Abstract
Glucocorticoids act directly on bone cells to decrease production of osteoblasts and osteoclasts, increase osteoblast and osteocyte apoptosis, and prolong osteoclast life span. Conversely, daily injections of PTH decrease osteoblast and osteocyte apoptosis and increase bone formation and strength. Using a mouse model, we investigated whether the recently demonstrated efficacy of PTH in glucocorticoid-induced bone disease results from the ability of this therapeutic modality to counteract at least some of the direct effects of glucocorticoids on bone cells. Glucocorticoid administration to 5- to 6-month-old Swiss-Webster mice for 28 d increased the prevalence of osteoblast and osteocyte apoptosis and decreased osteoblast number, activation frequency, and bone formation rate, resulting in reduced osteoid, wall and trabecular width, bone mineral density, and bone strength. In contrast, daily injections of PTH caused a decrease in osteoblast and osteocyte apoptosis and an increase in osteoblast number, activation frequency, bone formation rate, bone mineral density, and bone strength. The decreased osteocyte apoptosis was associated with increased bone strength. When the two agents were combined, all the adverse effects of glucocorticoid excess on bone were prevented. Likewise, in cultured osteoblastic cells, PTH attenuated the adverse effects of glucocorticoids on osteoblast survival and Wnt signaling via an Akt phosphorylation-dependent mechanism. We conclude that intermittent PTH administration directly counteracts the key pathogenetic mechanisms of glucocorticoid excess on bone, thus providing a mechanistic explanation of its efficacy against glucocorticoid-induced osteoporosis.
PTH directly targets the key pathogenetic mechanisms associated with chronic glucocorticoid therapy by antagonizing glucocorticoid-induced attenuation of Akt phosphorylation and decreased β-catenin\TCF-mediated transcription, and increased apoptosis.
Daily injections of PTH are an effective treatment for patients with osteoporosis including those with the glucocorticoid-induced form of the disease (1,2,3,4,5). However, the exact mechanism(s) responsible for the efficacy of this therapy remains unclear. The prevailing explanations for the increase in osteoblast number associated with this treatment are increased osteoblastogenesis, attenuation of osteoblast apoptosis, activation of quiescent lining cells, or some combination of the three (6). The efficacy of this treatment also seems diminished or delayed with previous or concurrent bisphosphonate therapy, most likely because bisphosphonates decrease bone remodeling and thereby the number of osteoblasts, although some disagreement on this issue persists (7,8,9,10,11). However, the decreased bone remodeling typically found in glucocorticoid-induced osteoporosis does not seem to prevent a vigorous anabolic response to PTH, but the mechanism(s) underlying this response has yet to be determined (3,4,5).
Histological studies in patients receiving long-term glucocorticoid treatment consistently show reduced numbers of osteoblasts on cancellous bone (12,13,14,15). Decreased osteoblast numbers are due to reduced production of new osteoblast precursors as well as premature apoptosis of mature, matrix-secreting osteoblasts (13). These features result in the most characteristic finding in glucocorticoid-induced osteoporosis: a decrease in the amount of bone formed during the remodeling cycle (12,13,14). This finding is manifested by a reduction in the distance from a cement line to a quiescent cancellous perimeter, known as the wall width, and a decrease in the rate of bone formation as determined by the separation between two time-spaced tetracycline labels multiplied by the linear extent of the labels (15). Inadequate numbers of osteoblasts and reduced wall width are also the cause of the reduction in trabecular width in glucocorticoid-induced osteoporosis, a result of incomplete cavity repair during bone remodeling (13). With glucocorticoid excess, the production of osteoclasts decreases but the number of these cells is maintained due to prolongation of osteoclast life span (16). Quite a different set of events occurs with daily injections of PTH. Indeed, we previously reported that daily injections of PTH increased the life span of mature osteoblasts by decreasing the prevalence of osteoblast apoptosis and that this antiapoptotic effect of PTH was responsible for the increase in wall width, trabecular width, bone formation rate, and bone mass (17).
In studies described herein using the mouse model, we investigated the mechanistic basis of the efficacy of intermittent PTH administration in the setting of glucocorticoid excess. We report that the expected increase in the prevalence of osteoblast and osteocyte apoptosis and the decrease in osteoblast number, bone formation, and strength were prevented by simultaneous PTH administration. Decreased osteoblast apoptosis was related to an increase in the rate of bone formation and decreased osteocyte apoptosis was related to preservation of bone strength. Consistent with these findings, PTH abrogated the suppressive effect of glucocorticoids on protein kinase B (Akt) phosphorylation and Wnt signaling (wingless signaling pathway).
Materials and Methods
Animals
Mice were electronically tagged (Biomedic Data System Inc., Maywood, NJ) and kept in plastic cages (one animal per cage) under standard laboratory conditions with a 12-h dark, 12-h light cycle and a constant temperature of 20 C and humidity of 48%. Mice were fed a standard rodent diet (Agway RMH 3000, Arlington Heights, IL) containing 22% protein, 5% fat, 5% fiber, 6% ash, 3.5 kcal/g, 1.0 IU vitamin D3 per gram, 0.97% calcium, and 0.85% phosphorus with water ad libitum. The Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences and the Animal Care and Use Committee of the Central Arkansas Veterans Healthcare System approved these studies. Five- to 6-month-old Swiss-Webster male mice were purchased from Harlan Inc. (Indianapolis, IN). We implanted slow-release pellets (Innovative Research of America, Sarasota, FL) of placebo or 2.1 mg/kg · d of prednisolone for 28 d, a dose equivalent to 20 mg/d prednisolone to a human according to the relationship with body size, kilograms3/4 known as the metabolic weight (13,16,18). Human PTH 1-34 (Bachem, Torrance, CA) or vehicle was given as daily injections of 100 ng/g · d (17). There were four groups of animals (n = 5–11/group): placebo and vehicle, prednisolone and vehicle, placebo and PTH, and prednisolone and PTH.
Bone mass measurements
Spinal bone mineral density (BMD) of L1-4 was measured using a QDR-1000 with customized murine software (Hologic, Inc., Bedford, MA) as previously described (13). Reproducibility was assessed by repeated measurement of a plastic-embedded murine phantom and the coefficient of variation was 2.32%. BMD measurements were obtained at the beginning of the experiment and 28 d after pellet implantation. Before scanning, animals were sedated with 120 mg per 10 g body weight sodium pentobarbital (im) (Sigma-Aldrich, St. Louis, MO). Microcomputed tomography (micro-CT) analysis of the distal femora was done after the bones were dissected, cleaned, fixed in 10% Millonig's formalin, transferred to ethanol, loaded into 12.3-mm-diameter scanning tubes, and imaged (μCT40; Scanco Medical, Basserdorf, Switzerland). Scans were integrated into three-dimensional (3-D) voxel images (1024 × 1024 pixel matrices for each individual planar stack). A Gaussian filter (sigma = 0.8, support = 1) was used to reduce signal noise and a threshold of 200 was applied to all analyzed scans. Scans were done at 12 μm resolution (E = 55 kVp, I = 145 μA, integration time = 200 msec). In the distal femur, 151 transverse slices were taken from the epicondyles and extending toward the proximal end of the femur. Manual analysis excluded the cortical bone and the primary spongiosa from the analysis of the cancellous bone tissue. All trabecular measurements were made by manually drawing contours every 10–20 slices and using voxel counting and sphere filling distance transformation indices. Cortical thickness was measured at the femoral middiaphysis. Micro-CT measurements were expressed in 3-D nomenclature (19).
Bone strength measurements
The load-bearing properties of L6 were measured using a single-column material testing machine and a calibrated tension/compression load cell (model 5542; Instron Corp., Canton, MA) as previously described (20,21). Load cell calibration was verified in accordance with American Society for Testing and Materials E74-02 standards and traceable to the National Institute of Standards and Technology. Data were recorded and analyzed using the Merlin IX software package (Instron). The L6 specimens were cleaned of surrounding soft tissue, wrapped in gauze soaked in 37 ± 0.5 C normal saline, and tested on the day the animals were killed. The length, width, and depth of the bones were recorded with a digital caliper at a resolution of 0.01 mm (Mitutoyo no. 500-196; Ace Tools, Ft. Smith, AR). The cross-sectional area was assumed to be an ellipse and calculated as A = 0.25 π (width) (depth). Articular and spinous processes that would interfere with compression were excised using an iris scissors. After preseating with less than 0.5 newtons of applied load, vertebrae were compressed between screw-driven loading platens using a lower-platen, customized miniature spherical seat that minimizes shear by adjusting to irregularities in the end plates of the specimens. Best seating was obtained with the load applied along the caudocephalad axis at a speed of 0.5 mm/min until failure. Standard materials (200 mg coated tablets of generic ibuprofen) for compression were run before each set of determinations. The maximum load at the breaking point was normalized for bone size and expressed in megapascals (or newtons per square millimeter).
Bone histomorphometry
The lumbar vertebrae (L1-L4) were fixed and embedded undecalcified in methylmethacrylate, and the histomorphometric examination was done on longitudinal sections with a digitizer tablet (OsteoMetrics, Inc., Decatur, GA) interfaced to a Zeiss Axioscope (Carl Zeiss, Thornwood, NY) with a drawing tube attachment, as previously described (13,17,20). Apoptosis of osteoblasts was detected by in situ nick-end labeling using the Klenow enzyme (EMD Chemicals Inc., Gibbstown, NJ) in sections counterstained with 2% methyl green as previously described (21). Apoptotic osteoblasts were identified as cells with brown, pyknotic nuclei lining the cancellous bone perimeter and apoptotic osteocytes as brown, pyknotic cells buried in lacunae within mineralized bone. To calculate the extent of apoptosis, an average of 1020 ± 480 (sd) osteoblasts and 2800 ± 340 osteocytes were counted per vertebral bone section (22). Osteocyte density was expressed as the total number of osteocytes per cancellous bone area, as previously reported (17). Vertebral cancellous bone measurements were restricted to the secondary spongiosa and expressed in two-dimensional nomenclature (19). Osteoclasts were identified by their morphology and positive tartrate-resistant acid phosphatase staining. Bone formation rate was defined as the distance between the double-tetracycline labels multiplied by the sum of the double-labeled perimeter plus half of the single-labeled perimeter and expressed using the cancellous bone perimeter as a referent. Wall width was measured as the mean distance from the cement line to the overlying quiescent cancellous bone perimeter. The probability that a new cycle of remodeling will be initiated at any point on the cancellous bone perimeter or activation frequency was calculated by dividing the bone formation rate by the wall width.
Plasmids (p), transfections, and luciferase assay
pcDNA and enhanced green fluorescent protein (pEGFP) were purchased from Invitrogen (Carlsbad, CA) and CLONTECH (Mountain View, CA), respectively. A reporter plasmid carrying three T cell factor (TCF) binding sites upstream of a minimal c-fos promoter driving the firefly luciferase gene (TOPFLASH) (23) was provided by B. Vogelstein (Johns Hopkins University Medical Institutions, Baltimore, MD). The cDNAs for wild-type Akt and dominant-negative (dn) Akt were provided by M. E. Greenberg (Harvard Medical School, Boston, MA). The dnAkt is a catalytically inactive mutant with a K179M substitution (24).
To assay β-catenin/TCF-mediated transcription, cells were transfected with 0.2 μg TOPFLASH, 0.1–0.2 μg pcDNA, or 0.1 μg of each constitutive active or dnAkt mutants using Lipofectamine Plus (Invitrogen) to a total of 0.4 μg of DNA. TCF-luciferase activity was determined 24 h later using the dual-luciferase reporter assay system (Promega, Madison, WI), according to the manufacturer's instructions. Light intensity was measured with a luminometer and luciferase activity was divided by the Renilla activity (control reporter) to normalize for transfection efficiency.
Cell culture and in vitro apoptosis assays
OB-6 (25) and UAMS-32P (26) cells, two osteoblastic murine bone marrow-derived cell lines developed in our laboratory, as well as osteoblastic UMR-106 cells (27) were cultured in α-MEM (Invitrogen) supplemented with 10% fetal calf serum (Hyclone, Logan, UT), penicillin (100 U/ml), streptomycin (100 μg/ml), and glutamine (292 μg/ml). For the quantification of apoptosis, medium in the cultures was changed to 2% serum before the initiation of the treatments. Apoptosis was determined in OB-6 cells by direct visualization of changes in nuclear morphology in EGFP-labeled cells as described previously (28). The visualization of pyknotic or fragmented nuclei was facilitated by cotransfection of cells with an EGFP-expressing construct.
Western blot
Phosphorylated Akt was assayed by immunoblotting using a rabbit polyclonal antibody recognizing Ser473 phosphorylated Akt (Cell Signaling Technology, Beverly, MA). Protein levels of β-actin were analyzed using a mouse monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Quantification of the intensity of the bands in the autoradiograms was performed using a VersaDoc imaging system (Bio-Rad, Berkeley, CA).
Statistics
To investigate treatment effects for the various measurements between all four groups, one-way ANOVA and Sidak's correction for multiple comparisons was used and P < 0.05 was considered significant (Stata statistical software; StatCorp, College Station, TX) (29). Comparisons of interest were specified a priori. Bartlett's test was conducted to determine the homogeneity of the variance assumption. To confirm previously observed differences between the placebo control group and either the prednisone alone or PTH alone groups (13,17), two-group comparisons were analyzed using the unpaired Student's t test. Pearson correlation coefficients were calculated to test for an association between two independently measured variables. The data are shown as the means ± sd.
Results
Intermittent PTH administration prevents the decrease in BMD, microarchitecture, and strength in mice receiving prednisolone
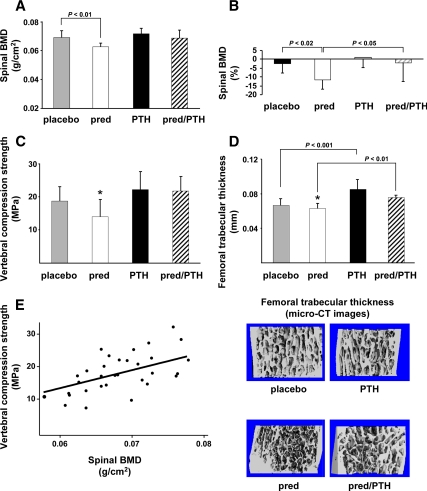
In mice receiving prednisolone alone for 28 d, mean spinal BMD was 10% less and the percent decrease in BMD was 3.2-fold more than in mice receiving placebo (Fig. 1, A and B). In line with these findings, prednisolone administration also caused a 25% decrease in vertebral compression strength (Fig. 1C). Micro-CT analysis of the femur revealed that prednisolone administration alone caused an 8.5% reduction in distal femoral trabecular thickness (P < 0.05) (Fig. 1D). There were no significant changes in distal femoral cancellous bone volume divided by tissue volume, trabecular number, trabecular separation, or cortical thickness. Conversely, administration of daily PTH injections caused an anabolic effect on the distal femoral trabecular thickness compared with animals receiving placebo (P < 0.001) (Fig. 1D). Mice receiving both prednisolone and PTH demonstrated preservation of spinal BMD and femoral trabecular thickness compared with animals receiving prednisolone alone (Fig 1, B and D). PTH treatment not only protected the loss of bone mass caused by glucocorticoids but also prevented the loss of bone strength (Fig. 1C). There was a strong relationship between spinal BMD and vertebral compression strength (Fig. 1E).
Figure 1.
PTH prevents glucocorticoid-induced loss of bone mass and strength in 5- to 6-month-old Swiss-Webster mice. A, Final spinal BMD values measured by dual-energy x-ray absorptiometry. B, Percent change in spinal BMD from baseline to the end of the 28-d experiment. C, Vertebral compression strength. D, Distal femoral cancellous trabecular thickness (3-D) measured by micro-CT. Representative images are shown below the graph. E, Vertebral compression strength is directly related to spinal BMD (r = 0.61, P < 0.001). Brackets indicate changes by ANOVA. *, P < 0.04 for comparison between the placebo and prednisolone groups by Student's t test. Pred, Prednisolone; MPa, megapascals.
PTH prevents the decrease in histomorphometric vertebral cancellous composition and architecture in mice receiving prednisolone
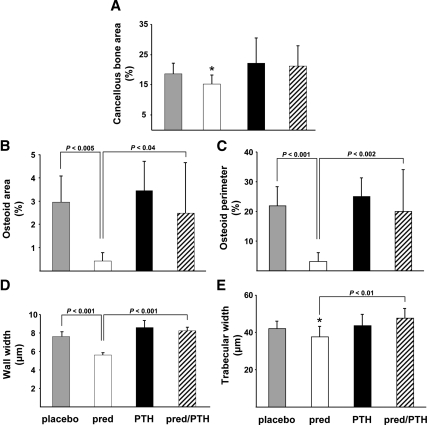
Mice receiving prednisolone alone had 18% less cancellous bone area compared with the animals receiving placebo (Fig. 2A). Furthermore, these mice exhibited a dramatic decrease in osteoid as evidenced by a reduction of osteoid area and perimeter to 14–15% of the values found in the mice receiving the placebo (Fig. 2, B and C). The decrease in osteoid was accompanied by a 26% decrease in the wall width (Fig. 2D). Consistent with the decrease in wall width, animals receiving prednisolone had an 11% decrease in histomorphometrically measured trabecular width (Fig. 2E). Each of these histological findings characteristic of glucocorticoid-induced osteoporosis was prevented in mice receiving glucocorticoids and PTH simultaneously.
Figure 2.
PTH preserves murine vertebral cancellous bone, osteoid, wall width, and trabecular width. A, Cancellous bone area. B, Osteoid width. C, Osteoid perimeter. D, Wall width. E, Trabecular width (two dimensional). Brackets indicate changes by ANOVA. *, P < 0.02 for comparison between the placebo and prednisolone (pred) groups by Student's t test.
PTH prevents the prednisolone-induced changes in osteoblast apoptosis, bone formation rate, and activation frequency
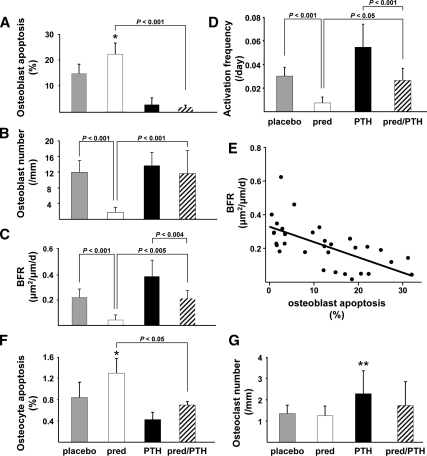
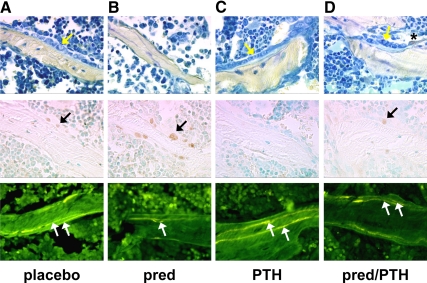
The prevalence of osteoblast apoptosis was 153% greater in the mice receiving prednisolone alone compared with mice receiving placebo (Figs. 3A and 4, A and B). In contrast, osteoblast apoptosis in animals receiving PTH alone or both prednisolone and PTH was dramatically decreased to only 14–21% of that found in mice receiving placebo (Figs. 3A and 4, C and D). Concordant with these findings, osteoblast number in mice receiving prednisolone alone was only 15% of that found in animals receiving placebo, whereas osteoblast number in the mice receiving both prednisolone and PTH was the same as in the animals receiving placebo (Fig. 3B and Fig. 4, A–D). Moreover, the glucocorticoid-induced decrease in bone formation rate and birth rate of new remodeling units (activation frequency) was prevented by the administration of PTH (Figs. 3, C and D, and 4, A–D). That this prevention required increases in the osteoblast number and activation frequency is indicated by the evidence that the increase in osteoblast number was tightly linked to an increase in the activation frequency (r = 0.73, P < 0.001), similar to what we previously reported after orchidectomy (30). In support of the finding that an increase in the osteoblast number accompanies the increase in new bone remodeling, a significant negative correlation was found between the bone formation rate and the prevalence of osteoblast apoptosis (Fig. 3E). Changes in the prevalence of osteoblast apoptosis accounted for 41% of the changes in the bone formation rate (r2 = 41%). Importantly, bone formation rate and activation frequency were significantly greater in the mice receiving PTH alone as compared with the animals receiving both prednisolone and PTH (Fig. 3, C and D).
Figure 3.
PTH prevents glucocorticoid-induced osteoblast and osteocyte apoptosis and preserves the bone formation rate in murine vertebral cancellous bone tissue. A, The prevalence of osteoblast apoptosis. B, Osteoblast number per cancellous perimeter. C, The bone formation rate (BFR) on a bone perimeter referent. D, Activation frequency in episodes per day. Pred, Prednisolone. E, BFR correlates inversely with the prevalence of osteoblast apoptosis (r = −0.64, P < 0.001). F, The prevalence of osteocyte apoptosis. G, Osteoclast number per cancellous perimeter. Brackets indicate changes by ANOVA. *, P < 0.05 for comparison between the placebo and prednisolone groups; **, P < 0.01 for comparison between the placebo and PTH alone groups by Student's t test.
Figure 4.
PTH preserves murine vertebral cancellous osteoblast numbers, prevents osteoblast apoptosis, and maintains bone formation rate. A, Placebo and vehicle. B, Prednisolone (pred) alone. C, PTH alone. D, Prednisolone and PTH. Treatment was given for 28 d. In the first row, osteoblasts were stained using toluidine blue (yellow arrows) and the asterisk indicates a tartrate-resistant acid phosphatase-positive osteoclast. In the second row, apoptotic osteoblasts were identified as brown, in situ nick-end labeling-positive cells located at the cancellous bone perimeter (black arrows). In the third row, the bone formation rate was determined using tetracycline labels (white arrows). Photomicrographs are at ×400 magnification.
Osteoclast number in mice receiving prednisolone alone was maintained at the same level as in the animals receiving placebo. However, in the group receiving PTH alone, osteoclast number was 170% greater than in the placebo group (Fig. 3G), highlighting the dramatic tilting of the balance between resorption and formation in favor of the latter with intermittent PTH.
PTH prevents the prednisolone-induced decrease in osteocyte apoptosis and preserves bone strength
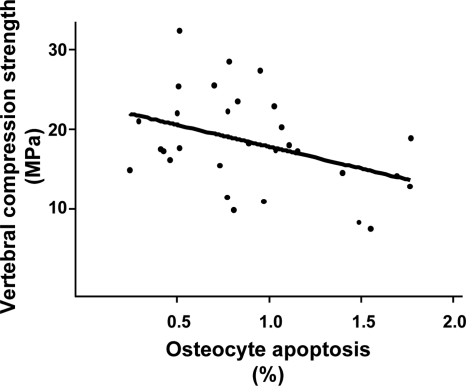
Osteocyte apoptosis was 148% greater in the mice receiving prednisolone compared with mice receiving placebo, and this increase was prevented in the animals receiving PTH along with prednisolone (Fig. 3F). In addition, when compared with the mice receiving placebo, animals receiving PTH alone exhibited increased osteocyte density (79.1 ± 19.0 vs. 48.2 ± 15.7 sd, P < 0.02), a finding consistent with the evidence that PTH decreases osteoblast apoptosis (17). The contribution of osteocyte viability to strength was strongly suggested by an inverse relationship between the prevalence of osteocyte apoptosis and vertebral compression strength (r = −0.40, P < 0.04) (Fig. 5).
Figure 5.
Vertebral compression strength is inversely related to the prevalence of osteocyte apoptosis (r = −0.40, P < 0.04). MPa, Megapascals.
Role of Akt in PTH prevention of glucocorticoid-induced apoptosis and suppression of Wnt signaling in osteoblastic cells
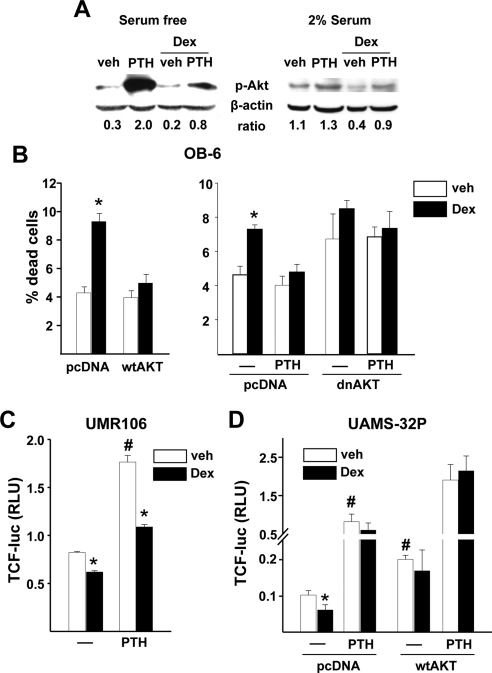
To explore potential mechanisms underlying our in vivo findings, we investigated the effects of glucocorticoids and PTH on Akt phosphorylation and Wnt signaling, two critical regulators of osteoblast differentiation and survival known to be down-regulated by glucocorticoids (31). PTH stimulated, and dexamethasone inhibited, phosphorylation of Akt in osteoblastic OB-6 cells maintained in the absence of serum (to minimize growth factor stimulated phosphorylation) as well as the presence of serum (Fig. 6A). More importantly, pretreatment with PTH partially blocked the dexamethasone-induced decrease in Akt phosphorylation. The role of Akt in the regulation of osteoblast apoptosis was confirmed by demonstrating that dexamethasone-induced apoptosis of OB-6 cells is prevented by overexpression of Akt (Fig. 6B). A dominant-negative form of Akt increased apoptosis and addition of dexamethasone had no further effect, indicating that glucocorticoid-induced osteoblast apoptosis is mediated by suppression of Akt phosphorylation.
Figure 6.
PTH prevents dexamethasone-induced attenuation of Akt phosphorylation, decreased β-catenin/TCF-mediated transcription, and increased osteoblast apoptosis. A, Phosphorylated Akt levels by Western blot in OB-6 cells serum starved for 4 h or cultured in α-MEM containing 2% serum and then pretreated for 30 min with 50 nm PTH followed by 100 nm dexamethasone for 30 min. B, Percentage of dead OB-6 cells transfected with a vector control (pcDNA) or wild-type Akt plasmid (wtAKT), along with an EGFP plasmid, and treated with vehicle (veh) or 100 nm dexamethasone for 6 h (left panel). In a similar experiment, cells were cotransfected with dnAkt and pretreated for 1 h with 50 nm PTH (right panel). The number of apoptotic cells was determined by examining the nuclear morphology of fluorescent cells. Bars, means ± sd of triplicate determinations. C, Luciferase activity in UMR-106 cells transfected with a TCF-luciferase (luc) reporter construct and pretreated for 1 h with 50 nm PTH followed by 100 nm dexamethasone for 24 h. Bars, Means ± sd of triplicate determinations of relative luciferase units (RLU) normalized for Renilla activity. D, Luciferase activity in UAMS-32P cells transfected with a TCF-luc reporter construct and cotransfected with a vector control (pcDNA) or wild-type Akt plasmid and treated as in C. *, P < 0.05 vs. respective vehicle; #, P < 0.05 vs. vehicle in untreated cells. Dex, Dexamethasone.
Dexamethasone also inhibited and PTH stimulated Wnt signaling in osteoblastic UMR-106 cells and UAMS-32P cells as measured by a TCF-luciferase reporter construct (Fig. 6, C and D). In support of the results with Akt phosphorylation in OB6 cells, pretreatment with PTH partially, or completely, prevented the negative effects of dexamethasone on Wnt signaling in UMR-106 and UAMS-32P cells, respectively. Overexpression of Akt in UAMS-32P cells prevented the dexamethasone-induced decrease in Wnt signaling under both basal conditions and after addition of PTH, suggesting that Akt phosphorylation plays a central role in the ability of PTH to attenuate the negative effects of glucocorticoids on Wnt signaling.
Discussion
Hyperparathyroidism was once considered a potential pathogenetic factor in glucocorticoid-induced osteoporosis (32). However, it is now clear that secondary increases in PTH do not occur in glucocorticoid-induced osteoporosis and epidemiological, densitometric, and histomorphometric evidence is against abnormal PTH regulation in glucocorticoid-induced osteoporosis (33). Moreover, intermittent PTH administration represents the first rational approach to the therapy of glucocorticoid-induced osteoporosis by counteracting several aspects of the pathophysiology of this disorder (23,34).
The animal studies described in this paper demonstrate that intermittent administration of PTH counteracts the increase in the prevalence of osteoblast apoptosis caused by glucocorticoid excess and that this effect of PTH contributes to its bone anabolic efficacy. Specifically, the results of our studies strongly suggest that protection of osteoblasts from the proapoptotic effect of glucocorticoids by PTH is the main mechanism responsible for the maintenance of osteoblast number, osteoid area and perimeter, wall and trabecular width, and BMD with this therapeutic agent. PTH also protected against glucocorticoid-induced changes in activation frequency and bone formation rate as well as BMD and bone strength. Hence, intermittent PTH administration is an effective treatment for glucocorticoid-induced osteoporosis because it directly counteracts the key pathogenetic mechanisms of glucocorticoid excess.
In contrast to PTH, antiresorptive therapies, the current standard of care for glucocorticoid-induced osteoporosis, do not affect the suppressed osteoblast number and bone formation, which are the principal features of the disease (12,13,14,15). Furthermore, patients with glucocorticoid-induced osteoporosis treated with PTH for 36 months have greater increases in BMD and fewer new vertebral fractures than similar patients treated with bisphosphonates (5). Nonetheless, it is important to note that the anabolic effect of PTH is compromised by concurrent glucocorticoid therapy as reported by others and as demonstrated here by the greater bone formation rate and activation frequency in mice receiving PTH alone as compared with mice receiving the combination of prednisolone and PTH (Fig. 3, C and D) (35). Dose and duration of glucocorticoid treatment as well as host factors such as severity of the underlying illness, weight loss, concurrent medications, renal function, and IGF-I levels may also contribute to a diminished efficacy of PTH in patients with glucocorticoid-induced bone disease compared with patients with osteoporosis from other causes (6,9).
PTH stimulates many of the osteogenic and prosurvival pathways known to be negatively affected by glucocorticoids, notably Akt phosphorylation (31,36,37) and Wnt signaling (38,39,40,41,42,43,44,45). Akt phosphorylation promotes Wnt signaling by inhibiting glycogen synthase kinase-3β and thereby stabilizes β-catenin leading to β-catenin/TCF-dependent transcription (45). The important role of suppressed Wnt signaling in the pathogenesis of glucocorticoid-induced osteoporosis is supported by the evidence that a synthetic inhibitor of glycogen synthase kinase-3β attenuates glucocorticoid-induced bone loss (31,46). In vitro studies presented in this manuscript demonstrate that PTH stimulates Akt phosphorylation and β-catenin/TCF-stimulated transcription in osteoblastic cells and abrogates the negative effects of glucocorticoids on these important proosteogenic and prosurvival pathways. By expressing excess wild-type or a dominant-negative form of Akt, we confirmed that PTH stimulation of Akt phosphorylation plays an important role in the ability of the hormone to attenuate both glucocorticoid-induced osteoblast apoptosis and glucocorticoid-induced suppression of Wnt signaling. These findings support the contention that PTH attenuation of glucocorticoid-induced bone loss involves stimulation of Akt phosphorylation and Wnt signaling, which promote the differentiation and survival of osteoblasts.
Cancellous bone is more susceptible than cortical bone to glucocorticoid-induced damage (12,13,14,15). Consistent with this, we found decreased trabecular width but no changes in cortical thickness in the mice with glucocorticoid excess. Also in agreement with prior observations (14,15,17), we found that PTH increases the width of existing trabeculae rather than creating new trabecular profiles. The maintenance of bone strength with PTH administration in animals receiving glucocorticoids indicates that this trabecular widening was structurally effective. However, it is very likely that besides architectural changes other effects of intermittent PTH administration may contribute to the preservation of bone strength. We have previously reported that prevention of osteocyte apoptosis via transgenic expression of 11β-hydroxysteroid dehydrogenase type 2, an enzyme that inactivates glucocorticoids, prevents glucocorticoid-induced loss of bone strength, independent of bone loss (19). Therefore, the decrease in the prevalence of osteocyte apoptosis with intermittent PTH has most likely played also a role in the preservation of bone strength. Strong support for this contention was provided by the inverse relationship between the prevalence of osteocyte apoptosis and vertebral compression strength revealed by our studies (Fig. 5).
Preservation of bone strength with the administration of PTH in animals receiving glucocorticoids may also be related to changes in bone vascularity and hydration. Recently we reported that part of the explanation for the greater decline in bone strength than in loss of bone mass with glucocorticoid excess may be due to disruption of bone vasculature and diminished bone hydraulic support (47,48). In addition, we obtained evidence that PTH increases the number of bone blood vessels (49). Further work is necessary to determine whether PTH-induced increases in vascularity contribute to bone strength in glucocorticoid-induced osteoporosis.
In conclusion, the evidence presented here indicates that the administration of intermittent PTH prevents glucocorticoid-induced osteoblast and osteocyte apoptosis and preserves the rate of bone formation, BMD, and strength, at least in part, by antagonizing glucocorticoid-induced attenuation of Akt phosphorylation and decreased β-catenin/TCF-mediated transcription. Hence, the ability of intermittent PTH administration to directly target the key pathogenetic mechanisms associated with chronic glucocorticoid therapy makes it a mechanistically rational treatment for glucocorticoid-induced osteoporosis.
Acknowledgments
The authors thank W. Webb, C. Wicker III, S. Berryhill, T. Chambers, E. Hogan, R. Shelton, L. Han, L. Climer, and X. Qiu (University of Arkansas for Medical Sciences) for their technical assistance.
Footnotes
This work was supported by Veterans Affairs Merit Review grants from the Office of Research and Development, Department of Veterans Affairs, National Institutes of Health (P01-AG13918) and Tobacco Settlement Funds provided by the University of Arkansas for Medical Sciences College of Medicine.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 21, 2010
Abbreviations: Akt, Protein kinase B; BFR, bone formation rate; BMD, bone mineral density; 3-D, three dimensional; dn, dominant negative; EGFP, enhanced green fluorescent protein; L, lumbar; micro-CT, micro-computed tomography; p, plasmid; TCF, T cell factor; Wnt, wingless signaling pathway.
References
- Reeve J, Williams D, Hesp R, Hulme P, Klenerman L, Zanelli JM, Darby AJ, Tregear GW, Parsons JA 1976 Anabolic effect of low doses of a fragment of human parathyroid hormone on the skeleton in postmenopausal osteoporosis. Lancet 1:1035–1038 [DOI] [PubMed] [Google Scholar]
- Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK 2005 Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev 26:688–703 [DOI] [PubMed] [Google Scholar]
- Lane NE, Sanchez S, Modin GW, Genant HK, Pierini E, Arnaud CD 1998 Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest 102:1627–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saag KG, Shane E, Boonen S, Marín F, Donley DW, Taylor KA, Dalsky GP, Marcus R 2007 Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 357:2028–2039 [DOI] [PubMed] [Google Scholar]
- Saag KG, Zanchetta JR, Devogelaer JP, Adler RA, Eastell R, See K, Krege JH, Krohn K, Warner MR 2009 Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum 60:3346–3355 [DOI] [PubMed] [Google Scholar]
- Jilka RL 2007 Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone 40:1434–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser JA, Kneissel M, Thomsen JS, Mosekilde L 2000 PTH and interactions with bisphosphonates. J Musculoskelet Neuronal Interact 1:53–56 [PubMed] [Google Scholar]
- Delmas PD, Vergnaud P, Arlot ME, Pastoureau P, Meunier PJ, Nilssen MH 1995 The anabolic effect of human PTH (1–34) on bone formation is blunted when bone resorption is inhibited by the bisphosphonate tiludronate—is activated resorption a prerequisite for the in vivo effect of PTH on formation in a remodeling system? Bone 16:603–610 [DOI] [PubMed] [Google Scholar]
- Cosman F, Lindsay R 1998 Is parathyroid hormone a therapeutic option for osteoporosis? A review of the clinical evidence. Calcif Tissue Int 62:475–480 [DOI] [PubMed] [Google Scholar]
- Ma YL, Bryant HU, Zeng Q, Schmidt A, Hoover J, Cole HW, Yao W, Jee WS, Sato M 2003 New bone formation with teriparatide [human parathyroid hormone-(1–34)] is not retarded by long-term pretreatment with alendronate, estrogen, or raloxifene in ovariectomized rats. Endocrinology 144:2008–2015 [DOI] [PubMed] [Google Scholar]
- Samadfam R, Xia Q, Goltzman D 2007 Co-treatment of PTH with osteoprotegerin or alendronate increases its anabolic effect on the skeleton of oophorectomized mice. J Bone Miner Res 22:55–63 [DOI] [PubMed] [Google Scholar]
- Weinstein RS 2001 Glucocorticoid-induced osteoporosis. Rev Endocr Metab Disord 2:65–73 [DOI] [PubMed] [Google Scholar]
- Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC 1998 Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of the deleterious effects on bone. J Clin Invest 102:274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster DW 1989 Bone histomorphometry in glucocorticoid-induced osteoporosis. J Bone Miner Res 4:137–141 [DOI] [PubMed] [Google Scholar]
- Dempster DW, Arlot MA, Meunier PJ 1983 Mean wall thickness and formation periods of trabecular bone packets in corticosteroid-induced osteoporosis. Calcif Tissue Int 35:410–417 [DOI] [PubMed] [Google Scholar]
- Weinstein RS, Chen JR, Powers CC, Stewart SA, Landes RD, Bellido T, Jilka RL, Parfitt AM, Manolagas SC 2002 Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest 109:1041–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC 1999 Increased bone formation by prevention of osteoblast apoptosis with PTH. J Clin Invest 104:439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiber M 1961 The fire of life: an introduction to animal energetics. Chaps 10, 11. New York: John Wiley, Sons, Inc. [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR 1987 Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res 2:595–610 [DOI] [PubMed] [Google Scholar]
- O'Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS 2004 Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 145:1835–1841 [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC 2007 Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285–27297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Parfitt AM, Manolagas SC 2007 Perspective—quantifying osteoblast and osteocyte apoptosis: challenges and rewards. J Bone Miner Res 22:1492–1501 [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW 1998 Identification of c-MYC as a target of the APC pathway. Science 281:1509–1512 [DOI] [PubMed] [Google Scholar]
- Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN 1995 The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81:727–736 [DOI] [PubMed] [Google Scholar]
- Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL 1999 Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARγ2. J Cell Biochem 74:357–371 [PubMed] [Google Scholar]
- Fu Q, Jilka RL, Manolagas SC, O'Brien CA 2002 Parathyroid hormone stimulates receptor activator of NFκB ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP-response element-binding protein. J Biol Chem 277:48868–48875 [DOI] [PubMed] [Google Scholar]
- Partridge NC, Alcorn D, Michelangeli VP, Ryan G, Martin TJ 1983 Morphological and biochemical characterization of four clonal osteogenic sarcoma cell lines of rat origin. Cancer Res 43:4308–4314 [PubMed] [Google Scholar]
- Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T 1999 Prevention of osteocyte and osteoblasts apoptosis by bisphosphonates and calcitonin. J Clin Invest 104:1363–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- StatCorp 2005 Stata statistical software, release 8.2. College Station, TX: Stata Corp. [Google Scholar]
- Weinstein RS, Jia D, Powers CC, Stewart SA, Jilka RL, Parfitt AM, Manolagas SC 2004 The skeletal effects of glucocorticoid excess override those of orchidectomy in mice. Endocrinology 145:1980–1987 [DOI] [PubMed] [Google Scholar]
- Smith E, Frenkel B 2005 Glucocorticoids inhibit the transcriptional activity of LEF/TCF in differentiating osteoblasts in a glycogen synthase kinase-3β-dependent and -independent manner. J Biol Chem 280:2388–2394 [DOI] [PubMed] [Google Scholar]
- Stevenson JC 1998 Management of corticosteroid-induced osteoporosis. Lancet 352:1327–1328 [DOI] [PubMed] [Google Scholar]
- Rubin MR, Bilezikian JP 2002 The role of parathyroid hormone in the pathogenesis of glucocorticoid-induced osteoporosis: a re-examination of the evidence. J Clin Endocrinol Metab 87:4033–4041 [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Weinstein RS, Jilka RL, Parfitt AM 1998 Parathyroid hormone and corticosteroid-induced osteoporosis. Lancet 352:1940 [DOI] [PubMed] [Google Scholar]
- Oxlund H, Ortoft G, Thomsen JS, Danielsen CC, Ejersted C, Andreassen TT 2006 The anabolic effect of PTH on bone is attenuated by simultaneous glucocorticoid treatment. Bone 39:244–252 [DOI] [PubMed] [Google Scholar]
- Liu X, Bruxvoort KJ, Zylstra CR, Liu J, Cichowski R, Faugere MC, Bouxsein ML, Wan C, Williams BO, Clemens TL 2007 Lifelong accumulation of bone in mice lacking Pten in osteoblasts. Proc Natl Acad Sci USA 104:2259–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Kambe F, Cao X, Lu X, Ishiguro N, Seo H 2007 Parathyroid hormone activates phosphoinositide 3-kinase-Akt-Bad cascade in osteoblast-like cells. Bone 40:354–359 [DOI] [PubMed] [Google Scholar]
- Yao W, Cheng Z, Pham A, Busse C, Zimmermann EA, Ritchie RO, Lane NE 2008 Glucocorticoid-induced bone loss in mice can be reversed by the actions of parathyroid hormone and risedronate on different pathways for bone formation and mineralization. Arthritis Rheum 58:3485–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Yamaguchi T, Yano S, Kanazawa I, Yamauchi M, Yamamoto M, Sugimoto T 2009 BMP/Wnt antagonists are upregulated by dexamethasone in osteoblast and reversed by alendronate and PTH: potential therapeutic targets for glucocorticoid-induced osteoporosis. Biochem Biophys Res Commun 379:261–266 [DOI] [PubMed] [Google Scholar]
- Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, Manolagas SC, Jilka RL 2005 Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146:4577–4583 [DOI] [PubMed] [Google Scholar]
- O'Brien CA, Plotkin LI, Vyas K, Cazer PE, Gortazar AR, Goellner JJ, Chen J, Shleton R, Weinstein RS, Schipani E, Jilka RL, Manolagas SC, Bellido T 2006 Activation of PTH receptor 1 specifically in osteocytes suppresses Sost expression and increases bone mass in transgenic mice. J Bone Miner Res 20:S4 [Google Scholar]
- Wan M, Yang C, Li J, Wu X, Yuan H, Ma H, He X, Nie S, Chang C, Cao X 2008 Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev 22:2968–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer I, Keller H, Leupin O, Kneissel M 2010 Does osteocytic SOST suppression mediate PTH bone anabolism? Trends Endocrinol Metab 21:237–244 [DOI] [PubMed] [Google Scholar]
- Kramer I, Loots GG, Studer A, Keller H, Kneissel M 2010 Parathyroid hormone (PTH) induced bone gain is blunted in SOST overexpressing and deficient mice. J Bone Miner Res 25:178–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S 2005 Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by β-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem 280:41342–41351 [DOI] [PubMed] [Google Scholar]
- Wang FS, Ko JY, Weng LH, Yeh DW, Ke HJ, Wu SL 2009 Inhibition of glycogen synthase kinase-3β attenuates glucocorticoid-induced bone loss. Life Sci 85:685–692 [DOI] [PubMed] [Google Scholar]
- Weinstein RS 2010 Glucocorticoids, osteocytes, and skeletal fragility: the role of bone vascularity. Bone 46:564–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O'Brien CA, Roberson PK, Boskey AL, Clemens TL, Manolagas SC 2010 Endogenous glucocorticoids decrease vascularity and increase skeletal fragility in aged mice. Aging Cell 9:147–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka R, Climer L, DeLoose A, Nagarajan R, O'Brien CA, Weinstein R, Manolagas S 2009 Balanced remodeling in response to sustained PTH elevation requires osteoclastic bone resorption, sclerostin suppression, as well as increased angiogenesis. J Bone Miner Res 24(Suppl 1) A09001656 (Abstract) [Google Scholar]