Abstract
Bisphenol-A (BPA) is an endocrine-disrupting chemical used in the production of plastic food and beverage containers, leading to ubiquitous low-dose human exposure. It has been suggested that exposure to even low doses of BPA during development may be associated with increased susceptibility to obesity and diabetes later in life. Despite growing public concern, the existing empirical data are equivocal, prompting The Endocrine Society, the National Institute of Environmental Health Sciences, and others to call for further research. In this study, we tested the hypothesis that perinatal exposure to an ecologically relevant dose of BPA (1 part per billion via the diet) results in increased susceptibility to high-fat diet-induced obesity and glucose intolerance in adult CD-1 mice. The data did not support this hypothesis. In agreement with previous reports, we find that weanling mice exposed to BPA during gestation and lactation are heavier compared with control mice. We also find that BPA mice are longer than controls at 4 wk of age, but these differences are no longer apparent when the mice reach adulthood, even when tested on a high-fat diet. We conclude that this larger size-for-age represents a faster rate of growth early in development rather than an obese, diabetic phenotype in adulthood.
Perinatal exposure to an ecologically relevant dose of bisphenol-A via the diet does not increase susceptibility to diet-induced obesity or glucose intolerance in mice.
Bisphenol-A (BPA) is a chemical plasticizer and xenoestrogen used in the production of polycarbonate plastics and epoxy resins. Human exposure to BPA is ubiquitous at low levels and occurs primarily through the diet, including by migration from food and beverage containers (1,2). Although it is widely agreed that high-dose exposure to BPA can have adverse physiological effects, the consequences of ecologically relevant levels of exposure have been contentious (3,4,5). Low-dose exposure is of greatest concern at early developmental stages because the relative level of exposure as a function of body weight is higher for fetuses and infants compared with adults, and because small changes in circulating estrogen during normal development have important organizational effects (3). Consequently, even small changes in estrogenic tone induced by low-dose exposure to BPA could potentially lead to large phenotypic effects in adulthood.
Diethylstilbestrol (DES) is an estrogen receptor (ER) agonist that was once widely prescribed to expectant mothers for the prevention of miscarriage. The use of DES by pregnant women was discontinued when deleterious physiological consequences, primarily affecting the reproductive organs, were observed in their adult children (6,7,8). Experiments in rodents have indicated that exposure to relatively low doses of DES during gestation and lactation can lead to malformations and cancers of the reproductive tract in adulthood (9,10). In addition to its negative effects on the developing reproductive tract, it has also been reported that low-dose perinatal exposure to DES is associated with obesity in adult mice (11,12).
Because both BPA and DES are ER agonists, it has been proposed that the DES system may provide a predictive model for potential risks of low-dose environmental BPA exposure (3,12,13,14). Several groups have reported that low-dose developmental exposure to BPA, similar to exposure to DES, has adverse consequences for the developing reproductive tract in adult rodents (14,15,16,17). However, other groups have observed no such effects (18). It has also been reported that pre- and perinatal exposure to low-dose BPA leads to increased body weight in adult rodents (19,20,21), although it is not clear to what extent this increased growth reflects an obese phenotype. Again, other groups report either no effect (15,17,22,23) or reduced adult body weight (24) in similar experiments (3,4,5,25).
In response to public worry over potential consequences of environmental exposure to BPA, the National Toxicology Program (NTP) Center for the Evaluation of Risks to Human Reproduction (CERHR) recently conducted an evaluation of the potential for BPA to cause adverse effects on reproduction and development (2008). The NTP report (4,5) concluded that the existing data regarding low-dose exposure to BPA were inconsistent, that interpretation was often hampered by inadequate experimental design and data analysis, and that further study was warranted. Regarding the connection between BPA and obesity, the report states that “there is currently insufficient evidence to conclude that BPA exposure during development predisposes laboratory animals to develop obesity or metabolic diseases such as diabetes, later in life” (see page 30 in Ref. 5).
In the present experiments, we tested the hypothesis that exposure to DES and/or to ecologically relevant levels of BPA during gestation and lactation leads to the development of obesity and diabetes in adult mice and that this effect is amplified under high-fat feeding conditions.
Materials and Methods
Animals, breeding, and treatment diets
All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati. CD-1 mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and housed in polycarbonate cages with polycarbonate water bottles and corncob bedding under 14-h light, 10-h dark cycle conditions with food and water available ad libitum. All animals were fed AIN93G control diet before breeding. Three females and one male were placed together in one cage for breeding. Mating was confirmed by the appearance of a vaginal plug on the following morning, designated embryonic d 0 (E0). Plugged females were housed separately and fed one of three treatment diets: 1) unmodified AIN93G control diet, 2) AIN93G containing 4 μg/kg · diet [4 parts per billion (ppb) DES, approximately 1 μg/kg · body weight (bw)/d, assuming a 20-g female consumes 5-g diet per day], or 3) AIN93G containing an ecologically relevant dose of BPA (4), [1 μg/kg · diet (1 ppb) BPA, approximately 0.25 μg/kg · bw/d].
Each diet was a purified, open-source diet formulated by Research Diets (New Brunswick, NJ). BPA (>99% pure; Sigma-Aldrich, St. Louis, MO) or DES (99% pure, Sigma-Aldrich) was first dissolved in a small volume of methanol. The appropriate amount of this solution was then pipetted into 1.5-kg oil and stirred overnight. The treated oils were provided to Research Diets for incorporation into the AIN93G-based diets.
Dams remained on the treatment diets until their pups were weaned at 21 d of age. Food intake was measured weekly from mating to weaning. Because psychogenic stress to dams and pups during the perinatal phase leads to differences in metabolic endpoints in adulthood (26), we were concerned with minimizing stress to the dams and pups before weaning. Therefore, to limit handling, dams were not weighed during pregnancy and lactation. Litters were recorded at birth, but pups were not weighed before weaning.
Weaning
On postnatal d 21 (p21), 1455 pups from 119 litters were sexed and weaned. Pups from litters of eight or fewer and 15 or greater were excluded from the study. One male and one female pup from each of the remaining litters (∼30 per maternal treatment) were chosen at random to continue in the long-term study. We accounted for variance due to differences in litter size by including it as a covariate in our statistical model, as suggested by Chapin (4,5). Sokal and Rohlf (27) state that analysis of covariance (ANCOVA) “attempts to simulate the results that would have been obtained had a constant value been used for the independent covariate.” We favored this method over culling the litters because it minimizes perinatal stress, minimizes the potential for perinatal overnutrition, and because it accounts for variance due to in utero differences in litter size. Each of these factors can affect the endpoints measured in this study (26,28,29).
These pups were weighed and singly housed in polycarbonate cages with corncob bedding and an automatic watering system under 12-h light, 12-h dark cycle conditions. All weanling mice were fed a purified low butter-fat diet (LFD) (10% fat by weight) (Research Diets) until they were 9 wk old. LFD intake was measured weekly from 4–7 wk of age; pelleted diets were placed on top of the wire-bar lids, and the lids plus food were weighed on a weekly basis. Body weight was measured weekly from wk 4–7 of age and again at 9 wk.
Body length
Body length, measured from nose to anus, was determined in a randomly chosen subset of 4-wk-old mice (n = 10 per treatment per sex).
Body composition
Magnetic resonance imaging (MRI) was performed on all mice at 7 wk of age to determine fat and lean body composition, using an Echo MRI whole-body composition analyzer (EchoMedical Systems, Houston, TX). Each mouse was tested in duplicate, and readings were accepted if they differed by less than 10%.
Glucose tolerance tests (GTTs)
GTTs were performed at 8 wk of age. Mice were fasted for 16 h, beginning 2 h into the dark phase of the light-dark cycle (as in Refs. 30,31,32,33). After a baseline blood sample was taken (0 min), 25% d-glucose (Phoenix Pharmaceutical, St. Joseph, MO) was injected ip. Blood glucose was measured at 0, 15, 30, 45, 60, and 120 min after glucose administration on duplicate samples using Accu-chek glucometers and test strips (Roche, Indianapolis, IN). All blood samples were obtained from the tip of the tail vein of freely moving mice. Because lean mass accounts for the majority of glucose uptake during a GTT, each male mouse received a fixed dose of glucose (34) determined from body composition parameters measured the week before the GTT. The average lean mass for all males (across groups) was calculated, and each male received a dose of ip glucose equal to 1.75 g/kg average lean mass. Likewise, each female mouse received a fixed dose of glucose. The average lean mass for all females (across groups) was calculated, and each female received a dose of glucose equal to 1.75 g/kg average lean mass. There were no differences in lean mass among any of the groups for either males or females. Female cycles were not monitored, and consequently, GTTs were conducted irrespective of estrous phase. Mice were excluded from the data analysis if they did not exhibit a rise in blood glucose of greater than 20 mg/dl in the first 15 min after injection or if they exhibited diarrhea because such symptoms indicate that the glucose injection did not hit the ip cavity. Given the practical constraints involved with measuring insulin in such a large group of animals, we do not report the corresponding insulin levels.
High butter-fat diet (HFD)
At 9 wk of age, the diet of half of the mice in each group was changed to a purified HFD (40% fat by weight; Research Diets) that is otherwise matched to the LFD in micronutrient content. The other half continued on the LFD. Food intake and body weight were measured weekly from 9–14 wk of age. Body composition of 14-wk-old mice was measured again with MRI, and at 15 wk of age, GTTs were repeated.
Statistical analyses
Data were analyzed using STATISTICA6 (StatSoft, Tulsa, OK) or GraphPad (Prism, San Diego, CA). Length of gestation, sex ratio, and litter size were analyzed using ANOVA. The remaining data were analyzed using the appropriate ANCOVA model, with litter size included as the covariate, followed by Tukey post hoc tests. Other potential litter effects were avoided in this study because only one individual of each sex from each litter was included in the experiment.
Results
Mating through weaning (e0–p21)
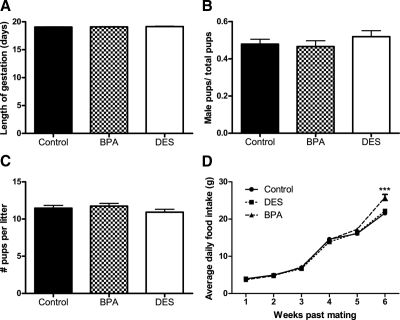
There was no effect of maternal diet on length of gestation, sex ratio, or litter size (Fig. 1, A–C). Likewise, there was no effect of maternal diet on maternal food intake from e0 to p14 (Fig. 1D). Cages containing mothers and pups exposed to BPA-supplemented diet consumed more calories from p14 to p21 compared with both the control (P < 0.001) and DES (P < 0.001) groups (Fig. 1D), and this remained significant when accounting for the effect of litter size (P < 0.000001).
Figure 1.
Effects of perinatal exposure to BPA and DES on traits of dams and their litters. Length of gestation in days (e0–p0) (A), sex ratio (B), and litter size (C) of dams eating either control diet (n = 31) or diet containing 4-ppb BPA (n = 34) or 1-ppb DES (n = 32). There were no significant differences among the groups for these endpoints (ANOVA, P > 0.05). D, Average daily food intake of dams (and their pups) exposed to BPA is higher at wk 6 compared with mice eating the control or DES diets [repeated-measures (RM) ANCOVA followed by Tukey post hoc tests: P (litter size) <0.000001, P (time × maternal treatment) <0.000001; within wk 6, P (BPA vs. control) <0.001, P (BPA vs. DES) <0.001]. ***, P < 0.001.
Traits of weanlings
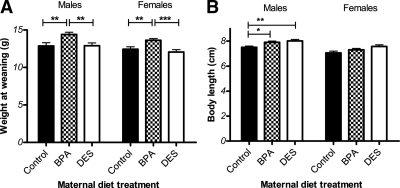
Pups whose dams had been exposed to BPA were heavier at weaning than either the control pups (P < 0.01) or the pups whose dams had been exposed to DES (P < 0.001) (Fig. 2A), and this remained the case after accounting for the effect of litter size (P < 0.000001). Body length was measured in a subset of pups at 4 wk of age. Pups exposed to either BPA or to DES tended to be longer compared with control pups (Fig. 2B). Among males, this difference was statistically significant for both BPA (P < 0.05) and DES (P < 0.01) pups, and litter size did not significantly predict body length. Among females, the trend was similar, but the effect of treatment group did not reach significance (P < 0.098) when litter size (P < 0.059) is included in the model.
Figure 2.
Body size of weanlings. A, Male and female BPA pups were heavier at 3 wk of age compared with control and DES pups [ANCOVA followed by Tukey post hoc tests: for males, P (maternal treatment) <0.000001, P (BPA vs. control) <0.01, P (BPA vs. DES) <0.01, P (litter size) <0.000001; for females, P (maternal treatment) <0.000001, P (BPA vs. control) <0.01, P (BPA vs. DES) <0.001, P (litter size) <0.000001]. B, Body length was measured in a subset of animals (n = 10 sex/maternal treatment) at 4 wk of age. Among males, control mice were significantly shorter than both BPA and DES mice. Among females, the trend was similar, but the differences were not significant when litter size was included in the model [ANCOVA followed by Tukey post hoc tests: for males, P (maternal treatment) <0.01, P (control vs. BPA) <0.05, P (control vs. DES) <0.01, P (litter size) <0.059; for females, P (maternal treatment) <0.098, P (litter size) <0.05]. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Growth while the pups were maintained on LFD, 3–9 wk of age
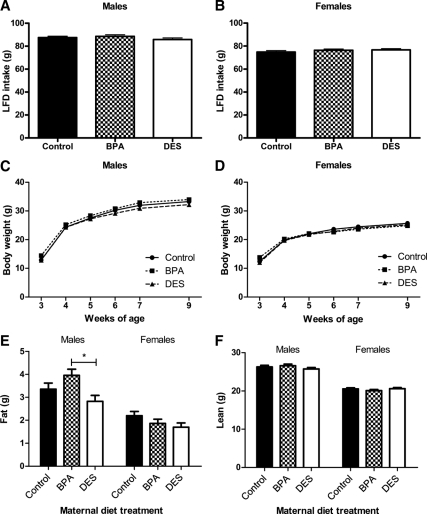
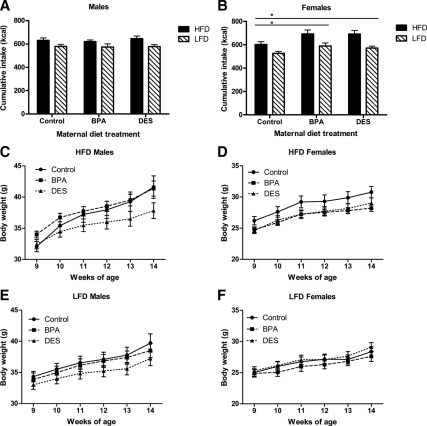
Maternal treatment had no effect on food intake (Fig. 3, A and B) or body weight (Fig. 3, C and D) among male or female mice. Litter size did not predict food intake; however, among both males and females, litter size was significantly associated with body weight (P < 0.01). Among males, mice exposed to BPA during development had more body fat at 7 wk of age compared with mice exposed to DES (P < 0.05), but there were no significant differences between animals in either treatment group compared with control mice (Fig. 3E). There were no differences among the groups in lean body mass (Fig. 3F). Among 7-wk-old females, maternal diet had no effect on body composition (Fig. 3, E and F). These outcomes do not change when body composition is expressed relative to body weight (data not shown).
Figure 3.
Growth on LFD from 4–9 wk of age. Cumulative LFD intake by male (A) and female (B) mice did not differ according to maternal treatment [ANCOVA: P (maternal treatment) >0.05, P (litter size) >0.05]. C, Among males, body weight differences among the maternal treatment groups were lost after weaning [repeated-measures (RM) ANCOVA: P (maternal treatment) >0.05, P (litter size) <0.01, P (litter size × time) <0.01]. D, Among females, body weight differences among the maternal treatment groups were lost after weaning [RM ANCOVA: P (maternal treatment) >0.05, P (litter size) <0.01, P (litter size × time) <0.001, P (maternal treatment × time) <0.000001]. E, At 7 wk of age, BPA males had more body fat than DES males [ANCOVA followed by Tukey post hoc tests: P (maternal treatment) <0.05, P (BPA vs. DES) <0.05, P (litter size) >0.05]. At 7 wk of age, there were no body fat differences among the females [ANCOVA: P (maternal treatment) >0.05, P (litter size) >0.05]. F, There were no differences in lean mass among the groups for either sex [ANCOVA: P (maternal treatment) >0.05, P (litter size) >0.05]. *, P < 0.05.
Glucose tolerance at 8 wk of age
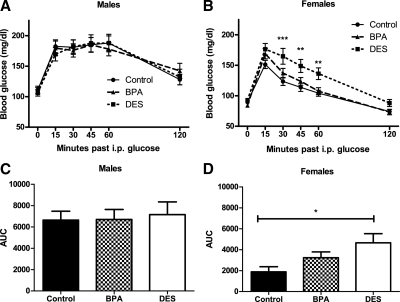
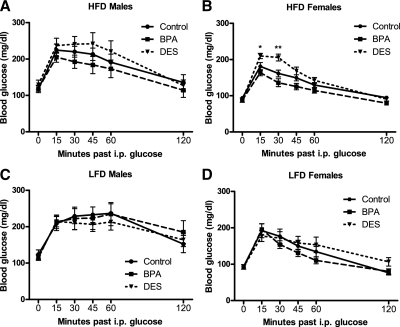
As we (35) and others (reviewed in Ref. 36) have reported elsewhere, female mice were relatively more glucose tolerant than males. There were no differences in glucose tolerance among male mice (Fig. 4A). Consistent with a previously reported trend (12), female mice exposed to DES during development were relatively glucose intolerant (P < 0.01; Fig. 4, B and D), and BPA females responded similarly to controls. Litter size was not associated with glucose tolerance in either sex.
Figure 4.
Blood glucose in 8-wk-old male and female mice after an ip glucose load. A and C, Among males, there were no differences in glucose tolerance [repeated-measures (RM) ANCOVA, ANCOVA]. B, DES females exhibited impaired glucose tolerance compared with control and BPA mice [RM ANCOVA followed by Tukey post hoc tests: P (maternal treatment) <0.01, P (litter size) >0.05, P (DES vs. control) <0.01, P (DES vs. BPA) <0.0.05]. Symbols indicate significant Tukey post hoc comparisons (*, P < 0.05; **, P < 0.01; ***, P < 0.001) vs. control. D, DES females exhibited impaired glucose tolerance as compared by area under the curve (AUC) [ANCOVA followed by Tukey post hoc tests: P (maternal treatment) <0.05, P (litter size) >0.05, P (DES vs. control <0.05)].
Growth when maintained on HFD, 9–14 wk of age
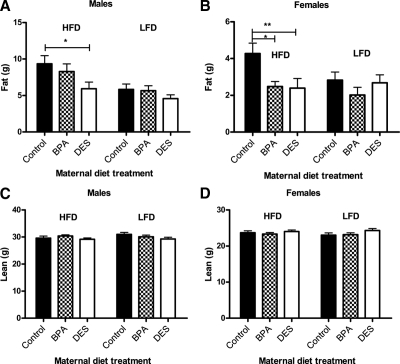
Among males eating either a HFD or LFD, there were no differences in caloric intake among the groups (Fig. 5A), although DES males had lower body weight compared with control (P < 0.058) and BPA males (P < 0.05; Fig. 5, C and E) and less body fat (P < 0.05; Fig. 6A) compared with control males. Among females eating HFD or LFD, control mice ate fewer calories than either BPA (P < 0.05) or DES mice (P < 0.05; Fig. 5B). However, they did not differ in body weight (Fig. 5, D and F), and contrary to our hypothesis, they actually had more body fat than either BPA (P < 0.01) or DES (P < 0.05) females (Fig. 6B). There were no differences among the groups in lean body mass for either males or females (Fig. 6, C and D). Litter size was significantly associated with caloric intake (P < 0.05), body weight (P < 0.05), and lean body mass (P < 0.05) in females but not in males. These outcomes do not change when body composition is expressed relative to body weight (data not shown).
Figure 5.
Growth on HFD vs. LFD from 9–14 wk of age. Cumulative caloric intake was increased in male and female mice eating a HFD vs. LFD. A, Among males, there was no effect of maternal treatment on caloric intake [ANCOVA: P (diet) <0.001, P (maternal treatment) >0.05, P (litter size) >0.05]. B, Among females, control mice ate fewer calories compared with either BPA or DES mice [ANCOVA followed by Tukey post hoc tests: P (diet) <0.0001, P (maternal treatment) <0.01, P (control vs. BPA) <0.05, P (control vs. DES) <0.05, P (litter size) <0.05]. Male mice gained more weight while eating a HFD (C) vs. LFD (E). DES males tended to be lighter than either control or BPA males, although this difference did not reach statistical significance [repeated-measures (RM) ANCOVA followed by Tukey post hoc tests: P (maternal treatment) <0.05, P (diet × time) <0.000001, P (maternal treatment × time) <0.0001, P (DES vs. control) <0.058, P (DES vs. BPA) <0.058, P (litter size) >0.05]. Female mice gained more weight while eating a HFD (D) vs. LFD (F). There were no differences in body weight among the groups [RM ANCOVA: P (maternal treatment) >0.05, P (diet × time) <0.001, P (litter size) <0.05, P (litter size × time) <0.05]. *, P < 0.05.
Figure 6.
Body composition after 5 wk of HFD vs. LFD feeding. A, Among 14-wk-old males, HFD-fed mice had more body fat than LFD-fed mice. Control males also had more body fat than DES males [ANCOVA followed by Tukey post hoc tests: P (diet) <0.001, P (maternal treatment) <0.05, P (control vs. DES) <0.05, P (litter size) >0.05]. B, Control females had more body fat than either BPA or DES females [ANCOVA followed by Tukey post hoc tests: P (maternal treatment) <0.01, P (control vs. BPA) <0.01, P (control vs. DES) <0.05, P (litter size) >0.05]. There were no differences in lean mass among the groups in either males (C) or females (D) (ANCOVA). However, litter size did significantly predict lean body mass in 14-wk-old females (P < 0.05). *, P < 0.05; **, P < 0.01.
Glucose tolerance at 15 wk of age
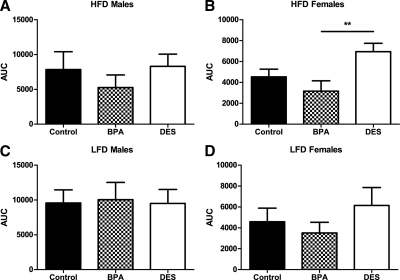
There were no differences in glucose tolerance among male mice under either HFD- or LFD-feeding conditions (Figs. 7, A and C, and 8, A and C). For females eating a LFD, there were no differences in glucose tolerance (Figs. 7D and 8D). However, among females eating the HFD (Figs. 7B and 8B), DES females were again relatively glucose intolerant (P < 0.05).
Figure 7.
Blood glucose in 15-wk-old male and female mice after an ip glucose load. Among males, there were no differences in glucose tolerance regardless of whether the mice ate a HFD (A) or LFD (C) [repeated-measures (RM) ANCOVA]. B, Among females eating HFD, DES females exhibited impaired glucose tolerance [RM ANCOVA: P (maternal treatment) <0.05, P (time × maternal treatment) <0.01; symbols indicate significant Tukey post hoc comparisons (*, P < 0.05; **, P < 0.01) vs. the control females]. D, Among females eating LFD, there were no differences in glucose tolerance (RM ANCOVA).
Figure 8.
Area under the curve (AUC) for 15-wk-old male and female mice after an ip glucose load. Among males, there were no differences in AUC regardless of whether the mice ate a HFD (A) or LFD (C) (ANCOVA). B, Among females eating a HFD, DES females had significantly higher AUC compared with BPA females [ANCOVA followed by Tukey post hoc comparisons: P (maternal treatment) <0.05, P (DES vs. BPA) <0.01]. D, Among females eating a LFD, there were no significant differences in AUC (ANCOVA). **, P < 0.01.
Discussion
The hypothesis that perinatal exposure to BPA leads to the development of metabolic syndrome in adults is compelling and has led to much speculation on the Internet and in the popular press, yet there is limited empirical support for such a relationship. We report here that exposure to an ecologically relevant dose of BPA from e0 to p21 leads to increased growth of CD-1 mouse pups around the time of weaning but does not result in accelerated body weight or body fat gain later in adulthood or impaired glucose regulation, even when the mice are maintained on a HFD.
We chose a maternal dose of BPA (1 ppb, approximately 0.25 μg/kg · bw/d via the diet) that is similar to the estimated range of adult dietary exposure in the United States, as reported independently by both the Food and Drug Administration (FDA) and the NTP-CERHR. The FDA (37) conservatively estimated that an adult in the United States is exposed to 0.185 μg/kg · bw/d BPA via the diet. This figure was based on in-house analyses of BPA levels in canned foods and beverages and assumes that the average 60-kg person consumes 3 kg food/d. The NTP-CERHR report estimated the range of adult exposure by back-calculating from urinary concentrations observed in the National Health and Nutrition Examination Survey dataset. The estimated range of dietary exposure for adults between 20 and 60 yr of age was 0.0179–0.3476 μg/kg · bw/d (4,5), which agrees with the figure calculated by the FDA. Developmental exposure to our 0.25 μg/kg dose of BPA has been previously reported to alter the development of brain sexual differentiation (38) and mammary gland (39) in CD-1 mice.
We found that perinatal exposure to a diet containing 1-ppb BPA leads to a roughly 20% increase in maternal food intake from p14 to 21 (Fig. 1D) and to increased body weight of the pups (12% for males, 10.5% for females) at p21 in CD-1 mice (Fig. 2A). We also observed an increase in body length of BPA mice at 4 wk of age (Fig. 2B). Additional studies would be necessary to further delineate the critical developmental timeframes or mechanisms underlying this increased size of BPA weanlings. Nonetheless, these data are consistent with a shifted growth curve but do not necessarily indicate an obese phenotype. To determine whether the increased size of BPA weanlings persists into adulthood, we weaned all of the mice onto LFD and assessed food intake and body weight over the period from 3–9 wk of age. We also determined body composition at 7 wk of age. For both sexes, the relationship between maternal treatment and body weight was lost during this time (Fig. 3, C and D). By 7 wk of age, there were no significant differences in LFD intake or body weight among the groups for either sex. Likewise, there were no significant differences in body composition between BPA and control mice (Fig. 3, E and F). Eight-week-old BPA mice exhibited normal glucose tolerance (Fig. 4, A–D).
Next, we tested the hypothesis that developmental exposure to BPA may increase susceptibility to weight gain caused by HFD by placing some cohorts of mice on a HFD during adulthood to assess susceptibility to diet-induced obesity (DIO) and resulting glucose intolerance. Half of the mice from each treatment group began eating a HFD that derives 40% of its calories from fat, with the major fat component being highly saturated butter fat, beginning at 9 wk of age. The remaining half continued eating the micronutrient-matched LFD (n = ∼15 mice per diet/treatment/sex for a total n = 182) (40). Although we hypothesized that BPA mice would be more susceptible to HFDIO, the data did not support this prediction. Among males, maintenance on the HFD significantly increased caloric intake (Fig. 5A) and there was a significant interaction between dietary fat and body weight over time, as we have observed in the past (41,42). However, there was no effect of BPA on caloric intake or on body weight (Fig. 5, A and C–E). Among females, there was a significant main effect of dietary fat content on caloric intake (Fig. 5B) and a significant interaction between dietary fat and body weight over time (Fig. 5, D and F). BPA females consumed more calories than control females, independent of diet (Fig. 5B). However, contrary to our hypothesis, BPA females had less body fat than control females (Fig. 6, B and D). Although this outcome may at first be counterintuitive, it is consistent with other mouse models of DIO resistance. MCH1R knockout (43) and ACC2 knockout mice (44), for example, are hyperphagic but DIO resistant due to increased activity and energy expenditure. Additional studies would be necessary to clarify the mechanism underlying DIO resistance in our BPA females. BPA mice did not exhibit the predicted impairment of glucose tolerance (Figs. 7 and 8). Collectively, these data do not suggest an obese BPA phenotype. We conclude that BPA mice have accelerated growth early in life, but that this increased size at weaning does not translate to increased susceptibility to DIO in adults.
The DES treatment group was included to provide a positive control for the possibility that BPA might exert effects by acting via a classical ER (ERα and ERβ) (3). It has been previously reported that perinatal exposure to 1 μg/kg · bw DES, the dose used in our study, is associated with obesity and impaired glucose tolerance in adult female CD-1 mice (12). In our hands, DES mice of both sexes had similar food intake throughout gestation and lactation (Fig. 1D) and similar weight at weaning compared with control mice (Fig. 2A). However, 4-wk-old DES mice were longer than control mice (Fig. 2B), suggesting a lean rather than an obese phenotype. From 4–7 wk of age, there were no differences in food intake or body weight among males or females while maintained on the LFD (Fig. 3, A–D). Seven-week-old DES males and females had similar body composition compared with control mice, but the DES males had significantly less fat mass compared with BPA males (Fig. 3, E and F). Eight-week-old DES females had significantly impaired glucose tolerance (Fig. 4, B and D), consistent with a previously reported trend (12). From 9–14 wk of age, DES males consumed the same number of calories as control mice maintained on either diet (Fig. 5A) but tended to gain less weight (Fig. 5, C and E) and accumulated less fat (Fig. 6A); 9- to 14-wk-old DES females consumed significantly more calories than control females (Fig. 5B) but gained the same amount of weight (Fig. 5, D and F) and had less fat (Fig. 6B). HFD-fed DES females had significantly impaired glucose tolerance at 15 wk of age (Fig. 7, B and D).
It has frequently been suggested that the relatively well-characterized DES system may provide a predictive model for potential consequences of low-dose developmental exposure to BPA (12,13,14). Our data highlight the difficulties with such an extrapolation. Although our experimental model was sensitive to the provided doses of both DES and BPA, indicating the success of our positive control, the two xenoestrogens induced different phenotypes in our hands. In addition to being a relatively weak ERα and ERβ agonist, BPA interacts with several other cellular targets (reviewed in Refs. 3,4,5 and 45), including the androgen receptor (46,47), aryl hydrocarbon receptor (48), the estrogen-related receptor-γ (49,50), the seven-transmembrane ER G protein-coupled receptor 30 (51), a nonclassical membrane bound form of the ER (52), and the thyroid hormone receptors (53). Consequently, although the DES model may predict some potential consequences of developmental exposure to BPA, and although it may provide an appropriate positive control for its actions at traditional ERs, we agree with Chapin et al. that it would be “overly simplistic” to interpret the data only within the context of their consistency with a classical estrogenic mechanism (see page 10 in Ref. 5).
The September 2008 NTP-CERHR monograph (5) provides a thorough and critical review of the existing literature. It cites several studies that report increased postnatal growth in rats and mice exposed to low-dose BPA during development (19,20,21,23) and other studies that report no differences (17,54,55) or even reduced postnatal growth (24). The NTP report concludes that the cause of such discrepancies is unclear, and it calls attention to methodological and/or statistical concerns regarding many low-dose BPA papers in the current literature. Of particular concern were experiments that: 1) administered BPA by ip injection or by osmotic minipump rather than using an oral route of administration that more closely mimics human exposure (including Ref. 24), 2) used nonpurified chow diets containing variable amounts of phytoestrogens, 3) had inadequate sample size (including Refs. 21 and 23), or 4) failed to use a positive control (including Refs. 20, 21, and 23). Finally, “the single most common technical shortcoming noted” was the “lack of experimental or statistical control for litter effects (p. 14)” (see page 14 in Ref. 5) (including Refs. 19,20,21 and 23).
The current study addresses each of these concerns and constraints by: 1) administering the BPA orally via the diet, 2) using only open-source, purified diets throughout the study, 3) including a relatively large sample size, n = approximately 15 animals/sex/maternal treatment/diet for a total of 182 mice, and 4) including the DES group as a positive control for the actions of BPA at traditional ERs. Most importantly, we also controlled for litter effects by including only one animal of each sex from each litter to participate in the long-term study. Differences in litter size were accommodated by including it as a cofactor in all statistical analyses. Consistent with the NTP report's cautions, litter size was a statistically significant cofactor in many analyses, even using endpoints as late as 15 wk of age, highlighting the strong association between litter size and the metabolic phenotype of adult rodents and the importance of taking litter effects into account in this and other studies.
Despite our adherence to the methodological and statistical concerns raised by the NTP, there are caveats to the interpretation of these data. First, the current study does not address the possibility that chronic adult exposure to BPA may lead to obesity and metabolic syndrome. We note that the increased food intake observed from p14 to p21 and the increased size of BPA mice at weaning may result at least in part from direct consumption of BPA diet by the young mice before weaning. It is therefore possible that continued exposure to BPA throughout adulthood may have sustained the phenotype, and additional studies would be necessary to address this hypothesis. Second, as with all rodent studies, extrapolating from these data to make predictions about human health can be challenging. Because the pharmacokinetics of BPA is different between humans and rodents (5), internal doses may differ between mice and humans receiving the same dietary exposure. Similarly, baseline levels from nonoral routes of exposure may differ; for example, the mice in our study may have some level of exposure (distributed evenly across the treatment groups) via the polycarbonate housing. Resolving these discrepancies by measuring circulating levels, particularly for low-dose studies, is problematic because the currently available assays may not be sufficiently sensitive (4,5). Finally, the present study uses only one dose of DES and one dose of BPA. Consequently, it does not address the possibility that a different dose of BPA would have elicited the predicted phenotype. Nonetheless, our data are consistent with previous reports that perinatal exposure to somewhat higher doses of BPA is associated with similarly increased body weight in weanling rodents. Howdeshell et al. (23), for example, reported about a 12% greater body weight in weanling mice exposed to a maternal dose of 2.4 μg BPA/kg · bw. Likewise, Miyawaki et al. (20) reported a 13% increase in body weight at p31 in female rats at a maternal dose of 260 μg/kg · bw. These studies report qualitatively similar phenotypes (12–13% increase in body weight compared with the 10.5% increase we observed) using doses of BPA that are 10- and 100-fold greater than the dose used here. Rubin et al. (19) also reported a similar increase in body weight at p21 in mice exposed to a maternal dose of 100 μg/kg · bw BPA; this larger size was sustained through 16 wk of age. The authors of the NTP report considered each of these studies to be of limited use due to several of the methodological and/or statistical limitations mentioned above. Furthermore, none of these studies reported body length limiting the interpretation of increased body weight. Additional studies would be necessary to confirm their findings.
The hypothesis that perinatal exposure to ecologically relevant doses of BPA predisposes individuals to obesity and glucose intolerance as adults is provocative and, given the important implications for public health, demands rigorous investigation. To date, few empirical studies have explicitly investigated this hypothesis. Here, we report, in a study not linked to entities with a vested interest in the outcome, that developmental exposure to 1-ppb BPA via the mother's diet results in accelerated growth early in life. However, we conclude that this increased size-for-age does not translate to increased weight or body fat gain or to impaired glucose regulation, even when exposed to a HFD later in life.
Acknowledgments
We thank David D'Alessio and Jackie Reed for helpful comments and discussion regarding experimental design and data analysis and Matej Bajzer, Jason Barrera, Adam Chambers, Michelle Foster, Ruth Guitierrez-Aguilar, Heinz Hoppert, Dong-Hoon Kim, Michelle Kirby, Rohit Kohli, Emily Orr, Ken Parks, Hilary Wilson Perez, Darleen Sandoval, Karen Scott, and Kathi Smith provided technical assistance during the GTTs.
Footnotes
This work was supported by National Institutes of Health Grants DK054890 and DK073505 (to R.J.S.) and DK082173 (to K.K.R.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 29, 2010
For editorial see page 2404
Abbreviations: ANCOVA, Analysis of covariance; BPA, bisphenol-A; bw, body weight; CERHR, Center for the Evaluation of Risks to Human Reproduction; DES, diethylstilbestrol; DIO, diet-induced obesity; e, embryonic day; ER, estrogen receptor; FDA, Food and Drug Administration; GTT, glucose tolerance test; HFD, high butter-fat diet; LFD, low butter-fat diet; MRI, magnetic resonance imaging; NTP, National Toxicology Program; p, postnatal day; ppb, parts per billion.
References
- Le HH, Carlson EM, Chua JP, Belcher SM 2008 Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Lett 176:149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV 2007 Human exposure to bisphenol A (BPA). Reprod Toxicol 24:139–177 [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM 2009 Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev 30:75–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin R, Adams J, Boekelheide K, Gray L, Hayward S, Lees P, McIntyre B, Portier K, Schnorr T, Selevan S, Vandenbergh J, Woskie S 2008 NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B 83:157–395 [DOI] [PubMed] [Google Scholar]
- Center for the Evaluation of Risks to Human Reproduction 2008 NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. Pittsburgh: National Toxicology Program, Department of Health and Human Services [Google Scholar]
- Palmer JR, Herbst AL, Noller KL, Boggs DA, Troisi R, Titus-Ernstoff L, Hatch EE, Wise LA, Strohsnitter WC, Hoover RN 2009 Urogenital abnormalities in men exposed to diethylstilbestrol in utero: a cohort study. Environ Health 8:37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst AL, Ulfelder H, Poskanzer DC 1971 Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med 284:878–881 [DOI] [PubMed] [Google Scholar]
- Veurink M, Koster M, Berg LT 2005 The history of DES, lessons to be learned. Pharm World Sci 27:139–143 [DOI] [PubMed] [Google Scholar]
- Marselos M, Tomatis L 1992 Diethylstilboestrol: II, pharmacology, toxicology and carcinogenicity in experimental animals. Eur J Cancer 29A:149–155 [DOI] [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Bullock BC 1980 Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res 40:3988–3999 [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, Snyder RJ, Jefferson WN 2005 Developmental exposure to estrogenic compounds and obesity. Birth Defects Res A Clin Mol Teratol 73:478–480 [DOI] [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, Snyder RJ, Phillips TM, Jefferson WN 2007 Developmental exposure to endocrine disruptors and the obesity epidemic. Reprod Toxicol 23:290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, Jefferson WN 2009 Environmental estrogens and obesity. Mol Cell Endocrinol 304:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, Padilla-Banks E 2009 Prenatal exposure to bisphenol A at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ Health Perspect 117:879–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, Padilla-Banks E 2007 Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol 24:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM 2005 Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod 72:1344–1351 [DOI] [PubMed] [Google Scholar]
- Durando M, Kass L, Piva J, Sonnenschein C, Soto AM, Luque EH, Muñoz-de-Toro M 2007 Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ Health Perspect 115:80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Shimomoto T, Katashima S, Watanabe G, Taya K, Maekawa A 2004 Maternal exposure to low doses of bisphenol A has no effects on development of female reproductive tract and uterine carcinogenesis in Donryu rats. J Reprod Devel 50:349–360 [DOI] [PubMed] [Google Scholar]
- Rubin BS, Murray MK, Damassa DA, King JC, Soto AM 2001 Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect 109:675–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki J, Sakayama K, Kato H, Yamamoto H, Masuno H 2007 Perinatal and postnatal exposure to bisphenol A increases adipose tissue mass and serum cholesterol level in mice. J Atheroscler Thromb 14:245–252 [DOI] [PubMed] [Google Scholar]
- Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP 2004 Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology 145:592–603 [DOI] [PubMed] [Google Scholar]
- Tinwell H, Haseman J, Lefevre PA, Wallis N, Ashby J 2002 Normal sexual development of two strains of rat exposed in utero to low doses of bisphenol A. Toxicol Sci 68:339–348 [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS 1999 Environmental toxins: exposure to bisphenol A advances puberty. Nature 401:763–764 [DOI] [PubMed] [Google Scholar]
- Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T 2002 Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod Toxicol 16:117–122 [DOI] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS 2007 In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol 24:199–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH 2009 Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes 58:1116–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf JF 1995 Biometry. 3rd ed. New York: W. H. Freeman and Co. [Google Scholar]
- Palmer AK, Ulbrich BC 1997 The cult of culling. Fund Appl Toxicol 38:7 [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Rake A, Voits M, Fink H, Rohde W, Dörner G 1999 Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome X-like alterations in adulthood of neonatally overfed rats. Brain Res 836:146–155 [DOI] [PubMed] [Google Scholar]
- Barros RP, Gabbi C, Morani A, Warner M, Gustafsson JA 2009 Participation of ERα and ERβ in glucose homeostasis in skeletal muscle and white adipose tissue. Am J Physiol Endocrinol Metab 297:E124–E133 [DOI] [PubMed] [Google Scholar]
- Qatanani M, Szwergold NR, Greaves DR, Ahima RS, Lazar MA 2009 Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J Clin Invest 119:531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasumi K, Esaki R, Iwachidow K, Yasuhara Y, Ogi K, Tanaka H, Nakata M, Yano T, Shimakawa K, Taketomi S, Takeuchi K, Odaka H, Kaisho Y 2009 Overexpression of GPR40 in pancreatic alpha Î2 cells augments glucose-stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice. Diabetes 58:1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly-Maitre B, Belgardt BF, Jordan SD, Coornaert B, von Freyend MJ, Kleinridders A, Mauer J, Cuddy M, Kress CL, Willmes D, Essig M, Hampel B, Protzer U, Reed JC, Brüning JC 2010 Hepatic Bax-inhibitor (BI)-1 inhibits IRE1α and protects from obesity-associated insulin resistance and glucose intolerance. J Biol Chem 285:6198–6207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness OP, Ayala JE, Laughlin MR, Wasserman DH 2009 NIH experiment in centralized mouse phenotyping: the Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. Am J Physiol Endocrinol Metab 297:E849–E855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Strader AD, Sorrell JE, Chambers JB, Woods SC, Seeley RJ 2008 Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis. Am J Physiol Endocrinol Metab 294:E630–E639 [DOI] [PubMed] [Google Scholar]
- Macotela Y, Boucher J, Tran TT, Kahn CR 2009 Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes 58:803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA 2008 Draft assessment of bisphenol-A for use in food contact applications. Silver Spring, MD: Food and Drug Administration [Google Scholar]
- Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM 2006 Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology 147:3681–3691 [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM 2007 Exposure to environmentally relevant doses of the xenoestrogen Bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology 148:116–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, D'Alessio DA, Tso P, Rushing PA, Clegg DJ, Benoit SC, Gotoh K, Liu M, Seeley RJ 2004 Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiol Behav 83:573–578 [DOI] [PubMed] [Google Scholar]
- Strader AD, Reizes O, Woods SC, Benoit SC, Seeley RJ 2004 Mice lacking the syndecan-3 gene are resistant to diet-induced obesity. J Clin Invest 114:1354–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Sandoval D, Reed JA, Matter EK, Tolod EG, Woods SC, Seeley RJ 2008 The role of GM-CSF in adipose tissue inflammation. Am J Physiol Endocrinol Metab 295:E1038–E1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS, Guan XM, Jiang MM, Feng Y, Camacho RE, Shen Z, Frazier EG, Yu H, Metzger JM, Kuca SJ, Shearman LP, Gopal-Truter S, MacNeil DJ, Strack AM, MacIntyre DE, Van der Ploeg LH, Qian S 2002 Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci USA 99:3240–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ 2001 Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science 291:2613–2616 [DOI] [PubMed] [Google Scholar]
- Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM 2007 In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol 24:178–198 [DOI] [PubMed] [Google Scholar]
- Sohoni P, Sumpter JP 1998 Several environmental oestrogens are also anti-androgens. J Endocrinol 158:327–339 [DOI] [PubMed] [Google Scholar]
- Xu LC, Sun H, Chen JF, Bian Q, Qian J, Song L, Wang XR 2005 Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicology 216:197–203 [DOI] [PubMed] [Google Scholar]
- Krüger T, Long M, Bonefeld-Jørgensen EC 2008 Plastic components affect the activation of the aryl hydrocarbon and the androgen receptor. Toxicology 246:112–123 [DOI] [PubMed] [Google Scholar]
- Takayanagi S, Tokunaga T, Liu X, Okada H, Matsushima A, Shimohigashi Y 2006 Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor γ (ERRγ) with high constitutive activity. Toxicol Lett 167:95–105 [DOI] [PubMed] [Google Scholar]
- Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, Tokunaga T, Kawabata S, Kimura M, Shimohigashi Y 2007 Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERRγ. J Biochem 142:517–524 [DOI] [PubMed] [Google Scholar]
- Thomas P, Dong J 2006 Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem 102:175 [DOI] [PubMed] [Google Scholar]
- Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B 2000 Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc Natl Acad Sci USA 97:11603–11608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, Hataya Y, Shimatsu A, Kuzuya H, Nakao K 2002 Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab 87:5185–5190 [DOI] [PubMed] [Google Scholar]
- Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S 2003 Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci Res 45:345–356 [DOI] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG 2006 Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm Behav 50:85–93 [DOI] [PubMed] [Google Scholar]