Abstract
Gonadotropin-stimulated estrogen receptor-β (ERβ)-null preovulatory follicles exhibit submaximal estradiol production, insufficient acquisition of LH receptor, and attenuated expression of essential ovulatory genes. These observations lead to low ovulatory rates compared with wild-type (WT) follicles. We hypothesize that insufficient LH receptor results in reduced cAMP production after an ovulatory stimulus. Individual preantral follicles were cultured with FSH for 4 d and then induced to ovulate with a single dose of human chorionic gonadotropin (hCG). cAMP levels 1 h after hCG were 50% lower in ERβ-null than WT follicles. To determine whether the lack of LH receptor, and resulting lack of cAMP, could be bypassed by direct activation of adenylyl cyclase, WT and ERβ-null follicles were induced to ovulate with forskolin. Ten micromolar forskolin doubled the ovulatory rate of ERβ-null follicles compared with treatment with hCG (∼50 vs. 25%, respectively). In WT follicles, 10 μm forskolin reduced the ovulation rate compared with hCG (14 vs. 83%, respectively), indicating that high doses of forskolin inhibited WT ovulation. A 10 μm concentration of forskolin induced cAMP levels in ERβ-null follicles that were comparable to levels produced in WT follicles after hCG and either partially or completely rescued the attenuated expression of LH-responsive genes. These data indicate that direct activation of adenylyl cyclase, resulting in increased production of cAMP, partially rescues the ovulatory response of ERβ-null follicles, suggesting that insufficient LH receptor and low cAMP levels contribute to their poor ovulatory rates. We also determined that ERβ-null ovaries exhibit an alteration in the activation of ERK1/2. Our evaluation of the ERβ-null ovarian phenotype indicates that ERβ plays a role in facilitating folliculogenesis. We show that expression of ERβ in preovulatory follicles is required for adequate cAMP production and propose that an optimal level of cAMP is required for hCG-stimulated ovulation.
ERβ functions are necessary to ensure sufficient lutenizing hormone (LH) receptor expression in preovulatory granulosa cells and maximum cAMP synthesis in response to the ovulatory LH surge.
As functional units of the ovary, follicles rely on the actions of pituitary gonadotropins for growth, maturation, and ovulation. FSH is critical to the induction of granulosa cell proliferation, estradiol synthesis, antrum formation, and acquisition of LH receptor expression, all of which characterize a healthy, mature follicle (1). FSH actions alone, however, are insufficient and must synergize with locally derived estradiol to provide maximal expression of steroidogenic enzymes and LH receptor (2,3,4). If the follicle survives to reach the preovulatory stage, a synchronous bolus of LH from the pituitary stimulates ovulation and thereby makes the oocyte available for fertilization.
The phenotypes of estrogen receptor (ER)-null mice strongly indicate that ERβ, but not ERα, is critical to mediating the synergistic effects of estradiol during follicle maturation (5,6). This is consistent with the former receptor type being the predominant nuclear ER form expressed in granulosa cells of growing follicles (7,8). Follicles in ERβ-null mice exhibit lower expression of aromatase (Cyp19), decreased estradiol production, and reduced ovulation rates, even when superovulated with exogenous gonadotropins. These defects converge to produce a subfertile phenotype, indicating a fundamental role for ERβ in ovarian function (5,6). We have replicated the ovarian phenotype of ERβ-null mice at the level of individual follicles cultivated to maturity in vitro (6). These studies, supported by in vivo characterizations of the ERβ-null ovary, have led us to hypothesize that poor ovulatory rates in ERβ-null mice are due to insufficient estradiol synthesis and submaximal expression of LH receptor in the granulosa cells of preovulatory follicles (5).
LH receptors are G protein-coupled membrane-bound receptors (9,10), and as such, their activation leads to increased adenylyl cyclase activity, cAMP production, and activation of cAMP-dependent protein kinases [protein kinase A (PKA)] (11). In addition to PKA activation, activation of MAPK, PKC, and AKT/PKB pathways in response to LH or human chorionic gonadotropin (hCG) treatment in the preovulatory follicle are described (12,13). In the current study, we again employ in vitro follicle culture to further evaluate the impact of insufficient acquisition of LH receptor in preovulatory follicles of ERβ-null mice and to assess whether this phenotype is the principal cause of the poor ovulatory rates observed in this model. Relative to wild type (WT), individually cultured ERβ-null preovulatory follicles clearly exhibited reduced LH receptor levels, leading to lower cAMP synthesis when challenged with an ovulatory dose of hCG. However, cAMP synthesis in ERβ-null follicles does rise to WT-like levels when we substitute forskolin for hCG, the former being able to directly activate adenylyl cyclase independent of LH receptor. Concurrent with increased cAMP synthesis after stimulation with forskolin were considerably improved patterns of gene expression and ovulation rates among in vitro cultured ERβ-null follicles. Furthermore, we found that MAPK activation after hCG treatment in vivo is impaired in the ERβ-null ovary, suggesting that this pathway may also be disrupted in ERβ-null follicles. These findings further support our hypothesis that the reduced ovulatory rate in ERβ-null follicles is primarily due to a failure to acquire maximum LH receptor expression upon reaching the preovulatory state. This study further demonstrates that once sufficiently stimulated, the processes necessary to mediate follicle rupture are generally intact in ERβ-null follicles.
Materials and Methods
Mice
All animal procedures were approved by the National Institute of Environmental Health Sciences (NIEHS) Animal Care and Use Committee and are in compliance with an NIEHS-approved animal study proposal. The generation of ERβ-null mice has been previously described by Krege et al. (14). Breeding pairs on a C57BL6 background were obtained from Taconic Farms (Germantown, NY). Animals were maintained under a 12-h light, 12-h dark cycle schedule and fed NIH-31 mouse chow. Offspring were genotyped by PCR analysis as previously described (14). All animals used for follicle isolation were unstimulated prepubertal mice (21–23 d old). Animals used for ovarian collections were stimulated prepubertal mice treated by ip injections with 5 IU pregnant mare serum gonadotropin (PMSG) (Sigma-Aldrich, St. Louis, MO) and 48 h later with 5 IU hCG (Sigma-Aldrich).
Follicle isolation and culture
Follicles were collected as previously described with minor modifications (6). Briefly, prepubertal (21–23 d old) ERβ-null and WT female mice were euthanized by CO2 aspiration. The ovaries were extracted and transferred to glass slides in Leibovitz medium (Invitrogen Corp., Carlsbad, CA) supplemented with insulin (5 μg/ml; Sigma-Aldrich), transferrin (10 μg/ml; Sigma-Aldrich), selenium (2 ng/ml; Sigma-Aldrich), ascorbic acid (50 μg/ml; Sigma-Aldrich), and 0.3% BSA (Sigma-Aldrich). The same criterion for follicle selection was used as described by Emmen et al. (6). Large preantral follicles (200 ± 10 μm in diameter) possessing two or more layers of thecal cells were carefully dissected under a dissecting scope from the ovaries using 25-gauge needles. Selected follicles were then collected and pooled (per animal) in Nunc four-well dishes (Nalge, Nunc International, Rochester, NY) containing α-MEM (Invitrogen) supplemented as above and with penicillin (100 U/ml; Life Technologies), streptomycin (100 μg/ml; Life Technologies), and 5% fetal bovine serum (Hyclone, Logan, UT) and then held in a humidified incubator at 37 C in 5% CO2. Individual follicles were then distributed in 24-well plates containing Millipore CM culture plate inserts (Millipore, Bedford, MA) and 250 μl α-MEM supplemented as above and with 100 mIU/ml FSH (Gonadal-F; Serono, Rockland, MA). Follicles were cultured at 37 C in 5% CO2. After 24 h (d 1), 150 μl medium was replaced with fresh α-MEM supplemented as described above. Care to avoid temperature or pH fluctuations was taken when manipulating plates containing follicles. Follicles that did not increase in diameter or exhibited darkening of the granulosa cell mass or had spontaneously extruded their oocyte were excluded from any further analysis. Follicular architecture is preserved in this culture system because the follicles maintain their three-dimensional structure and the basement membrane remains intact. Follicles do not attach to the bottom of the well; hence, they maintain their morphology, antrum integrity is not disrupted, and theca and granulosa cell interactions are preserved throughout the culture period. Ovulation was assessed by the presence of a released oocyte 18 h after hCG treatment.
Treatments
Follicles that reached at least 340 μm in diameter on d 3 of culture (d 0 is collection day) were stimulated to ovulate with 4 IU/ml hCG (Pregnyl; Serono) or forskolin (Sigma-Aldrich) diluted in dimethylsulfoxide (DMSO) and used at a concentration of 5, 10, or 15 μm. The final concentration of DMSO in wells never exceeded 0.01%. Because of the low number of follicles that are collected and available for treatment, experiments involving ovulatory treatments using hCG or forskolin were repeated eight times. Vehicle (DMSO, 0.01%) and positive control groups (hCG 4 IU/ml) were included in each experiment. Results from all the experiments were comparable, and data were pooled.
cAMP measurements and estradiol assay
Follicles were treated with an ovulatory stimulus (either hCG or different concentrations of forskolin), and the follicles and media were collected and individually frozen at different time points after treatment (0, 30, 60, 120, and 180 min). No isobutylmethylxanthine was used. cAMP was measured in follicles or media (as indicated) using the cAMP Direct Biotrak EIA (GE Healthcare, Piscataway, NJ). Individual follicles were lysed or media used directly following manufacturer's instructions.
For measuring the amount of estradiol produced by follicles while in culture, spent medium was collected after treatments as indicated and stored at −80 C until assayed. Estradiol was measured using a double-antibody RIA kit (Diagnostics Systems Laboratories, Webster, TX).
Gene expression analyses
Follicles were snap frozen before the ovulatory treatment (hCG or forskolin) or 4 h after treatments and stored at −80 C. Total RNA was isolated from individual follicles using the PicoPure RNA isolation kit (Arcturus, Mountain View, CA) according to the manufacturer's protocol. All RNA preparations were treated for contaminating DNA using ribonuclease-free deoxyribonuclease (QIAGEN, Valencia, CA) according to the manufacturer's protocol. Because of the low concentration of RNA obtained from each individual follicle, the cDNA preparation was generated using the whole preparation of RNA (a volume of 10 μl) in a 25-μl reaction using random hexamers and the Superscript II cDNA synthesis system (Invitrogen Corp., Carlsbad, CA) according to the manufacturer's protocol. cDNA was treated with ribonuclease and diluted with water to a final volume of 125 μl. Real-time RT-PCR assessment was performed. Each reaction included 5 μl cDNA, 10 pmol primer, and 1× Power SYBR Green Master Mix (Applied Biosystems, Foster City, CA) in a total reaction volume of 25 μl. TaqMan gene expression assay (Applied Biosystems) probe/primer sets were used to analyze FSHr expression (Mm00442819). Fold changes in gene expression were determined by quantitation of cDNA from target (treated) samples relative to a calibrator sample (vehicle). For normalization purposes, an identical set of reactions was prepared using primers specific for the ribosomal L7 (rPL7). For TaqMan analysis, a TaqMan rPL7 probe was used. Amplification was carried out in an ABI PRISM 7900 sequence detection system (Applied Biosystems) as follows: 50 C for 2 min and 95 C for 10 min (once) and 95 C for 15 sec and 60 C for 1 min (40 times). Quantitative differences in the cDNA target between samples were determined using the mathematical model of Pfaffl (15) as previously described (16) Sequences for Sult1e1 were previously published by Gershon et al. (17). Androgen receptor primers were obtained from SABiosciences (Frederick, MD). The sequence for the epiregulin primers was obtained from PrimerBank (ID 6679683a1). All other sequences of primers used are listed in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Western blot analysis
To be able to extract sufficient protein to perform Western blot analysis, whole ovaries rather than individual follicles were used. Mice were treated with 5 IU PMSG and 48 h later were injected with 5 IU hCG. Ovaries were collected at different times after hCG treatment. Protein extracts were prepared using the M-per kit (Pierce Biotechnology, Rockford, IL). All antibodies used were from Cell Signaling Technology, Inc.(Danvers, MA) diluted 1:1000. Protein (5 μg) was separated in a 10% NuPage gel (Invitrogen) and transferred to a nitrocellulose membrane blocked for 1 h in 5% BSA in Tris-buffered saline with Tween 20. The membranes were then incubated overnight at 4 C with one of the following antibodies: p44/42 MAPK (Erk1/2; no. 9102) or phospho-p44/42 MAPK (Erk1/2; Thr202/Tyr204; no. 9101). After washing, the membranes were incubated with an antirabbit IgG antibody (no. 7074). The ECL Plus detection system (GE Healthcare) was used.
Statistical analysis
Fisher exact and χ2 tests were use to detect differences in ovulation rates between genotypes within treatments. ANOVA and t test were used to detect differences in gene expression analysis. Results for ovulation rates are presented as percentages of follicles treated, and data were pooled between replicates. Experiments were repeated two to three times unless otherwise noted. Results from gene expression analysis and cAMP production are presented as average ± se.
Results
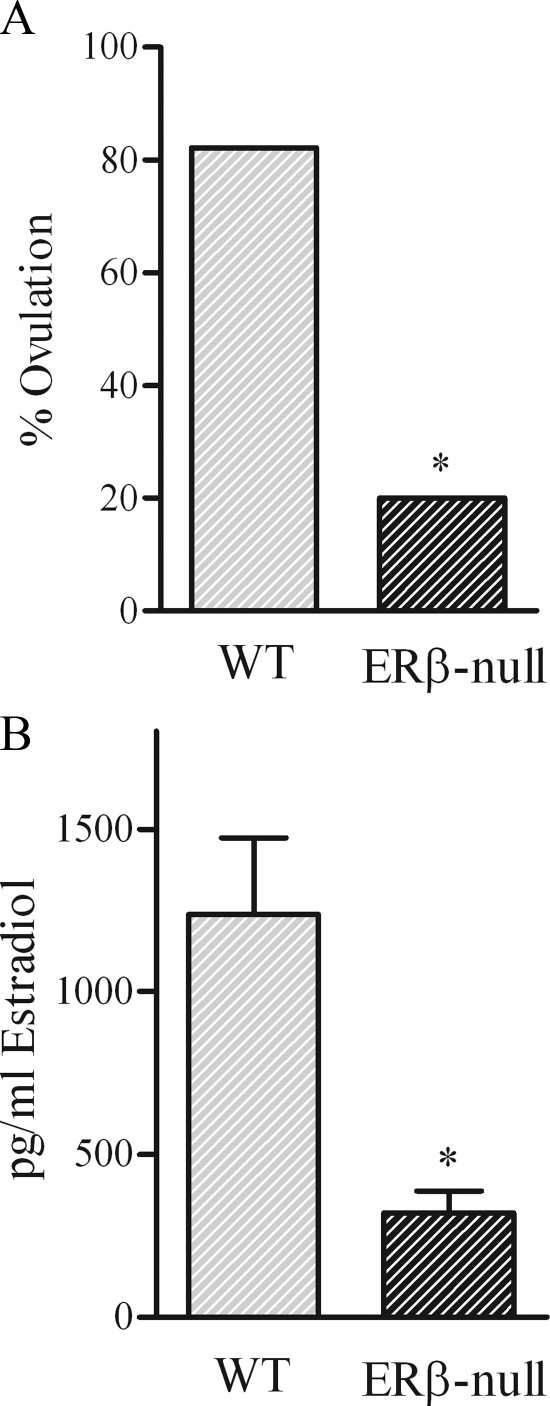
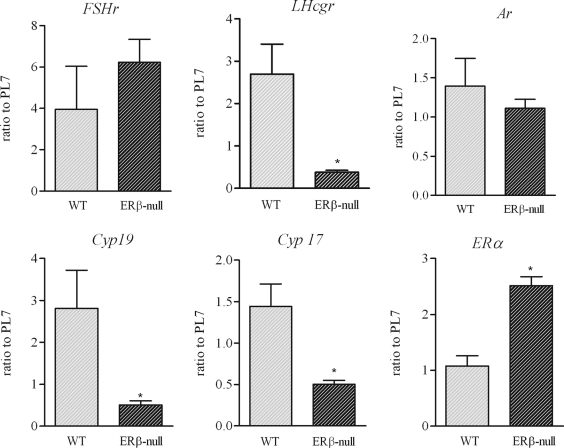
To set the benchmark for our experiments, we again evaluated the ovulatory rates of WT and ERβ-null follicles after growth and maturation in culture. In agreement with previous reports, only approximately 20% of ERβ-null follicles exhibited successful ovulation after hCG stimulation, compared with more than 80% of follicles from WT and heterozygous mice (P < 0.05, Fig. 1A). In addition, the average estradiol accumulation in culture media during the 48 h leading up to ovulation was 75% lower in ERβ-null follicles compared with WT (321 ± 151 vs. 1238 ± 234 pg/ml, respectively, P < 0.001; Fig. 1B). Gene expression analyses of follicles cultured up to the preovulatory stage, but then harvested rather than stimulated to ovulate, demonstrated no difference in androgen receptor (Ar) between genotypes. In addition, no differences were detected in Fshr expression between WT and ERβ-null follicles (Fig. 2), consistent with past reports comparing expression in whole ovary (5) and isolated granulosa cells from ERβ-null mice (18). However, ERβ-null preovulatory follicles did exhibit significantly lower expression of the FSH-regulated genes Cyp19 (P < 0.05) and Lhcgr (P < 0.05) relative to WT (Fig. 2). Interestingly, expression of Cyp17, a thecal cell-specific gene, was also reduced by approximately 65% in ERβ-null follicles relative to WT. In turn, Esr1 (ERα), also considered to be primarily limited to the thecal cells, was increased 2.5-fold in preovulatory ERβ-null follicles relative to WT (Fig. 2).
Figure 1.
Ovulation rates after hCG treatments (A) and estradiol production by preovulatory follicles (B). A, Follicles that reached at least 340 μm in diameter were induced to ovulate by treatment with (4 IU) hCG. Ovulation rates were significantly lower in ERβ-null follicles compared with WT follicles (*, P < 0.05; n = 14–16 per genotype). B, Estradiol was measured in the spent medium after a 48-h accumulation period (before hCG treatment). WT follicles produce significantly higher amounts of estradiol during culture compared with ERβ-null follicles (*, P < 0.001; n = 8 per genotype).
Figure 2.
Gene expression in preovulatory follicles. Preantral follicles from WT (light bars) and ERβ-null (dark bars) mice were isolated and cultured in the presence of FSH until they reached 340 μm in diameter. Follicles were frozen, and gene expression was assessed by quantitative PCR. Expression of Lhcgr, Cyp19a, and Cyp17 was significantly lower in ERβ-null follicles compared with WT follicles (*, P < 0.05; n = 19 pooled from three experiments). Expression of ERα was found to be significantly increased in ERβ-null preovulatory follicles (*, P < 0.001; n = 7 and n = 14 follicles for WT and ERβ-null, respectively).
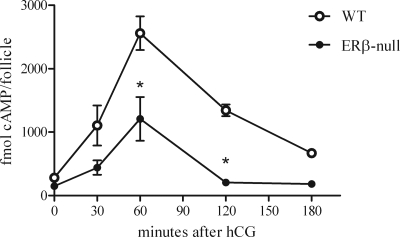
The invariably lower Lhcgr expression in ERβ-null follicles led us to hypothesize that cAMP synthesis after hCG stimulation of the LH receptor pathway is disrupted. We therefore measured cAMP levels at 0, 30, 60, 120, and 180 min after an hCG ovulatory stimulus in individually cultured WT and ERβ-null preovulatory follicles (Fig. 3). Both genotypes exhibited a similar pattern of cAMP production, with a peak at 60 min after induction followed by a precipitous decline (Fig. 3). However, the average peak cAMP level among ERβ-null follicles was only 50% that of WT (1209 ± 345 vs. 2559 ± 265 fmol/follicle, P < 0.05). Furthermore, cAMP levels in ERβ-null follicles had returned to baseline within 120 min of stimulation, whereas WT cAMP levels still remained elevated at 50% peak levels at this time and had not yet returned to baseline levels as far out as 180 min after stimulation.
Figure 3.
Time course of cAMP levels produced by individual follicles after hCG treatment. Preantral follicles were cultured in the presence of FSH. Follicles that reached 340 μm in diameter were treated with hCG and frozen at either 0, 30, 60, 120, and 180 min after treatment. Individual follicles were lysed, and cAMP content was measured. WT follicles produced significantly higher levels of cAMP compared with ERβ-null follicles at 60 min, and levels of cAMP remained high in WT follicles 120 min after hCG (*, P < 0.05; n = 6–8 follicles per time point).
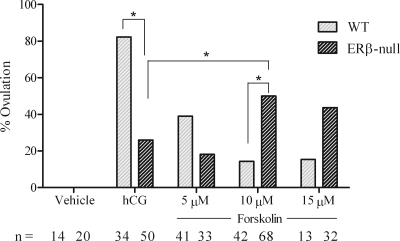
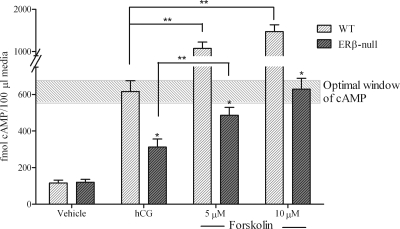
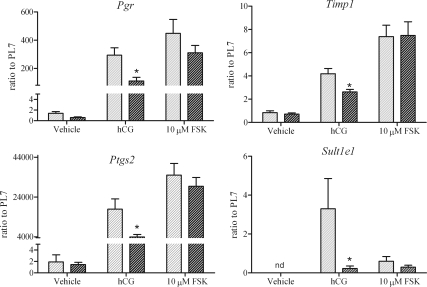
The data thus far support our hypothesis that reduced Lhcgr expression in preovulatory ERβ-null follicles leads to significant reductions in both the peak and duration of cAMP activity after hCG stimulation. To test this hypothesis, we measured these same end points after treatment with forskolin, which directly activates adenylyl cyclase and thereby circumvents any genotypic differences in LH receptor expression. The data presented represent results pooled from eight independent experiments (Fig. 4). After stimulation with 10 μm forskolin, ERβ-null follicles exhibited a 2-fold higher cAMP level at 60 min relative to those stimulated with hCG (Fig. 5). WT follicles also responded to forskolin and exhibited an approximately 1.5- and 2-fold higher cAMP level with forskolin at 5 and 10 μm, respectively, relative to hCG (Fig. 5). The induction of genes known to be critical to the ovulatory cascade also exhibited remarkable improvement in ERβ-null follicles when substituting forskolin for hCG. Due to the difficulty in obtaining sufficient number of follicles for analysis at all doses, and based on the dose response (Figs. 4 and 5), only forskolin at 10 μm was used in experiments designed for gene expression analyses. As shown in Fig. 6, 10 μm forskolin induced the expression of prostaglandin endoperoxide synthase 2 (Ptgs2), tissue inhibitor of metalloproteinases I (Timp-1), and progesterone receptor (Pgr) in ERβ-null follicles to levels that were significantly higher than those with hCG and, more importantly, to levels that reached or surpassed the benchmarks consistent with ovulation set by hCG-stimulated WT follicles. Interestingly, Sult1e1 was not induced in ERβ-null follicles after hCG treatment, and expression was not induced by forskolin in either genotype (Fig. 6).
Figure 4.
Ovulation after forskolin treatment. Preantral follicles were collected and cultured with FSH as described. WT and ERβ-null preovulatory follicles were treated with vehicle (0.01% DMSO), hCG (4 IU), or 5, 10, or 15 μm forskolin. Ovulation was assessed 18 h after treatment. Asterisks represent differences between groups as shown. ERβ-null follicles treated with 10 μm forskolin exhibited a significant increase (P = 0.013) in ovulatory rates compared with hCG treatment. WT follicles treated with either concentrations of forskolin exhibited a significantly (P < 0.001) decreased ovulatory rate compared with follicles treated with hCG. This experiment was repeated eight times, and the total number of follicles per group is listed.
Figure 5.
cAMP levels after forskolin treatment. Preovulatory WT and ERβ-null follicles (collected and cultured as previously described) were treated with vehicle (0.01% DMSO), hCG (4 IU), or 5 or 10 μm forskolin. Medium was collected 1 h after treatment for measurement of cAMP. *, Significant difference between genotypes within treatments (P < 0.05); **, significant difference between treatment within genotype (P < 0.05). The experiment was repeated two times and included a total of eight to 15 follicles per treatment. The shaded box highlights the optimal window of cAMP levels.
Figure 6.
Gene expression 4 h after hCG or forskolin treatment. Preovulatory WT (light bars) and ERβ-null (dark bars) follicles were treated with vehicle (0.01% DMSO), hCG (4 IU), or 10 μm forskolin (FSK). Follicles were frozen 4 h after treatment, and gene expression was assessed by quantitative PCR. Gene expression was calculated based on WT vehicle expression for all genes except for Sult1e1 where expression was not detected (nd) in the vehicle group, and expression was calculated based on WT treated with hCG. The experiment was repeated three times and included a total of 12–18 follicles per treatment. ERβ-null follicles exhibited lower gene expression after hCG treatment compared with WT follicles (*, P < 0.05). Forskolin treatment resulted in an increase in gene expression in ERβ-null follicles that was comparable to the level observed in WT follicles treated with hCG.
The improved induction of cAMP synthesis in ERβ-null follicles after activation of adenylyl cyclase came with 2-fold increase in the ovulation rate in ERβ-null follicles in vitro (Fig. 4). Interestingly, WT follicles did not respond as well to forskolin as a substitute for hCG and exhibited a dose-dependent decrease in ovulatory rates after stimulation. The data illustrated in Figs. 4 and 5 suggest that peak cAMP levels that are most conducive to ovulation in vitro lie within a narrow range of approximately 600 fmol/100 μl medium. Peak cAMP levels that either fail to reach or surpass these levels are not conducive to follicle rupture. Consistent with this hypothesis, ERβ-null follicles exhibited greater ovulation rates when cAMP levels are within the range of 600 fmol/100 μl. We hypothesize that insufficient LH receptor levels in a majority of preovulatory ERβ-null follicles prohibit their ability to attain sufficient cAMP levels when stimulated by hCG, and presumably by LH, but this deficiency can be overcome considerably when the LH receptor is bypassed by direct activation of the adenylyl cyclase system.
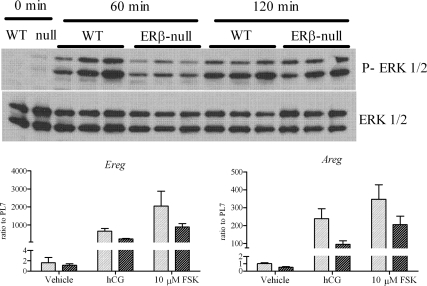
Although forskolin treatment remarkably improved cAMP levels in preovulatory ERβ-null follicles, levels never matched those found in similarly treated WT follicles. These data support the long-held hypothesis that LH signaling leading to ovulation is not solely mediated by adenylyl cylase and cAMP. This is illustrated herein by the striking induction of Sult1e1 expression by hCG but not forskolin in WT follicles (Fig. 6) and failure of either factor to induce stimulation in ERβ-null follicles. Several recent reports provide convincing evidence of the role for MAPK1/3 (ERK1/2) signaling in follicle rupture (19). We therefore sought to evaluate ERK1/2 signaling, but because isolating measurable amounts of protein from individual follicles is not feasible, these assays were done in whole ovary samples from each genotype. WT and ERβ-null mice were treated with 5 IU PMSG and 48 h later injected with 5 IU hCG and the ovaries collected 60 and 120 min after hCG. ERK1/2 activation in harvested ovaries was then measured by Western blot (Fig. 7). Within 60 min of hCG treatment, ERK1/2 activation was higher in WT ovaries relative to ERβ-null ovaries. By 120 min, however, ERK1/2 levels had risen and were comparable in both genotypes. Expression of epidermal growth factor-like factors amphiregulin (Areg) and epiregulin (Ereg), also known to be critical for ovulation (20,21) and involved in the ERK1/2 pathway (19,22), trend to be lower in ERβ-null follicles but were not significantly different compared with WT after hCG treatment (Fig. 7). After forskolin treatment, the expression of Areg and Ereg improved in both WT and ERβ-null follicles relative to when treated with hCG (Fig. 7).
Figure 7.
Phosphorylation of ERK1/2 in vivo and expression of Areg and Ereg. Prepubertal mice, WT or ERβ-null, were treated with PMSG, and 48 h later, ovaries were collected (0 min), or they were treated with hCG and their ovaries collected 60 or 120 min after hCG. The experiment was repeated twice with three mice per experiment per time point. Expression of Areg and Ereg was measured in six to eight follicles per genotype (WT, light bars; ERβ-null, dark bars) per treatment and were treated as described. FSK, Forskolin.
Discussion
All evidence to date indicates that subfertility in ERβ-null mice is the result of inefficient ovulation due primarily to a reduced capacity of preovulatory follicles to respond to an ovulatory bolus of LH or its analog hCG (5,6,18). The controlled environment uniquely afforded by in vitro culture of individual follicles has allowed us to further demonstrate that the poor ovulatory rate among ERβ-null follicles coincides with a significantly reduced level of cAMP, the primary intracellular second messenger of LH signaling, when challenged with an ovulatory dose of hCG. Because the response to hCG is principally dependent on the level of LH receptor among the granulosa cells of a preovulatory follicle, and LH receptor expression is known to be significantly reduced in ERβ-null follicles, we attempted to test our hypothesis by determining whether ovulatory rates in ER-null follicles are improved when substituting forskolin for hCG. Forskolin, like LH and hCG, relies on cAMP as the intracellular second messenger signaling; however, unlike the latter, forskolin does not require the LH receptor but instead is able to directly activate adenylyl cyclase to produce cAMP. Our data demonstrate that forskolin, when used instead of hCG, improves the ovulatory rate of ERβ-null follicles, and this improvement occurs in the context of WT-like levels of cAMP production and expression of genes known to be critical to ovulation. These findings build upon the growing evidence that ERβ actions are critical to the acquisition of sufficient LH receptor levels during follicle maturation to the preovulatory stage.
Interestingly, we found that maximum ovulation rates require peak cAMP levels to fall within a relatively narrow range after stimulation with hCG or forskolin. In our culture system, a dose of 4 IU hCG is optimal for inducing ovulation in WT follicles in culture and leads to cAMP concentrations in the media that approximate 600 fmol/100 μl medium. In contrast, ERβ-null follicles exhibit approximately 300 fmol/100 μl medium in response to this same dose of hCG and, as such, exhibit reduced ovulation rates. We have previously shown that increasing the dose of hCG has no measurable impact on the ovulatory rates of ERβ-null follicles either in vivo (5) or in vitro (6), further indicating that LH receptor levels are limiting in ERβ-null follicles. However, when cAMP production in ERβ-null follicles is improved to more than 600 fmol/100 μl medium, only possible through bypassing the LH receptor and using forskolin as the ovulatory stimulus, we saw a significant increase in the ovulatory rates and gene expression among ERβ-null follicles. These observations, when combined, indicate that optimal cAMP levels after the ovulatory stimulus fall within a narrow range and that levels below or above this range are less likely to lead to successful follicle rupture. Indeed, it has been proposed in humans that folliculogenesis is adversely affected when LH stimulation crosses a maximum ceiling and that this leads to either arrested follicular growth (23,24) or premature luteinization of the follicular wall and trapping of the oocyte within the follicle (25). Our results may provide a mechanistic explanation for this clinical phenomenon.
The average induction of LH target genes exhibited by ERβ-null follicles after hCG treatment is lower than in WT follicles (Fig. 6). However, we must assume that expression of these genes in some ERβ-null follicles is sufficient to mediate follicle rupture, thereby accounting for the approximately 20% of ERβ-null follicles that successfully ovulate. It is impossible, however, to measure both preovulatory gene expression and ovulation in the same follicle in our in vitro follicle culture system. Therefore, we are not able to associate gene expression levels with success or failure to ovulate in individual follicles.
Although forskolin as an ovulatory stimulus was able to induce more optimal levels of cAMP production and improve the overall ovulation rate among ERβ-null follicles, the treatment did not provide a full rescue of ovulation rates to the levels observed in WT follicles under optimal conditions. It also cannot be discounted that potential differences in phosphodiesterase (PDE) expression may also contribute to the lower cAMP levels and gene expression observed in ERβ-null follicles. Deroo et al. (18) reported the level of expression of the different PDEs expressed in cultured granulosa cells and concluded that PDE1c expression is higher in ERβ-null granulosa cells when treated with FSH. We attempted to measure expression of PDE1c but were unable to detect measurable levels in individual preovulatory follicles cultured in our system (data not shown). This potential difference in cAMP degradation may contribute to the overall lower level of cAMP in ERβ-null follicles after an ovulatory treatment because our treatments did not include isobutylmethylxanthine.
At none of the doses tested was forskolin as effective as hCG in inducing ovulation in WT follicles. Although this latter observation may be attributed to forskolin causing excess production of cAMP as described above, these findings also support the widely held concept that LH and hCG stimulation are not solely dependent on activation of adenylyl cyclase and cAMP for intracellular signaling. Herein we show that indeed, Sult1e1 is significantly induced by hCG but not forskolin in WT follicles, indicating that this induction is dependent on activation of the LH receptor but involves signaling pathways other than cAMP. In addition to cAMP induction of PKA, activation of LH receptors has also been shown to stimulate PKC and tyrosine kinase pathways independent of cAMP production (1,26,27). Recent evidence that MAPK in granulosa cells is required for ovulation in mice (19) further indicates the complexity of the ovulatory cascade. We show that MAPK1/3 (ERK1/2) activation 60 min after an hCG challenge is considerably attenuated in ERβ-null ovaries relative to similarly treated WT. Furthermore, recent studies have demonstrated that LH stimulation induces the activation of the EGF network, particularly the expression of Areg and Ereg (20,21), and by 4 h, their expression is dependent on ERK1/2 expression in vivo (19). Furthermore, the induction of Pgr by LH receptor activation has been described to be upstream from ERK1/2 activation and Areg and Ereg expression (22). The expression of Areg and Ereg in ERβ-null preovulatory follicles treated with hCG tended to be lower relative to WT treated with hCG, which corresponds to the lower ERK1/2 phosphorylation observed (Fig. 7). Interestingly, even with the improvement of Pgr, Areg, and Ereg expression after forskolin treatment, the ovulatory response of ERβ-null follicles remained lower than the response obtained from WT follicles treated with hCG. It is conceivable that use of a combination of phorbol myristate acetate plus forskolin could provide activation of a wider spectrum of second messenger pathways and improve ovulatory rates of ERβ-null follicles even further. If true, such findings would remain consistent with insufficient acquisition of LH receptor before receiving an LH bolus as the primary defect in ERβ-null follicles.
The failure of ERβ-null follicles to acquire maximum expression of Cyp19 and Lhcgr does not appear to be due to defects in FSH signaling, on which both are known to be dependent. The levels of cAMP in ERβ-null granulosa cells cultured with FSH are reportedly not different from WT granulosa cells (18). Expression levels of the FSH receptor gene (Fshr) is comparable between WT and ERβ-null follicles (Fig. 2). Furthermore, overall follicle growth leading up to the preovulatory state, another index of FSH action, is comparable between WT and ERβ-null follicles (data not shown). This is in contrast to an earlier report that indicated ERβ-null follicles do not exhibit normal growth rates during maturation (6). We attribute our observation to a series of improvements made to the in vitro follicle culture methods that resulted in an equal percentage of WT and ERβ-null follicles being able to reach the 340-μm diameter we use to trigger ovulation. Despite improved growth rates, however, Cyp19 expression, estradiol production, and acquisition of LH receptors continued to be considerably below normal in ERβ-null follicles.
Interestingly, we also describe for the first time a significant reduction in the expression of Cyp17, a thecal cell-specific gene, in ERβ-null follicles in culture. This occurs in the context of a more than 2-fold increase in Esr1 (ERα) expression in ERβ-null follicles relative to WT. The increased ERα level in ERβ-null follicles is presumed to be localized to the theca cell compartment. The reduced Cyp17 expression, perhaps due to ERα-mediated repression (28), is likely also contributing to the reduced estradiol production in ERβ-null follicles because androgen synthesis is the rate-limiting factor for estradiol synthesis and solely dependent on thecal cell Cyp17 activity. Nevertheless, these observations indicate that ERβ may also play an indirect role in the proper differentiation of the theca cell layer in preovulatory follicles.
In conclusion, our study indicates that the failure of ERβ-null follicles to acquire maximal LH receptor expression upon reaching the preovulatory state is the primary cause of the reduced ovulatory rate observed in these mice. These data build upon the growing evidence that ERβ actions in the follicle are critical to its reaching the preovulatory stage in healthy condition and properly staged to receive the ovulatory dose of LH. To a considerable extent, this deficit can be overcome artificially in culture by stimulating ovulation via direct activation of adenylyl cyclase using forskolin instead of LH, which is dependent on sufficient LH receptors. Furthermore, these findings indicate that the machinery necessary to mediate follicle rupture is generally intact in ERβ-null preovulatory follicles once sufficiently stimulated, indicating that ERβ plays no direct role in ovulation.
Supplementary Material
Acknowledgments
We thank Dr. Grace Kissling for assistance in performing the statistical analysis and Dr. Mary Hunzicker-Dunn, Dr. Bonnie Deroo, and members of the Receptor Biology Section for reviewing the manuscript and providing helpful comments.
Footnotes
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences Z01ES70065 (to K.S.K.).
Current address for J.F.C.: Taconic Inc., Albany, New York.
Current address for F.L.J.: Duke University Medical Center, Durham, NC.
Current address for F.T.: Tottori University School of Medicine, Yonago, Japan.
Disclosure Summary: All authors have nothing to declare.
First Published Online April 8, 2010
Abbreviations: DMSO, Dimethylsulfoxide; ER, estrogen receptor; hCG, human chorionic gonadotropin; PDE, phosphodiesterase; PKA, protein kinase A; PMSG, pregnant mare serum gonadotropin; WT, wild type.
References
- Richards JS, Fitzpatrick SL, Clemens JW, Morris JK, Alliston T, Sirois J 1995 Ovarian cell differentiation: a cascade of multiple hormones, cellular signals, and regulated genes. Recent Prog Horm Res 50:223–254 [DOI] [PubMed] [Google Scholar]
- Knecht M, Brodie AM, Catt KJ 1985 Aromatase inhibitors prevent granulosa cell differentiation: an obligatory role for estrogens in luteinizing hormone receptor expression. Endocrinology 117:1156– 1161 [DOI] [PubMed] [Google Scholar]
- Kessel B, Liu YX, Jia XC, Hsueh AJ 1985 Autocrine role of estrogens in the augmentation of luteinizing hormone receptor formation in cultured rat granulosa cells. Biol Reprod 32:1038–1050 [DOI] [PubMed] [Google Scholar]
- Segaloff DL, Wang HY, Richards JS 1990 Hormonal regulation of luteinizing hormone/chorionic gonadotropin receptor mRNA in rat ovarian cells during follicular development and luteinization. Mol Endocrinol 4:1856–1865 [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Deroo BJ, Korach KS 2005 Estrogen receptor-β is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology 146:3247–3262 [DOI] [PubMed] [Google Scholar]
- Emmen JM, Couse JF, Elmore SA, Yates MM, Kissling GE, Korach KS 2005 In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER)α and ERβ null mice indicate a role for ERβ in follicular maturation. Endocrinology 146:2817–2826 [DOI] [PubMed] [Google Scholar]
- Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS 1997 Tissue distribution and quantitative analysis of estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) messenger ribonucleic acid in the wild-type and ERα-knockout mouse. Endocrinology 138:4613–4621 [DOI] [PubMed] [Google Scholar]
- Couse JF, Curtis SW, Washburn TF, Eddy EM, Schomberg DW, Korach KS 1995 Disruption of the mouse oestrogen receptor gene: resulting phenotypes and experimental findings. Biochem Soc Transact 23:929–935 [DOI] [PubMed] [Google Scholar]
- Ascoli M, Fanelli F, Segaloff DL 2002 The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev 23:141–174 [DOI] [PubMed] [Google Scholar]
- Simoni M, Gromoll J, Nieschlag E 1997 The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev 18:739–773 [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Birnbaumer L 1976 Adenylyl cyclase activities in ovarian tissues. II. Regulation of responsiveness to LH, FSH, and PGE1 in the rabbit. Endocrinology 99:185–197 [DOI] [PubMed] [Google Scholar]
- Richards JS 2001 New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol Endocrinol 15:209–218 [DOI] [PubMed] [Google Scholar]
- Panigone S, Hsieh M, Fu M, Persani L, Conti M 2008 Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol 22:924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O 1998 Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW 2001 A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroo BJ, Hewitt SC, Collins JB, Grissom SF, Hamilton KJ, Korach KS 2009 Profile of estrogen-responsive genes in an estrogen-specific mammary gland outgrowth model. Mol Reprod Dev 76:733–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon E, Hourvitz A, Reikhav S, Maman E, Dekel N 2007 Low expression of COX-2, reduced cumulus expansion, and impaired ovulation in SULT1E1-deficient mice. FASEB J 21:1893–1901 [DOI] [PubMed] [Google Scholar]
- Deroo BJ, Rodriguez KF, Couse JF, Hamilton KJ, Collins JB, Grissom SF, Korach KS 2009 Estrogen receptor β is required for optimal cAMP production in mouse granulosa cells. Mol Endocrinol 23:955–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS 2009 MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 324:938–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M 2004 EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303:682–684 [DOI] [PubMed] [Google Scholar]
- Conti M, Hsieh M, Park JY, Su YQ 2006 Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol 20:715–723 [DOI] [PubMed] [Google Scholar]
- Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS 2006 Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol 20:1352–1365 [DOI] [PubMed] [Google Scholar]
- Hillier SG 1994 Current concepts of the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum Reprod 9:188–191 [DOI] [PubMed] [Google Scholar]
- Loumaye E, Engrand P, Shoham Z, Hillier SG, Baird DT 2003 Clinical evidence for an LH ‘ceiling' effect induced by administration of recombinant human LH during the late follicular phase of stimulated cycles in World Health Organization type I and type II anovulation. Hum Reprod 18:314–322 [DOI] [PubMed] [Google Scholar]
- Birkenfeld A, Mor-Joseph S, Ezra J, Simon A, Navot D 1990 Preovulatory luteinization during induction of follicular maturation with menotrophin and menotrophin-clomiphene combination. Hum Reprod 5:561–564 [DOI] [PubMed] [Google Scholar]
- Morris JK, Richards JS 1993 Hormone induction of luteinization and prostaglandin endoperoxide synthase-2 involves multiple cellular signaling pathways. Endocrinology 133:770–779 [DOI] [PubMed] [Google Scholar]
- Morris JK, Richards JS 1995 Luteinizing hormone induces prostaglandin endoperoxide synthase-2 and luteinization in vitro by A-kinase and C-kinase pathways. Endocrinology 136:1549–1558 [DOI] [PubMed] [Google Scholar]
- Taniguchi F, Couse JF, Rodriguez KF, Emmen JM, Poirier D, Korach KS 2007 Estrogen receptor-α mediates an intraovarian negative feedback loop on thecal cell steroidogenesis via modulation of Cyp17a1 (cytochrome P450, steroid 17α-hydroxylase/17,20 lyase) expression. FASEB J 21:586–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.