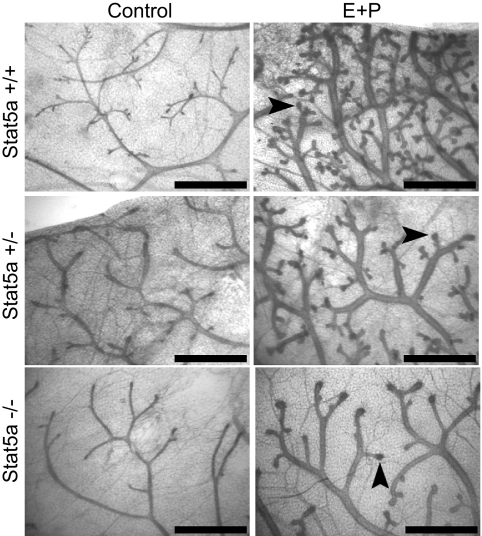
We previously demonstrated that Stat5a expression in mammary epithelial cells requires E and P (11). Because E and P are critical regulators of mammary gland development, and because proliferation is required for gland development and ductal branching, we focused on the relationship between Stat5a and the proliferative response to E+P. OVX 18-wk virgin Stat5a−/−, +/−, and +/+ mice were treated with a combination of E and P for 5 d. As expected, 5 d of E+P treatment generated a visible morphological response in wild-type mice. This included the development of side branches, and the formation of enlarged ductal tips, which are precursors to alveoli, at the ends of both ducts and side branches (Fig. 2). As in adult virgin mice, there were significantly fewer side branches in Stat5a−/− compared with wild-type glands, and Stat5a+/− mice contained an intermediate number. However, all genotypes exhibited enlarged tips at the ends of virtually all ducts and side branches.
Figure 2.
Morphological response of mammary glands to hormone treatment. Mammary gland whole mounts were prepared from 18-wk-old OVX Stat5a+/+, Stat5a+/−, and Stat5a−/− mice treated for 5 d with E+P. Arrows indicate enlarged ductal tips where alveoli will form. Scale bar, 1 mm.
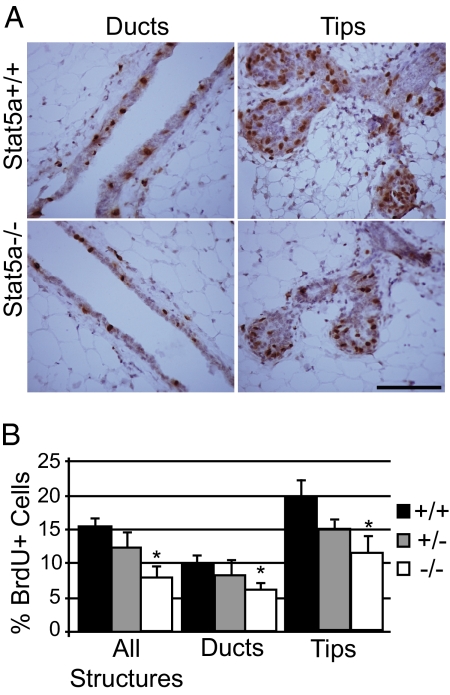
Previous studies have demonstrated that transplanted Stat5a−/−, b−/− mammary epithelia exhibit a defective proliferative response to E+P treatment (7). To determine whether Stat5a is specifically required, wild-type, null, and heterozygous OVX mice were treated with E+P for 5 d, then administered a pulse of BrdU 2 h before they were killed. In wild-type glands, 15 ± 1% of all epithelial cells were BrdU+, and this number was decreased approximately 50% (7 ± 1%) in glands from Stat5a−/− mice (Fig. 3). The percentage of BrdU+ cells in heterozygous mice was intermediate (12 ± 2%) but was not significantly different from either the wild type or mutant. Although BrdU+ cells were present throughout the entire mammary gland, the areas of highest proliferation were at the enlarged tips of ducts and side branches, regardless of the Stat5a genotype (Fig. 3). When duct and tip structures were analyzed independently, the defect in the percentage of BrdU+ cells in the knockout mice remained approximately 50% (Fig. 3B). Thus, Stat5a−/− mice displayed a proliferative defect in all structures analyzed.
Figure 3.
Impaired proliferative response to hormones in Stat5a−/− mammary glands. Immunoperoxidase detection of incorporated BrdU was performed on mammary gland sections from 18-wk-old OVX mice treated for 5 d with E+P. A, Representative images from BrdU staining are shown for Stat5a+/+ and Stat5a−/− ducts and alveolar buds. Scale bar, 30 μm. B, Quantitation of the percent BrdU+ luminal epithelial cells. Results are shown for quantitation carried out on all structures, as well as duct and alveolar bud structures independently. The values represent the mean ± sem from five mice per group with a minimum of 1000 cells analyzed per mouse. *, P < 0.05 that there were fewer BrdU+ cells in glands of Stat5a−/− mice compared with Stat5a+/+ glands.
Identification of RANKL as a Stat5a target in the mammary gland
Stat5a positive cells rarely proliferate in response to E+P treatment, indicating that Stat5a must stimulate proliferation via a paracrine mediator. One potential mediator of Stat5a's effects in the mammary gland is the TNF family member RANKL, a secreted protein that is essential for the development of side branches (14) and alveologenesis (15). Prolactin treatment induces RANKL expression in the mammary gland (15), and studies in cell culture suggest that this occurs through the Janus kinase 2/Stat5a pathway (16). We previously reported that Stat5a and RANKL are highly colocalized in mammary luminal epithelial cells in vivo after E+P treatment, which would support a model in which RANKL is downstream of Stat5a (11). However, although the combination of E and P are required for Stat5a expression in BALB/c mice (11), P alone can induce RANKL and alveologenesis in the BALB/c strain (14), suggesting that RANKL can also be induced in a Stat5a independent manner.
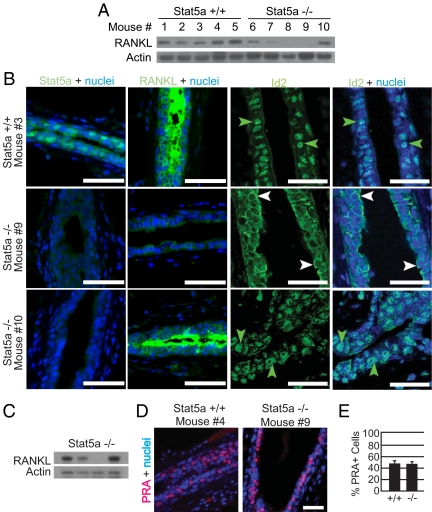
To directly test whether RANKL expression is dependent on Stat5a, Western blot analysis was carried out using mammary gland homogenates from individual wild-type and knockout mice. RANKL expression was not detectable in glands from control OVX mice (data not shown), and was induced by E+P in all wild-type mice examined (Fig. 4A). In contrast, RANKL expression was highly variable in E+P-treated Stat5a−/− mice. In some mice (such as nos. 8 and 9), RANKL was undetectable, whereas in others (such as nos. 6 and 10), the level was similar to that seen in the wild type. These findings were confirmed by immunofluorescent staining of mammary gland sections from the same mice (Fig. 4B).
Figure 4.
RANKL expression and Id2 localization are downstream targets of Stat5a in the mammary gland. A, Western blot analysis of RANKL levels in whole mammary gland homogenates from OVX 18-wk-old Stat5a+/+ and Stat5a−/− mice treated for 5 d with E+P. Each lane represents a single mammary gland from an individual mouse. B, Immunofluorescent staining for Stat5a, RANKL, and Id2 was performed on mammary gland sections from the mice described in A. Representative images of Stat5a, RANKL, and Id2 staining (green) are shown. For sections stained for Stat5a and RANKL, nuclei were counter-stained with DAPI (blue), whereas nuclei in sections stained for Id2 were counterstained with TO-PRO (blue). Scale bar, 25 μm. Note the nuclear localization of Id2 in mice nos. 3 and 10 (green arrows) and the cytoplasmic localization of Id2 in mouse no. 9 (white arrows). C, Western blot analysis of RANKL levels in whole mammary gland homogenates from OVX 18-wk-old Stat5a−/− mice treated for 5 d with P. Each lane represents a single mammary gland from an individual mouse. D, Immunofluorescent detection of PRA (red) in mammary gland samples from the mice described in A. Nuclei were stained with DAPI (blue). Representative images from sections of Stat5a+/+ and Stat5a−/− mammary glands are shown. Scale bar, 30 μm. E, Quantitation of the percentage of PRA-positive epithelial cells was conducted. The values represent the mean ± sem from five mice per group, with a minimum of 1000 cells analyzed per mouse.
The combination of E+P is required to induce Stat5a in BALB/c mice (11), but P alone can induce RANKL in this strain (14). We therefore hypothesized that there are two pathways that can activate the RANKL gene. One would require only P, and would be independent of Stat5a. The second would be Stat5a dependent and would therefore require both E and P. If this is correct, and Stat5a−/− mice differ in their ability to use the Stat5a-independent pathway, we would expect P alone to induce RANKL expression in some, but not all, Stat5a−/− mice. To determine whether this is the case, OVX Stat5a−/− mice were treated with P for 5 d, and Western blot analysis was carried out. As predicted, RANKL was induced in some but not all animals (Fig. 4C).
We considered several possible explanations for the differences in RANKL regulation in individual Stat5a−/− mice. Because PR is required for RANKL expression in the mouse mammary gland (14), differences in PR expression could be a contributing factor. To test this, staining for PRA was carried out on E+P-treated Stat5a−/− and Stat5a+/+ mammary gland sections. Analysis of the PRB isoform was not carried out, because this protein is not readily detectable in the mammary gland until approximately 10 d of E+P treatment (21). The two genotypes examined exhibited similar PRA staining intensities (Stat5a+/+ no. 4 and Stat5a−/− no. 9 are shown) (Fig. 4D) and contained a similar percentage of PRA+ cells (∼47%) (Fig. 4E), and no significant variability in PRA expression was detected among the Stat5a-null animals. Thus, the inability of some Stat5a−/− animals to induce RANKL is not due to a lack of PRA expression. Stat5b can compensate for the absence of Stat5a, because increased Stat5b expression rescues the Stat5a−/− mammary phenotype after multiple pregnancies (12,13). We therefore investigated whether increased Stat5b was present in some E+P-treated Stat5a−/− mammary glands. Stat5b was present at uniformly low levels in all mice (data not shown), indicating that the high levels of RANKL seen in mice nos. 6 and 10 are not due to increased Stat5b signaling.
Identification of Id2 as a RANKL target in the mammary gland
The variable induction of RANKL by E+P in Stat5a-deficient mice provided an opportunity to examine potential downstream targets of this secreted protein in the mammary gland. One such target is Id2. The Id proteins are inhibitors of basic helix-loop-helix transcription factors that contain a helix-loop-helix motif but lack a DNA-binding domain and are involved in regulating both cell proliferation and differentiation (22,23). Previous studies have reported that nuclear accumulation of Id2 requires RANKL signaling in vivo and is critical for RANKL-mediated proliferation in primary mouse mammary epithelial cells in vitro (18). To determine whether Id2 nuclear localization is dependent on Stat5a, immunohistochemical staining was carried out on mammary gland sections from the E+P-treated mice used for the RANKL analyses. In wild-type mice, 79 ± 1.7% of mammary epithelial cells were Id2 positive, and the protein was predominantly nuclear in both luminal and myoepithelial cells (Fig. 4B and Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). The subcellular localization of Id2 differed among Stat5a−/− animals and corresponded closely with the levels of RANKL observed. Id2 was predominantly cytoplasmic in Stat5a−/− mice with low RANKL levels, (Fig. 4B, mouse no. 9 is shown) but predominantly nuclear in mice displaying RANKL levels similar to wild type (mouse no. 10 is shown). These findings confirm that nuclear accumulation of Id2 requires RANKL signaling and suggest that both induction of RANKL and nuclear localization of Id2 in response to E+P treatment require Stat5a in some but not all animals.
Expression of cell cycle regulators is altered in E+P-treated Stat5a−/− mammary glands
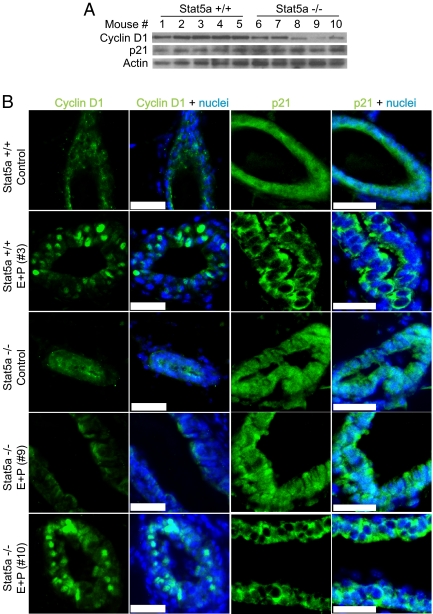
Having identified a defect in both RANKL and Id2 expression in Stat5a−/− mice, we investigated potential effects on cell cycle regulators that might explain the decreased proliferation observed in Stat5a knockout mammary glands after E+P treatment. Cyclin D1 expression is reportedly regulated by RANK-driven nuclear factor-κB signaling in the mammary gland (17,24). To determine whether the lack of RANKL in Stat5a−/− mice resulted in decreased in cyclin D1 expression, immunoblot analysis was carried out using total mammary gland homogenates from the E+P-treated Stat5a+/+ and Stat5a−/− mice described above. Cyclin D1 was expressed at equivalent levels in all five wild-type mice. Expression levels were generally lower in the Stat5a−/− mice but did not strictly correlate with the level of RANKL (Fig. 5A). Immunohistochemical staining of tissue sections revealed that control, OVX wild-type, and knockout mice had light diffuse staining distributed throughout all epithelial cells. After E+P treatment, wild-type mice exhibited strong nuclear staining in a subset of epithelial cells (Fig. 5B and Supplemental Fig. 2). E+P-treated Stat5a−/− mice previously identified as having high levels of RANKL expression also had cells with strong nuclear cyclin D1 staining (mouse no. 10 is shown), whereas little to no nuclear cyclin D1 was detected in knockout mice with low RANKL expression (mouse no. 9 is shown). Thus, the accumulation of nuclear cyclin D1 in response to E+P treatment is correlated with high RANKL expression.
Figure 5.
Cyclin D1 expression and p21 localization are altered in mammary glands from Stat5a knockout mice. A, Western blot analysis of cyclin D1 and p21 levels in whole mammary gland homogenates from 18-wk-old Stat5a+/+ and Stat5a−/− mice treated for 5 d with E+P. Each lane represents a single mammary gland from an individual mouse, corresponding to the same mice shown in Fig. 4. B, Immunofluorescent staining for cyclin D1 and p21 was performed on mammary gland sections from the mice described in A. Representative images of cyclin D1 and p21 staining (green) are shown. Nuclei were counterstained with DAPI (blue). Scale bar, 20 μm. Note the strong nuclear expression of cyclin D1 and the cytoplasmic localization of p21 in mice nos. 3 and 10. Additional cyclin D1 and p21 staining is shown in Supplemental Fig. 2.
Transcriptional repression of the p21 gene by Id2 has previously been reported in mammary epithelial cells in vitro (18). To determine whether the lack of nuclear Id2 observed in some Sta5a−/− mice resulted in decreased p21 protein levels, total mammary gland homogenates were examined by immunoblot analysis. No difference in the overall expression of p21 was detected between Stat5a+/+ and Stat5a−/− mice after E+P treatment (Fig. 5A). However, immunohistochemistry revealed a difference in the subcellular localization of p21 in the mammary epithelium of these animals. Diffuse staining was observed throughout epithelial cells in both control (OVX) wild-type and knockout mice. The protein was located primarily in the cytoplasm in the glands of E+P-treated wild-type mice and in the Stat5a−/− mice that expressed RANKL and contained nuclear Id2 (Fig. 5B and Supplemental Fig. 2). In contrast, mammary epithelial cells in Stat5a−/− mice containing inactive (cytoplasmic) Id2 exhibited diffuse p21 staining (mouse no. 9 is shown), resembling that observed in control animals. Thus, we propose that exclusion of p21 from the nucleus is a result of RANKL and/or Id2 signaling.
Discussion
To investigate Stat5a's role in early mammary gland development, we examined whole mounts from virgin Stat5a−/−, −/+, and +/+ mice at pubertal (6 wk) and mature (18 wk) stages of development. The results obtained demonstrate that Stat5a is dispensable for ductal elongation but is required for the development of secondary and side branches (Fig. 1). Primary branch points occur mainly through the bifurcation of terminal end buds, and we previously demonstrated that Stat5a is not expressed in these structures (11). In contrast, secondary and side branches are produced via proliferation and differentiation of epithelial cells within ducts, where Stat5a is present. It is therefore likely that the branching defect we have detected is limited to secondary and side branches. This is consistent with the fact that no significant difference was observed in the number of branch points in mammary glands from 6-wk-old Stat5a−/− mice, because the majority of branching occurring during puberty is primary branching. Our finding that Stat5a is required for branching supports the results of a previous study, in which a branching defect was detected in Stat5a-deficient epithelium transplanted into wild-type stroma (13).
In addition to studying virgin mammary glands, we compared the early response to pregnancy levels of E+P in wild-type and Stat5a−/− mice. It was previously reported that transplanted epithelia from Stat5a−/−, b−/− double knockout mice exhibit a defective proliferative response to acute E+P treatment (7,8). Our results indicate that the Stat5a isoform is specifically required, because there were approximately 50% fewer proliferating epithelial cells in E+P-treated Stat5a−/− than wild-type glands. The blunted proliferative response to E+P induced may explain the lack of side branches present in virgin Stat5a−/− mice, because the development of side branches requires proliferation that is induced by E+P during normal estrous cycles. It is also possible, however, that the branching defect is independent of Stat5a's role in proliferation.
The use of 5-d treatments allowed us to examine morphological responses to E+P. Overall, there was a defect in both side branching and alveolar formation in the Stat5a−/− animals. However, enlarged tips were present on the distal ends of both ducts and side branches in all genotypes after E+P treatment (Fig. 2). Because these enlarged tips are the precursors of alveoli, we propose that the defective alveolar development previously reported in pregnant Stat5a−/− mice (6,10) may in part be the result of fewer side branches on which alveoli can form.
Having established a role for Stat5a in the early response to E+P, we further investigated the mechanism(s) by which it acts. We previously demonstrated that Stat5a positive cells in vivo also express RANKL (11). RANKL and its receptor RANK are important regulators of mammary gland development, where they promote both proliferation and survival (15,25). Previous in vitro studies demonstrated that PRL induction of RANKL expression depends on Janus kinase 2 and Stat5a, and identified a Stat5 binding site (γ-interferon-activated sequence element) within the RANKL promoter (16). The results presented here indicate that Stat5a is an activator of RANKL expression in response to E+P treatment in vivo, because an overall defect in RANKL induction was observed in Stat5a−/− animals. However, RANKL expression was highly variable between animals, with some exhibiting extremely low and others containing near wild-type levels. We hypothesize that this variability is due to the mixed C57BL/6-129/SvJ genetic background of the Stat5a−/− mice, because there is previous evidence for strain specific differences in the regulation of RANKL expression. Expression of Stat5a requires both E and P in BALB/c (11) and C57BL/6 mammary glands (14). However, P alone can induce RANKL in BALB/c mice but not in C57BL/6 mice (14), indicating that RANKL can be induced independently of Stat5a in BALB/c mice, but not in C57BL/6 mice. Based on this, we propose that in the mixed background, mice with a predominant contribution from the C57BL/6 strain would require Stat5a to induce RANKL, whereas others would not. A strain-dependent requirement for Stat5a may also explain the discrepancies concerning a branching defect in Stat5 deficient mammary glands in previous reports (6,7,13).
The presence or absence of RANKL expression in Stat5a-deficient mice allowed us to identify Id2, cyclin D1, and p21 as possible in vivo targets of RANKL. RANKL has been reported to induce proliferation of primary mammary epithelial cells in vitro via nuclear translocation of Id2, and Id2-null epithelial cells in vivo exhibit impaired proliferation and remain poorly differentiated during pregnancy (18). Our results suggest a tight link between RANKL levels and Id2 localization after E+P treatment in vivo. Id2 was predominately nuclear in E+P-treated wild-type animals, as well as in the Stat5a−/− mice exhibiting high RANKL expression. Conversely, Id2 was primarily cytoplasmic in the Stat5a−/− mice with low RANKL levels. The mechanism regulating Id2 nuclear localization during mammary gland development has not been directly investigated, but in vitro studies using MCF-7 breast cancer cells containing a transfected HA-Id2 construct suggest that nuclear translocation of Id2 in response to RANKL stimulation requires phosphorylation of Id2 on serine 5 by cyclin E/cyclin-dependent kinase 2 complexes and is dependent on both phosphoinositide 3-kinase and MAPK signaling (18). It is interesting to note that nuclear Id2 was present in both luminal and myoepithelial cells after E+P treatment. This is consistent with the fact that RANK is expressed in both cell types and with the observation that myoepithelial cells proliferate in response to RANKL treatment in vitro (26).
Reported activities of nuclear Id2 include inhibiting retinoblastoma protein activity and inhibiting transcription of the cyclin-dependent kinase inhibitor p21 (27,28). Id2−/− mammary epithelial cells contain increased p21 levels compared with wild type in vivo (29) and fail to down-regulate p21 in response to RANKL treatment in vitro (18). In our experiments, we did not detect a consistent difference in p21 levels in animals expressing low vs. high levels of RANKL by Western blot analysis of whole mammary gland extracts [compare Stat5a−/− mice nos. 9 and 10, which express different levels of RANKL (Fig. 4A) but equivalent levels of p21) (Fig. 5A)]. A similar situation was seen with cyclin D1, whose expression has previously been reported to be regulated by RANKL signaling in the mammary gland (17), e.g. Stat5a−/− mouse no. 7 contains high levels of cyclin D1 (Fig. 5A), although RANKL expression is low (Fig. 4A). However, analysis of total mammary gland extracts by Western blot analysis may mask epithelium specific effects, and when mammary epithelial cells were examined specifically by immunohistochemistry, differences were seen in the pattern of both p21 and cyclin D1 staining. Cyclin D1 accumulated in the nucleus, and p21 was excluded from the nucleus in Stat5a−/− mice expressing RANKL but not in those that do not express RANKL (Fig. 5).
In summary, based on our results, we propose a mechanism in which E+P induce Stat5a, which in turn increases expression of RANKL. This requirement for Stat5a may be strain dependent, and there are likely additional, Stat5a-independent pathways that can also result in RANKL induction. Once expressed, secreted RANKL binds to its receptor RANK, leading to activation of signaling pathways that result in the nuclear accumulation of Id2 and cyclin D1, and cytoplasmic sequestration of p21, all of which would promote proliferation. Although proof of this model will require additional mechanistic studies, it is consistent with the fact that RANKL−/− mice display impaired nuclear localization of Id2 during pregnancy (18) and that RANKL−/−, Id2−/−, cyclin D1−/−, and Stat5a−/− mammary glands all display similar defects in alveolar development during pregnancy (6,10,15,30,31). It is interesting to note that, although Id2 is nuclear and p21 is cytoplasmic in a high percentage of cells in animals with high RANKL, a much smaller number of cells contain nuclear cyclin D1 and incorporate BrdU into DNA. This suggests that molecules in addition to Id2 and p21 are involved in the proliferative response to E+P. Such molecules may be downstream of RANKL or may be RANKL-independent targets of Stat5a. Determination of whether ectopic expression of RANKL in the developing mammary gland could overcome the Stat5a requirement would distinguish between these possibilities.
Supplementary Material
Acknowledgments
We thank Dr. James Ihle at St. Jude Children's Research Hospital (Memphis, TN) for providing us with Stat5a+/− mouse embryos and Elizabeth Starnes, Tuan Nguyen, Lynne Ngouajio, and May Tan for their technical assistance.
Footnotes
This work was supported by the Breast Cancer and the Environment Research Centers Grant U01 ES/CA 012800 from the National Institute of Environment Health Science and the National Cancer Institute, National Institutes of Health, Department of Health and Human Services. Support for this research was also provided by the Jean P. Schultz Endowed Oncology Research Fund to the Michigan State University College of Human Medicine.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 14, 2010
Abbreviations: BrdU, 5-Bromo-2′-deoxyuridine; DAPI, 4′,6-diamidino-2-phenylindole; E, estrogen; Id, inhibitor of DNA binding; OVX, ovariectomized; P, progesterone; p21, p21Waf1/Cip1; PBSA, PBS with azide; PR, P receptor; PRA, plasma renin activity; RANKL, receptor activator of nuclear factor-κB ligand; Stat, signal transducer and activator of transcription.
References
- Howlin J, McBryan J, Martin F 2006 Pubertal mammary gland development: insights from mouse models. J Mammary Gland Biol Neoplasia 11:283–297 [DOI] [PubMed] [Google Scholar]
- Brisken C, Rajaram RD 2006 Alveolar and lactogenic differentiation. J Mammary Gland Biol Neoplasia 11:239–248 [DOI] [PubMed] [Google Scholar]
- Darnell Jr JE 1997 STATs and gene regulation. Science 277:1630–1635 [DOI] [PubMed] [Google Scholar]
- Ihle JN 1996 STATs: signal transducers and activators of transcription. Cell 84:331–334 [DOI] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Gouilleux F, Groner B, Hennighausen L 1995 Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci USA 92:8831–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN 1998 Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93:841–850 [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Shillingford JM, Smith GH, Grimm SL, Wagner KU, Oka T, Rosen JM, Robinson GW, Hennighausen L 2001 Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J Cell Biol 155:531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, Robinson GW, Hennighausen L 2004 Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol 24:8037–8047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavnilovitch E, Cardiff RD, Groner B, Barash I 2004 Deregulation of Stat5 expression and activation causes mammary tumors in transgenic mice. Int J Cancer 112:607–619 [DOI] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L 1997 Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev 11:179–186 [DOI] [PubMed] [Google Scholar]
- Santos SJ, Haslam SZ, Conrad SE 2008 Estrogen and progesterone are critical regulators of Stat5a expression in the mouse mammary gland. Endocrinology 149:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen MT, Xie J, Bubendorf L, Wagner KU, Rui H 2002 Basal activation of transcription factor signal transducer and activator of transcription (Stat5) in nonpregnant mouse and human breast epithelium. Mol Endocrinol 16:1108–1124 [DOI] [PubMed] [Google Scholar]
- Liu X, Gallego MI, Smith GH, Robinson GW, Hennighausen L 1998 Functional rescue of Stat5a-null mammary tissue through the activation of compensating signals including Stat5b. Cell Growth Differ 9:795–803 [PubMed] [Google Scholar]
- Aupperlee MD, Drolet AA, Durairaj S, Wang W, Schwartz RC, Haslam SZ 2009 Strain-specific differences in the mechanisms of progesterone regulation of murine mammary gland development. Endocrinology 150:1485–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, Boyle WJ, Khokha R, Penninger JM 2000 The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103:41–50 [DOI] [PubMed] [Google Scholar]
- Srivastava S, Matsuda M, Hou Z, Bailey JP, Kitazawa R, Herbst MP, Horseman ND 2003 Receptor activator of NF-κB ligand induction via Jak2 and Stat5a in mammary epithelial cells. J Biol Chem 278:46171–46178 [DOI] [PubMed] [Google Scholar]
- Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, Karin M 2001 IKKα provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell 107:763–775 [DOI] [PubMed] [Google Scholar]
- Kim NS, Kim HJ, Koo BK, Kwon MC, Kim YW, Cho Y, Yokota Y, Penninger JM, Kong YY 2006 Receptor activator of NF-κB ligand regulates the proliferation of mammary epithelial cells via Id2. Mol Cell Biol 26:1002–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E 1994 Manipulating the mouse embryo: a laboratory manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Banerjee MR, Wood BG, Lin FK, Crump LR 1976 Organ culture of whole mammary gland of the mouse. Method Cell Sci 2:457–462 [Google Scholar]
- Aupperlee MD, Haslam SZ 2007 Differential hormonal regulation and function of progesterone receptor isoforms in normal adult mouse mammary gland. Endocrinology 148:2290–2300 [DOI] [PubMed] [Google Scholar]
- Ruzinova MB, Benezra R 2003 Id proteins in development, cell cycle and cancer. Trends Cell Biol 13:410–418 [DOI] [PubMed] [Google Scholar]
- Sikder HA, Devlin MK, Dunlap S, Ryu B, Alani RM 2003 Id proteins in cell growth and tumorigenesis. Cancer Cell 3:525–530 [DOI] [PubMed] [Google Scholar]
- Fernandez-Valdivia R, Mukherjee A, Ying Y, Li J, Paquet M, DeMayo FJ, Lydon JP 2009 The RANKL signaling axis is sufficient to elicit ductal side-branching and alveologenesis in the mammary gland of the virgin mouse. Dev Biol 328:127–139 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Suarez E, Branstetter D, Armstrong A, Dinh H, Blumberg H, Dougall WC 2007 RANK overexpression in transgenic mice with mouse mammary tumor virus promoter-controlled RANK increases proliferation and impairs alveolar differentiation in the mammary epithelia and disrupts lumen formation in cultured epithelial acini. Mol Cell Biol 27:1442–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam SZ, Drolet A, Smith K, Tan M, Aupperlee M 2008 Progestin-regulated luminal cell and myoepithelial cell-specific responses in mammary organoid culture. Endocrinology 149:2098–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavarone A, Garg P, Lasorella A, Hsu J, Israel MA 1994 The helix-loop-helix protein Id-2 enhances cell proliferation and binds to the retinoblastoma protein. Genes Dev 8:1270–1284 [DOI] [PubMed] [Google Scholar]
- Prabhu S, Ignatova A, Park ST, Sun XH 1997 Regulation of the expression of cyclin-dependent kinase inhibitor p21 by E2A and Id proteins. Mol Cell Biol 17:5888–5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Nishikawa SI, Yokota Y 2000 Lactation defect in mice lacking the helix-loop-helix inhibitor Id2. EMBO J 19:5772–5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K, Meyer B, Gruss P, Cui Y, Renou JP, Morgan FV, Smith GH, Reichenstein M, Shani M, Hennighausen L, Robinson GW 2002 Mammary epithelial cells are not able to undergo pregnancy-dependent differentiation in the absence of the helix-loop-helix inhibitor Id2. Mol Endocrinol 16:2892–2901 [DOI] [PubMed] [Google Scholar]
- Fantl V, Edwards PA, Steel JH, Vonderhaar BK, Dickson C 1999 Impaired mammary gland development in Cyl-1(−/−) mice during pregnancy and lactation is epithelial cell autonomous. Dev Biol 212:1–11 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.