Abstract
IGF regulates steroidogenesis in granulosa cells through expression of the cytochrome P450 side-chain cleavage enzyme (P450scc) (CYP11A1), the rate-limiting enzyme in this biosynthetic process. We showed previously that the polypyrimidine tract-binding protein-associated splicing factor (PSF) acts as a repressor, whereas Sp1 is an activator, of P450 gene expression. The aim of the present study was to investigate IGF-stimulated ERK signaling regulating P450scc gene expression in the immortalized porcine granulosa cell line JC-410. We used a reporter gene under control of the IGF response element from the P450scc promoter. Inhibition of ERK phosphorylation with U0126 [1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto)butadiene] blocked IGF-I induction of IGF response element reporter gene activity. Western blotting revealed that IGF-I treatment resulted in phosphorylation of ERK that was specifically inhibited by U0126. ERK activation led to phosphorylation of T739 (an ERK site) on Sp1 that was diminished by U0126 or overexpression of PSF. Coimmunoprecipitation and Western blotting of nuclear extracts showed that phosphorylated ERK (pERK) bound PSF under basal conditions. IGF-I caused dissociation of pERK from PSF. Finally, chromatin immunoprecipitation analysis showed that PSF and Sp1 constitutively occupy the P450scc promoter independent of IGF-I treatment. These events provide a potential molecular mechanism for release of PSF repression of P450scc expression by dissociation of pERK and subsequent pERK-mediated phosphorylation of Sp1 to drive transcriptional induction of the P450scc gene in the absence of altered binding of PSF or Sp1 to the promoter. Understanding IGF-I regulation of these critical ovarian signaling pathways is the first step to delineating ovarian hyperstimulation syndromes such as polycystic ovarian syndrome.
Regulatory mechanisms in reproductive biology are shown whereby IGF-I activates Erk signaling and disrupts multiprotein complexes between pErk and the steroidogenic transcriptional repressor, polypyrimidine tract-binding protein-associated splicing factor.
IGF-I regulates pleiotrophic processes, including follicular development, ovulation, luteinization, and ovarian steroidogenesis (1,2,3,4) in addition to cancer growth and metastasis (5,6). These diverse processes are mediated through complex multifactorial signaling pathways that engage downstream mediators, including ERK, phosphatidylinositol 3-kinase, and protein kinase A (PKA) (6,7). For example, in Leydig cells, IGF leads to phosphorylation-dependent activation of ERK through interactions with the PKA and protein kinase C (PKC) pathways (8,9,10,11). In granulosa cells, IGF-I regulates steroidogenesis through induction of the P450 side-chain cleavage enzyme (P450scc) (CYP11A1) gene (12) via mechanisms that include PKCι and ERK (13).
A key mediator of IGF action is the transcription factor Sp1 that activates expression of P450scc (14,15) and other pregnancy-related genes (16). The generalized actions of Sp1 in steroidogenesis are reflected by regulation of P450scc in gonads and adrenals, as well as the ovary (17). Multiple signaling pathways converge on Sp1, including ERK, Akt, and c-Jun N-terminal protein kinase (18,19). The first ERK-regulated phosphorylation sites on Sp1 were identified at T453 and T739 in response to growth factor regulation of the vascular endothelial growth factor gene (20). These sites have since been implicated in several signaling pathways, including fibroblast growth factor stimulation of ERK, phosphorylation on these ERK consensus sites on Sp1, and functional changes of Sp1 action on target gene expression (21).
Protein-associated splicing factor (PSF) is also an important mediator of IGF induction of steroidogenesis in which it has a powerful repressive effect (22,23). It is a multifunctional protein involved in many different processes originally identified as a component of the RNA splicing machinery (24). With the emergent elucidation of the tightly coupled relationship between gene transcription and RNA splicing, additional activities of PSF have been discovered. It is now clearly established that PSF also acts as a transcriptional corepressor of the nuclear receptors for thyroid hormone, progesterone, and androgens acting via diverse mechanisms that include proteosomal receptor degradation, inhibition of DNA binding, and/or recruitment of coactivators such as the histone deacetylase (HDAC) complex and mSin3A (25,26,27). We found that PSF represses IGF induction by directly binding to the IGF response element (IGFRE) (22) and, using chromatin immunoprecipitation (ChIP) assays, to the promoter of the endogenous P450scc gene. We further showed that PSF binds PKCι via a protein-protein interaction mechanism that is independent of PKC catalytic activity and results in derepression of PSF inhibition of transcription (28). Similar actions were described for PSF repression of androgen receptor (AR) binding to the AR response element by a ligand-independent mechanism (25). Furthermore, overexpression of PSF represses transcriptional activity of several steroid receptors through direct protein-protein interactions with several members of the transcriptional regulatory machinery (26). Finally, others have reported that PSF binding and repression of the IGFRE is relieved by microRNA retrotransposons, such as VL30, that induce release of PSF from the promoter, thereby leading to derepression of gene expression (23,29).
ERK is a key integrator of convergent signaling pathways to regulate gene expression in response to a wide variety of extracellular signals. We recently demonstrated the importance of IGF-I synergism with PKC in phosphorylation-dependent activation of ERK in induction of the P450scc in a porcine granulosa cell line and primary granulosa cells (13). ERK is often present in multiprotein complexes, typically with the upstream activator MAPK kinase (MEK) that acts as a nucleocytoplasmic shuttle to direct ERK to the nucleus in which it can impinge on the activity of transcription complexes (30,31,32).
These relationships between IGF, ERK, Sp1, and PSF led us to hypothesize that there was a direct link between these signaling intermediates that regulate expression of the P450scc gene in granulosa cell steroidogenesis. We first tested IGF-I in phosphorylation of ERK and activation of the IGFRE. This led to explorations on the actions of IGF-I on ERK-dependent phosphorylation and activation of Sp1, the composition of multiprotein complexes between pERK and these transcription factors, and regulation of PSF and Sp1 binding to the IGFRE of the endogenous P450scc promoter. We found a robust signaling pathway that provides multiple regulatory mechanisms through phosphorylation of stable proteins.
Materials and Methods
Materials
Human recombinant IGF-I was from Bachem Biosciences (King of Prussia, PA). U0126 [1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto)butadiene] (10 μm), an inhibitor of MEK (upstream activator of ERK), was from Promega (Madison, WI). Rabbit antihuman ERK, antihuman phosphorylated (p) ERK (T202/Y204), antimouse pS473 Akt, antihuman Akt, mouse antihuman pERK conjugated to agarose beads, and secondary antibodies conjugated to agarose beads for coimmunoprecipitation were from Cell Signaling Technologies (Beverley, MA). Mouse monoclonal Antihuman RNA Polymerase II (Pol II) was from Millipore (Billerica, MA). Peptide affinity-purified rabbit antihuman antibody to Sp1 phosphorylated on T739 (pT739 Sp1) was prepared as described previously (20). Mouse antihuman Sp1 was from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse antihuman PKCι was from BD Biosciences (San Jose, CA). A PSF peptide corresponding to human amino acids 687-698 (CRGREEYEGPNK) was designed in our laboratory and used for production of rabbit antihuman PSF antibody by Bio-Molecular Technology (Frederick, MD). Secondary antibodies were goat antirabbit IgG horseradish peroxidase (Southern Biotechnology Associates, Birmingham, AL) and sheep antimouse IgG horseradish peroxidase (GE Healthcare, Piscataway, NJ).
JC-410 cells
The JC-410 cell line was developed from a primary culture of porcine granulosa cells (33) and validated for studies in granulosa cell physiology in a number of previous investigations (28,34). JC-410 cells were grown to 85% confluence as described previously and placed in serum-free media overnight before pretreatment in serum-free media with inhibitors for 1 h and/or 20 nm IGF-I for 4 or 48 h.
Plasmids
The IGFRE-luciferase reporter gene contained the proximal P450scc promoter fused to luciferase as described previously (15,22,34). For PSF studies, plasmids contained the cytomegalovirus promoter without (mock) or with (PSF) the entire coding sequence of the human PSF gene (22).
Transient transfection in JC-410 cells and luciferase assay
JC-410 cells were grown in Eagle's MEM with 3% fetal bovine serum and 1 ng/ml insulin for 1 wk after thawing as reported previously (33). After an additional 2–3 wk in culture in the absence of insulin, cells were plated at 1.5 × 106 cells per 35-mm well in a six-well plate with 2 ml of Eagle's MEM and 3% fetal bovine serum per well. After overnight culture, cells were transfected with the indicated plasmids with LipofectAMINE 2000 (Invitrogen, Carlsbad, CA) as described previously (22). DNA-LipofectAMINE reagent complexes were left on cells for 6 h before removal and addition of treatment medium for an additional 48 h at 37 C. Cells were rinsed with PBS and assayed for luciferase activity with the Promega luciferase assay system or for Western blotting. Light production was measured with a Turner Designs (Sunnyvale, CA) TD-20e luminometer. The Bio-Rad (Hercules, CA) Protein DC Assay Reagent was used to measure protein concentrations of the lysates to normalize analyses.
Immunoprecipitation
Nuclear extracts (500 μg) were prepared as described previously and incubated overnight at 4 C on a rocker with 2 μg primary antibodies, followed by capture with secondary antibody-conjugated agarose beads (28). After washing, entire bead complexes were boiled in sodium dodecyl sulfate (SDS) sample buffer and analyzed by Western blotting.
Western blotting
Whole-cell extracts (20 μg), nuclear extracts (20 μg), or immunoprecipitated complexes were resolved by 10% SDS-PAGE under reducing conditions. After transfer to nitrocellulose, blots were blocked with 5% milk-Tris-buffered saline (TBS) and incubated overnight at 4 C with primary antibody in 5% milk-TBS. After washing, the species-appropriate secondary antibody conjugated to horseradish peroxidase was added in 5% milk-TBS for 1 h at room temperature. Bands were detected using enhanced chemiluminescence Western blotting reagent (GE Healthcare).
ChIP
ChIP was performed as described previously with minor modifications (34). Briefly, cells were cross-linked with 1% formaldehyde and treated in 100 mm glycine, and cell pellets were resuspended in SDS lysis buffer with protease inhibitors and sonicated on ice. Immunoprecipitation was performed overnight using Dynabeads Protein G (Invitrogen). After de-cross-linking, DNA was extracted and amplified by real-time PCR using primers for the IGFRE (sense, 5′ gaacctcacgctgcagaaat 3′; antisense, 5′ aatgttcacgtccttcctcct 3′) or internal coding sequences of the P450scc (sense, 5′ gagatggcacgcaacctgaag 3′; antisense, 5′ cttagtgtctccttgatgctggc 3′) and normalized to actin.
Statistical analysis
Analyze-it Statistics (www.analyze-it.com) was used to perform ANOVA with Dunnett's post hoc analysis to determine statistical significance for replicate experiments in each study. For statistical analyses, densitometric Western blot band intensities were normalized to those from control, untreated cells set equal to one.
Results
IGF-I regulation of phosphorylation of ERK and induction of IGFRE-driven reporter gene activity
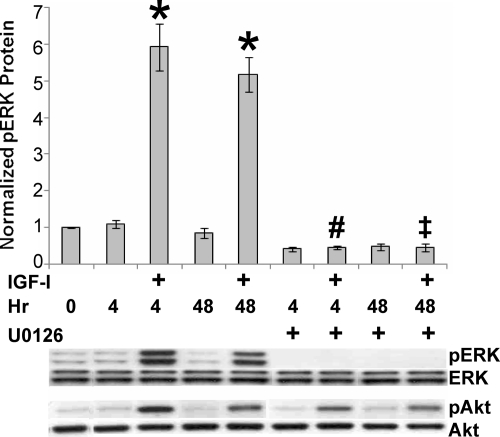
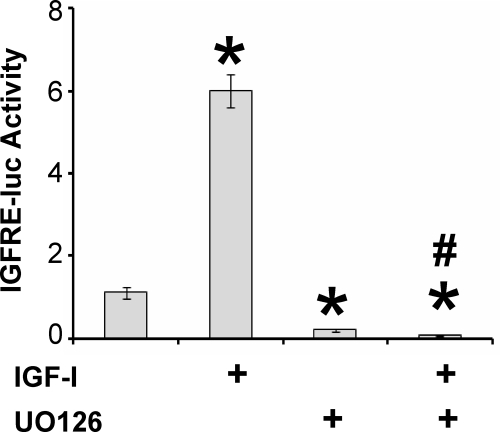
JC-410 cells were treated with IGF-I and ERK phosphorylation assessed by Western blotting. Phosphorylation of ERK was induced within 4 h and sustained, although slightly reduced, for 48 h (Fig. 1). Treatment with U0126, an inhibitor of the upstream ERK-activating kinase MEK, reduced both basal and IGF-I stimulated phosphorylation of ERK. The specificity of this response was shown by the lack of effect of U0126 on IGF-I induced phosphorylation of S473 on Akt (Fig. 1). Because we showed previously that IGF stimulated activity of the IGFRE, we tested the effects of ERK on this transcriptional response. Again, U0126 reduced both basal and IGF-I-induced reporter gene activity (Fig. 2).
Figure 1.
Regulation of IGF-I stimulated phosphorylation of ERK. JC-410 cells were grown in serum-free media for 24 h and pretreated with vehicle or U0126 (10 μm) for 1 h. Cells were then treated for 4 or 48 h without or with 20 nm IGF-I. Whole-cell extracts were then probed with the indicated antibodies showing representative results from one experiment. Error bars represent mean ± se of four independent experiments. ANOVA, P < 0.001, F(8,27) = 61.15. *, P < 0.001 compared with control (0 h). #, P < 0.001 compared with IGF (4 h); ‡, P < 0.001 compared with IGF (48 h).
Figure 2.
ERK mediation of IGF-I activation of the IGFRE. JC-410 cells were transfected with the IGRFE-luciferase reporter gene. After 1 d in serum-free media, cells were pretreated with vehicle or U0126 (10 μm) for 1 h, followed with or without IGF-I (20 nm) for 48 h. Luciferase activity was measured and normalized to basal, untreated controls. Error bars represent mean ± se of four independent experiments. ANOVA, P < 0.001, F(3,12) = 170.19. *, P < 0.001 compared with control (no IGF). #, P < 0.001 compared with IGF.
IGF-I regulation of ERK activation by PSF and ERK phosphorylation of Sp1
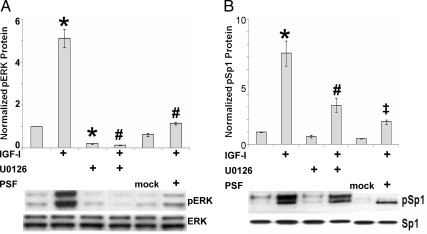
We showed previously that the transcription factor PSF represses IGF-I induction of the IGFRE (22). To explore the relationship between ERK activation and PSF, we transiently overexpressed PSF. The IGF-I increase in ERK phosphorylation was blocked by U0126 and inhibited by PSF overexpression compared with transfection with mock (empty) plasmid (Fig. 3A). At the same time, IGF-I induced phosphorylation of Sp1 on T739 (Fig. 3B), an established ERK site (20). This effect was also significantly inhibited by treatment with U0126 or PSF overexpression (Fig. 3B).
Figure 3.
Effects of ERK inhibition and PSF overexpression on IGF-I regulation of phosphorylation of ERK and Sp1. JC-410 cells were untransfected (no PSF) or transfected with mock (empty) or PSF plasmids. After 1 d in serum-free media, cells were pretreated for 1 h with vehicle or 10 μm U0126, followed with or without IGF-I (20 nm) for 48 h. Whole-cell extracts were then analyzed by Western blotting for ERK and pERK (A) or Sp1 and pT739 Sp1 (B) as shown in representative blots. Error bars are mean ± se from four independent experiments. A, ANOVA, P < 0.001, F(5,18) = 110.41. *, P < 0.001 compared with control (no IGF). #, P < 0.001 compared with IGF. B, ANOVA, P < 0.001, F(5,18) = 28.57. *, P < 0.001 compared with control (no IGF). #, P = 0.012 compared with IGF. ‡, P = 0.0018 compared with IGF.
Effect of IGF-I on multiprotein complexes
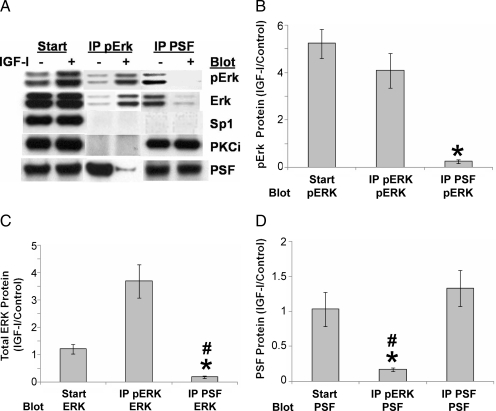
To better understand nuclear multiprotein complexes in IGF-I signaling, we performed coimmunoprecipitation/Western blotting of nuclear extracts. We showed previously that PSF and PKCι were associated in the nucleus in unstimulated JC-410 cells (28). The representative Western blot in Figure 4A shows that pERK was also associated with PSF under basal conditions but that IGF-I led to dissociation of pERK from PSF (middle two lanes, bottom pair), as well as the reciprocal experiment performing the immunoprecipitation first with PSF and then blotting pERK (right two lanes, top pair). We also found that pERK was not associated with either Sp1 or PKCι, or PSF with Sp1. Quantification of replicate experiments further demonstrated the robustness of IGF-I-induced multiprotein complex dissociation when protein-protein interactions were analyzed by examining the ratios from IGF-I treatment compared with control. Compared with control, IGF-I clearly reduced the amount of pERK associated with immunoprecipitated PSF (Fig. 4B), total ERK associated with PSF (Fig. 4C), and PSF associated with pERK (Fig. 4D).
Figure 4.
IGF-I regulation of nuclear multiprotein complexes. Cells grown for 24 h in serum-free media were treated with or without 20 nm IGF-I for 48 h and nuclear extracts immunoprecipitated (IP) with mouse antihuman pERK or rabbit antihuman PSF. The nuclear extracts (Start) and the immunoprecipitated complexes (IP pERK, middle; IP PSF, right) were resolved by SDS-PAGE and analyzed by Western blotting for pERK, total ERK, PSF, Sp1, or PKCι (A). For additional quantification and statistical analysis of replicate experiments, protein levels were normalized to IGF-treated cells over control cells (IGF-I/control) for pERK (B), total ERK (C), and PSF (D). Error bars represent mean ± se of three independent experiments. B, ANOVA, P = 0.0017, F(2,6) = 22.19; *, P = 0.0065 for IP PSF compared with IP pERK. C, ANOVA, P = 0.0012, F(2,6) = 24.85; *, P = 0.0038 for IP PSF compared with Start; #, P = 0.0044 for IP PSF compared with IP pERK. D, ANOVA, P = 0.0163, F(2,6) = 8.83; *, P = 0.0233 for IP pERK compared with Start; #, P = 0.0106 for IP pERK compared with IP PSF.
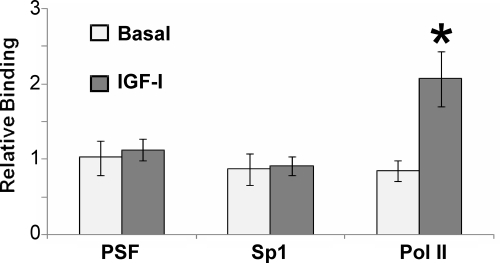
IGF-I regulation of PSF and Sp1 binding to the IGFRE of the endogenous P450scc promoter
Because we reported previously that PSF and Sp1 were bound to the IGFRE in the endogenous P450scc promoter under basal conditions in Y1 adrenal cells (34), we pursued promoter occupancy of both PSF and Sp1 in the JC-410 cell line in response to IGF-I. Figure 5 shows that both PSF and Sp1 also constitutively occupy the IGFRE of the endogenous P450scc promoter in the JC-410 cell line and that IGF-I treatment does not alter their occupancy. To confirm the lack of effect of IGF-I on PSF and Sp1 occupancy, we tested RNA Pol II binding to the internal coding sequences of the endogenous P450scc gene. IGF-I induced Pol II binding (Fig. 5) and demonstrated the established responsiveness to IGF-I that leads to increased mRNA production, protein expression, and steroidogenesis (12,28,34).
Figure 5.
IGF-I regulation of PSF and Sp1 binding to the IGFRE of the endogenous P450scc promoter. Cells grown for 24 h in serum-free media were treated with or without 20 nm IGF-I for 48 h and analyzed by ChIP assay for PSF or Sp1 binding to the IGFRE in the promoter of the endogenous P450scc or for RNA Pol II binding to the P450scc gene. Error bars represent mean ± se of three independent experiments. ANOVA, P = 0.0065, F(5,18) = 4.68; *, P = 0.0193 for Pol II after treatment with IGF-I compared with basal.
Discussion
We investigated regulatory mechanisms of IGF-I through ERK signaling on P450scc expression in the porcine JC-410 ovarian granulosa cell line. Experimentally, we were able to show a direct, specific link between IGF-I treatment, ERK phosphorylation, and phosphorylation of Sp1 on an ERK-dependent Sp1 phosphorylation site. Moreover, we were able to delineate PSF regulation of this signaling pathway by association with pERK. This significant level of regulation was achieved without altering the occupancy of PSF or S1 on the IGFRE in the promoter of the P450scc gene.
Our observations on ERK signaling that underlies the molecular mechanism of action of IGF-I are consistent with many previous observations in other settings (10,11,17) but raise some novel considerations for pathway cross talk in regulation of steroidogenesis in granulosa cells. The observations that ERK is phosphorylated in response to phorbol 12-myristate 13-acetate stimulation of PKCι in granulosa cells (13) and of PKA and PKC in Leydig cell steroidogenesis (8,9,10) illuminates a complex communication in the steroidogenic pathway. As explicated by these latter authors, such cross talk is stimulus, cell type, and promoter dependent, indicating the subtleties of information processing. In addition, the sustained activation of ERK in our studies may suggest that at least some of these effects are indirect. Our study is the first to link ERK signaling directly to the P450scc promoter.
We (15,22,34) and others (26) have shown that overexpression of PSF has a dominant-negative impact on target gene induction. Surprisingly, phosphorylation of both ERK itself and the ERK site at T739 on Sp1 were also inhibited by PSF overexpression. This indicates that PSF directly inhibits ERK phosphorylation, thus reducing subsequent downstream ERK-dependent phosphorylation of T739 on Sp1. Although PSF is an established repressor of transcription, the detailed molecular mechanisms for this activity remain unclear.
Although PSF association with pERK provides an intriguing mechanism of regulation, of equal interests are the mechanisms responsible for IGF-I-mediated dissociation of PSF and pERK. There is an abundance of evidence in the literature that PSF association with proteins is highly regulated. We reported previously (28) that PSF and PKCι were associated in nuclear complexes and that PSF repression was relieved by overexpression of either kinase-dead or constitutively active PKCι. PSF binding to PKCα in the nucleus of neuroblastoma cells was also reported to be independent of kinase catalytic activity (35). The binding of micro RNA retrotransposons, such as VL30, to the PSF RNA binding motif altered PSF binding affinities, thereby derepressing the gene and allowing transcription to proceed (23,29). Such actions are also reflected in Parkinson's disease in which DJ-1 colocalizes with, and coactivates, PSF to inhibit silencing (36). Additional regulatory events on chromatin involve DJ-1 inhibition of PSF sumoylation-dependent recruitment of HDAC1 (37). In the case of the PSF-AR complexes, repression is also reversed by HDAC inhibitors (25). Finally, interaction with other signaling pathways is critical because cAMP-dependent phosphorylation of PSF leads to dissociation of multiprotein complexes and removal of repression (38), whereas hyperphosphorylation of PSF on many sites is associated with regulation of protein-protein interactions and localization to hyperspeckled interchromatin granules (39). In the current context, IGF-I disruption of the pERK-PSF complexes could integrate many of these common themes to allow dissociated pERK to access, phosphorylate, and activate Sp1, thereby driving steroidogenesis.
In summary, in porcine JC-410 granulosa cells, IGF-I induction of P450scc expression is directly and specifically mediated through ERK phosphorylation and subsequent phosphorylation of Sp1. This signaling pathway is highly regulated by association of PSF with pERK. Additional delineation of the mechanisms controlling this novel association will expand our understanding of the pathways regulating steroidogenesis in granulosa cells.
Footnotes
This work was supported by National Institute of Child Health and Human Development Grant HD-36092 (to R.J.U.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 6, 2010
Abbreviations: AR, Androgen receptor; ChIP, chromatin immunoprecipitation; HDAC, histone deacetylase; IGFRE; IGF response element; MEK, MAPK kinase; p, phosphorylated; P450scc, P450 side-chain cleavage enzyme; PKA, protein kinase A; PKC, protein kinase C; PKCι, PKC inhibitor; PSF, polypyrimidine tract-binding protein-associated splicing factor; SDS, sodium dodecyl sulfate; TBS, Tris-buffered saline; U0126, 1,4-diamino-2,3-dicyano-1,4-bis(o- aminophenylmercapto)butadiene.
References
- Adashi EY, Resnick CE, Hurwitz A, Ricciarellie E, Hernandez ER, Roberts CT, Leroith D, Rosenfeld R 1992 The intra-ovarian IGF system. Growth Regul 2:10–15 [PubMed] [Google Scholar]
- Adashi EY 1992 Intraovarian regulation: the IGF-I example. Reprod Fertil Dev 4:497–504 [DOI] [PubMed] [Google Scholar]
- Adashi EY 1992 Intraovarian peptides. Stimulators and inhibitors of follicular growth and differentiation. Endocrinol Metab Clin North Am 21:1–17 [PubMed] [Google Scholar]
- Miller WL 1988 Molecular biology of steroid hormone synthesis. Endocr Rev 9:295–318 [DOI] [PubMed] [Google Scholar]
- Bentov I, Narla G, Schayek H, Akita K, Plymate SR, LeRoith D, Friedman SL, Werner H 2008 Insulin-like growth factor-I regulates Kruppel-like factor-6 gene expression in a p53-dependent manner. Endocrinology 149:1890–1897 [DOI] [PubMed] [Google Scholar]
- Samani AA, Yakar S, LeRoith D, Brodt P 2007 The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev 28:20–47 [DOI] [PubMed] [Google Scholar]
- Clemmons DR, Maile LA 2003 Minireview: integral membrane proteins that function coordinately with the insulin-like growth factor I receptor to regulate intracellular signaling. Endocrinology 144:1664–1670 [DOI] [PubMed] [Google Scholar]
- Manna PR, Jo Y, Stocco DM 2007 Regulation of Leydig cell steroidogenesis by extracellular signal-regulated kinase 1/2: role of protein kinase A and protein kinase C signaling. J Endocrinol 193:53–63 [DOI] [PubMed] [Google Scholar]
- Manna PR, Chandrala SP, Jo Y, Stocco DM 2006 cAMP-independent signaling regulates steroidogenesis in mouse Leydig cells in the absence of StAR phosphorylation. J Mol Endocrinol 37:81–95 [DOI] [PubMed] [Google Scholar]
- Manna PR, Chandrala SP, King SR, Jo Y, Counis R, Huhtaniemi IT, Stocco DM 2006 Molecular mechanisms of insulin-like growth factor-I mediated regulation of the steroidogenic acute regulatory protein in mouse leydig cells. Mol Endocrinol 20:362–378 [DOI] [PubMed] [Google Scholar]
- Stocco DM, Wang X, Jo Y, Manna PR 2005 Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol 19:2647–2659 [DOI] [PubMed] [Google Scholar]
- Urban RJ, Garmey JC, Shupnik MA, Veldhuis JD 1990 Insulin-like growth factor type I increases concentrations of messenger ribonucleic acid encoding cytochrome P450 cholesterol side-chain cleavage enzyme in primary cultures of porcine granulosa cells. Endocrinology 127:2481–2488 [DOI] [PubMed] [Google Scholar]
- Sriraman V, Modi SR, Bodenburg Y, Denner LA, Urban RJ 2008 Identification of ERK and JNK as signaling mediators on protein kinase C activation in cultured granulosa cells. Mol Cell Endocrinol 294:52–60 [DOI] [PubMed] [Google Scholar]
- Samson SL, Wong NC 2002 Role of Sp1 in insulin regulation of gene expression. J Mol Endocrinol 29:265–279 [DOI] [PubMed] [Google Scholar]
- Urban RJ, Bodenburg Y 2002 PTB-associated splicing factor regulates growth factor-stimulated gene expression in mammalian cells. Am J Physiol Endocrinol Metab 283:E794–E798 [DOI] [PubMed] [Google Scholar]
- Simmen RC, Zhang XL, Zhang D, Wang Y, Michel FJ, Simmen FA 2000 Expression and regulatory function of the transcription factor Sp1 in the uterine endometrium at early pregnancy: implications for epithelial phenotype. Mol Cell Endocrinol 159:159–170 [DOI] [PubMed] [Google Scholar]
- Guo IC, Shih MC, Lan HC, Hsu NC, Hu MC, Chung BC 2007 Transcriptional regulation of human CYP11A1 in gonads and adrenals. J Biomed Sci 14:509–515 [DOI] [PubMed] [Google Scholar]
- Sroka IC, Nagle RB, Bowden GT 2007 Membrane-type 1 matrix metalloproteinase is regulated by sp1 through the differential activation of AKT, JNK, and ERK pathways in human prostate tumor cells. Neoplasia 9:406–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak HJ, Park MJ, Cho H, Park CM, Moon SI, Lee HC, Park IC, Kim MS, Rhee CH, Hong SI 2006 Transforming growth factor-β1 induces tissue inhibitor of metalloproteinase-1 expression via activation of extracellular signal-regulated kinase and Sp1 in human fibrosarcoma cells. Mol Cancer Res 4:209–220 [DOI] [PubMed] [Google Scholar]
- Milanini-Mongiat J, Pouysségur J, Pagès G 2002 Identification of two Sp1 phosphorylation sites for p42/p44 mitogen-activated protein kinases: their implication in vascular endothelial growth factor gene transcription. J Biol Chem 277:20631–20639 [DOI] [PubMed] [Google Scholar]
- Bonello MR, Khachigian LM 2004 Fibroblast growth factor-2 represses platelet-derived growth factor receptor-α (PDGFR-α) transcription via ERK1/2-dependent Sp1 phosphorylation and an atypical cis-acting element in the proximal PDGFR-α promoter. J Biol Chem 279:2377–2382 [DOI] [PubMed] [Google Scholar]
- Urban RJ, Bodenburg Y, Kurosky A, Wood TG, Gasic S 2000 Polypyrimidine tract-binding protein-associated splicing factor is a negative regulator of transcriptional activity of the porcine p450scc insulin-like growth factor response element. Mol Endocrinol 14:774–782 [DOI] [PubMed] [Google Scholar]
- Song X, Sui A, Garen A 2004 Binding of mouse VL30 retrotransposon RNA to PSF protein induces genes repressed by PSF: effects on steroidogenesis and oncogenesis. Proc Natl Acad Sci USA 101:621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JG, Mayer SA, Tempst P, Nadal-Ginard B 1991 Characterization and molecular cloning of polypyrimidine tract-binding protein: a component of a complex necessary for pre-mRNA splicing. Genes Dev 5:1237–1251 [DOI] [PubMed] [Google Scholar]
- Dong X, Sweet J, Challis JR, Brown T, Lye SJ 2007 Transcriptional activity of androgen receptor is modulated by two RNA splicing factors, PSF and p54nrb. Mol Cell Biol 27:4863–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Shylnova O, Challis JR, Lye SJ 2005 Identification and characterization of the protein-associated splicing factor as a negative co-regulator of the progesterone receptor. J Biol Chem 280:13329–13340 [DOI] [PubMed] [Google Scholar]
- Mathur M, Tucker PW, Samuels HH 2001 PSF is a novel corepressor that mediates its effect through Sin3A and the DNA binding domain of nuclear hormone receptors. Mol Cell Biol 21:2298–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban RJ, Bodenburg YH, Jiang J, Denner L, Chedrese J 2004 Protein kinase Cι enhances the transcriptional activity of the porcine P-450 side-chain cleavage insulin-like response element. Am J Physiol Endocrinol Metab 286:E975–E979 [DOI] [PubMed] [Google Scholar]
- Song X, Sun Y, Garen A 2005 Roles of PSF protein and VL30 RNA in reversible gene regulation. Proc Natl Acad Sci USA 102:12189–12193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgermeister E, Seger R 2007 MAPK kinases as nucleo-cytoplasmic shuttles for PPARγ. Cell Cycle 6:1539–1548 [DOI] [PubMed] [Google Scholar]
- Yao Z, Flash I, Raviv Z, Yung Y, Asscher Y, Pleban S, Seger R 2001 Non-regulated and stimulated mechanisms cooperate in the nuclear accumulation of MEK1. Oncogene 20:7588–7596 [DOI] [PubMed] [Google Scholar]
- Volmat V, Camps M, Arkinstall S, Pouysségur J, Lenormand P 2001 The nucleus, a site for signal termination by sequestration and inactivation of p42/p44 MAP kinases. J Cell Sci 114:3433–3443 [DOI] [PubMed] [Google Scholar]
- Chedrese PJ, Rodway MR, Swan CL, Gillio-Meina C 1998 Establishment of a stable steroidogenic porcine granulosa cell line. J Mol Endocrinol 20:287–292 [DOI] [PubMed] [Google Scholar]
- Urban RJ, Bodenburg YH, Wood TG 2002 NH2 terminus of PTB-associated splicing factor binds to the porcine P450scc IGF-I response element. Am J Physiol Endocrinol Metab 283:E423–E427 [DOI] [PubMed] [Google Scholar]
- Rosenberger U, Lehmann I, Weise C, Franke P, Hucho F, Buchner K 2002 Identification of PSF as a protein kinase Cα-binding protein in the cell nucleus. J Cell Biochem 86:394–402 [DOI] [PubMed] [Google Scholar]
- Xu J, Zhong N, Wang H, Elias JE, Kim CY, Woldman I, Pifl C, Gygi SP, Geula C, Yankner BA 2005 The Parkinson's disease-associated DJ-1 protein is a transcriptional co-activator that protects against neuronal apoptosis. Hum Mol Genet 14:1231–1241 [DOI] [PubMed] [Google Scholar]
- Zhong N, Kim CY, Rizzu P, Geula C, Porter DR, Pothos EN, Squitieri F, Heutink P, Xu J 2006 DJ-1 transcriptionally up-regulates the human tyrosine hydroxylase by inhibiting the sumoylation of pyrimidine tract-binding protein-associated splicing factor. J Biol Chem 281:20940–20948 [DOI] [PubMed] [Google Scholar]
- Sewer MB, Nguyen VQ, Huang CJ, Tucker PW, Kagawa N, Waterman MR 2002 Transcriptional activation of human CYP17 in H295R adrenocortical cells depends on complex formation among p54(nrb)/NonO, protein-associated splicing factor, and SF-1, a complex that also participates in repression of transcription. Endocrinology 143:1280–1290 [DOI] [PubMed] [Google Scholar]
- Shav-Tal Y, Cohen M, Lapter S, Dye B, Patton JG, Vandekerckhove J, Zipori D 2001 Nuclear relocalization of the pre-mRNA splicing factor PSF during apoptosis involves hyperphosphorylation, masking of antigenic epitopes, and changes in protein interactions. Mol Biol Cell 12:2328–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]