Abstract
Gestational exposure to maternal overweight (OW) influences the risk of obesity in adult life. Male offspring from OW dams gain greater body weight and fat mass and develop insulin resistance when fed high-fat diets (45% fat). In this report, we identify molecular targets of maternal OW-induced programming at postnatal d 21 before challenge with the high-fat diet. We conducted global transcriptome profiling, gene/protein expression analyses, and characterization of downstream signaling of insulin and adiponectin pathways in conjunction with endocrine and biochemical characterization. Offspring born to OW dams displayed increased serum insulin, leptin, and resistin levels (P < 0.05) at postnatal d 21 preceding changes in body composition. A lipogenic transcriptome signature in the liver, before development of obesity, was evident in OW-dam offspring. A coordinated locus of 20 sterol regulatory element-binding protein-1-regulated target genes was induced by maternal OW. Increased nuclear levels of sterol regulatory element-binding protein-1 and recruitment to the fatty acid synthase promoter were confirmed via ELISA and chromatin immunoprecipitation analyses, respectively. Higher fatty acid synthase and acetyl coenzyme A carboxylase protein and pAKT (Thr308) and phospho-insulin receptor-β were confirmed via immunoblotting. Maternal OW also attenuated AMP kinase/peroxisome proliferator-activated receptor-α signaling in the offspring liver, including transcriptional down-regulation of several peroxisome proliferator-activated receptor-α-regulated genes. Hepatic mRNA and circulating fibroblast growth factor-21 levels were significantly lower in OW-dam offspring. Furthermore, serum levels of high-molecular-weight adiponectin (P < 0.05) were decreased in OW-dam offspring. Phosphorylation of hepatic AMP-kinase (Thr172) was significantly decreased in OW-dam offspring, along with lower AdipoR1 mRNA. Our results strongly suggest that gestational exposure to maternal obesity programs multiple aspects of energy-balance regulation in the offspring.
Maternal obesity programs systemic changes in insulin and adiponectin levels in the offspring and causes global changes in the hepatic transcriptome revealing a reprogramming of lipogenic and lipid degradative pathways.
The global rise in the prevalence of overweight (OW) and obesity is paralleled by an alarming increase in the incidence of OW among pregnant women (1). Being OW during pregnancy has significant impact on the health of both the mother and the offspring. Pregravid OW increases the risk of preeclampsia, gestational diabetes, and other labor-related complications (2,3). For the offspring, exposure to maternal OW increases the risk of being large for gestational age at birth, which substantially increases the risk of OW in adulthood (3,4,5). Moreover, exposure to maternal obesity at conception and during pregnancy has been hypothesized to lead to developmental programming of excessive weight and adiposity gain in the offspring (6,7). Several lines of evidence from epidemiological and clinical studies support this contention. These include the stronger association of maternal body mass index (BMI) (compared with paternal BMI) with offspring BMI (8), the decreased risk of obesity in children born to obese women after weight loss after bariatric surgery (9,10), and the positive relationship between maternal weight gain between pregnancies and risk of OW in offspring compared with their siblings (11).
Using a model of gestational OW in the rat, we previously demonstrated that maternal obesity programs increased sensitivity to weight gain in the offspring in the absence of changes in birth weights (12). In this model, exposure to maternal OW is limited in utero and results in marked increase in the weight gain in the adult offspring after weaning to an obesogenic high-fat diet (HFD). Moreover maternal obesity per se leads to hyperinsulinemia, increased percent liver weight, and adipocyte hypertrophy in the offspring, suggesting the programming of increased lipogenic responses (12). However, the changes in offspring endocrinology at weaning [i.e. postnatal d 21 (PND21)], which may lead to increased lipogenesis and obesity risk are poorly appreciated. In this report, we focus on the effect of maternal OW on the programming of key endocrine signaling (insulin and adiponectin) in the offspring. Although the majority of studies examining maternal obesity have used energy-dense (high fat) or highly palatable diets to produce gestational OW, relatively little is known about the effects of overconsumption of calories per se. Self-limiting consumption of diets due to satiety has been the primary limitation in development of obesity in animal models, necessitating use of energy-dense diets. We employed controlled feeding of liquid diets via total enteral nutrition (TEN) as a mechanistic tool to overcome this limitation.
The present study had three objectives. First, we examined the effect of maternal OW on offspring endocrine parameters, before changes in body weight or adiposity in the offspring. Specifically, we examined whether maternal OW in utero impacts hormones involved in energy balance and lipogenesis. Second, we elucidate global transcriptome changes in the liver of the offspring at weaning using microarrays to identify loci of programming. Third, we examine cellular signaling pathways regulated by insulin and adiponectin in the liver leading to altered hepatic physiology in the offspring of OW dams. Our data strongly suggest that exposure to maternal OW from conception to birth programs systemic changes in insulin and adiponectin levels and alters a diverse suite of genes involved in carbohydrate metabolism, lipid biosynthesis, and fatty acid catabolism.
Materials and Methods
Animals and chemicals
Female Sprague Dawley rats (150–175 g) were obtained from Charles River Laboratories (Wilmington, MA). Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-approved animal facility. All experimental treatments were conducted in accordance with the guidelines established and approved by the Institutional Animal Care and Use Committee at University of Arkansas for Medical Sciences. Unless specified otherwise, all chemicals were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO).
Experimental protocol
Virgin female Sprague Dawley rats were intragastrically cannulated and allowed to recover for 10 d as previously described (12,13,14,15,16). Rats were fed liquid diets at either 155 kcal/kg3/4 · d (referred to as lean dams) or at 220 kcal/kg3/4 · d (40% excess calories, referred to as OW dams). Caloric intake for the lean group was determined from preliminary studies and designed to mimic body weights and body composition of rats consuming standard commercial diets ad libitum (12). TEN diets met National Research Council nutrient recommendations, including essential fatty acids, and were 20% protein (casein), 75% carbohydrate (dextrose and maltodextrin), and 5% fat (corn oil) as percentage of total calories (12,13,16). These diets have been previously used by our group in a number of studies (12,13,14,15,16,17,18,19). Infusion of diets was carried out 23 h/d using computer-controlled syringe pumps for 3 wk. Animals had ad libitum access to drinking water throughout. Body weights were monitored three times per week. At the end of 3 wk, body composition was noninvasively estimated using nuclear magnetic resonance (NMR) (12).
After 3 wk of overfeeding, lean and OW rats (n = 18 per group) were allowed to mate for 1 wk. Each female rat was housed with one male and allowed ad libitum access to AIN-93G diet for this period. After mating, all female rats (lean and OW) resumed receiving diets at 220 kcal/kg3/4 · d (National Research Council-recommended caloric intake for pregnancy in rats). All rats were allowed to give birth naturally. Numbers and sex of pups, birth weight, and crown-to-rump and anogenital distance were measured for each pup on PND1. On PND2, four males and four female pups from each litter were cross-fostered to dams that had been previously time-impregnated to give birth on the same day as the dams receiving infusion diets. Cross-fostered dams were not cannulated and had ad libitum access to AIN-93G pelleted diets throughout lactation. Using this experimental paradigm, we ensured that offspring's exposure to maternal OW was limited to only intrauterine development (12). Female offspring of lean and OW dams were used for separate experiments, and only data from male offspring are reported here. Male offspring were euthanized under anesthesia at PND21 (n = 8 per group). Blood, liver, kidneys, and adipose tissues (retroperitoneal and gonadal depots) were weighed and collected. Samples were fixed in neutral-buffered formalin for histological analyses, and remaining tissues were frozen in liquid nitrogen and stored at −70 C for RNA and protein analyses. Serum was obtained by centrifugation of blood samples and stored at −20 C for endocrine and metabolic assessments.
Body composition analyses
Body composition of dams and offspring was assessed via NMR (Echo Medical Systems, Houston, TX), x-ray computerized tomography (CT) (LaTheta LCT-100; Echo Medical Systems), and postmortem dissected weights of retroperitoneal and gonadal adipose tissues. NMR was performed in conscious unanesthetized rats in duplicate, and indices of percent fat and lean mass were derived using this technique (12,16). For CT analyses, approximately 90 sections, 1 mm apart, were acquired encompassing the entire visceral region of the animal under anesthesia. Indices of percent fat ratio (ratio of volume occupied by fat/volume occupied by lean tissue), percent fat mass, and percent lean mass were calculated using Aloka CT software (Tokyo, Japan) as described previously (12,16).
Serum biochemistry, liver histology, and endocrine status
Serum glucose, triglycerides, fibroblast growth factor-21 (FGF21), nonesterified fatty acids (NEFA), insulin, leptin, adiponectin, and resistin was measured as described in Supplemental Materials and Methods published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. Hepatic glycogen was assessed using a commercially available glycogen assay kit (BioVision Inc., Mountain View, CA). Liver sections were stained with hematoxylin and eosin or Oil Red O as previously described (15,16). The separation of serum adiponectin complexes was performed using the method described by Schraw et al. (20). Serum (25 μl) was separated on a Superdex 200 10/300 GL column (GE Healthcare Biosciences, Piscataway, NJ) using an ÄKTA fast protein liquid chromatography (FPLC) system (GE Healthcare) in a HEPES/Ca2+ buffer. Thirty 0.215-ml fractions (from 7–14 ml) were collected. Aliquots of fractions were heat denatured in loading buffer containing dithiothreitol and were separated using SDS-PAGE. Immunoblotting was carried out using standard procedures, and adiponectin monomers (∼30 kDa) were quantitated using Western blotting. Using this technique, we ascertained that fractions containing high molecular weight (HMW) adiponectin complexes (fractions 6–13). Quantitative analyses of the percentage of HMW adiponectin relative to total adiponectin in serum was performed using ELISA (B-Bridge International, Sunnyvale, CA). Serum from individual offspring from lean and OW dams (n = 8 per group) was separated using FPLC as described above. After separation, adiponectin levels in whole serum and HMW adiponectin-containing fractions (6,7,8,9,10,11,12,13) was assessed using ELISA on the same plate. Data are expressed as percentage of HMW adiponectin in serum relative to total adiponectin.
Hepatic gene expression analyses
RNA isolation and microarray analyses
Total RNA was isolated from liver of offspring at PND21 (n = 8 per group) using the RNeasy kit (QIAGEN, Valencia, CA), including on-column deoxyribonuclease digestion. Three microarrays (GeneChip Rat 230 2.0; Affymetrix, Santa Clara, CA) were used for each group. Pools of equal amounts of RNA from two to three rats were used for analyses per microarray. Thus, eight rats per group were represented over the three microarrays. cRNA synthesis, labeling, hybridization, and scanning were carried out using the manufacturer's instructions (16,18,21).
Microarray data normalization and analysis
Microarray data analyses were carried out using GeneSpring version 7.3X software (Agilent Technologies, Santa Clara, CA) (16,21). The .CEL files containing probe level intensities were processed using the robust multiarray analysis algorithm for background adjustment, normalization, and log2 transformation of perfect match values (22). Subsequently, the data were subjected to normalization by setting measurements less than 0.01 to 0.01 and by per-chip and per-gene normalization using GeneSpring. The normalized data were used to generate a list of differentially expressed genes between offspring of OW and lean dams at PND21. Genes were filtered based on minimum ±1.8-fold change (OW vs. lean) and P value ≤0.05 using Student's t test. Corrections for multiple testing were performed using the false discovery rate method (23). A list of transcripts that were differentially expressed as a function of maternal OW was generated, and correlation-based hierarchical clustering between treatment groups was performed. Known biological functions of genes were queried using Affymetrix NetAffx and gene ontology analyses performed using GeneSpring (16,21). Furthermore, the list of genes affected in offspring liver by maternal OW was analyzed using Ingenuity pathway analysis (IPA).
Real-time RT-PCR
Total RNA from liver and retroperitoneal adipose tissues from offspring at PND21 (same offspring that were used for microarray analyses) was isolated using RNeasy columns (QIAGEN). One microgram of total RNA was reverse transcribed (n = 8 per group for PND21) using IScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Real-time PCR analysis was performed as described previously. Gene-specific primers were designed using Primer Express Software (Supplemental Table 1). Relative amounts of mRNA were quantitated using a standard curve and normalized to the expression of cyclophilin A mRNA (16,18).
Immunoblotting, immunoprecipitation, and TransAM ELISA
Immunoblotting was carried out using standard procedures (16). A detailed description is provided in Supplemental Materials and Methods. Immunoblotting was performed for AMP-activated protein kinase (AMPK), phospho-AMPK Thr172, acetyl coenzyme A carboxylase, carnitine palmitoyl transferase-1a (Cpt-1a), insulin receptor (IR)-β, IR substrate (IRS)-1, phospho-AKT Ser473, phospho-AKT Thr308, AKT, ERK1/2, pERK1/2, fatty acid synthase (FASN), glyceraldehyde-3-phosphate dehydrogenase, lamin A, and sterol regulatory element-binding protein-1 (SREBP-1) proteins in total lysates or extracts from nuclear or mitochondrial fractions. Immunoprecipitation was performed in triplicate using 500 μg protein from pooled liver lysates (each pool representing two to three separate animals). After overnight incubation with either anti-phosphotyrosine antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or nonspecific IgG, immune complexes were pulled down using protein G magnetic beads, washed, and solubilized in 2× Laemmli buffer. Aliquots were separated using SDS-PAGE, and immunoblotting was carried out using anti-IR-β antibody (Cell Signaling Technology, Beverly, MA). TransAM ELISA (Active Motif, Carlsbad, CA) was used to assess abundance of SREBP-1 protein in nuclear extracts. Hepatic nuclear protein (40 μg) from individual animals was used and the procedures carried out according to manufacturer's instructions. Controls such as recombinant SREBP-1 protein and competition with consensus oligonucleotides (not bound to the plate) were included in the assay. Abundance of SRE-bound SREBP-1 is represented as absorbance values at A450 nm.
Chromatin immunoprecipitation (ChIP)
Recruitment of SREBP-1 and peroxisome proliferator activated receptor (PPAR)-α on the promoters of respective target genes was assessed using ChIP. The ChIP-IT enzymatic kit (Active Motif) was used with minor modifications for in vivo samples and described previously (24). Three pools of liver samples (with each pool representing two to three separate animals) were used for the analyses. Details of the procedure are described in the Supplemental Materials and Methods. Immunoprecipitation was performed using 5 μg of either anti-SREBP-1 (Novus Biologicals, Littleton, CO), anti-PPAR-α (Abcam, Cambridge, MA), or matched nonspecific IgG. Target binding regions on the FASN and FGF21 promoters were amplified by PCR for SREBP-1 and PPAR-α, respectively.
Statistical analysis
Data are expressed as means ± sem. Statistical differences between lean and OW rats before conception or dams during gestation were determined using two-tailed Student's t test. Similarly, differences between offspring of lean and OW dams at PND21 were determined using two-tailed Student's t test. Statistical significance was set at P < 0.05. Statistical analyses were performed using SigmaStat 3.3 software (Systat Software Inc., San Jose, CA).
Results
In utero programming of offspring metabolism by maternal OW
Overfeeding female rats at 220 kcal/kg3/4 · d via TEN for 3 wk resulted in approximately 120% greater body weight gain (P < 0.001, Supplemental Fig. 1A). Body composition assessed via NMR revealed that total body fat in OW females was 156% greater than lean controls (P < 0.001) and was accompanied by a significantly lower lean mass (P < 0.001, Supplemental Fig. 1B). Body weights and adiposity of rats fed diets at 155 kcal/kg3/4 · d (lean group) were similar to ad libitum-fed controls at the start of the study (232 ± 1.3 g ad libitum-fed rats and 235 ± 1.5 g in the lean group; n = 13 and n = 18, respectively) and at 3 wk of feeding via TEN (265 ± 2.4 g ad libitum-fed rats and 267 ± 1.9 g in the lean group; n = 13 and n = 18, respectively). We previously reported the development of significant fasting hyperinsulinemia, hyperleptinemia, and elevated serum triglyceride and NEFA concentrations in OW dams (12). Between wk 3 and 4 (from the beginning of diets), female rats were housed with one male breeder rat and given ad libitum access to AIN-93G diets (pellets). After 1 wk of mating, both lean and OW dams resumed receiving the TEN feeding at 220 kcal/kg3/4 · d. During this period, the rate of pregnancy-related weight gain (gestation d 8–21) was the same for both lean and OW dams (Supplemental Fig. 1C). However, the OW dams remained heavier, and the body composition differences between the groups persisted throughout gestation.
Offspring were nursed (starting PND1) by noncannulated surrogate dams that received ad libitum access to AIN-93G-based pellet diets. Hence, exposure to maternal OW was limited to conception and gestation. Birth weights (lean dams, 6.5 ± 0.1 g, vs. OW dams, 6.2 ± 0.2 g), number of pups (lean dams, 11.7 ± 0.3, vs. OW dams, 10.7 ± 1.0), male-to-female ratio (lean dams, 0.99 ± 0.1, vs. OW dams, 1.0 ± 0.1), crown-to-rump distance (lean dams, 1.5 ± 0.03 in., vs. OW dams, 1.5 ± 0.02 in.), and anogenital distance (lean dams, 0.11 ± 0.005 in., vs. OW dams, 0.11 ± 0.005 in.) did not differ between offspring of lean- and OW-dam groups. To examine the consequences of fetal programming due to maternal OW, we assessed metabolic and endocrine parameters at weaning (PND21) (Table 1). Body weights of male offspring did not differ at weaning (Table 1). However, percent liver weight (P < 0.001) in the offspring of OW dams was approximately 125% greater compared with their lean dam counterparts. Dissected weights of the kidneys and visceral adipose tissues (retroperitoneal and gonadal depots) at weaning did not differ between groups. Lack of increase in body adiposity at weaning in the offspring of OW dams was also confirmed by quantitative NMR (Table 1) and x-ray CT analyses. Despite no changes in body weights or adiposity, serum insulin, leptin, and resistin concentrations were significantly elevated (140, 200, and 180%, respectively) in the offspring of OW dams (P < 0.05) (Table 1). Serum triglyceride levels remained unchanged, whereas circulating NEFA levels were significantly lower in OW-dam offspring (Table 1). Consistent with increased liver weight, hepatic glycogen levels were also increased to approximately 159% compared with lean dam offspring (Table 1).
Table 1.
Effect of maternal OW on offspring metabolic and endocrine parameters at weaning
| Parameter | Lean | OW | P value |
|---|---|---|---|
| Body weight (g) | 68 ± 3.1 | 66 ± 2.0 | 0.70 |
| Liver weight (g) | 2.3 ± 0.13 | 2.8 ± 0.11 | <0.05 |
| % Liver weight (g) | 3.4 ± 0.09 | 4.2 ± 0.1 | <0.0001 |
| % Visceral fat weight | 1.06 ± 0.06 | 0.85 ± 0.07 | 0.07 |
| % Kidney weight | 1.04 ± 0.02 | 1.05 ± 0.01 | 0.63 |
| % Lean mass | 66 ± 1.84 | 64 ± 0.99 | 0.31 |
| % Fat mass | 12.8 ± 0.83 | 11.9 ± 0.52 | 0.36 |
| Glucose (mg/dl) | 134 ± 8.9 | 141 ± 4.9 | 0.55 |
| Insulin (ng/ml) | 0.76 ± 0.02 | 1.07 ± 0.03 | <0.05 |
| Leptin (ng/ml) | 1.82 ± 0.66 | 3.65 ± 0.44 | <0.05 |
| Adiponectin (μg/ml) | 6.05 ± 0.8 | 3.92 ± 0.89 | 0.09 |
| Resistin (ng/ml) | 13.9 ± 0.82 | 25.1 ± 2.1 | <0.001 |
| Triglyceride (mg/dl) | 227 ± 32 | 221 ± 26 | 0.90 |
| NEFA (μm) | 249 ± 26 | 135 ± 20 | <0.005 |
| Liver glycogen (μg/mg) | 143 ± 17 | 228 ± 17 | <0.005 |
Data were obtained from offspring of lean or OW dams at PND21 (n = 8 per group). Lean and OW dams were fed via TEN as described in Materials and Methods. Data are expressed as means ± sem. Indices of percent lean and fat mass were determined using noninvasive quantitative NMR. Weights of liver, kidney, and visceral adipose tissues (retroperitoneal plus gonadal fat depots) were assessed at the time of euthanasia. P values were determined using Student's t test.
Maternal OW alters hepatic transcriptome in the offspring
To assess whether in utero exposure to maternal OW altered hepatic gene expression in the offspring at weaning, we performed gene expression analyses. Unsupervised global condition clustering revealed clustering of expression profiles between offspring based on their maternal phenotypes, suggesting significant treatment effect on global gene expression (Supplemental Fig. 2). After normalization, 147 transcripts were identified to be differentially expressed in offspring of OW vs. lean dams (±1.8-fold, P ≤ 0.05, Supplemental Table 2). Correlation-based hierarchical clustering of the genes affected by maternal OW is depicted in Fig. 1A. These transcripts were used for gene ontology analyses based on molecular function, biological function, and pathway analyses. Altered genes possessed binding or catalytic functions (46% each), transporter activity (5%), or signal transduction or regulated transcription (4% each) (Supplemental Fig. 3). Of the 147 transcripts altered, 11 were expressed sequence tag sequences with poorly defined biological functions. Approximately 26 transcripts were identified based on sequence similarity via the Rat Genome Database (Supplemental Table 2).
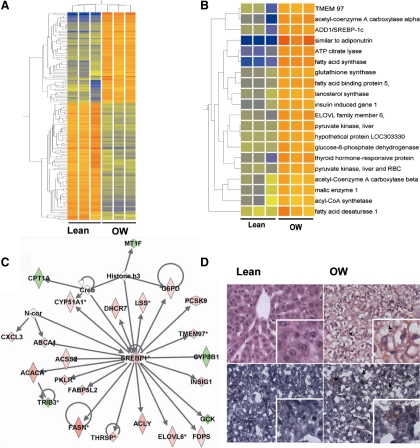
Figure 1.
A, Hierarchical clustering of 147 transcripts altered by maternal OW in offspring liver. Gene expression was assessed in offspring liver at PND21 using Rat Genome 230 2.0 microarrays (Affymetrix) (n = 3 microarrays per group). Genes were filtered based on a minimum ±1.8-fold change (OW vs. lean) and P value ≤0.05 using Student's t test. B, Correlation-based clustering of genes regulated by SREBP-1 with functions in carbohydrate or lipid metabolism derived from the list of genes altered by maternal OW. Heat maps were generated using GeneSpring Gx. Orange, yellow, and blue represent up-regulation, no relative effect, and down-regulation of transcripts, respectively. C, IPA gene network of highest significance identified using IPA software from the list of altered genes. A set of SREBP-1-regulated lipogenic genes and those involved in fatty acid/cholesterol metabolism is evident. Colors green and red represent down-regulation and up-regulation respectively. D, Representative photomicrographs of liver tissues from offspring of lean and OW dams at PND21. Top panel, H&E-stained sections; bottom panel, Oil Red O-stained sections. Magnification, ×400.
Of the genes with known biological functions, we identified 33 genes involved in carbohydrate/lipid metabolism and fatty acid biosynthesis, whose expression was altered in offspring of OW dams (Supplemental Table 2). Additionally, we found suites of genes with known roles in electron transport and cholesterol metabolism (Supplemental Table 2). We further used pathway analysis software (IPA) to identify common regulators of the altered genes. Two transcription factors, SREBP-1 and PPAR-α, were identified as critical nodes of regulation, consistent with increased hepatic lipid accumulation (Fig. 1C). Correlation-based hierarchical clustering of known SREBP-1 target genes involved in lipid biosynthesis is shown in Fig. 1B. A uniform induction of 20 SREBP-1-regulated genes, primarily involved in lipid biosynthesis, including SREBP-1, was revealed. Consistent with increased percent liver weight and lipogenic gene expression, histological examination of hematoxylin- and eosin-stained liver sections revealed enlarged hepatocytes and lipid accumulation (in Oil Red O-stained sections), characteristic of hepatic steatosis (Fig. 1D).
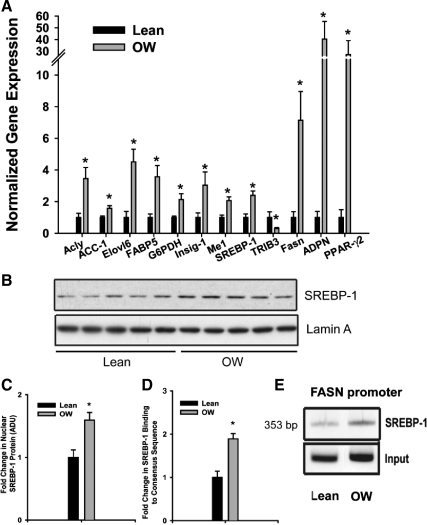
We performed independent verification of 11 lipogenesis-related genes using real-time RT-PCR (Fig. 2A). To further understand the induction in lipogenic genes, we also assessed expression of PPAR-γ2 (Fig. 2A). mRNA expression of all transcripts identified via microarray analyses was confirmed via real-time PCR. Most remarkably, expression of SREBP-1 (2.1-fold), ATP-citrate lyase (3.6-fold), FASN (7-fold), adiponutrin (40-fold), and PPAR-γ2 (30-fold) was significantly induced (P < 0.01) in livers from offspring of OW dams (Fig. 2A). Next we attempted to ascertain whether changes in SREBP-1 mRNA were translated to higher protein levels. Immunoblot analyses of hepatic nuclear extracts clearly showed ∼1.7-fold higher (P < 0.05) SREBP-1 protein levels in the OW-dam offspring compared with lean cohorts (Fig. 2, B and C). Greater nuclear SREBP-1 was also confirmed via TransAM ELISA, which uses binding to an SREBP-1 binding site followed by immunodetection. Consistent with immunoblotting results, TransAM ELISA revealed approximately 1.9-fold (P < 0.05) greater SREBP-1 protein levels in hepatic nuclear extracts from OW-dam offspring (Fig. 2D). Finally, we used ChIP assay to assess recruitment of SREBP-1 on binding sites on the FASN promoter. In addition to being induced transcriptionally in OW-dam offspring, FASN is recognized as a SREBP-1 target gene and has a well-characterized proximal promoter with defined SREBP-1 binding sites (25). Recruitment of SREBP-1 on the FASN promoter was increased approximately 2-fold (P < 0.05) in OW-dam offspring relative to lean controls, consistent with results from both gene expression and nuclear protein assessments (Fig. 2E).
Figure 2.
A, Hepatic mRNA expression of genes from offspring of lean and OW dams at PND21 (n = 8 per group). B–D, Gene expression was assessed via real-time RT-PCR. Hepatic SREBP-1 in nuclear extracts using Western blots (n = 5 per group) (B and C) and TransAM ELISA (n = 8 per group) (D) from offspring lean and OW dams at PND21. E, ChIP analyses of SREBP-1 recruitment on FASN promoter. Statistical differences were determined using a Student's t test. *, P < 0.05. ADU, Arbitrary density units.
Insulin-responsive lipogenic proteins are induced in offspring of OW dams
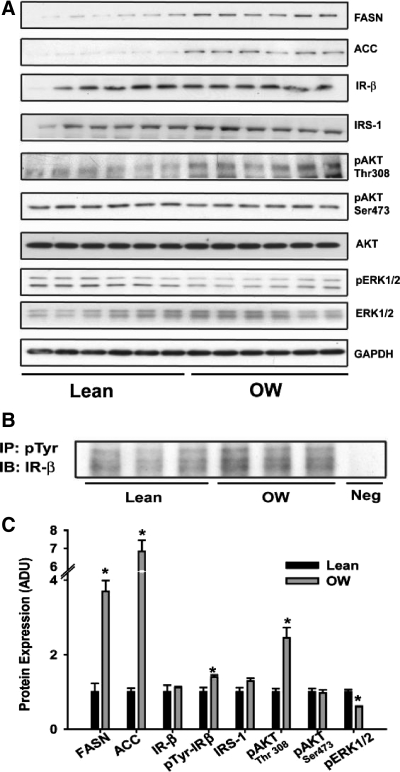
Because both serum insulin levels and hepatic expression of lipogenic genes (downstream of insulin-responsive SREBP-1) were elevated in offspring of OW dams, we examined whether components of insulin signaling were altered. First, protein expression of key lipogenic enzymes, FASN and acetyl coenzyme A carboxylase, were significantly induced (4- and 7-fold, respectively; Fig. 3, A and C), confirming both gene expression and hepatic steatosis data. No differences were observed in protein levels of IR-β, IRS-1, or total Akt levels (Fig. 3, A and C). However, tyrosine phosphorylation of IR-β and the phosphorylation of Akt at Thr308 were significantly increased (P < 0.05, Fig. 3, A–C) in liver of OW-dam offspring, consistent with modestly increased insulin signaling. We also observed significantly decreased phosphorylation of ERK1/2 in OW offspring (Fig. 3, A and C).
Figure 3.
A, Expression of lipogenic enzymes and signaling proteins in total lysates from livers of offspring from lean and OW dams at PND21 by Western blotting (n = 6 per group). B, Immunoprecipitation (IP) of phosphotyrosine in total liver lysates from offspring at PND21 (n = 3 pools representing a total of eight animals per group). Immunoblotting (IB) was performed using anti-IR-β antibody. C, Densitometric quantitation of immunoblots from offspring at PND21. Statistical differences were determined using a Student's t test. *, P < 0.05. ADU, Arbitrary density units.
Maternal OW decreases PPAR-α-AMPK signaling in offspring liver
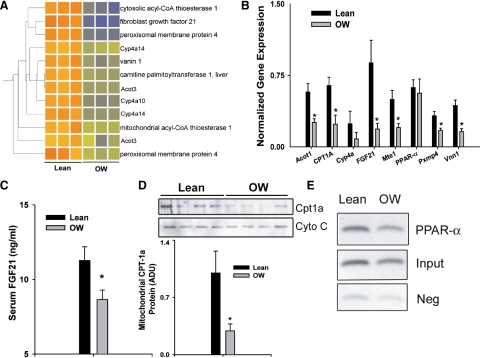
Examination of microarray data suggested that mRNA expression of at least eight PPAR-α-regulated genes was decreased in livers of OW-dam offspring at PND21. Because PPAR-α is a key regulator of lipid catabolic processes, we further examined whether maternal OW programmed decreased PPAR-α in offspring liver. Hierarchical clustering of these genes is depicted in Fig. 4A. Using real-time PCR, we confirmed decreased gene expression of at least six genes (Fig. 4B). Most genes were involved in ω-hydroxylation or peroxisomal β-oxidation of fatty acids. Importantly, gene expression of FGF21 and Cpt-1a was decreased approximately 5- and 2-fold, respectively (P < 0.05, Fig. 4B). Serum concentrations of FGF21 were also significantly lower (P < 0.05) in offspring of OW dams (Fig. 4C). Mitochondrial Cpt-1a protein in OW-dam offspring was decreased to 30% of levels in lean-dam offspring, confirming gene expression results (Fig. 4D). Moreover, ChIP analyses revealed that recruitment of PPAR-α to the FGF21 promoter was significantly lower in livers of OW-dam offspring (P < 0.05, Fig. 4E). These data strongly suggest that offspring of OW dams demonstrate decreased hepatic PPAR-α signaling.
Figure 4.
A, Correlation-based clustering of genes regulated by PPAR-α from the list of transcripts altered by maternal OW. B, mRNA expression of PPAR-α-regulated genes in livers of offspring from lean and OW dams at PND21 (n = 8 per group). Gene expression was assessed via real-time RT-PCR. C, Serum FGF21 levels determined by RIA in offspring of lean and OW dams at PND21 (n = 8 per group). D, Mitochondrial Cpt-1a protein levels and densitometric quantitation in liver of offspring at PND21 (n = 4 per group, each pool represents two separate animals). E, ChIP analyses showing PPAR-α recruitment on FGF21 promoter. Statistical differences were determined using a Student's t test. *, P < 0.05. ADU, Arbitrary density units.
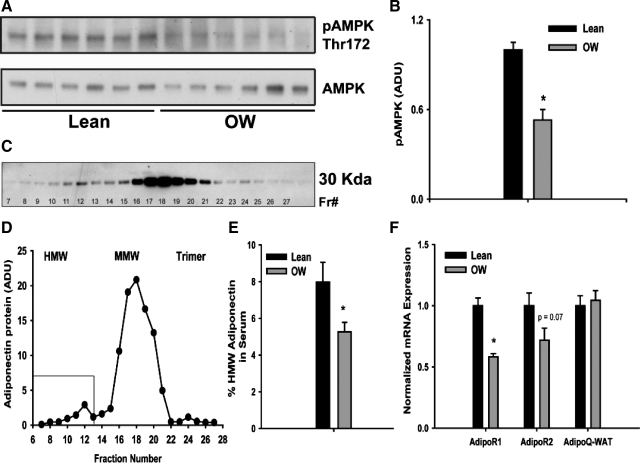
AMPK, a critical regulator of fatty acid catabolic processes, also cooperates with PPAR-α signaling. We hence examined phosphorylation status of AMPK in liver lysates from lean- and OW-dam offspring. Although total AMPK protein levels remained unchanged, phosphorylation of AMPK (at Thr172) was decreased to 50% in OW-dam offspring (P < 0.05, Fig. 5, A and B). Circulating adiponectin has been implicated in regulating both AMPK and PPAR-α signaling. Moreover, recent studies have suggested that HMW forms of adiponectin may be important in metabolic signaling (20,26,27). Serum concentrations of total adiponectin were modestly lowered in OW-dam offspring, albeit not significantly (Table 1). FPLC-based separation of serum was used to specifically quantitate HMW forms of circulating adiponectin (Fig. 5, C and D). Offspring from OW dams showed a 32% decrease in HMW adiponectin levels (P < 0.05) compared with offspring of lean dams at weaning (Fig. 5E). Adiponectin mRNA levels in retroperitoneal adipose tissues were unchanged, suggesting that maternal OW affects either packaging and/or secretion of adiponectin from adipocytes (Fig. 5F). Hepatic expression of AdipoR1 mRNA was also significantly (P < 0.05) lower in offspring of OW dams with modest nonsignificant decrease observed in AdipoR2 mRNA expression (Fig. 5F). These findings collectively suggest that exposure to maternal OW decreased adiponectin-AMPK-PPAR-α signaling along with several downstream targets involved in lipid catabolism.
Figure 5.
A and B, Expression and densitometric quantitation of hepatic phosphorylated (Thr172) and total AMPK in total liver lysates from offspring of lean and OW dams at PND21. C and D, Analyses of adiponectin monomers by immunoblotting after FPLC-based separation of serum from offspring at PND21. Thirty fractions (0.215 ml) from over the retention volume of the complexes (7–14 ml) were collected. Aliquots of each fraction were denatured and separated by SDS-PAGE. E, Percentage of HMW adiponectin in serum of offspring from lean or OW dams at PND21 (n = 8 per group). F, mRNA expression of AdipoR1 and AdipoR2 in liver and adiponectin in retroperitoneal white adipose tissue in offspring at PND21 (n = 8 per group). Gene expression was assessed via real-time RT-PCR. Statistical differences were determined using Student's t test. *, P < 0.05. ADU, Arbitrary density units.
Discussion
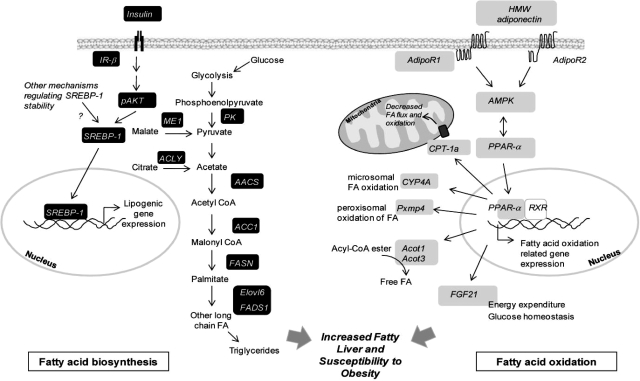
The risk of obesity in adulthood is subject to programming beginning at conception. Although several reports have confirmed similar programming of offspring obesity risk, much remains to be known about the underlying mechanisms (28,29,30,31,32,33,34,35). We aimed at identifying critical endocrine and transcriptome changes before the development of overt obesity in the offspring. Several novel findings are evident from the present studies. In the absence of changes in body weights at weaning, offspring of OW dams demonstrated 1) underlying endocrine abnormalities in systemic insulin, resistin, and adiponectin levels; 2) global changes in the hepatic transcriptome, revealing a reprogramming of lipogenic and lipid degradative pathways; and 3) changes in critical signaling pathways regulating nutrient utilization (AMPK-PPAR-α) and lipogenesis (SREBP-1) in the offspring. A schematic summarizing the changes in OW-dam offspring is presented in Fig. 6.
Figure 6.
Schematic summarizing changes in genes regulating lipid biosynthesis and oxidation in offspring liver at PND21. Genes represented in black and gray boxes are up-regulated and down-regulated, respectively (either transcriptionally or via phosphorylation). Overall, genes involved in lipid biosynthesis and insulin signaling are up-regulated, and lipid oxidation regulating genes via PPAR-α and AMPK are down-regulated.
We used TEN for its ability to overfeed rats in a controlled manner while maintaining dietary composition. This is a distinctive aspect of the present experimental design. Using this model, we replicated many of the metabolic and endocrine features of OW individuals in TEN-fed OW dams, including hyperinsulinemia, hyperleptinemia, insulin resistance, and increased serum triglyceride and NEFA concentrations (12). Our data are consistent with results from White et al. (31) suggesting that maternal adiposity is a key determinant of fetal programming. Although high-fat consumption certainly contributes to increased weight gain, obesity in the population at large results from a variety of meal and activity patterns. The precise control of gestational weight gain is another important aspect of the experimental design. Because high gestational weight gain significantly increases the risk of childhood obesity (36), we matched weight gains in both the lean and OW rats. Our data suggest that maternal OW even in the absence of excessive weight gain has significant impact on offspring metabolism at weaning. Finally, by limiting the exposure of offspring to maternal OW specifically to gestation, we excluded confounding variables such as changes in lactation efficiency and milk quality in OW dams. Hence, the differences between offspring of lean and OW dams are direct consequences of programming events initiated in utero.
A central finding of our studies is the identification of two loci controlling fatty acid metabolism, SREBP-1 and PPAR-α, as potential targets of programming. SREBP-1 is exquisitely sensitive to circulating insulin and serves as a critical effector of the lipogenic arm of insulin (37,38,39). Importantly, our results highlight that not only is SREBP-1 mRNA induced, but also a battery of (∼20) downstream targets involved in lipid and cholesterol biosynthesis regulated via SREBP-1 are coordinately increased. These are supported by definitive data showing increased recruitment of SREBP-1 to one of its target promoters. Recently, using a mouse model of diet-induced obesity, Bruce et al. (28) also reported increased hepatic gene expression of SREBP-1 and FASN in 15-wk-old offspring. Our results corroborate these findings and further suggest that lipogenic gene expression occurs before onset of obesity in the offspring of OW dams. Our data do not unequivocally address whether the observed hyperinsulinemia and increased hepatic insulin signaling underlie greater SREBP-1 mRNA/protein and subsequent lipogenic gene expression. However, offspring of OW dams demonstrate modestly higher circulating insulin levels and increased postreceptor signaling of some aspects in the liver. Insulin signaling is orchestrated through a complex network of mediators linked to two main signaling pathways: the phosphatidylinositol 3-kinase-AKT pathway and the Ras-MAPK pathway activated via growth factor receptor-bound protein 2/son of sevenless (Grb2/SOS). The former pathway is responsible for most of the metabolic aspects of insulin, whereas the latter regulates mitogenic responses and differentiation (40,41). Although generally considered distinct, there is cross talk between these pathways via ERK-mediated negative regulation of phosphatidylinositol 3-kinase through phosphorylation of IRS-1 at Ser636 (42). Offspring of OW dams demonstrated increased phosphorylation of AKT; however, phosphorylation of ERK1/2 was decreased, suggesting that specific aspects of insulin signaling may be targeted in the offspring. Increases in serum leptin concentrations were observed in offspring of OW dams, independent of changes in body adiposity. Because insulin is an important regulator of leptin gene expression and secretion (43,44), the increase in serum leptin may be the result of hyperinsulinemia observed in offspring of OW dams. Although speculative at this stage, it is also possible that higher leptin levels are a result of early leptin resistance in the offspring of OW dams.
Hepatic lipogenic signaling was enhanced in offspring of OW dams. However, we caution against an interpretation of systemic sensitization to insulin, because it is possible that insulin signaling pathways in the skeletal muscle may be inhibited in OW-dam offspring. Examples of such divergent insulin signaling between skeletal muscle and other insulin-sensitive tissues, such as liver (and adipose tissue), have been reported. Mice with muscle-specific deletion of IR (MIRKO mice) develop mild hyperinsulinemia without hyperglycemia (45). Although insulin-mediated glucose uptake in the muscle is decreased by 74%, uptake of glucose in the adipose tissue is increased 3-fold in these mice (45). Similarly, MIRKO mice demonstrate increased hepatic glycogen synthesis and glucose uptake in the liver (46). Furthermore, studies in rats infused with insulin chronically using osmotic mini-pumps suggest that although insulin-induced glucose utilization is decreased in muscles of insulinized rats, lipogenesis and glycogen synthesis in liver and white adipose tissues are increased (47,48). These studies demonstrate that mild hyperinsulinemia systemically may be sufficient to drive hepatic steatosis and obesity if the muscle is not responsive to insulin. In fact, data from our own studies in older offspring (at PND130) clearly reveal significant insulin resistance in the offspring of OW dams, despite increased lipogenic expression in liver and adipose tissues (12). Recent findings from two models of maternal OW also suggest that skeletal muscle insulin signaling is decreased in offspring in utero and at weaning (33,49). Furthermore, decreased circulating adiponectin and higher resistin levels are likely to decrease insulin sensitivity and glucose uptake in skeletal muscles (26,50). Hence, it appears that maternal OW may selectively increase lipogenic signaling via insulin in liver and adipose tissues.
Gestational exposure to OW is likely to alter signaling in multiple tissues in the developing offspring, and changes in circulating levels of HMW adiponectin might be one such target. Decreases in HMW adiponectin levels occurred in the absence of changes in adiponectin mRNA in the adipose tissue. The formation of higher-order complexes occurs in the adipocyte and is regulated mainly at the level of secretion (51,52). Hence, it is plausible that gestational OW may influence mechanisms regulating adiponectin complex formation. Binding of adiponectin to its receptors (AdipoR1 or AdipoR2) increases their interaction with the adaptor protein APPL1 (adaptor protein, PH domain, and leucine zipper containing 1), which promotes the phosphorylation of AMPK (53). Activated AMPK mediates its pleiotropic functions in target tissues, acting as an energy sensor, increasing fatty acid oxidation and insulin sensitivity, and inhibiting lipogenesis (26,54). Similarly, the nuclear receptor PPAR-α plays critical roles in mobilizing energy during energy-deficient states (55,56). AMPK cooperatively stimulates PPAR-α-dependent gene expression especially in the liver to increase expression of critical components of the peroxisomal and mitochondrial fatty oxidation pathways (57,58). Offspring of OW dams displayed a distinct down-regulation of both AMPK phosphorylation and PPAR-α-dependent target genes such as Cpt-1a and Cyp4A. Our results of lower hepatic AMPK phosphorylation are consistent with decreased HMW adiponectin levels and with previous results (33). In studies employing an overnourished sheep model of maternal obesity, Zhu and colleagues elegantly demonstrated that phosphorylation of AMPK in offspring skeletal muscle was decreased in late gestation (33). The present data demonstrating decreased AMPK phosphorylation in the liver are in line with their findings and suggest a global decrease in AMPK signaling due to maternal obesity.
In the present studies, FGF21 was identified as a novel target influenced by maternal OW. FGF21 is a hepatic hormone recently identified for its critical role to orchestrate integrative responses during fasting by promoting lipolysis in adipose tissue (59). Expression of FGF21 is regulated by PPAR-α (60,61), and recent studies have increasingly recognized its function as a regulator of whole-body energy balance (62). Mice overexpressing FGF21 develop resistance to diet-induced adiposity, and exogenous administration of FGF21 to mice ameliorates both genetically driven and diet-induced obesity, without decreasing caloric intake (59,63). Taken together, it is appealing to speculate that sustained decreases in FGF21 in offspring of OW dams might contribute to increased adiposity and adipose hypertrophy, as observed previously.
In conclusion, we have demonstrated that exposure to maternal obesity, specifically during gestation, results in hepatic steatosis and extensive gene expression changes in the liver of the offspring at weaning. Increased expression of a number of genes regulating lipid biosynthesis appears to be coordinated via the lipogenic transcription factor SREBP-1, associated with increased systemic insulin levels. Offspring born to OW dams also display lower circulating HMW adiponectin levels and decreased hepatic AMPK phosphorylation. Several downstream PPAR-α signaling targets including FGF21 are decreased in OW-dam offspring, consistent with hepatic steatosis and increased susceptibility to obesity. These results suggest targeting lipogenic pathways may be an effective strategy in mitigating increased adiposity.
Supplementary Material
Acknowledgments
We thank Matt Ferguson and the members of the Arkansas Children's Nutrition Center Animal Research Core Facility for their assistance with TEN. We thank Michael Blackburn, Jamie Badeaux, Renee Till, Crystal Combs, and Michèle Perry for their technical assistance. We gratefully acknowledge Dr. Victoria Esser (University of Texas Southwestern Medical Center, Dallas, TX) for providing the anti-Cpt-1a antibody.
Footnotes
This work was supported by National Institutes of Health Grant R01-DK084225 (to K.S.) and U.S. Department of Agriculture-ARS CRIS (Agricultural Research Service-Current Research Information System) 6251-51000-005-00D.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 6, 2010
Abbreviations: AMPK, AMP-activated protein kinase; BMI, body mass index; ChIP, chromatin immunoprecipitation; Cpt-1a, carnitine palmitoyl transferase-1a; CT, computerized tomography; FASN, fatty acid synthase; FGF21, fibroblast growth factor-21; FPLC, fast protein liquid chromatography; HFD, high-fat diet; HMW, high molecular weight; IPA, Ingenuity pathway analysis; IR, insulin receptor; IRS, IR substrate; NEFA, nonesterified fatty acids; NMR, nuclear magnetic resonance; OW, overweight; PND21, postnatal d 21; PPAR, peroxisome proliferator-activated receptor; SREBP-1, sterol regulatory element-binding protein-1; TEN, total enteral nutrition.
References
- Yeh J, Shelton JA 2005 Increasing prepregnancy body mass index: analysis of trends and contributing variables. Am J Obstet Gynecol 193:1994–1998 [DOI] [PubMed] [Google Scholar]
- King JC 2006 Maternal obesity, metabolism, and pregnancy outcomes. Annu Rev Nutr 26:271–291 [DOI] [PubMed] [Google Scholar]
- Castro LC, Avina RL 2002 Maternal obesity and pregnancy outcomes. Curr Opin Obstet Gynecol 14:601–606 [DOI] [PubMed] [Google Scholar]
- Mei Z, Grummer-Strawn LM, Scanlon KS 2003 Does overweight in infancy persist through the preschool years? An analysis of CDC Pediatric Nutrition Surveillance System data. Soz Praventivmed 48:161–167 [DOI] [PubMed] [Google Scholar]
- Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, Regan L, Robinson S 2001 Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord 25:1175–1182 [DOI] [PubMed] [Google Scholar]
- Levin BE 2000 The obesity epidemic: metabolic imprinting on genetically susceptible neural circuits. Obes Res 8:342–347 [DOI] [PubMed] [Google Scholar]
- Mingrone G, Manco M, Mora ME, Guidone C, Iaconelli A, Gniuli D, Leccesi L, Chiellini C, Ghirlanda G 2008 Influence of maternal obesity on insulin sensitivity and secretion in offspring. Diabetes Care 31:1872–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielzik S, Langnäse K, Mast M, Spethmann C, Müller MJ 2002 Impact of parental BMI on the manifestation of overweight 5–7 year old children. Eur J Nutr 41:132–138 [DOI] [PubMed] [Google Scholar]
- Kral JG, Biron S, Simard S, Hould FS, Lebel S, Marceau S, Marceau P 2006 Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics 118:e1644–e1649 [DOI] [PubMed] [Google Scholar]
- Smith J, Cianflone K, Biron S, Hould FS, Lebel S, Marceau S, Lescelleur O, Biertho L, Simard S, Kral JG, Marceau P 2009 Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab 94:4275–4283 [DOI] [PubMed] [Google Scholar]
- Villamor E, Cnattingius S 2006 Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet 368:1164–1170 [DOI] [PubMed] [Google Scholar]
- Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM 2008 Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol 294:R528–R538 [DOI] [PubMed] [Google Scholar]
- Badger TM, Ronis MJ, Lumpkin CK, Valentine CR, Shahare M, Irby D, Huang J, Mercado C, Thomas P, Ingelman-Sundberg M 1993 Effects of chronic ethanol on growth hormone secretion and hepatic cytochrome P450 isozymes of the rat. J Pharmacol Exp Ther 264:438–447 [PubMed] [Google Scholar]
- Badger TM, Crouch J, Irby D, Hakkak R, Shahare M 1993 Episodic excretion of ethanol during chronic intragastric ethanol infusion in the male rat: continuous vs. cyclic ethanol and nutrient infusions. J Pharmacol Exp Ther 264:938–943 [PubMed] [Google Scholar]
- Baumgardner JN, Shankar K, Hennings L, Badger TM, Ronis MJ 2008 A new model for nonalcoholic steatohepatitis in the rat utilizing total enteral nutrition to overfeed a high-polyunsaturated fat diet. Am J Physiol Gastrointest Liver Physiol 294:G27–G38 [DOI] [PubMed] [Google Scholar]
- Shankar K, Harrell A, Kang P, Singhal R, Ronis MJ, Badger TM 2010 Carbohydrate-responsive gene expression in the adipose tissue of rats. Endocrinology 151:153–164 [DOI] [PubMed] [Google Scholar]
- Korourian S, Hakkak R, Ronis MJ, Shelnutt SR, Waldron J, Ingelman-Sundberg M, Badger TM 1999 Diet and risk of ethanol-induced hepatotoxicity: carbohydrate-fat relationships in rats. Toxicol Sci 47:110–117 [DOI] [PubMed] [Google Scholar]
- Shankar K, Hidestrand M, Liu X, Xiao R, Skinner CM, Simmen FA, Badger TM, Ronis MJ 2006 Physiologic and genomic analyses of nutrition-ethanol interactions during gestation: Implications for fetal ethanol toxicity. Exp Biol Med (Maywood) 231:1379–1397 [DOI] [PubMed] [Google Scholar]
- Shankar K, Liu X, Singhal R, Chen JR, Nagarajan S, Badger TM, Ronis MJ 2008 Chronic ethanol consumption leads to disruption of vitamin D3 homeostasis associated with induction of renal 1,25 dihydroxyvitamin D3-24-hydroxylase (CYP24A1). Endocrinology 149:1748–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE 2008 Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology 149:2270–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Shankar K, Simmen RC 2009 Early soy exposure via maternal diet regulates rat mammary epithelial differentiation by paracrine signaling from stromal adipocytes. J Nutr 139:945–951 [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP 2003 Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264 [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y 1990 More powerful procedures for multiple significance testing. Stat Med 9:811–818 [DOI] [PubMed] [Google Scholar]
- Singhal R, Shankar K, Badger TM, Ronis MJ 2008 Estrogenic status modulates aryl hydrocarbon receptor-mediated hepatic gene expression and carcinogenicity. Carcinogenesis 29:227–236 [DOI] [PubMed] [Google Scholar]
- Wang Y, Jones Voy B, Urs S, Kim S, Soltani-Bejnood M, Quigley N, Heo YR, Standridge M, Andersen B, Dhar M, Joshi R, Wortman P, Taylor JW, Chun J, Leuze M, Claycombe K, Saxton AM, Moustaid-Moussa N 2004 The human fatty acid synthase gene and de novo lipogenesis are coordinately regulated in human adipose tissue. J Nutr 134:1032–1038 [DOI] [PubMed] [Google Scholar]
- Shetty S, Kusminski CM, Scherer PE 2009 Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol Sci 30:234–239 [DOI] [PubMed] [Google Scholar]
- Wang Y, Lam KS, Yau MH, Xu A 2008 Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J 409:623–633 [DOI] [PubMed] [Google Scholar]
- Bruce KD, Cagampang FR, Argenton M, Zhang J, Ethirajan PL, Burdge GC, Bateman AC, Clough GF, Poston L, Hanson MA, McConnell JM, Byrne CD 2009 Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 50:1796–1808 [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD 2008 Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51:383–392 [DOI] [PubMed] [Google Scholar]
- Waterland RA, Travisano M, Tahiliani KG, Rached MT, Mirza S 2008 Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes (Lond) 32:1373–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CL, Purpera MN, Morrison CD 2009 Maternal obesity is necessary for programming effect of high-fat diet on offspring. Am J Physiol Regul Integr Comp Physiol 296:R1464–R1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Yan X, Tong JF, Zhao J, Zhu MJ 2010 Maternal obesity, inflammation, and fetal skeletal muscle development. Biol Reprod 82:4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MJ, Han B, Tong J, Ma C, Kimzey JM, Underwood KR, Xiao Y, Hess BW, Ford SP, Nathanielsz PW, Du M 2008 AMP-activated protein kinase signalling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J Physiol 586:2651–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Govek E 1998 Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol 275:R1374–R1379 [DOI] [PubMed] [Google Scholar]
- McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL 2009 Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 119:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW 2007 Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol 196:322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M, Guichard C, Ferré P, Foufelle F 1999 Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci USA 96:12737–12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimano H, Yahagi N, Amemiya-Kudo M, Hasty AH, Osuga J, Tamura Y, Shionoiri F, Iizuka Y, Ohashi K, Harada K, Gotoda T, Ishibashi S, Yamada N 1999 Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J Biol Chem 274:35832–35839 [DOI] [PubMed] [Google Scholar]
- Griffin MJ, Sul HS 2004 Insulin regulation of fatty acid synthase gene transcription: roles of USF and SREBP-1c. IUBMB Life 56:595–600 [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR 2006 Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7:85–96 [DOI] [PubMed] [Google Scholar]
- Virkamäki A, Ueki K, Kahn CR 1999 Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J Clin Invest 103:931–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzakri K, Roques M, Gual P, Espinosa S, Guebre-Egziabher F, Riou JP, Laville M, Le Marchand-Brustel Y, Tanti JF, Vidal H 2003 Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes 52:1319–1325 [DOI] [PubMed] [Google Scholar]
- Leroy P, Dessolin S, Villageois P, Moon BC, Friedman JM, Ailhaud G, Dani C 1996 Expression of ob gene in adipose cells. Regulation by insulin. J Biol Chem 271:2365–2368 [DOI] [PubMed] [Google Scholar]
- Bradley RL, Cheatham B 1999 Regulation of ob gene expression and leptin secretion by insulin and dexamethasone in rat adipocytes. Diabetes 48:272–278 [DOI] [PubMed] [Google Scholar]
- Kim JK, Michael MD, Previs SF, Peroni OD, Mauvais-Jarvis F, Neschen S, Kahn BB, Kahn CR, Shulman GI 2000 Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle. J Clin Invest 105:1791–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Virkamaki A, Michael MD, Winnay JN, Zisman A, Kulkarni RN, Kahn CR 2000 A model to explore the interaction between muscle insulin resistance and β-cell dysfunction in the development of type 2 diabetes. Diabetes 49:2126–2134 [DOI] [PubMed] [Google Scholar]
- Cusin I, Terrettaz J, Rohner-Jeanrenaud F, Jeanrenaud B 1990 Metabolic consequences of hyperinsulinaemia imposed on normal rats on glucose handling by white adipose tissue, muscles and liver. Biochem J 267:99–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusin I, Rohner-Jeanrenaud F, Terrettaz J, Jeanrenaud B 1992 Hyperinsulinemia and its impact on obesity and insulin resistance. Int J Obes Relat Metab Disord 16(Suppl 4):S1–S11 [PubMed] [Google Scholar]
- Bayol SA, Simbi BH, Stickland NC 2005 A maternal cafeteria diet during gestation and lactation promotes adiposity and impairs skeletal muscle development and metabolism in rat offspring at weaning. J Physiol 567:951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppan CM, Lazar MA 2002 Resistin and obesity-associated insulin resistance. Trends Endocrinol Metab 13:18–23 [DOI] [PubMed] [Google Scholar]
- Wang ZV, Schraw TD, Kim JY, Khan T, Rajala MW, Follenzi A, Scherer PE 2007 Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol 27:3716–3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Wang H, Farmer SR 2007 Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-Lα. Mol Cell Biol 27:4698–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ 2006 APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 8:516–523 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T 2003 Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423:762–769 [DOI] [PubMed] [Google Scholar]
- Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W 1999 Peroxisome proliferator-activated receptor α mediates the adaptive response to fasting. J Clin Invest 103:1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon M 2009 The role of PPARα in lipid metabolism and obesity: focusing on the effects of estrogen on PPARα actions. Pharmacol Res 60:151–159 [DOI] [PubMed] [Google Scholar]
- Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB 2006 Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor α. Diabetes 55:2562–2570 [DOI] [PubMed] [Google Scholar]
- Bronner M, Hertz R, Bar-Tana J 2004 Kinase-independent transcriptional co-activation of peroxisome proliferator-activated receptor α by AMP-activated protein kinase. Biochem J 384:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB 2005 FGF-21 as a novel metabolic regulator. J Clin Invest 115:1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E 2007 Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5:426–437 [DOI] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA 2007 Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab 5:415–425 [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Mangelsdorf DJ 2010 Fibroblast growth factor 21: from pharmacology to physiology. Am J Clin Nutr 91:254S–257S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A 2008 Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149:6018–6027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.