Abstract
Vitamin D insufficiency is a global health issue. Although classically associated with rickets, low vitamin D levels have also been linked to aberrant immune function and associated health problems such as inflammatory bowel disease (IBD). To test the hypothesis that impaired vitamin D status predisposes to IBD, 8-wk-old C57BL/6 mice were raised from weaning on vitamin D-deficient or vitamin D-sufficient diets and then treated with dextran sodium sulphate (DSS) to induce colitis. Vitamin D-deficient mice showed decreased serum levels of precursor 25-hydroxyvitamin D3 (2.5 ± 0.1 vs. 24.4 ± 1.8 ng/ml) and active 1,25-dihydroxyvitamin D3 (28.8 ± 3.1 vs. 45.6 ± 4.2 pg/ml), greater DSS-induced weight loss (9 vs. 5%), increased colitis (4.71 ± 0.85 vs. 1.57 ± 0.18), and splenomegaly relative to mice on vitamin D-sufficient chow. DNA array analysis of colon tissue (n = 4 mice) identified 27 genes consistently (P < 0.05) up-regulated or down-regulated more than 2-fold in vitamin D-deficient vs. vitamin D-sufficient mice, in the absence of DSS-induced colitis. This included angiogenin-4, an antimicrobial protein involved in host containment of enteric bacteria. Immunohistochemistry confirmed that colonic angiogenin-4 protein was significantly decreased in vitamin D-deficient mice even in the absence of colitis. Moreover, the same animals showed elevated levels (50-fold) of bacteria in colonic tissue. These data show for the first time that simple vitamin D deficiency predisposes mice to colitis via dysregulated colonic antimicrobial activity and impaired homeostasis of enteric bacteria. This may be a pivotal mechanism linking vitamin D status with IBD in humans.
Vitamin D deficiency predisposes mice to an experimental form of inflammatory bowel disease which involves abnormal containment of enteric bacteria.
Impaired vitamin D status has long been recognized as a complication of inflammatory bowel disease (IBD) and Crohn's disease in particular (1,2,3,4,5,6). For many years, this was considered to be due primarily to dietary malabsorption after gastrointestinal (GI) inflammation (7,8,9). As a result, the principal clinical challenge for IBD patients with vitamin D deficiency has been the management of associated bone disease (10). However, more recent studies have shed new light on the link between vitamin D and IBD. In particular, it has been hypothesized that dysregulation of vitamin D function may contribute to the pathogenesis of IBD, whereas concommitantly, vitamin D supplementation may provide a novel prevention or treatment strategy for this prevalent health problem.
Several strands of evidence support a more causal role for vitamin D status in IBD. In the first instance, recent studies have underlined the potent effects of vitamin D as a modulator of both innate and adaptive immunity (11,12). These actions appear to depend on local (intracrine or paracrine) conversion of prohormone 25-hydroxyvitamin D3 (25OHD3) to active 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] (13). Given that 25OHD3 is the major circulating form of vitamin D, it is likely that impaired vitamin D status will compromise the immune activity of vitamin D (11,12). This has been postulated as a susceptibility factor for infectious diseases such as tuberculosis (14), but decreased serum 25OHD3 has also been linked to autoimmune diseases such as type 1 diabetes (15) and multiple sclerosis (16). A role for vitamin D in IBD is also supported by recent studies showing that 1,25(OH)2D3 is a potent stimulator of nucleotide-binding oligomerization domain-containing 2 (NOD2), an important pattern recognition receptor within the immune system (17). NOD2 deficiency due to mutations in its gene has been linked to the pathogenesis of Crohn's disease (18,19,20), a form of IBD, but it now appears that impaired NOD2 function may also occur under conditions of vitamin D deficiency.
A functional role for vitamin D in IBD is further endorsed by expression of the enzyme 1α-hydroxylase (CYP27B1) by macrophages (13), dendritic cells (21), and colonic epithelial cells (22). CYP27B1 catalyzes the conversion of 25OHD3 to 1,25(OH)2D3 and is therefore essential for tissue-specific responses to alterations in vitamin D status. Moreover, previous studies from our group have shown that expression of CYP27B1 is up-regulated in disease-affected tissue from patients with Crohn's disease (23). In this case, dysregulated colonic expression of CYP27b1 was associated with increased circulating levels of 1,25(OH)2D3, indicating that localized synthesis of this metabolite can spill over into the general circulation under conditions of persistent disease (23).
Animal models have also supported a link between vitamin D and IBD. Knockout mice for the gene encoding the receptor for 1,25(OH)2D3 [vitamin D receptor (VDR)] are more susceptible to experimental forms of colitis (24,25,26,27,28). In a similar fashion, we have shown recently that CYP27B1 knockout mice are also more susceptible to colitis (29). However, as yet, there have been no equivalent studies to assess the impact of impaired vitamin D status on animal models of IBD. In view of the fact that local synthesis of 1,25(OH)2D3 in the colon can act to support immunomodulation at this barrier site, we hypothesized that low vitamin D (25OHD3) status may compromise this immunity, leading to IBD. In the current study, we have tested this hypothesis by inducing experimental colitis in mice raised on normal or vitamin D-deficient diets.
Materials and Methods
Animals
The Institutional Animal Care and Use Committee at the University of California Los Angeles approved the protocol for the use of mice in the study. Weight and sex matched 3-wk-old C57BL/6 mice (The Jackson Laboratories, West Sacramento, CA) were either raised on a normal diet (n = 16) or vitamin D-deficient diet (n = 16) for 6 wk. All diets were procured from Research Diets, Inc. (New Brunswick, NJ). Some tissues were obtained from C57BL/6 mice (The Jackson Laboratories) maintained at Cedars-Sinai Medical Center on sufficient or vitamin D deficient diets.
Experimental colitis
Colitis was induced in mice by administration of 2.5% dextran sodium sulfate (DSS) in drinking water ad libitum for 6 d, after which the mice were resumed on water for the remainder of the experiment. Mice receiving only tap water served as controls. Body weight and animal symptoms, including the extent of diarrhea and rectal bleeding, were closely monitored during and after DSS treatment.
Tissue collection and analysis
Immediately after killing, blood was removed from mice by cardiac puncture, and the resulting serum stored at −80 C before analysis of circulating levels of 25OHD3 and 1,25(OH)2D3 (B. Hollis; Medical University of South Carolina, Charleston, SC). Serum calcium levels were determined by atomic absorption spectroscopy (University of California Los Angeles Department of Pathology). Small and large intestines were also removed from killed mice, washed to remove fecal matter, and then either: 1) preserved in 10% formaldehyde before embedding in paraffin blocks; 2) divided into sections corresponding to small intestine, proximal colon (caecum to midtransverse colon) and distal colon (midtransverse colon to anus), then snap frozen. Kidneys and spleen were also snap frozen before RNA extraction.
Clinical colitis scores
Assessment of mouse health was determined by quantifying body weight loss, stool consistency (diarrhea), hemoccult positivity, or gross bleeding as described previously (30). Briefly, no weight loss was scored as 0, weight loss of 1–5% from baseline as 1, 5–10% as 2, 10–20% as 3, and more than 20% as 4. For stool consistency, a score of 0 points was assigned for well-formed pellets, 2 points for pasty and semiformed stools that did not adhere to the anus, and 4 points for liquid stools that did adhere to the anus. For bleeding, a score of 0 points was assigned for no blood, 2 points for positive hemoccult, and 4 points for gross bleeding. These scores were added together and divided by three, resulting in a total clinical score ranging from 0 (healthy) to 4 (maximal activity of colitis).
Histological colitis scoring
Postmortem, the entire colon was removed, from the cecum to the anus, and colon length and weight measured as markers of inflammation. A segment of the proximal and distal colon was fixed in 10% formalin, processed, and embedded in paraffin; 4-μm-thick sections of this tissue were stained with hematoxylin and eosin. Histological scoring was performed in a blinded fashion by a pathologist, with a combined score for inflammatory cell infiltration (score, 0–3), ulceration, and tissue damage (score, 0–3). The presence of occasional inflammatory cells in the lamina propria was assigned a value of 0, increased numbers of inflammatory cells in the lamina propria as 1, confluence of inflammatory cells, extending into the submucosa, as 2, and transmural extension of the infiltrate as 3. For tissue damage, no mucosal damage was scored as 0, discrete lymphoepithelial lesions were scored as 1, surface mucosal erosion or focal ulceration was scored as 2, and extensive mucosal damage and extension into deeper structures of the bowel wall were scored as 3. The scores for each piece of tissue were totalled and divided by the number of pieces. The combined histological score ranged from 0 (no changes) to 6 (extensive cell infiltration and tissue damage).
Flow cytometry
Spleens were pressed through a nylon cell strainer to isolate single cell suspensions. Cells were washed twice and counted before analysis for flow cytometry. Flow cytometry followed routine procedures, using 1 × 106 cells per sample. The cells were preincubated with Mouse T Lymphocyte Subset Antibody Cocktail (PE-Cy7 CD3e, PE CD4, and FITC CD8) and FITC antimouse CD19 (BD Pharmingen, San Diego, CA) for 20 min on ice. Staining with biotinylated antibody was followed by staining with respective streptavidin (BD Pharmingen). Flow cytometric analysis was conducted on a BD-LSR I Analytic Flow Cytometer (Becton Dickinson, Mountainview, CA) and analyzed by using the BD CELLQUEST analysis program (Becton Dickinson).
Quantitative real-time RT-PCR amplification of cDNAs
RNA was extracted from mouse tissues using the RNeasy Total RNA extraction kit as detailed by the manufacturer (QIAGEN, Valencia, CA) and 1.5 μg aliquots used for cDNA synthesis by Superscript II RT and oligo-dT and random hexamer primers (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations. Quantitative real-time RT-PCR was performed in a Stratagene cycler (Stratagene, La Jolla, CA), using TaqMan probes and primers (Applied Biosystems, Foster City, CA). Expression of mRNAs for mouse Cyp27b1, IL-1α, IL-10, IL-17, and interferon (IFN)γ was assessed using the following TaqMan mouse gene expression assays: CYP27b1 (Mm01165922), IFNγ (Mm00801778_m1), IL-1α (00439620), IL-10 (Mn00439616), IL-17 (Mm00439619), and 18S rRNA (4352339E) (Applied Biosystems). Aliquots (50 ng) of cDNA were amplified under the following conditions: 50 C for 2 min, 95 C for 10 min, followed by 40 cycles of 95 C for 15 sec and 60 C for 1 min. All reactions were performed in triplicate, and each target gene expression was normalized to 18S rRNA expression. The relative amount of target gene in each sample was estimated using the ΔCT method as described previously (29).
DNA microarray analysis of differential gene expression
RNA extracted from colon tissue of control and vitamin D-deficient mice (n = 4) was used to define differential gene expression under conditions of impaired vitamin D status. Affymetrix mouse 430 2.0 arrays were probed using the resulting cDNA samples and differentially expressed genes detected using a fold change cut-off (≥2) and multiple testing correction (P ≤ 0.05). Preprocessing (background correction and normalization), detection of differentially expressed genes, and cluster analysis were performed using dChip software. Validation of nine differentially expressed genes was performed using real-time PCR as described below using specific TaqMan (Applied Biosystems) probes and primers (see Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Real-time PCR measurement of bacterial DNA
DNA was extracted from colonic tissues (DNAeasy kit; QIAGEN) after washing to remove residual feces. For the quantification of bacterial, a pair of universal primers specific for conserved regions of 16S rDNA [TaqMan primers and probes from Applied Biosystems: forward primer, 5′-tcctacgggaggcagcagt-3′; reverse primer, 5′-ggactaccagggtatctaatcctgtt-3′; and probe, (6-FAM)-5′-cgtattaccgcggctgctggcac-3′-(TAMRA)] were used to amplify this bacterial gene. Primers specific to 18S rRNA were used as an endogenous control to normalize loading between samples. The relative amount of 16S rDNA in each sample was estimated using the ΔΔCT method.
Immunohistochemical analysis of angiogenin-4 (Ang4)
Immunohistochemical analysis of Ang4 protein expression in paraffin-embedded tissue sections was carried out using methods described previously (31). Briefly, sections were dewaxed through a series of xylene and graded ethanol washes, and antigens were retrieved by 20-min boiling in 10 mm citrate (pH 6.0). The slides were stained with anti-Ang4 antiserum (kind gift from Lora Hooper, University of Texas Southwestern Medical Center, Dallas, TX). After incubation with peroxidase-conjugated secondary antibody, signals were visualized with a diaminobenzidine peroxidase substrate kit (Vector Laboratories, Burlingame, CA).
Statistical analysis
Data were analyzed by Student's t test or one-way ANOVA with Fisher's post hoc test, using Statview version 10.00 for Windows.
Results
DSS-induced colitis is more severe in vitamin D-deficient mice
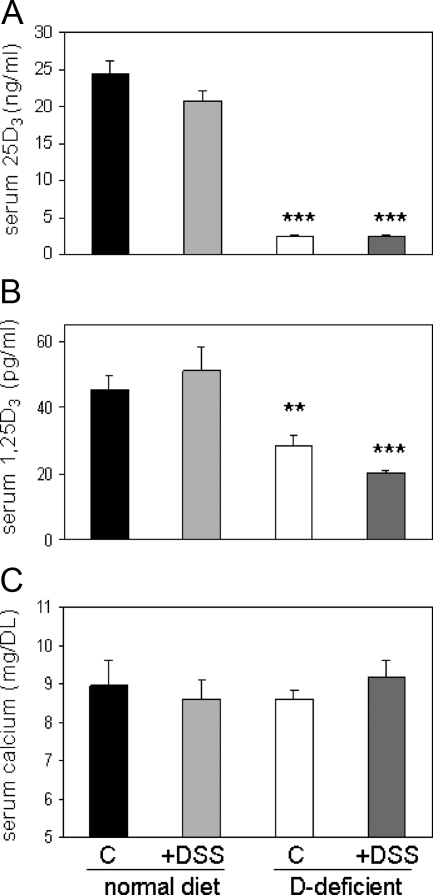
Mice raised from weaning on a vitamin D-deficient diet for 5 wk and then treated with tap water for 10 d showed decreased circulating levels of 25OHD3 (2.5 ± 0.1 vs. 24.4 ± 1.8 ng/ml, P < 0.001) and 1,25(OH)2D3 (28.8 ± 3.1 vs. 45.6 ± 4.2 pg/ml, P < 0.001) compared with mice on a vitamin D-sufficient control diet (Fig. 1, A and B). No significant change in these levels was observed for mice treated with 2.5% DSS relative to tap water controls (Fig. 1, A and B). Similarly, none of the changes in serum vitamin D metabolites observed for mice on the vitamin D-deficient diet was associated with altered serum levels of calcium (Fig. 1C).
Figure 1.
Serum vitamin D metabolites and calcium in mice raised on vitamin D-sufficient and vitamin D-deficient diets. A, Serum concentrations of 25OHD3 (25D3) (ng/ml) in C57BL/6 mice raised on vitamin D-sufficient (normal) or vitamin D-deficient (D-deficient) diets from weaning (wk 4) until wk 9. At wk 8, mice were exposed to either regular tap water (control, C) or water with 2.5% dextran sodium sulfate (DSS) for 1 wk. B, Serum concentrations of 1,25(OH)2D3 (1,25D3) (pg/ml). Panel C, Serum concentrations of calcium (mg/dl). Values are means ± sem (n = 8). ***, Statistically different from corresponding vitamin D-sufficient mice (normal diet), P < 0.001; **, P < 0.01.
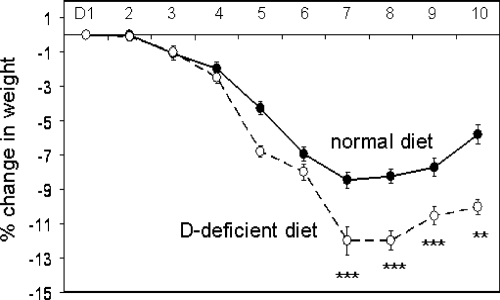
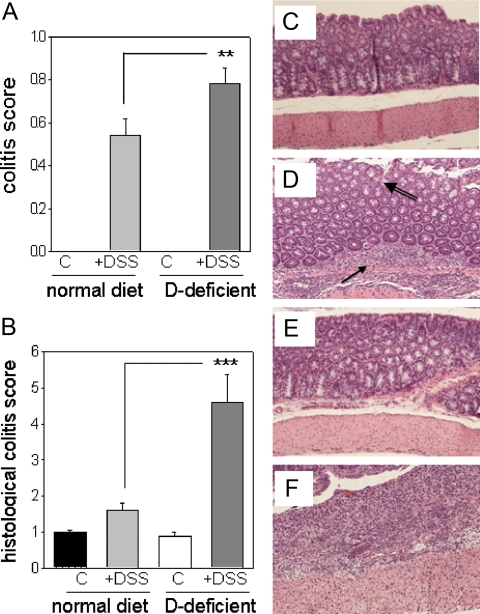
When treated with 2.5% DSS for 7 d followed by 3 d of recovery, mice raised on vitamin D-deficient or vitamin D-sufficient diets lost weight relative to tap water controls. However, this was more pronounced for vitamin D-deficient mice, which showed a maximal weight loss of 12.0% ± 2.2 on d 7 of DSS treatment compared with 8.0% ± 1.2 for vitamin D-sufficient mice (Fig. 2). Analysis of parameters of murine health (Fig. 3A), and colonic histology (Fig. 3B), demonstrated increased severity of colitis in DSS-treated vitamin D-deficient mice relative to DSS-treated vitamin D-sufficient mice. There was no difference in colitis scoring between vitamin D-deficient and vitamin D-sufficient mice treated with tap water (Fig. 3, A and B). H&E staining of colonic sections revealed the presence of severe ulceration, granulation, and inflammation in DSS-treated mice on the vitamin D-sufficient diet (Fig. 3D). This was exacerbated still further in DSS-treated vitamin D-deficient mice, with extensive inflammatory infiltration being evident (Fig. 3F).
Figure 2.
DSS-induced weight loss in mice raised on vitamin D-sufficient and vitamin D-deficient diets. Eight-week-old mice raised on vitamin D-sufficient (normal) or vitamin D-deficient (D-deficient) diets were exposed to either regular tap water (C) or water with 2.5% DSS. Changes in body weight for DSS-treated mice relative to control (tap water) equivalents for mice raised on vitamin D-sufficient (normal,filled circles) and vitamin D-deficient (D-deficient, open circles) diets (% change).
Figure 3.
DSS-induced colitis in mice raised on vitamin D-sufficient and vitamin D-deficient diets. Eight-week-old mice raised on vitamin D-sufficient (normal) or vitamin D-deficient (D-deficient) diets were exposed to either regular tap water (C) or water + DSS. A, Animal health as determined by clinical scores. B, Histological scoring of colitis using colonic tissue sections. C–F, Example H&E staining for vitamin D-sufficient mice receiving tap water (control) (C) and vitamin D-sufficient mice receiving water with 2.5% DSS (control + DSS) (D). Arrow indicates immune infiltrate, double-lined arrow indicates loss of colonic epithelial morphology. E, Vitamin D-deficient mice receiving tap water (D-deficient). F, Vitamin D-deficient mice receiving water with 2.5% DSS (D-deficient + DSS). Values are means ± sem (n = 8). ***, Statistically different from corresponding vitamin D-sufficient mice (normal diet), P < 0.001; **, P < 0.01.
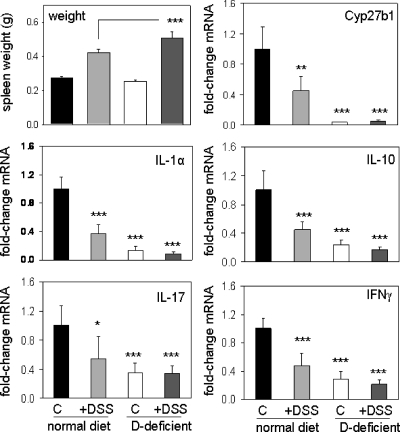
Vitamin D deficiency in mice leads to altered splenic function
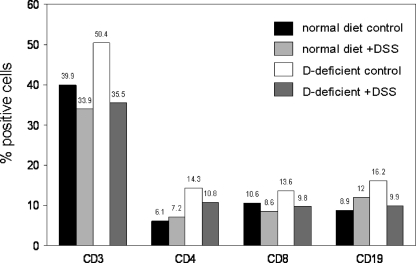
Mice raised on vitamin D-sufficient or vitamin D-deficient diets showed increased splenic weight when treated with DSS (Fig. 4). This was accompanied by altered expression of mRNAs for key inflammatory cytokines such as IL-1, IL-10, IL-17, and IFNγ, as well as expression of the vitamin D-activating enzyme CYP27B1 (Fig. 4). In the absence of DSS, the vitamin D-deficient diet had no effect on splenic weight but nevertheless led to a pronounced suppression of cytokine expression. To investigate further these effects of altered vitamin D status, flow cytometry was carried out using cells isolated from mouse spleens. Data in Fig. 5 revealed elevated levels of CD3+, CD4+, CD8+, and CD19+ cells in spleens from vitamin D-deficient mice in the absence of any DSS challenge.
Figure 4.
Dysregulation of splenic cytokines in mice raised on vitamin D-sufficient and vitamin D-deficient diets. Wild-type C57BL/6 mice were raised on either a normal diet or vitamin D-deficient chow from weaning until 9 wk of age when they were treated either with regular water or water + dextran sodium sulfate (DSS). Data show changes in: 1) spleen weight, 2) mRNA expression for IL-1α, 3) IL-17, 4) Cyp27b1, 5) IL-10, and 6) IFNγ. Values are means ± sem (n = 8). ***, Statistically different from corresponding vitamin D-sufficient mice (normal diet), ***, P < 0.001; **, P < 0.01; *, P < 0.05.
Figure 5.
Severe IBD in vitamin D deficient mice is associated with significantly altered T-cell and B-cell proportions. Splenocytes from mice raised on either a normal or vitamin D-deficient diet treated with or without dextran sodium sulfate (DSS) were analyzed by flow cytometry for expression of T-cell (CD3), B-cell (CD19), and T-cell subset (CD4 and CD8) makers. Data are shown as the percentage of cells positive for each marker.
Analysis of the effects of vitamin D-deficiency on colonic gene expression
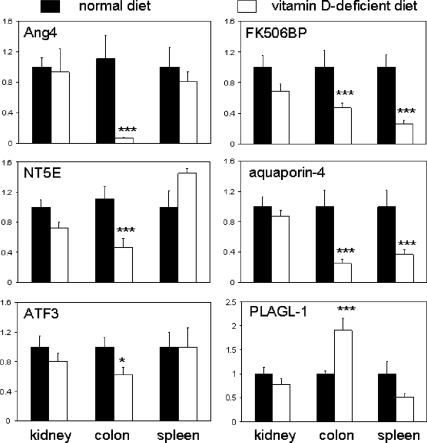
In view of the fact that vitamin D deficiency alone was sufficient to alter splenic gene expression and cell composition (Figs. 4 and 5), we postulated that similar changes could also occur in the colon. Colonic RNA from vitamin D-sufficient and vitamin D-deficient mice (in the absence of DSS treatment) was used in DNA array analyses to define genes with either increased or decreased expression under conditions of impaired vitamin D status. Quantification of n = 4 samples from each group of samples revealed 27 genes with a statistically significant more than 2-fold altered expression in vitamin D-deficient colon tissue relative to vitamin D-sufficient mice (Table 1). Of these, six were assessed by real-time RT-PCR to validate differential mRNA expression, with levels in colonic tissue compared with kidney and spleen (Fig. 6). A further 229 genes showed more than 1.5-fold altered expression in vitamin D-deficient colon tissue relative to vitamin D-sufficient mice (see Supplemental Table 2). Of these, three were validated for differential mRNA expression (Supplemental Fig. 1).
Table 1.
Differential colonic gene expression in vitamin D-deficient vs. vitamin D-sufficient mice
| Gene accession no. | Gene name | Fold change | |
|---|---|---|---|
| 1 | AK003232 | Carbonyl reductase 3 (CBR3) | −3.02 |
| 2 | AV273591 | 5′-Nucleotidase, ecto (NT5E) | −2.84 |
| 3 | AW489155 | AQP4 | −2.49 |
| 4 | NM_054084 | Calcitonin-related polypeptide, β (CALCB) | −2.47 |
| 5 | AY008277 | Chloride channel calcium activated 4 (CLCA4) | −2.41 |
| 6 | NM_024169 | FK506 binding protein 11 (FK506BP) | −2.37 |
| 7 | NM_008012 | Aldo-keto reductase family 1, member B8 (AKR1B8) | −2.36 |
| 8 | NM_009695 | Apolipoprotein C-II (APOC2) | −2.27 |
| 9 | NM_022324 | Stromal cell-derived factor 2-like 1 (SDF2L1) | −2.23 |
| 10 | NM_013473 | Annexin A8 (ANXA8) | −2.22 |
| 11 | BM214338 | RIKEN cDNA 1700019G17 gene | −2.2 |
| 12 | BC019946 | Activating transcription factor 3 (ATF3) | −2.18 |
| 13 | NM_026268 | Dual specificity phosphatase 6 (DUSP6) | −2.17 |
| 14 | AV060923 | Ang4 | −2.17 |
| 15 | BB698398 | GTP cyclohydrolase 1 (GTP-CH1) | −2.16 |
| 16 | NM_007675 | CEA-related cell adhesion molecule 10 (CEACAM1) | −2.16 |
| 17 | BC024598 | Syntaxin binding protein 6 (amisyn) (STXBP6) | −2.1 |
| 18 | BM241342 | Tripartite motif protein 30-like (TRIM30) | −2.08 |
| 19 | NM_010278 | Growth factor independent 1 (GFI1) | −2.07 |
| 20 | L07264 | Heparin-binding EGF-like growth factor (HB-EGF) | −2.07 |
| 21 | NM_009034 | Retinol binding protein 2, cellular (RBP2) | −2.07 |
| 22 | BB376947 | Transcribed locus | −2.07 |
| 23 | NM_011369 | Shc SH2-domain binding protein 1 (SHCBP1) | −2.03 |
| 24 | AK002622 | Phospholamban (PLN) | 2.14 |
| 25 | BB138877 | Ataxin 7-like 4 (ATXN7L4) | 2.14 |
| 26 | AF147785 | Pleiomorphic adenoma gene-like 1 (PLAGL-1) | 2.25 |
| 27 | BQ175713 | RIKEN cDNA D930050A07 gene | 2.36 |
Data show DNA microarray analyses using n = 4 vitamin D-deficient vs. vitamin D-sufficient mice for genes with more than 2-fold differential expression in the colon of vitamin D-deficient vs. vitamin D-sufficient mice. Genes in bold type were validated by real-time RT-PCR analyses.
Figure 6.
Validation of differentially expressed genes in kidney, colon, and spleen tissue from vitamin D-sufficient vs. vitamin D-deficient mice. Real-time RT-PCR analysis of mRNA expression for genes showing differential patterns of colonic expression in vitamin D-deficient mice (open bars) relative to mice on a normal diet (closed bars). Genes were selected from those originally identified by DNA array analysis as showing more than 2-fold change in expression (see genes highlighted in bold type in Table 1). PLAGL-1, Pleiomorphic adenoma gene-like 1; ATF3, activating transcription factor 3; NT5E, 5′ nucleotidase, ecto; FK506BP, FK506 binding protein 11. Values are represented as mean ± sem (n = 8) and significantly different as follows: ***, statistically different from corresponding vitamin D-sufficient mice (normal diet), P < 0.001; *, P < 0.05.
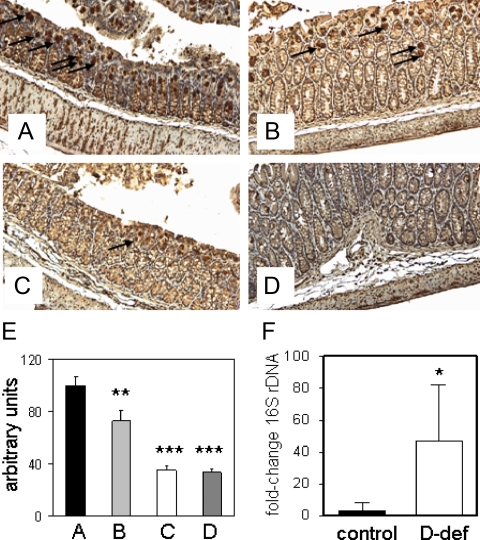
Colonic expression of Ang4 is decreased in vitamin D-deficient mice
To further characterize altered expression of the antimicrobial protein Ang4 associated with vitamin D deficiency, immunohistochemistry was carried out using sections of mouse colon (Fig. 7, A–D). Quantification of Ang4 expression showed that colonic expression of this protein was down-regulated by treatment with DSS (Fig. 7E). However, staining for Ang4 was even lower in vitamin D-deficient mice receiving either regular tap water (control) or DSS (Fig. 7E).
Figure 7.
The effects of vitamin D deficiency and DSS treatment on colonic expression of Ang4 protein and colonic bacterial load. A–D, Immunohistochemical analysis of colonic Ang4 protein expression in vitamin D-sufficient or vitamin D-deficient mice. A, Vitamin D-sufficient mice receiving tap water (control). B, Vitamin D-sufficient mice receiving water with 2.5% DSS (+DSS). C, Vitamin D-deficient mice receiving tap water (d-def). D, Vitamin D-deficient mice receiving water with 2.5% DSS (D-def + DSS). E, quantification of Ang4 expression (% expression relative to control samples). F, Bacterial load in vitamin D-sufficient (control) and vitamin D-deficient (D-def) colon tissue quantified by real-time PCR for 16S rDNA. All quantification using n = 6 separate colons. Values are represented as mean ± sem. ***, Statistically different from vitamin D-sufficient control mice, P < 0.001; **, P < 0.01; *, P < 0.05.
Vitamin D-deficient mice show increased bacterial infiltration of colonic tissue
Because Ang4 is known to exhibit bacteriocidal activity in the GI tract (31), we reasoned that decreased expression of this protein in the colon of vitamin D-deficient mice might lead to homeostatic dysregulation of enteric bacteria in this tissue. Analysis of the presence of 16S rDNA in colon tissue revealed 50-fold ± 20.0 higher levels of bacterial infiltration in vitamin D-deficient mice compared with vitamin D-sufficient mice (Fig. 7F).
Discussion
Immunomodulatory properties of the active form of vitamin D, 1,25(OH)2D3, have been recognized for almost 25 yr, but it is only recently that these actions have been linked to variations in vitamin D status. Specifically, the induction of CYP27B1 and VDR after toll-like receptor (TLR) induction of monocytes has revealed a sensitive intracrine system that is entirely dependent on the substrate for CYP27B1, namely 25OHD3 (13,32). In this instance, the ability of monocytes to launch TLR-mediated innate immune activity via the antimicrobial protein cathelicidin correlated with donor serum vitamin D status (25OHD3 concentration) (13,32). Based on these and other observations, it has been postulated that susceptibility to infectious diseases such as tuberculosis may be increased in patients with suboptimal vitamin D status (33). In humans, vitamin D deficiency has also been associated with IBD (1,2,3,4,5). However, the extent to which this is a cause of IBD or a consequence has yet to be fully clarified. To determine whether impaired vitamin D status, as opposed to ablation of VDR or CYP27B1 gene expression, alters susceptibility to IBD, we have used a mouse model of dietary vitamin D deficiency in combination with an experimental colitis protocol. In addition, DNA array analysis to define genes specifically dysregulated in the colon of vitamin D-deficient mice has indentified a novel innate immunity target for vitamin D that may play a pivotal role in microbiota homeostasis and susceptibility to IBD.
Previous studies have shown that dysregulation of vitamin D metabolism (29) or receptor signaling (24,25,26,27,28) predisposes mice to experimental IBD. The most commonly employed model for these studies has been DSS-induced colitis (24,28,29). Although not fully representative of human IBD forms such as Crohn's disease, DSS-induced colitis involves prevalent innate immune responses (34). Given the link between vitamin D and stimulation of innate immune activity (11,12), we reasoned that this would be an appropriate model for analysis of response to altered vitamin D status. In the current study, we have shown that simple deficiency of the main circulating form of vitamin D, 25OHD3, has similar effects on DSS-induced colitis as VDR or CYP27B1 gene knockout. Crucially, even in the absence of experimental colitis, vitamin D-deficient mice showed dysregulated colonic containment of enteric bacteria, suggesting that this may be a key mechanism that predisposes these mice to subsequent colitis. Dietary vitamin D restriction in mice resulted in serum 25OHD3 concentrations that were less than 20 ng/ml (50 nm) and therefore consistent with human parameters for vitamin D “deficiency” (35,36). Although the serum 25OHD3 levels in the vitamin D-deficient mice were low relative to normal human values, it is important to recognize that as many as 70% of adult patients with Crohn's disease have been reported to have circulating levels of 25OHD3 that are lower than 15 ng/ml (37.5 nm) (reviewed in Ref. 3). Moreover, although serum levels of active 1,25(OH)2D3 were decreased in the vitamin D-deficient mice relative to the vitamin D-sufficient mice, they remained within accepted normal ranges for this metabolite (20–65 pg/ml, 50–160 pm in humans). Adequacy of 1,25(OH)2D3 is required for the normal endocrine function of vitamin D with respect to renal and bone mineral homeostasis. Prolonged exposure to the vitamin D-restricted diet will eventually lead to 1,25(OH)2D3 deficiency, hypocalcemia, alterations in parathyroid function, and aberrant bone function. It was therefore important to note that within the time frame of this study, the vitamin D-deficient animals showed no significant alteration in serum calcium levels, consistent with normal endocrine function of vitamin D.
As with VDR and CYP27B1 knockout mice, the vitamin D-deficient animals were more susceptible to DSS-induced colitis. In view of the fact that we have previously demonstrated expression of the vitamin D-activating enzyme CYP27B1 in human (22,23) and mouse colon (29), it is tempting to speculate that the effect of low 25OHD3 levels is manifested through decreased localized generation of 1,25(OH)2D3 within the colon itself. However, as outlined above, it is also possible that decreased systemic levels of 1,25(OH)2D3 will contribute to colitis susceptibility through as yet unknown mechanisms. The fact that animal health and histological consequences of vitamin D deficiency were only observed in DSS-treated mice suggests that the effects of low 25OHD3 levels might only be of significance after an immune challenge. This would be consistent with the TLR-mediated mechanism we have described previously for monocytes, where CYP27B1 and VDR expression are enhanced to facilitate intracrine response to 25OHD3 (13,32). Thus, it is possible that impaired vitamin D status is particularly important after the onset of inflammation, with locally synthesized 1,25(OH)2D3 acting as a modulator of adaptive immune responses (11). Studies using VDR knockout mouse have shown clearly that different facets of T-cell function are dysregulated in these animals after the induction of colitis (26,27,28), including the aberrant colonic homing of intraepithelial lymphocytes (25).
Although animal health and histological scoring suggested that effects of vitamin D deficiency were only manifested in animals with induced colitis, analysis of spleen tissue provided a different perspective. Data for splenic gene expression and lymphocyte composition indicated that vitamin D deficiency alone was sufficient to produce a significant change in immune function at this site. The precise mechanism by which this occurs is unclear. However, the spleen data prompted a more detailed analysis of the effects of vitamin D deficiency with the colon itself. By using DNA array analysis for multiple colon tissue samples, we were able to identify genes that were consistently up-regulated or down-regulated in vitamin D-deficient mice in the absence of any DSS-induced tissue damage or inflammation.
Genes down-regulated in the colon of vitamin D-deficient mice included 5′ ectonucleotidase (NT5E/CD73), a surface marker of regulatory T cells that imparts a specific biochemical signature, characterized by adenosine generation, that has functional relevance for cellular immunoregulation (37). Expression of CD73 by two distinct CD4+ T-cell subsets, uncommitted primed precursor T helper and regulatory T cells, confers a potential antiinflammatory function by generating adenosine, dampening excessive immune responsiveness (38). Suppression of the colonic epithelial water channel aquaporin4 (Aqp4) in the colon of vitamin D-deficient mice is interesting given that previous reports have also documented suppression of Aqp4 in murine models of colitis and human patients with ulcerative colitis, Crohn's disease, and infectious colitis (39). However, it is important to recognize that decreased colonic expression of Aqp4 has also been linked to other GI disorders such as allergic diarrhea (40). Recent studies have identified adrenomedullin as potent antiinflammatory factor with the capacity to deactivate the intestinal inflammatory response and restore mucosal immune tolerance at multiple levels (41). It is therefore noteworthy that colonic expression of this hormone was significantly down-regulated in vitamin D-deficient mice. Conversely, specific up-regulation of the IL-7 receptor (IL-7R) under conditions of vitamin D deficiency is interesting in view of the essential role of IL-7 in the development and persistence of chronic colitis (42) and the fact that mucosal CD4+ T cells expressing high levels of IL-7R are intimately involved in promoting chronic colitis (43). Thus, it is tempting to speculate that up-regulation of colonic IL-7R expression in vitamin D-deficient mice may act to prime subsequent inflammatory activity after the experimental induction of colitis. Collectively, these observations suggest that vitamin D deficiency alone is sufficient to alter expression of key colonic genes. This, in turn, may increase susceptibility to colitis or enhance severity of the disease.
Ang4 was also specifically suppressed in colon tissue from vitamin D-deficient mice. Although classically implicated in tumor-associated angiogenesis, Ang4 is also known to promote bactericidal innate immune activity against intestinal microbes (31). Recent studies by other groups have implicated aberrant innate immune handling of enteric microbiota as an initiator of the adaptive immune damage associated with Crohn's disease (44). Thus, in the current study, we focused on Ang4, which is strongly expressed in the colon (31), but which is also a key component of Paneth cells, specialized crypt cells in the small intestine, which secrete lysosomal and antimicrobial proteins (31). Paneth cells play an essential role in regulating GI microbial status by maintaining submucosal autophagy, lysosomal activity, and elimination of pathogens (45,46). Several recent studies have proposed that dysregulation of innate immunity is a key factor in the pathogenesis of Crohn's disease (45,46,47,48). In view of the link between vitamin D status and antibacterial activity in various cell types, it was interesting to note the suppression of Ang4 mRNA and protein in colon tissue from vitamin D-deficient mice (Figs. 6 and 7). Previous studies demonstrating the induction of Ang4 by commensal bacteria (31) are consistent with a role for this protein in controlling access of microbes to the gut epithelium. Thus, it is possible that localized colonic metabolism of 25OHD3 to 1,25(OH)2D3 supports the regulation of enteric bacteria by promoting Ang4 expression, with this activity being compromised under conditions of low vitamin D status. The mechanism by which vitamin D influences expression Ang4 expression has yet to be defined. Putative vitamin D response elements are located within the promoter for the Ang4 gene (data not shown), but as yet, it is unclear whether there is direct transcriptional regulation at these sites. Indeed, given recent studies showing that 1,25(OH)2D3-induced expression of the antimicrobial protein β-defensin 4 involves up-regulation of the NOD2 intracellular pathogen-recognition protein (17), it is possible that the link between vitamin D and Ang4 is also complex.
In conclusion, we hypothesize that impaired vitamin D status may predispose to IBD as a consequence of dysregulated innate immune surveillance of enteric bacteria. In the animal model of colitis used in the current study, the effects of vitamin D deficiency exacerbated DSS-induced colonic inflammation. Because primates express the product of a single angiogenin gene, it is unclear whether humans will demonstrate a similar pathway linking vitamin D deficiency, angiogenin gene, and IBD. However, in view of the potent effects of vitamin D in promoting other multifunctional antibacterial proteins such as cathelicidin at other barrier sites, including the skin (49) and placenta (50,51), it seems likely that similar responses will occur in the GI tract. In this way, management of microbiota homeostasis may be an important facet of vitamin D function in the gut. Given the current interest in how the enteric bacteria can influence colonic immunity, it is tempting to speculate that alterations in vitamin D status may be a key factor in the disruption of normal microbiota function, with important consequences for the development of IBD.
Supplementary Material
Acknowledgments
We thank Dr. Lora Hooper (University of Texas Southwestern Medical Center, Dallas, TX) for help in providing antiserum and advice for imunohistochemical analysis of Ang4 expression.
Footnotes
This work was supported by the National Institutes of Health Grant AR050626 (to M.H.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 14, 2010
Abbreviations: Ang4, Angiogenin-4; Aqp4, aquaporin4; DSS, dextran sodium sulfate; GI, gastrointestinal; IBD, inflammatory bowel disease; IFN, interferon; IL-7R, IL-7 receptor; NOD2, nucleotide-binding oligomerization domain-containing 2; 25OHD3, 25-hydroxyvitamin D3; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; TLR, toll-like receptor; VDR, vitamin D receptor.
References
- Harries AD, Brown R, Heatley RV, Williams LA, Woodhead S, Rhodes J 1985 Vitamin D status in Crohn's disease: association with nutrition and disease activity. Gut 26:1197–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagianos K, Bector S, McConnell J, Bernstein CN 2007 Nutrition assessment of patients with inflammatory bowel disease. J Parenter Enteral Nutr 31:311–319 [DOI] [PubMed] [Google Scholar]
- Pappa HM, Grand RJ, Gordon CM 2006 Report on the vitamin D status of adult and pediatric patients with inflammatory bowel disease and its significance for bone health and disease. Inflamm Bowel Dis 12:1162–1174 [DOI] [PubMed] [Google Scholar]
- Pappa HM, Gordon CM, Saslowsky TM, Zholudev A, Horr B, Shih MC, Grand RJ 2006 Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics 118:1950–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen J, Falch JA, Mowinckel P, Aadland E 2002 Vitamin D status, parathyroid hormone and bone mineral density in patients with inflammatory bowel disease. Scand J Gastroenterol 37:192–199 [DOI] [PubMed] [Google Scholar]
- Gilman J, Shanahan F, Cashman KD 2006 Determinants of vitamin D status in adult Crohn's disease patients, with particular emphasis on supplemental vitamin D use. Eur J Clin Nutr 60:889–896 [DOI] [PubMed] [Google Scholar]
- Leichtmann GA, Bengoa JM, Bolt MJ, Sitrin MD 1991 Intestinal absorption of cholecalciferol and 25-hydroxycholecalciferol in patients with both Crohn's disease and intestinal resection. Am J Clin Nutr 54:548–552 [DOI] [PubMed] [Google Scholar]
- Lo CW, Paris PW, Clemens TL, Nolan J, Holick MF 1985 Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr 42:644–649 [DOI] [PubMed] [Google Scholar]
- Davies M, Mawer EB, Krawitt EL 1980 Comparative absorption of vitamin D3 and 25-hydroxyvitamin D3 in intestinal disease. Gut 21:287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie WD, Miller N, Rogala L, Bernstein CN 2008 Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD cohort study. Am J Gastroenterol 103:1451–1459 [DOI] [PubMed] [Google Scholar]
- Adams JS, Hewison M 2008 Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab 4:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewison M 2008 Vitamin D and innate immunity. Curr Opin Investig Drugs 9:485–490 [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL 2006 Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773 [DOI] [PubMed] [Google Scholar]
- Liu PT, Modlin RL 2008 Human macrophage host defense against Mycobacterium tuberculosis. Curr Opin Immunol 20:371–376 [DOI] [PubMed] [Google Scholar]
- Mathieu C, Gysemans C, Giulietti A, Bouillon R 2005 Vitamin D and diabetes. Diabetologia 48:1247–1257 [DOI] [PubMed] [Google Scholar]
- Cantorna MT 2008 Vitamin D and multiple sclerosis: an update. Nutr Rev 66:S135–S138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, Dionne S, Servant MJ, Bitton A, Seidman EG, Mader S, Behr MA, White JH 2010 Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-β defensin 2 innate immune pathway defective in Crohn's disease. J Biol Chem 285:2227–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nuñez G, Cho JH 2001 A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411:603–606 [DOI] [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G 2001 Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411:599–603 [DOI] [PubMed] [Google Scholar]
- Cuthbert AP, Fisher SA, Mirza MM, King K, Hampe J, Croucher PJ, Mascheretti S, Sanderson J, Forbes A, Mansfield J, Schreiber S, Lewis CM, Mathew CG 2002 The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology 122:867–874 [DOI] [PubMed] [Google Scholar]
- Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, Kilby MD, Moss PA, Chakraverty R 2003 Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol 170:5382–5390 [DOI] [PubMed] [Google Scholar]
- Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M 2001 Extrarenal expression of 25-hydroxyvitamin d(3)-1 α-hydroxylase. J Clin Endocrinol Metab 86:888–894 [DOI] [PubMed] [Google Scholar]
- Abreu MT, Kantorovich V, Vasiliauskas EA, Gruntmanis U, Matuk R, Daigle K, Chen S, Zehnder D, Lin YC, Yang H, Hewison M, Adams JS 2004 Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn's disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut 53:1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC 2008 Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 294:G208–G216 [DOI] [PubMed] [Google Scholar]
- Yu S, Bruce D, Froicu M, Weaver V, Cantorna MT 2008 Failure of T cell homing, reduced CD4/CD8αα intraepithelial lymphocytes, and inflammation in the gut of vitamin D receptor KO mice. Proc Natl Acad Sci USA 105:20834–20839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT 2003 A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol 17:2386–2392 [DOI] [PubMed] [Google Scholar]
- Froicu M, Zhu Y, Cantorna MT 2006 Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology 117:310–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froicu M, Cantorna MT 2007 Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol 8:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Nguyen L, Chun RF, Lagishetty V, Ren S, Wu S, Hollis B, DeLuca HF, Adams JS, Hewison M 2008 Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology 149:4799–4808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund B, Lehr HA, Fantuzzi G 2002 Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology 122:2011–2025 [DOI] [PubMed] [Google Scholar]
- Hooper LV, Stappenbeck TS, Hong CV, Gordon JI 2003 Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol 4:269–273 [DOI] [PubMed] [Google Scholar]
- Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M 2009 Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol 182:4289–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JS, Liu PT, Chun R, Modlin RL, Hewison M 2007 Vitamin D in defense of the human immune response. Ann NY Acad Sci 1117:94–105 [DOI] [PubMed] [Google Scholar]
- Wirtz S, Neurath MF 2007 Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev 59:1073–1083 [DOI] [PubMed] [Google Scholar]
- Holick MF 2009 Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol 19:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF 2007 Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC 2007 Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204:1257–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR 2006 T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol 177:6780–6786 [DOI] [PubMed] [Google Scholar]
- Hardin JA, Wallace LE, Wong JF, O'Loughlin EV, Urbanski SJ, Gall DG, MacNaughton WK, Beck PL 2004 Aquaporin expression is downregulated in a murine model of colitis and in patients with ulcerative colitis, Crohn's disease and infectious colitis. Cell Tissue Res 318:313–323 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kuramoto H, Kadowaki M 2007 Downregulation in aquaporin 4 and aquaporin 8 expression of the colon associated with the induction of allergic diarrhea in a mouse model of food allergy. Life Sci 81:115–120 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Delgado M 2006 Therapeutic effect of urocortin and adrenomedullin in a murine model of Crohn's disease. Gut 55:824–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsuka T, Kanai T, Nemoto Y, Makita S, Okamoto R, Tsuchiya K, Watanabe M 2007 IL-7 is essential for the development and the persistence of chronic colitis. J Immunol 178:4737–4748 [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Yajima T, Tanabe M, Fukui K, Okada E, Okamoto R, Oshima S, Nakamura T, Kanai T, Uehira M, Takeuchi T, Ishikawa H, Hibi T, Watanabe M 2003 Mucosal T cells expressing high levels of IL-7 receptor are potential targets for treatment of chronic colitis. J Immunol 171:1556–1563 [DOI] [PubMed] [Google Scholar]
- Packey CD, Sartor RB 2009 Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr Opin Infect Dis 22:292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman NH, Underwood MA, Bevins CL 2007 Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol 19:70–83 [DOI] [PubMed] [Google Scholar]
- Klionsky DJ 2009 Crohn's disease, autophagy, and the Paneth cell. N Engl J Med 360:1785–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersemann M, Wehkamp J, Fellermann K, Stange EF 2008 Crohn's disease—defect in innate defence. World J Gastroenterol 14:5499–5503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehkamp J, Schmid M, Stange EF 2007 Defensins and other antimicrobial peptides in inflammatory bowel disease. Curr Opin Gastroenterol 23:370–378 [DOI] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, Coda AB, Büchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zügel U, Bikle DD, Modlin RL, Gallo RL 2007 Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest 117:803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Kaplan AT, Low J, Nguyen L, Liu GY, Equils O, Hewison M 2009 Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol Reprod 80:398–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KN, Nguyen L, Chan J, Innes BA, Bulmer JN, Kilby MD, Hewison M 2006 Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol Reprod 75:816–822 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.