Abstract
Phospholamban (PLB) is a critical regulator of Ca2+ cycling in heart muscle cells, and its gene expression is markedly down-regulated by T3. Nonetheless, little is known about the molecular mechanisms of T3-dependent gene silencing in cardiac muscle, and it remains unclear whether thyroid hormone receptors (TRs) directly bind at the PLB gene in vivo and facilitate transcriptional repression. To investigate the regulatory role of TRs in PLB transcription, we used a physiological murine heart muscle cell line (HL-1) that retains cardiac electrophysiological properties, expresses both TRα1 and TRβ1 subtypes, and exhibits T3-dependent silencing of PLB expression. By performing RNA interference assays with HL-1 cells, we found that TRα1, but not TRβ1, is essential for T3-dependent PLB gene repression. Interestingly, a PLB reporter gene containing only the core promoter sequences −156 to +64 displayed robust T3-dependent silencing in HL-1 cells, thus suggesting that transcriptional repression is facilitated by TRα1 via the PLB core promoter, a regulatory region highly conserved in mammals. Consistent with this notion, chromatin immunoprecipitation and in vitro binding assays show that TRα1 directly binds at the PLB core promoter region. Furthermore, addition of T3 triggered alterations in covalent histone modifications at the PLB promoter that are associated with gene silencing, namely a pronounced decrease in both histone H3 acetylation and histone H3 lysine 4 methylation. Taken together, our data reveal that T3-dependent repression of PLB in cardiac myocytes is directly facilitated by TRα1 and involves the hormone-dependent recruitment of histone-modifying enzymes associated with transcriptional silencing.
Thyroid hormone-dependent repression of phospholamban gene expression in heart muscle cells is facilitated by thyroid hormone receptor α1 and involves the direct recruitment of histone modifying enzymes at the phospholamban gene core promoter.
Changes in thyroid hormone levels markedly influence heart function (1,2). Elevated levels enhance the speed and force of myocardial contractions, increase cardiac output, and accelerate the heart rate. The molecular basis for these actions is largely due to altered gene expression of several key proteins that regulate intracellular calcium homeostasis in cardiac myocytes. Of special importance, elevated thyroid hormone levels activate the gene expression of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2a) and concomitantly repress the gene expression of phospholamban (PLB), a potent inhibitor of SERCA2a (reviewed in Ref. 3). Consequently, these thyroid hormone-induced changes enhance active transport of cytosolic Ca2+ into the sarcoplasmic reticulum and promote myocyte Ca2+ cycling, thereby exerting a strong positive effect on the force and rates of cardiac contraction and relaxation (4). Decreases in thyroid hormone trigger the opposite changes in the levels of these two proteins leading to reduced myocyte Ca2+ cycling and lowered cardiac contractility.
The physiological action of T3 in the heart is mediated primarily through thyroid hormone receptors (TRs), members of the nuclear hormone receptor superfamily that regulate transcription from target genes bearing T3-response elements (TREs) (5,6). There are two different yet highly homologous TR subtypes, TRα1 and TRβ1, both of which are expressed in the heart (7). TREs linked to positively regulated T3-responsive target genes (e.g. SERCA2a) generally contain two or more copies of the hexamer AGGTCA arranged as a tandem direct repeat (6). In the presence of T3, TRs bind to positive TREs as a heterodimer with the retinoid X receptor (RXR) and activate transcription by recruiting coactivators (5,6). The best-characterized TR coactivators include members of the p160/SRC family that act as platforms for the recruitment of potent histone lysine acetyltransferases (8) and histone arginine methyltransferases (9,10). Importantly, acetylation of lysine residues and methylation of arginine residues on histones H3 and H4, near the positive TREs of target genes, results in a modified chromatin structure that facilitates transcriptional activation (8,11). In the absence of T3, TRs remains bound at positive TREs and can repress transcription by recruiting the corepressors nuclear receptor corepressor (NCoR) or silencing mediator of retinoid and thyroid receptors (SMRT) that are in turn associated with histone deacetylases (HDACs) (6,12). The histone deacetylation subsequently leads to a chromatin structure that silences transcription (11,12).
Paradoxically, TRs can also facilitate T3-dependent transcriptional repression but only from distinct target gene promoters containing negative TREs (nTREs). nTRES are poorly defined DNA elements generally containing one or more TR-binding half-sites and are usually located close to, and sometimes downstream from, the transcription start site (5,13,14,15,16). The molecular events involved in T3-dependent gene silencing remain poorly understood despite the apparently large number of negatively regulated target genes (5,16,17,18). Studies examining T3-dependent gene repression in the pituitary gland suggest that T3-liganded TRs may recruit HDACs to nTREs of target gene promoters, thus repressing transcription (19), whereas other studies suggest that in the absence of T3, TR-NCoR/SMRT complexes may sequester HDACs away from the promoter (20). More recent studies suggest that liganded TRs might also bind to Ligand-dependent Corepressor (LCoR) (21), a component of the corepressor of transcription factor REST-carboxy terminal binding protein (CoREST-CtBP) complex that also contains HDACs and the histone lysine demethylase (HDM) lysine specific demethylase 1 (LSD1) (22,23). Relevant to a possible mechanism of T3-dependent repression, LSD1 specifically demethylates histone H3 lysine 4 (H3K4) (24), thus removing a histone mark that is strongly associated with transcriptional activation (25).
Although it is clearly established that T3 suppresses PLB gene expression in the heart (26,27,28,29), it remains unclear whether TRs actually bind at the PLB gene and facilitate transcriptional silencing or whether the repression mechanism involves changes in covalent histone modifications and chromatin structure at the PLB promoter. To address these issues here, we used a cardiac muscle cell line (termed HL-1) (30) that retains differentiated cardiac electrophysiological properties as a model system to study T3-regulated PLB gene expression. T3 treatment of HL-1 cells results in a marked decrease in PLB mRNA and protein expression as well as alterations in Ca2+ handling that would be expected due to increased activity of SERCA2a. By performing RNA interference knockdown assays in HL-1 cells, we found that TRα1, but not TRβ1, is essential for T3-dependent PLB gene repression. Moreover, by carrying out HL-1 cell chromatin immunoprecipitation (ChIP), as well as in vitro immobilized template assays, we found that TRα1 binds to a highly conserved core promoter region of the PLB gene. We further demonstrate that T3 stimulation triggers a pronounced decrease in both histone H3 acetylation and histone H3K4 methylation at the PLB promoter. Our findings show that T3-dependent repression of PLB in cardiac myocytes is directly facilitated by TRα1 and involves the hormone-dependent recruitment of histone modifying enzymes associated with transcriptional silencing.
Materials and Methods
Cell culture
The murine HL-1 cardiac myocyte cell line was provided by Dr. William Claycomb (Louisiana State University, New Orleans, LA) (30). HL-1 cells were cultured under a 5% CO2 atmosphere in Claycomb medium (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS; Gemini Bio-Products, West Sacramento, CA), 2 mm glutamine, 100 U/ml penicillin/streptomycin (Invitrogen), and 10 μm norepinephrine (Sigma). The medium was changed approximately every 24 h. Cells were grown in 10-cm culture dishes that were precoated with 5 mg/ml fibronectin (Sigma) in 0.02% gelatin (BD Biosciences, San Jose, CA) for 4 h at room temperature.
Antibodies, plasmids, and reagents
Antibodies against TRα1, SERCA2a, and α-tubulin were from Santa Cruz Biotechnology (Santa Cruz, CA), antibodies against acetylated-H3 were from Millipore (Billerica, MA), antibodies against H3K4–2me were from Abcam (Cambridge, MA), and antibodies against phospholamban were from Thermo Scientific (Waltham, MA). The rat pGL3-PLB (−156 to +64) promoter luciferase reporter gene was provided by Dr. H. Kirk Hammond (University of California, San Diego, CA) and was described previously (31). The empty pGL3 vector and luciferase assay kit were from Promega (Madison, WI). T3 was purchased from Sigma-Aldrich (St. Louis, MO).
RNA interference and electroporation
Smart pool small interfering RNA (siRNA) specific for mouse TRα1 and TRβ1 were purchased from Dharmacon (Lafayette, CO). A scrambled siRNA smart pool (Dharmacon) was used as a control. For electroporation, HL-1 cells were grown up to 70% confluence in fibronectin-coated plates. Cells were trypsinized, washed one time with PBS and one time with gene pulser electroporation buffer (Bio-Rad Laboratories, Hercules, CA). Then 1 × 106 cells were resuspended in 0.4 ml of electroporation buffer and transferred into 4 mm sterile electroporation cuvettes (Bio-Rad). Two hundred nanomoles of siRNA were then added and gently mixed by pipetting. The cells were electroporated at 200 V, 1500 μF for 25 msec. The cells were immediately resuspended in 1 ml of Claycomb medium containing 5% charcoal/dextran-stripped (CDS)-FBS and transferred to fibronectin-coated six-well culture plates. Forty-eight hours after electroporation, the cells were treated either without or with T3 (1 μm) for 24 h in 5% CDS-FBS containing culture medium and processed for RT-PCR.
RNA extraction, reverse transcription, and real-time PCR
Total RNA was extracted from HL-1 cells using TRIzol reagent (Invitrogen, Carlsbad, CA) according to manufacturer protocol. RNA concentration was measured by spectrophotometry at 260 nm. Reverse transcription was performed using 1 μg of total RNA, 1 U Moloney murine leukemia virus-reverse transcriptase (Invitrogen), 10 pm oligo-deoxythymidine, and 200 mm deoxynucleoside triphosphates as indicated by the manufacturer. Semiquantitative PCR was performed using Taq polymerase (Denville Scientific, Metuchen, NJ) with specific primers (below) for 30 cycles. Real-time PCR was performed using the Opticon continuous fluorescence detection system (MJ Research, Waltham, MA) using a power SYBR Green PCR mix (Applied Biosystems, Warrington, UK). The following primers were used for both semiquantitative and real-time PCR: PLB forward, 5′-ATG ACG ACG ATT CAA ATC TCT TGG-3′ and reverse, 5′-TGG GTT TGC AAA GTT AGG CAT AA-3′; mTRα1 forward, 5′-TTT ACC AAG ATC ATC ACC CC-3′ and reverse, 5′-TTG ACA TTA GCA GCA CAG CC-3′; mTRβ1 forward, 5′-ATG CAT CTA TGT TGG CAT GG-3′ and reverse, 5′-ATG ATC TGG TCT TCA CAT GG-3′; β-actin forward, 5′-ATG GAT GAC GAT ATC GCT G-3′ and reverse, 5′-ATG AGG TAG TCT GTC AGG T-3′.
Luciferase reporter gene assays
HL-1 cells (5 × 104) were seeded in 12-well plates in Claycomb medium containing 5% CDS-FBS (Gemini) without antibiotics 24 h before transfection. The cells were then transfected with 100 ng of either the rat pGL3-PLB (−156 to +64) promoter luciferase reporter gene or an empty pGL3 vector along with 100 ng of pSV-β-gal (Promega) as an internal control using Lipofectamine 2000 (Invitrogen) in culture medium containing 5% CDS-FBS. Twenty-four hours after transfection, the medium was replaced with fresh medium containing or lacking T3 (1 μm) and incubated for additional 24 h in 5% CDS-FBS Claycomb medium. Cells were then harvested, and an equal amount of protein content was assayed for luciferase activity using a luciferase assay kit (Promega) via a luminometer. Luciferase activity was normalized for both protein concentration and β-galactosidase activity.
ChIP
ChIP was carried out as described earlier (32). In brief, HL-1 cells were cultured in 5% CDS-FBS Claycomb media for 3 d followed by incubation with or without T3 (1 μm) for 24 h. The cells were then treated with 1% formaldehyde and the chromatin harvested via sonication. The sonicated chromatin was then subjected to immunoprecipitation using specific antibodies against TRα1, acetylated histone H3 (H3-Ac), dimethylated histone H3K4 (H3K4–2me), or nonspecific rabbit IgG as a control. The immunoprecipitated DNA was then analyzed by semiquantitative PCR using primers spanning different PLB promoter and open reading frame regions: PLB-A forward, 5′-TAG CAA GAG CCA AAT GCA CTG C-3′ and reverse, 5′-CAT AGC TCA GGC CCT TGA A-3′; PLB-B forward, 5′-TAT GTT AGC TCT TCC ACT CC-3′ and reverse, 5′-ATC GTC ACA GTG CAG AGC-3′; and PLB-C forward, 5′-ATA GCA GCA TGG CTG ATA AGC-3′ and reverse, 5′-ATA GCT TCA CAA GAG ACA GC-3′. All ChIP experiments were carried out at least three times. Image processing was performed via gel documentation using the Quantity One software (Bio-Rad) and quantitation was performed using National Institutes of Health Image software (Bethesda, MD).
Confocal microscopy measurement of spontaneous calcium waves
HL-1 cells were cultured ΔTC3 glass-bottomed dishes (Bioptechs, Butler, PA) for confocal imaging of Fluo-4 fluorescence to measure Ca2+ signaling. Cells were treated with 1 μm T3 for 72 h before being loaded in the dark with 10 μm Fluo-4-AM fluorescent calcium indicator (Invitrogen) in culture media for 60 min at 37 C. After the loading period, cells were washed four times by replacement of 50% of the loading media with Tyrode solution consisting of (in millimoles) 140 NaCl, 5 KCl, 2.5 CaCl2, 2 MgCl2, 10 HEPES (pH 7.2), with a measured osmolarity of 290 mOsm. Measurements of intracellular calcium handling were performed at room temperature on a Bio-Rad Radiance-2100 confocal microscope equipped with an argon (488 nm) laser on a Nikon Eclipse TE2000S inverted microscope using a ×60, 1.4 NA oil immersion objective. Fluorescent images were collected using Bio-Rad imaging software in line scan mode. A single pixel line was placed along the center of a randomly selected field (512 pixels × 512 pixels), and fluorescence intensity was recorded along that line once every 2 msec for 60 sec. From each dish 10–12 random fields were selected, and duplicate dishes were used for each experiment. Each experiment was repeated three times. Resulting images of Fluo-4 fluorescence were analyzed using a custom IDL (ITT Visual Information Solutions, Boulder, CO) routine (33) that fits the trace of the fluorescence data from each individual Ca2+ wave with a double-exponential function. From these exponential functions, various kinetic properties of each event can be calculated, including the decay time constant, the full duration at half-maximal, and the maximum amplitude of the fluorescence signal. Statistical analysis was conducted using Origin 8 software (OriginLab, Northampton, MA).
Immobilized template assay
The FLAG-tagged full-length human TRα1 and RXRα proteins expressed and purified from recombinant baculovirus-infected insect Sf9 cells was carried out as previously reported (34). The 220-bp rat PLB core promoter fragment (−156 to +64) was PCR amplified from the rat pGL3-PLB plasmid using the 5′ biotinylated primer 5′-TGA AGC ACA ACA TGT TAC CG-3′ and the reverse primer 5′-CAA TGC AGA CTG TTT AGT TGT G-3′. The 306-bp PLB intragenic region (+3001 to +3307) was PCR amplified from the sonicated genomic HL-1 DNA using the 5′ biotinylated primer 5′-ATA GCA GCA TGG CTG ATA AGC-3′ and the reverse primer 5′-ATA GCT TCA CAA GAG ACA GC-3′. The template DNA was purified using a gel extraction kit (QIAGEN, Valencia, CA) and quantitated by spectrophotometry. M-280 Streptavidin Dynal beads (Invitrogen) were resuspended in equilibration buffer (5 mm Tris-HCl, pH 7.5; 1 mm EDTA; 1M NaCl; 0.003% Nonidet P-40) and then conjugated with 100 ng biotinylated template for 1 h at room temperature with constant agitation. The immobilized templates were then concentrated with a magnetic particle concentrator (MPC; Dynal), washed once with equilibration buffer, and then once with binding buffer (20 mm HEPES, pH 7.6; 4 mm MgCl2; 60 mm KCl; 0.08 mm EDTA; 8 mm dithiothreitol; 10% glycerol; 0.4 mg BSA per milliliter; and 0.05% Nonidet P-40). The beads were then concentrated by MPC and then resuspended in 50 μl binding buffer along with 50 ng of TRα1, 50 ng RXRα, and 100 nm T3 for 1 h at room temperature as indicated in the text. The Dynal beads were then concentrated by MPC, washed three times with binding buffer, resuspended in 2× sodium dodecyl sulfate-loading buffer, resolved by 8% SDS-PAGE, and then analyzed via immunoblotting.
Immunoblotting
HL-1 whole-cell lysate preparation and immunoblotting were carried out essentially as described earlier (32) using 25 μg of protein per lane. For immunoblots involving phospholamban expression, whole-cell lysates were fractionated on a 15% polyacrylamide sodium dodecyl sulfate gel.
Statistical analysis
Data are presented as mean ± sd. Means for the various treatment groups were compared by Student t test. P < 0.05 was considered significant.
Results
HL-1 cells express TRs and exhibit T3-dependent silencing of PLB mRNA expression
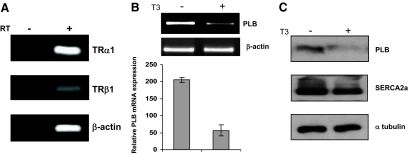
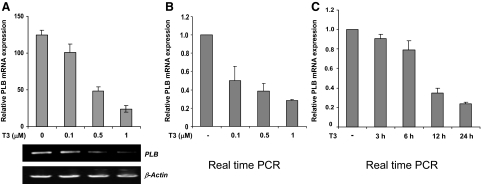
With the goal of studying T3-regulated gene expression in the heart, we asked whether mouse heart muscle-derived HL-1 cells (30) might be used as a physiological cardiomyocyte model system. To confirm that HL-1 cells express TRs and are thus appropriate for these studies, total RNA was extracted and then assayed by RT-PCR for TRα1 or TRβ1 mRNA expression. Consistent with previous TR immunohistochemistry studies in the mouse heart (7), mRNAs specific for both TRα1 and TRβ1 were detected with TRα1 being the more predominant subtype (Fig. 1A). Given the precedent for T3-dependent repression of PLB expression in the heart in vivo, we next asked whether T3 specifically represses PLB expression in HL-1 cells. Accordingly, HL-1 cells cultured with or without T3 were analyzed by RT-PCR for PLB mRNA expression and via immunoblot for PLB protein expression. Importantly, addition of T3 repressed PLB mRNA expression in HL-1 cells nearly 4-fold but had no significant effect on β-actin expression (Fig. 1B). We further found that T3 silenced PLB expression in a dose-dependent manner (Fig. 2, A and B) and that maximal repression occurs about 24 h after T3 treatment (Fig. 2C; data not shown). As expected, immunoblot analyses showed that addition of T3 decreased PLB protein expression in HL-1 cells nearly 3-fold yet had no significant inhibitory effect on SERCA2a or α-tubulin protein expression (Fig. 1C).
Figure 1.
HL-1 cells express TRs and exhibit T3-dependent silencing of PLB mRNA and protein expression. A, Total RNA was extracted from HL-1 cells and then assayed by semiquantitative RT-PCR using primers specific for TRα1, TRβ1, or β-actin. As a negative control, reverse transcriptase (RT) was omitted as indicated. B, T3 inhibits PLB mRNA expression. HL-1 cells cultured with or without T3 (1 μm) for 24 h were analyzed by RT-PCR using primers specific for PLB or β-actin. The PCR signals were quantitated using by gel documentation, normalized against β-actin expression, and then displayed graphically. Error bars represent the ±sd calculated from three separate experiments. C, Whole-cell lysate was prepared from HL-1 cells cultured with or without T3 (1 μm) for 72 h and then probed by immunoblot with antibodies against PLB, SERCA2a, or α-tubulin.
Figure 2.
T3 inhibits PLB gene expression in a dose- and time-dependent fashion. HL-1 cells were cultured with different concentrations of T3 for 24 h and then analyzed by RT-PCR semiquantitatively (A) or in real time (B). The semiquantitative RT-PCR results (A) were quantitated and displayed graphically as described in the Fig. 1 legend. C, HL-1 cells cultured with or without T3 (1 μm) for different lengths of time were analyzed by real-time RT-PCR. For both real-time RT-PCR experiments (A and B), values were normalized to β-actin expression. Error bars represent the ±sd calculated from three separate experiments. T3-untreated controls were set at 1.
T3-dependent increase in SERCA2a activity in HL-1 cells
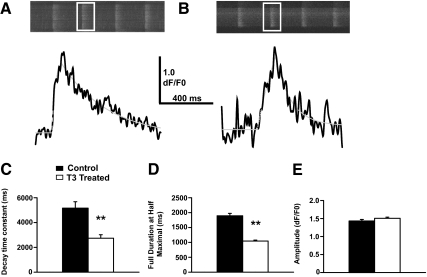
In the mammalian heart, PLB inhibits the activity of SERCA2a, the sarco/endoplasmic reticulum Ca2+-ATPase that regulates Ca2+ homeostasis in cardiomyocytes by pumping Ca2+ from the cytosol into the sarcoplasmic reticulum (3). Given our findings showing that T3 represses PLB gene expression in HL-1 cells, we investigated whether T3 treatment would alter intracellular Ca2+ homeostasis in ways that would be expected with decreased inhibition of SERCA2a activity. HL-1 cells display spontaneous Ca2+ waves that provide a useful index of the Ca2+ handling characteristics of this cell line when they undergo experimental manipulation (35). Fluo-4 Ca2+ indicator dye was used to image individual Ca2+ waves by confocal microscopy in HL-1 cells treated with vehicle control (Fig. 3A) or T3 (Fig. 3B). Measurement of the kinetic properties of these Ca2+ waves shows that their decay time constant decreased considerably in T3-treated cells compared with those treated with vehicle control (Fig. 3C). This indicates that the decay phase of the Ca2+ waves in T3-treated cells reduces at a higher rate, suggesting that increased SERCA2a activity accelerates Ca2+ uptake into the sarcoplasmic reticulum. Additionally, the full duration at half-maximal amplitude of Ca2+ waves decreases in the T3-treated cells (Fig. 3D) which is consistent with increased SERCA2a activity facilitating more rapid removal of Ca2+ from the cytosol. Although there are other potential modifications that could account for the observed changes in Ca2+ handling, the fact that the amplitude of the Ca2+ waves does not change (Fig. 3E) suggests that changes in the sarcoplasmic reticulum Ca2+ store or the Ca2+ release machinery do not contribute to the altered Ca2+ signaling in T3-treated cells. Taken together with the data in Figs. 1 and 2, our findings show that HL-1 cells accurately exhibit T3-dependent silencing of PLB gene expression and concomitantly exhibit physiological changes in SERCA2a activity analogous to that observed in the mammalian heart in vivo.
Figure 3.
Acceleration of decay kinetics in spontaneous Ca2+ waves indicates increased SERCA2a function in T3-treated HL-1 cells. Spontaneous Ca2+ waves in HL-1 cells that were either treated with T3 (1 μm) or vehicle control for 72 h were analyzed for their kinetic properties. A, Representative images of the fluo-4 fluorescent signal from control Hl-1 cells (top panels) with a trace (bottom panels, black line) of the mean fluorescent signal from the white boxed area. The trace data presented has been smoothed by a fast Fourier transform filter (4 point). A gray dashed line shows the function used to fit the fluorescence signal trace for analysis of kinetic properties. B, Same as in A for T3-treated HL-1 cells. C, T3 treatment (open bar) resulted in a decrease in the decay time constant (DTC) of Ca2+ waves when compared with a vehicle control (black bars). This suggests that Ca2+ clearance from the cytosol was accelerated due to T3 treatment, which is consistent with an increase of SERCA2a activity, resulting in increased uptake of Ca2+ into the sarcoplasmic reticulum. D, T3-treated cells also displayed a decreased duration of individual Ca2+ waves, which is also consistent with accelerated Ca2+ clearance for the cytosol due to elevated SERCA2a activity. E, The amplitude of individual Ca2+ waves in T3-treated cells was not significantly altered from those of vehicle-treated cells. Therefore, it is not likely that the alterations to the kinetics of the Ca2+ waves is due to alterations in the Ca2+ release machinery of, Ca2+ storage within the sarcoplasmic reticulum. Data are presented as mean ± sem. Statistical significance is determined by t test (**, P < 0.01).
TRα1 is required for PLB repression
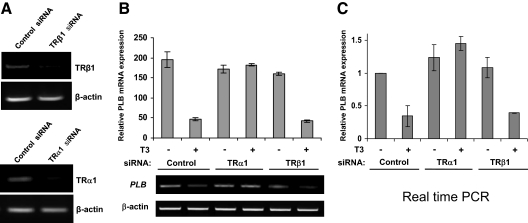
It remains unclear whether TRs are directly involved in T3-dependent repression of PLB gene expression in cardiomyocytes. To address this issue, we generated siRNA duplexes specific for either TRα1 or TRβ1 and then introduced the siRNAs into HL-1 cells via electroporation. Scrambled nonspecific siRNAs were transfected in parallel as a control. As shown in Fig. 4A, the knockdown efficiency of the TRα1 and TRβ1 siRNAs in HL-1 cells was greater than 80% as determined by RT-PCR. Interestingly, we found that knockdown of TRα1, but not TRβ1, completely abolished T3-dependent PLB gene repression in HL-1 cells (Fig. 4, B and C). Indeed, loss of TRα1 resulted in a modest T3-induced increase in PLB basal expression, possibly revealing a specific transcriptional stimulatory action of T3 that is manifest only in the absence of TRα1. These data show for the first time that TRα1 is specifically required for T3-dependent repression of PLB in cardiomyocytes and further suggest that TRα1 may be facilitating transcriptional repression by binding directly at the PLB gene promoter.
Figure 4.
Loss of TRα1 expression abolishes T3-dependent repression of PLB gene expression. A, HL-1 cells were electroporated with siRNA specific for either TRα1 or TRβ1 or with a nonspecific control siRNA. Total RNA was then extracted and assayed by semiquantitative RT-PCR using primers specific for TRα1, TRβ1, or β-actin. B and C, HL-1 cells were electroporated with siRNA specific for either TRα1 or TRβ1 or with a nonspecific control siRNA. The cells were then cultured with or without T3 (1 μm) for an additional 24 h. Total RNA was then extracted and analyzed by RT-PCR for PLB mRNA expression semiquantitatively (B) or in real time (C). Error bars represent the ±sd calculated from three separate experiments. In C, T3-untreated controls were set at 1. Quantitation and graphical representation were as outlined in the Fig. 2 legend.
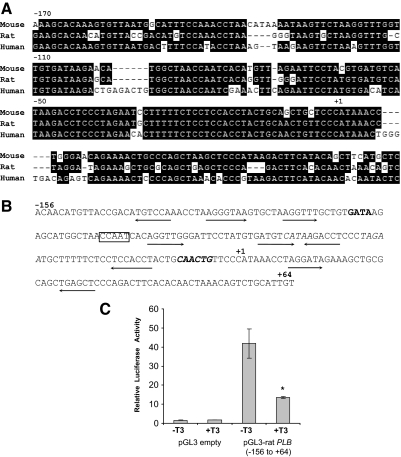
T3-dependent silencing of PLB gene expression is localized to the conserved core promoter region
Alignment of the promoter sequences from the mouse, rat, and human PLB gene reveal significant sequence conservation in the core promoter region (i.e. the promoter region immediately flanking the transcriptional start site) (36,37,38) (Fig. 5A). Indeed, the rat PLB core promoter fragment −159 to +64, containing conserved CCAAT, E-box, and GATA elements, was shown to be essential for basal PLB promoter activity in neonatal rat cardiomyocytes (31,39). Interestingly, we detected at least nine potential TR-binding half-sites within the rat −159 to +64 PLB promoter fragment, possibly implicating this region in negative T3-dependent regulation (Fig. 5B). To test this region for T3-dependent transcriptional regulation, we transfected a luciferase reporter gene driven by the rat −159 to +64 PLB promoter fragment into HL-1 cells and then measured transcription in the presence or absence of T3. Similar to the level of T3-dependent repression observed for endogenous PLB gene expression, addition of T3 repressed transcription of the PLB reporter gene nearly 3-fold (Fig. 5C). These results suggest that that PLB core promoter functions as a specific negative regulatory region for T3-dependent repression.
Figure 5.
T3-dependent silencing of PLB gene expression is localized to the conserved core promoter region. A, Alignment of mammalian PLB core promoter sequences. Nucleotide sequences from mouse (36), rat (37), and human (38) are shown. Numbers indicate relative nucleotide positions upstream of the transcription start site (+1). B, rat PLB core promoter sequences showing potential TR-binding half-sites (underlined arrows), potential TATA boxes (italics), GATA element (bold), CCAAT element (boxed), and an E-box (bold and italics). Numbers indicate relative nucleotide positions up- and downstream of the transcription start site (+1). C, HL-1 cells were transfected with either the rat pGL3-PLB (−156 to +64) promoter luciferase vector or an empty pGL3 vector along with pSV-β-gal as an internal control. The cells were then cultured with or without T3 (1 μm) for additional 24 h and then harvested for determination of luciferase activity via a luminometer. Luciferase activity was normalized for both protein concentration as well as β-galactosidase activity. Error bars represent the ±sd calculated from three separate experiments. *, Statistical significant difference, P < 0.01.
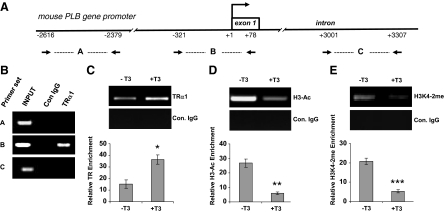
TRα1 directly binds at the PLB core promoter in vivo, and addition of T3 triggers changes in covalent histone modifications associated with transcriptional repression
Whether TRs directly bind at the PLB gene promoter in mammalian cells has remained unresolved. To begin to clarify this issue here, we performed ChIP analyses at the endogenous mouse PLB promoter using HL-1 cells together with an antibody specifically recognizing TRα1. Three different PCR primer sets were generated: one spanning an upstream PLB promoter region, another spanning a downstream intragenic region, and a third set spanning the PLB core promoter region (Fig. 6A). Consistent with the PLB promoter reporter gene assays, we clearly detected specific TRα1 occupancy at the core promoter region but not at the upstream or downstream promoter regions (Fig. 6B). Interestingly, occupancy of TRα1 at the core promoter was greater in the presence of T3 (∼2-fold), thus suggesting that T3 promotes TRα1 binding (Fig. 6C).
Figure 6.
TRα1 directly binds at the PLB core promoter in HL-1 cells, and addition of T3 triggers changes in covalent histone modifications associated with transcriptional repression. A, Schematic representation of the mouse PLB promoter including the relative positions of the three PCR primer sets. B and C, TRα1 binds at the PLB core promoter region and its occupancy increases in the presence of T3. Formaldehyde cross-linked chromatin was prepared from HL-1 cells cultured in normal serum (B) or additionally cultured in the presence of T3 (1 μm) for 24 h (C). ChIP was then carried out using an antibody specific for TRα1 or a nonspecific control (Con.) rabbit IgG and then analyzed by semiquantitative PCR using all three PCR primer sets (B) or primer set B alone (C). D and E, T3-dependent histone H3 deacetylation and demethylation at the PLB core promoter. ChIP was carried out on formaldehyde cross-linked chromatin prepared from HL-1 cells cultured with or without T3 using antibodies specific for H3-Ac or H3K4–2me and then analyzed by PCR using primer set B. The ethidium bromide-stained PCR bands in C–E were quantitated by gel documentation and are presented as the fold enrichment over the PCR signal generated by the control IgG. Error bars represent the ±sd calculated from three separate experiments. Statistical significant difference: *, P < 0.001; **, P < 0.005; ***, P < 0.004.
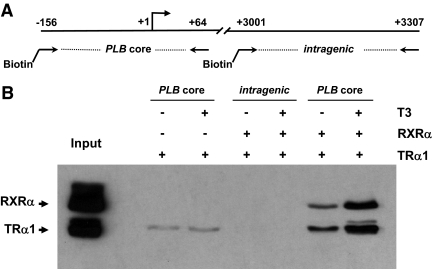
Because the resolution of ChIP in our laboratory is limited to 600- to 800-bp regions, we set out to more precisely test whether TRα1 directly binds at the PLB core promoter region by using a more defined in vitro immobilized template assay. Accordingly, we generated a 220-bp biotinylated rat PLB core promoter fragment (−156 to +64) and then incubated the template with recombinant baculovirus-expressed TRα1 or RXRα in the presence or absence of T3 (Fig. 7A). As a control, a biotinylated PLB intragenic region fragment (−3001 to −3307) was tested for binding in parallel. After precipitation of the templates via streptavidin beads and subsequent washing, the bound protein complexes were analyzed by immunoblot. In agreement with the ChIP assays, we found that TRα1 directly bound at the PLB core promoter (Fig. 7B). Moreover, we found that addition of RXRα markedly enhanced TRα1 binding as did the addition of T3. By contrast, no TRα1 or RXRα binding was detected at the PLB intragenic region in the presence or absence of T3. These data reinforce the notion that TRα1, apparently as a heterodimer with RXRα, directly binds at the PLB core promoter region and facilitates transcriptional repression in the presence of T3 in cardiomyocytes.
Figure 7.
TRα1 and RXRα directly bind to the PLB core promoter region in vitro. A, Schematic representation of the PLB promoter and intragenic region including the relative positions of the two biotinylated templates. B, TRα1 binds at the PLB core promoter, and its occupancy increases in the presence of RXRα and T3. The biotinylated PLB core promoter and intragenic templates were conjugated to streptavidin beads and then incubated with recombinant baculovirus-expressed FLAG-tagged TRα1 or RXRα in the presence or absence of T3 (10−7 m). The DNA-protein complexes were isolated, washed, fractionated by SDS-PAGE, and then probed by immunoblot using anti-FLAG antibodies.
Acetylation of lysine residues of histone H3 and H4 are covalent posttranslational modifications commonly associated with transcriptional activation (11), whereas deacetylation reverses this effect (8,25). Similarly, methylation of H3K4 is typically associated with active transcription, and loss of this mark is connected with transcriptional repression (25). Considering the fact that transcriptional regulation by TRs, both in the presence or absence of T3, is generally associated with changes in covalent histone modifications at target gene promoters (5,6), we again performed ChIP with HL-1 cells, this time using antibodies specific for acetylated H3 (H3-Ac) or dimethylated H3K4 (H3K4-2me) together with PCR primers spanning the core PLB promoter region. Interestingly, T3 triggered a pronounced decrease in both H3 acetylation (Fig. 6D) and H3K4 methylation (Fig. 6E) at the PLB promoter. Taken together, these findings indicate that distinct HDAC and HDM activities are recruited to the PLB gene promoter via TRα1 in a T3-dependent manner.
Discussion
Hyper- and hypothyroidism are among the most common endocrine disorders in the United States, affecting nearly 5% of the population (40). In turn, pathological alterations in T3 levels can exert profound effects on the heart and cardiovascular system (1,2). The molecular basis for these effects involve changes in the expression of multiple proteins including membrane ion channels and proteins involved in the regulation of intracellular Ca2+ homeostasis (3). Significantly, T3-dependent repression of PLB gene expression in cardiomyocytes is believed to contribute to the pathology of various heart disorders commonly associated with hyperthyroidism (e.g. tachycardia, atrial fibrillation, and heart failure) by enhancing Ca2+ cycling in the myocardium, thus leading to abnormal increases in the contractile force and rate of relaxation (3,4). Contrarily, pathological decreases in T3 (hypothyroidism) can elevate PLB expression, thus inhibiting Ca2+ cycling in cardiomyocytes and lowering cardiac contractility. Despite the clinical importance of T3-regulated PLB gene expression in the heart, very little is known about the T3-induced repression mechanism. Indeed, whether TRs directly bind at the PLB gene in vivo and facilitate transcriptional repression, and whether the putative repression mechanism involves changes in covalent histone modifications typically associated with transcriptional silencing, have remained unclear.
In this study, we used the mouse cardiomyocyte cell line HL-1 to investigate the mechanism of T3-regulated PLB gene expression in the heart. Whereas HL-1 cells retain several cardiac-like properties in culture (30), they only approximate the function of adult cardiomyocytes that are terminally differentiated. Nonetheless, using the HL-1 model system, we demonstrate for the first time that TRα1 is specifically required for T3-dependent repression of the PLB gene and that TRα1 directly binds at the PLB core promoter region. Furthermore, we show that addition of T3 triggers alterations in covalent histone modifications at the PLB promoter that are associated with gene silencing, namely a pronounced decrease in histone H3 acetylation and histone H3 lysine 4 methylation.
The identification of TRα1 as the primary TR subtype required for T3-dependent repression of cardiac PLB is consistent with previous TRα1 and TRβ1 knockout studies in mice showing that TRα1 has the more predominant contractile and electrophysiologic function in the heart (41,42,43). Furthermore, TRα1 is the more predominantly expressed TR subtype in the mammalian heart accounting for more than 70% of all TRs present in mouse cardiomyocytes (7,42). This estimate is in line with the relative mRNA expression levels of TRα1 and TRβ1 in HL-1 cells (Fig. 1A). Nonetheless, it is interesting to note that TRβ1was recently shown to specifically facilitate T3-dependent repression of the cardiac KCND3 potassium channel gene in rat cardiomyocytes via a distinct TR binding half-site in the core promoter region (44). Thus, structural differences between the PLB and KCND3 promoter regions, or distinct differences between the nTREs in each core promoter, likely influence the differential binding of specific TR subtypes (as well as their associated negative cofactors) at cardiac target genes. Indeed, our data showing TRα1/RXRα heterodimers can bind at the PLB core promoter in vitro contrasts the situation at the KCND3 gene in which TRβ1 apparently binds as a monomer or homodimer (43). Importantly, our findings showing that detectable levels of TRα1 are present at the PLB promoter in the absence of T3 further suggest that unliganded TRα1 may play a positive regulatory role in basal PLB gene expression.
Although TREs on positively regulated TR target genes have been well characterized (5,6), the TR-binding elements facilitating T3-dependent negative regulation (i.e. nTREs) are poorly defined and a consensus nTRE sequence has remained elusive. In general, nTREs comprise one or more TR-binding half-sites (loosely based on the consensus sequence AGGTCA) and are commonly found clustered in regions proximal to the transcription start site (13,14,15,16,44,45,46). We show here that T3-dependent repression of a rat PLB promoter reporter gene can be localized to a core promoter region containing the sequences −156 to +64 relative to the transcription start site. Importantly, in vitro immobilized template assays show that TRα1 directly binds to this region, either alone or as a heterodimer with RXRα, and that its binding efficiency is enhanced by T3 (Fig. 7B). Although the precise TRα1 binding site(s) remain undefined, at least nine potential TR-binding half-sites are present in the rat PLB core promoter, four of which are highly conserved in the both mouse and human PLB core promoters. In this connection, we found that in mouse HL-1 cardiomyocytes, TRα1 directly bound at the core PLB promoter and that its occupancy was enhanced by T3, consistent with the notion that that T3 promotes TRα1 binding at the PLB gene. Given the striking homology between the mouse, rat, rabbit, dog, and human PLB core promoter sequences (36,37,38,39) (Fig. 5A), it is conceivable that other conserved transcription factor binding elements in this region (CCAAT, GATA, and the E-box) may serve to facilitate TRα1 or TRα1/RXRα binding at the core promoter. Notably in this regard, binding sites for the GATA family of transcription factors are enriched proximal to androgen receptor-binding sites in the genome and are thought to cooperatively promote androgen receptor binding (47,48). Future studies will more precisely delineate the precise TRα1 binding elements in the PLB core promoter and reveal whether single or multiple nTREs are used TRα1 and/or TRα1/RXRα heterodimers.
We are aware that the T3 levels used in this study to suppress PLB gene expression in HL-1 cells are supraphysiological. This likely reflects poor uptake of T3/T4 into HL-1 cells in vitro, possibly due to reduced, absent, or ineffective expression of key thyroid hormone transporters upon adaption of the cardiac muscle cells to culture. Additionally, we and others (49,50) have routinely observed that T3/T4 levels required for gene repression in various cells lines in culture is typically higher than that required for gene activation. It is interesting to speculate that there may be a molecular basis for the relatively higher T3 levels required to observe TR-mediated repression in cell lines (e.g. hormone induced stabilization of the binding of TR and or TR/RXR to a weak nTRE).
Posttranslational covalent modifications on the amino-terminal tail domains of core histones markedly influence transcriptional activation and repression (8,11,25). Indeed, transcription regulation by TRs is typically associated with changes in covalent histone modifications at target gene promoters (5,6). We demonstrate here that T3 affects a pronounced decrease in both histone H3 acetylation and H3K4 methylation at the PLB promoter in cultured cardiomyocytes (Fig. 6, D and E). Both of these alterations are commonly associated with gene silencing (25). Our findings thus implicate TRα1 in the recruitment of HDAC and HDM activities to the PLB gene promoter in a T3-dependent manner in which the enzymes act as transcriptional corepressors. Previous studies examining transcriptional regulation of the thyroid-stimulating hormone-β gene in pituitary cells have led to two contrasting models by which TRs might facilitate T3-dependent repression. The first suggests that liganded TRs directly recruit HDAC-2 to the nTRE of the target promoter, thus silencing transcription (19), whereas the second model proposes unliganded TR-NCoR/SMRT complexes recruit HDACs away from the target promoter in the absence of T3 and allow HDACs to return to the promoter in the presence of T3 (20). Alternatively, T3-liganded TRs might also bind to ligand-dependent corepressor, a component of the corepressor of transcription factor REST-carboxy terminal binding protein corepressor complex that also contains HDAC-1 and -2 and the HDM LSD1 that can specifically demethylate H3K4 (21,22,23,24). Thus, it is conceivable that TRα1 might recruit HDAC and HDM activities to the PLB promoter in a single complex.
Whereas the findings here indicate that the T3-dependent reduction in histone H3 acetylation and H3K4 methylation may be important functional events that mediate PLB transcriptional silencing, other more simpler mechanisms may play contributing roles. For example, and in view of the number of putative nTREs found proximal to the PLB transcription start site, TRα1 binding at the core promoter region may inhibit the binding of other distinct general transcription factors commonly required by RNA polymerase II for transcriptional initiation (51). Future mutagenesis studies should precisely delineate the negative elements in the cardiac PLB core promoter that serve as binding sites for TRα1. Furthermore, future ChIP assays with T3-treated HL-1 cells should resolve between alternative repression mechanisms and additionally identify the specific corepressors, histone-modifying enzymes, and basal transcription factors that are recruited to, or dissociated from, the PLB promoter in a T3-dependent manner. Significantly, identifying the mechanisms and cofactors involved in T3-dependent PLB silencing in the heart may have important implications for treating hyper- and hypothyroid disorders and could lead to the development of novel chemical compounds that act therapeutically by blocking TR-cofactor binding or inhibit the functional (e.g. enzymatic) actions of the cofactors.
Acknowledgments
The authors acknowledge Dr. William Claycomb for the HL-1 cell line and Dr. H. Kirk Hammond for the rat pGL3-PLB reporter vector.
Footnotes
This work was supported by Grant 0855941D from the American Heart Association (to J.D.F.) and Grant AR054793 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (to N.W.).
Disclosure Summary: M.B., J.S., and P.S.R. have nothing to declare. J.D.F. received grant support (July 1, 2008–June 30, 2011) from the American Heart Association. N.W. received grant support (July 1, 2008–June 30, 2013) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
First Published Online April 14, 2010
Abbreviations: CDS, Charcoal/dextran-stripped; ChIP, chromatin immunoprecipitation; FBS, fetal bovine serum; H3-Ac, acetylated histone H3; HDAC, histone deacetylase; HDM, histone lysine demethylase; H3K4, histone H3 lysine 4; H3K4-2me, dimethylated histone H3K4; LSD1, lysine specific demethylase 1; MPC, magnetic particle concentrator; NCoR, nuclear receptor corepressor; nTRE, negative TRE; PLB, phospholamban; RXR, retinoid X receptor; SERCA2a, sarco/endoplasmic reticulum Ca2+-ATPase; siRNA, small interfering RNA; SMRT, silencing mediator of retinoid and thyroid receptor; TR, thyroid hormone receptor; TRE, T3-response element.
References
- Kahaly GJ, Dillmann WH 2005 Thyroid hormone action in the heart. Endocr Rev 26:704–728 [DOI] [PubMed] [Google Scholar]
- Klein I, Ojamaa K 2001 Thyroid hormone and the cardiovascular system. N Engl J Med 344:501–509 [DOI] [PubMed] [Google Scholar]
- Carr AN, Kranias EG 2002 Thyroid hormone regulation of calcium cycling proteins. Thyroid 12:453–457 [DOI] [PubMed] [Google Scholar]
- Fazio S, Palmieri EA, Lombardi G, Biondi B 2004 Effects of thyroid hormone on the cardiovascular system. Recent Prog Horm Res 59:31–50 [DOI] [PubMed] [Google Scholar]
- Yen PM 2001 Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- Zhang J, Lazar MA 2000 The mechanism of action of thyroid hormones. Annu Rev Physiol 62:439–466 [DOI] [PubMed] [Google Scholar]
- Stoykov I, Zandieh-Doulabi B, Moorman AF, Christoffels V, Wiersinga WM, Bakker O 2006 Expression pattern and ontogenesis of thyroid hormone receptor isoforms in the mouse heart. J Endocrinol 189:231–245 [DOI] [PubMed] [Google Scholar]
- Tsai CC, Fondell JD 2004 Nuclear receptor recruitment of histone-modifying enzymes to target gene promoters. Vitam Horm 68:93–122 [DOI] [PubMed] [Google Scholar]
- Stallcup MR 2001 Role of protein methylation in chromatin remodeling and transcriptional regulation. Oncogene 20:3014–3020 [DOI] [PubMed] [Google Scholar]
- Wysocka J, Allis CD, Coonrod S 2006 Histone arginine methylation and its dynamic regulation. Front Biosci 11:344–355 [DOI] [PubMed] [Google Scholar]
- Glass CK, Rosenfeld MG 2000 The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 14:121–141 [PubMed] [Google Scholar]
- Hu X, Lazar MA 2000 Transcriptional repression by nuclear hormone receptors. Trends Endocrinol Metab 11:6–10 [DOI] [PubMed] [Google Scholar]
- Hollenberg AN, Monden T, Flynn TR, Boers ME, Cohen O, Wondisford FE 1995 The human thyrotropin-releasing hormone gene is regulated by thyroid hormone through two distinct classes of negative thyroid hormone response elements. Mol Endocrinol 9:540–550 [DOI] [PubMed] [Google Scholar]
- Shen X, Li QL, Brent GA, Friedman TC 2004 Thyroid hormone regulation of prohormone convertase 1 (PC1): regional expression in rat brain and in vitro characterization of negative thyroid hormone response elements. J Mol Endocrinol 33:21–33 [DOI] [PubMed] [Google Scholar]
- Villa A, Santiago J, Belandia B, Pascual A 2004 A response unit in the first exon of the β-amyloid precursor protein gene containing thyroid hormone receptor and Sp1 binding sites mediates negative regulation by 3,5,3′-triiodothyronine. Mol Endocrinol 18:863–873 [DOI] [PubMed] [Google Scholar]
- Williams GR, Brent GA 1995 Thyroid hormone response elements. In: B.D. Weintraub, ed. Molecular endocrinology: basic concepts and clinical correlations. New York: Raven Press; 217–239 [Google Scholar]
- Feng X, Jiang Y, Meltzer P, Yen PM 2000 Thyroid hormone regulation of hepatic genes in vivo detected by complementary DNA microarray. Mol Endocrinol 14:947–955 [DOI] [PubMed] [Google Scholar]
- Oppenheimer JH, Schwartz HL, Mariash CN, Kinlaw WB, Wong NC, Freake HC 1987 Advances in our understanding of thyroid hormone action at the cellular level. Endocr Rev 8:288–308 [DOI] [PubMed] [Google Scholar]
- Sasaki S, Lesoon-Wood LA, Dey A, Kuwata T, Weintraub BD, Humphrey G, Yang WM, Seto E, Yen PM, Howard BH, Ozato K 1999 Ligand-induced recruitment of a histone deacetylase in the negative-feedback regulation of the thyrotropin β gene. EMBO J 18:5389–5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami T, Madison LD, Nagaya T, Jameson JL 1997 Nuclear receptor corepressors activate rather than suppress basal transcription of genes that are negatively regulated by thyroid hormone. Mol Cell Biol 17:2642–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes I, Bastien Y, Wai T, Nygard K, Lin R, Cormier O, Lee HS, Eng F, Bertos NR, Pelletier N, Mader S, Han VK, Yang XJ, White JH 2003 Ligand-dependent nuclear receptor corepressor LCoR functions by histone deacetylase-dependent and -independent mechanisms. Mol Cell 11:139–150 [DOI] [PubMed] [Google Scholar]
- Shi Y, Sawada J, Sui G, Affar el B, Whetstine JR, Lan F, Ogawa H, Luke MP, Nakatani Y, Shi Y 2003 Coordinated histone modifications mediated by a CtBP co-repressor complex. Nature 422:735–738 [DOI] [PubMed] [Google Scholar]
- Wang J, Scully K, Zhu X, Cai L, Zhang J, Prefontaine GG, Krones A, Ohgi KA, Zhu P, Garcia-Bassets I, Liu F, Taylor H, Lozach J, Jayes FL, Korach KS, Glass CK, Fu XD, Rosenfeld MG 2007 Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nature 446:882–887 [DOI] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y 2004 Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119:941–953 [DOI] [PubMed] [Google Scholar]
- Berger SL 2007 The complex language of chromatin regulation during transcription. Nature 447:407–412 [DOI] [PubMed] [Google Scholar]
- Arai M, Otsu K, MacLennan DH, Alpert NR, Periasamy M 1991 Effect of thyroid hormone on the expression of mRNA encoding sarcoplasmic reticulum proteins. Circ Res 69:266–276 [DOI] [PubMed] [Google Scholar]
- Kimura Y, Otsu K, Nishida K, Kuzuya T, Tada M 1994 Thyroid hormone enhances Ca2+ pumping activity of the cardiac sarcoplasmic reticulum by increasing Ca2+ ATPase and decreasing phospholamban expression. J Mol Cell Cardiol 26:1145–1154 [DOI] [PubMed] [Google Scholar]
- Kiss E, Jakab G, Kranias EG, Edes I 1994 Thyroid hormone-induced alterations in phospholamban protein expression. Regulatory effects on sarcoplasmic reticulum Ca2+ transport and myocardial relaxation. Circ Res 75:245–251 [DOI] [PubMed] [Google Scholar]
- Nagai R, Zarain-Herzberg A, Brandl CJ, Fujii J, Tada M, MacLennan DH, Alpert NR, Periasamy M 1989 Regulation of myocardial Ca2+-ATPase and phospholamban mRNA expression in response to pressure overload and thyroid hormone. Proc Natl Acad Sci USA 86:2966–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb WC, Lanson Jr NA, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo Jr NJ 1998 HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 95:2979–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao MH, Tang T, Guo T, Sun SQ, Feramisco JR, Hammond HK 2004 Adenylyl cyclase type VI gene transfer reduces phospholamban expression in cardiac myocytes via activating transcription factor 3. J Biol Chem 279:38797–38802 [DOI] [PubMed] [Google Scholar]
- Sharma D, Fondell JD 2002 Ordered recruitment of histone acetyltransferases and the TRAP/Mediator complex to thyroid hormone-responsive promoters in vivo. Proc Natl Acad Sci USA 99:7934–7939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisleder N, Ferrante C, Hirata Y, Collet C, Chu Y, Cheng H, Takeshima H, Ma J 2007 Systemic ablation of RyR3 alters Ca2+ spark signaling in adult skeletal muscle. Cell Calcium 42:548–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondell JD 2002 Gene activation by the thyroid hormone receptor in vitro and purification of the TRAP coactivator complex. Methods Mol Biol 202:195–214 [DOI] [PubMed] [Google Scholar]
- Landstrom AP, Weisleder N, Batalden KB, Bos JM, Tester DJ, Ommen SR, Wehrens XH, Claycomb WC, Ko JK, Hwang M, Pan Z, Ma J, Ackerman MJ 2007 Mutations in JPH2-encoded junctophilin-2 associated with hypertrophic cardiomyopathy in humans. J Mol Cell Cardiol 42:1026–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi K, Kadambi VJ, Koss KL, Luo W, Harrer JM, Ponniah S, Zhou Z, Kranias EG 1997 In vitro and in vivo promoter analyses of the mouse phospholamban gene. Gene 203:199–207 [DOI] [PubMed] [Google Scholar]
- Johns DC, Feldman AM 1992 Identification of a highly conserved region at the 5′ flank of the phospholamban gene. Biochem Biophys Res Commun 188:927–933 [DOI] [PubMed] [Google Scholar]
- McTiernan CF, Frye CS, Lemster BH, Kinder EA, Ogletree-Hughes ML, Moravec CS, Feldman AM 1999 The human phospholamban gene: structure and expression. J Mol Cell Cardiol 31:679–692 [DOI] [PubMed] [Google Scholar]
- McTiernan CF, Lemster BH, Frye CS, Johns DC, Feldman AM 1999 Characterization of proximal transcription regulatory elements in the rat phospholamban promoter. J Mol Cell Cardiol 31:2137–2153 [DOI] [PubMed] [Google Scholar]
- Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR 2007 Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002). Thyroid 17:1211–1223 [DOI] [PubMed] [Google Scholar]
- Forrest D, Vennström B 2000 Functions of thyroid hormone receptors in mice. Thyroid 10:41–52 [DOI] [PubMed] [Google Scholar]
- Gloss B, Trost S, Bluhm W, Swanson E, Clark R, Winkfein R, Janzen K, Giles W, Chassande O, Samarut J, Dillmann W 2001 Cardiac ion channel expression and contractile function in mice with deletion of thyroid hormone receptor α or β. Endocrinology 142:544–550 [DOI] [PubMed] [Google Scholar]
- Wikströom L, Johansson C, Saltó C, Barlow C, Campos Barros A, Baas F, Forrest D, Thorén P, Vennström B 1998 Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor α1. EMBO J 17:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassanov N, Er F, Michels G, Zagidullin N, Brandt MC, Hoppe UC 2009 Divergent regulation of cardiac KCND3 potassium channel expression by the thyroid hormone receptors α1 and β1. J Physiol 587:1319–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters KM, Miller CW, Ntambi JM 1997 Localization of a negative thyroid hormone-response region in hepatic stearoyl-CoA desaturase gene 1. Biochem Biophys Res Commun 233:838–843 [DOI] [PubMed] [Google Scholar]
- Wood WM, Kao MY, Gordon DF, Ridgway EC 1989 Thyroid hormone regulates the mouse thyrotropin β-subunit gene promoter in transfected primary thyrotropes. J Biol Chem 264:14840–14847 [PubMed] [Google Scholar]
- Masuda K, Werner T, Maheshwari S, Frisch M, Oh S, Petrovics G, May K, Srikantan V, Srivastava S, Dobi A 2005 Androgen receptor binding sites identified by a GREF_GATA model. J Mol Biol 353:763–771 [DOI] [PubMed] [Google Scholar]
- Wang Q, Carroll JS, Brown M 2005 Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol Cell 19:631–642 [DOI] [PubMed] [Google Scholar]
- Matsushita A, Sasaki S, Kashiwabara Y, Nagayama K, Ohba K, Iwaki H, Misawa H, Ishizuka K, Nakamura H 2007 Essential role of GATA2 in the negative regulation of thyrotropin β gene by thyroid hormone and its receptors. Mol Endocrinol 21:865–884 [DOI] [PubMed] [Google Scholar]
- Bogazzi F, Bartalena L, Brogioni S, Burelli A, Raggi F, Ultimieri F, Cosci C, Vitale M, Fenzi G, Martino E 2001 Desethylamiodarone antagonizes the effect of thyroid hormone at the molecular level. Eur J Endocrinol 145:59–64 [DOI] [PubMed] [Google Scholar]
- Chatterjee VK, Lee JK, Rentoumis A, Jameson JL 1989 Negative regulation of the thyroid-stimulating hormone α gene by thyroid hormone: receptor interaction adjacent to the TATA box. Proc Natl Acad Sci USA 86:9114–9118 [DOI] [PMC free article] [PubMed] [Google Scholar]