Abstract
Sorbitol-fermenting Bifidobacteria (SFB) proved to be an excellent indicator of very recent human faecal pollution (hours to days) in the investigated tropical stream and groundwater habitats. SFB were recovered from human faeces and sources potentially contaminated with human excreta. SFB were undetectable in animal faeces and environmental samples not contaminated with human faeces. Microcosm studies demonstrated a rapid die-off rate in groundwater (T90 value 0.6 days) and stream water (T90 value 0.9–1.7 days). Discrimination sensitivity analysis, including E. coli, faecal coliforms, total coliforms and Clostridium perfringens spores, revealed high ability of SFB to distinguish differing levels of faecal pollution especially for streams although high background levels of interfering bacteria can complicate its recovery on the used medium. Due to its faster die-off, as compared to many waterborne pathogens, SFB cannot replace microbiological standard parameters for routine water quality monitoring but it is highly recommendable as a specific and complementary tool when human faecal pollution has to be localized or verified. Because of its exclusive faecal origin and human specificity it seems also worthwhile to include SFB in future risk evaluation studies at tropical water resources in order to evaluate under which situations risks of infection may be indicated.
Keywords: microbial faecal pollution, microbial source tracking, sorbitol-fermenting Bifidobacteria, standard and alternative faecal indicators, tropical water bodies, urban areas, water quality
INTRODUCTION
Maintaining the microbial quality of water resources requires target-oriented management strategies. Microbial faecal source tracking can help to identify the perpetrators of environmental pollution, to establish best management practices and to prevent any further contamination (Jagals & Grabow 1996; Parveen et al. 1999; Scott et al. 2002; Nebra et al. 2003; Bonjoch et al. 2004; Reischer et al. 2008). The traditional microbial indicators (faecal coliforms, Escherichia coli, enterococci) do not allow pollution source differentiation, as they occur in animal as well as human faeces. Efforts to type strain libraries of traditional faecal indicators by genotypical or phenotypical methods are hindered by poor adaptation of these bacteria and laborious procedures (Orskov & Orskov 1981; Parveen et al. 1999; Scott et al. 2002; Nebra et al. 2003; Bonjoch et al. 2004). In the tropics, these classical indicators are also suspected to originate from non-faecal sources (such as soil) and to proliferate in tropical aquatic habitats under favourable situations and thus can be detectable at levels which may not reflect the original extent of faecal contamination (Carrillo et al. 1985; Rivera et al. 1988; Jimenez et al. 1989; Perez-Rosas & Hazen 1989; Wright 1989; Hazen & Toranzos 1990; Solo-Gabriele et al. 2000; Desmarais et al. 2002). As a result, microbial standard indicators may yield biased results. Alternative indicators are thus needed in order to complement or replace standard indicators under situations where its (single) use is no longer justified in tropical waters.
Non-sporing sorbitol-fermenting Bifidobacteria (SFB) are among the potential indicators for specific detection of human faecal contamination (Mara & Oragui 1983). SFB have been isolated only in human faeces with the development of Human Bifid Sorbitol Agar (HBSA). HBSA is specific for isolation and enumeration of sorbitol-fermenting strains of Bifidobacterium adolescentis and B. breve, which together constitute approximately 90% of human Bifidobacteria isolates (Mara & Oragui 1983; Sinton et al. 1998; Rhodes & Kator 1999). Studies on the use of HBSA to recover SFB from various contaminated waters have been conducted under field conditions (Mara & Oragui 1983; Jagals & Grabow 1996; Sinton et al. 1998; Rhodes & Kator 1999; Lynch et al. 2002; Scott et al. 2002; Long et al. 2003). However, these approaches were developed and tested comprehensively in temperate regions where the biological, physicochemical and socio-economic characteristics differ greatly from those of tropical regions (Toranzos & McFeters 1997). Very few SFB studies exist in developed tropical countries (Carrillo et al. 1985; Toranzos & McFeters 1997) and none in most tropical developing countries despite the problem of frequent outbreaks of waterborne diseases resulting from human source of the type of enteric pathogens such as Salmonella enterica serovar Typhi, Shigella spp. or Hepatitis A virus (Parveen et al. 1999; Scott et al. 2002). Additionally, bacterial flora of faeces from people living in widely different circumstances in different parts of the world may have differences in the type, number and frequency of isolation of bacterial groups (Drasar 1974). This could limit their use as indicators of recent and human specific faecal contamination. Furthermore, most SFB studies have been on surface waters such as streams, rivers and reservoirs (Mara & Oragui 1983; Carrillo et al. 1985; Long et al. 2003); studies on the suitability of SFB in assessing human faecal contamination of groundwater have not been done so far.
The aim of this study was to determine the presence of SFB in faecal sources including human and animal faeces of different host groups and environmental samples and furthermore to evaluate its use as a human-specific faecal indicator to monitor the microbiological water quality of aquatic resources in tropical and developing countries such as eastern Africa. We hypothesized that SFB exist only in human-specific faecal sources or in habitats where human faecal pollution was happening very recently. Soil samples from different locations within the study area were also investigated for the presence of SFB in order to have information on whether they originate from or regrow in the soil. In addition, the detection of SFB, standard coliforms (E. coli, faecal coliforms, total coliforms) and alternative Clostridium perfringens spores were compared in presumptively differently polluted stream and groundwater sources using the established and so-called ‘faecal pollution gradient approach’ of Byamukama et al. (2005). Furthermore, the survival rate for SFB in the stream and groundwater was evaluated by microcosm experiments to complement the information from the field study and to enable a better understanding of their ecology in tropical aquatic environments of eastern Africa.
MATERIALS AND METHODS
Description of the study area and sites
Dar es Salaam City is located on the coastal area of Tanzania, between 6°51′30″S and 6°47′30″S and 39°15′E and 39°17′E with an area of about 135 km2 (Figure 1). The city experiences both tropical and coastal climate with mean daily temperatures varying between 17° and 34°C and average humidity of about 67–96%. The annual rainfall averages between 1,000 and 1,400 mm, with the wettest period of the year being March to May. The evaporation rate is over 2,100 mm per annum. Being a coastal area, the city is characterized by sandy soils overlaying sandstone and limestone bedrock that allow fast percolation of the surface water, especially during rainy periods.
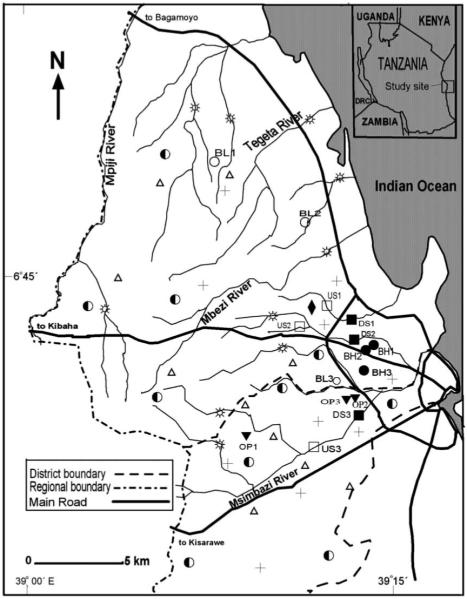
Figure 1.
The study area in Tanzania showing the locations of the 112 and 15 environmental faecal and water sampling sites, respectively: ○, Low-risk boreholes (BL1 to BL3); ●, Medium-risk boreholes (BH1 to BH3); ▼, High-risk borehole (OP1 to OP3); □, Upstream (US1 to US3); ■, Downstream (DS1 to DS 3); + , Human faeces; ◆, Sewage; △, Animal faeces;  , Polluted water; ◐ Tap water; DRC, Democratic Republic of Congo.
, Polluted water; ◐ Tap water; DRC, Democratic Republic of Congo.
In this study, 15 sampling sites were selected from streams and groundwater sources located in two municipalities of Dar es Salaam City (Figure 1); these included nine (9) boreholes and six (6) stream sites (selected from 3 streams). The samples were collected twice a month at each site from May to July 2005, during the time of the year when a mixture of rainy and dry patterns is evident. Over the whole sampling period, six (6) samples were collected from each sampling site, reflecting a total of 90 water samples. The borehole risk evaluation questionnaire described in WHO (1997) was used to select a gradient of boreholes differing in the probability of faecal contamination. The questionnaire contained ten sanitary conditions from which the boreholes were assessed. To each borehole, the risk scores were computed and later used to classify the borehole to low, medium or high risk (WHO 1997).
Three motorized boreholes (BL1, BL2, BL3) had risk scores of between 0 and 2. The boreholes were from the periphery of the city characterized by a low human population density. Although the boreholes were not fenced, there were no observable sources of contamination during the sampling period and they were thus classified as low-risk boreholes. Three other motorized boreholes (BH1, BH2, BH3) had risk scores of between 5 and 6. The boreholes were located at the squatter area in the city and characterized by the presence of pit latrines and/or septic tanks at a distance of 3 to 5 m from the boreholes. The boreholes were also located very close to the road and human residences; they were not fenced and stagnant waste water pools were observable in close proximity. These were classified as medium-risk boreholes. Open boreholes (OP1, OP2, OP3) had a risk score of between 8 and 10. The boreholes were located in the squatter areas and close to either pit latrines or septic tanks (about 3–5 m). The boreholes were neither fenced nor protected from surface run-off and their walls had cracks, which allowed the inward and outward movement of water. Water for domestic use is drawn from these boreholes using a plastic or rubber pail attached to a rope, which is left lying on the ground in between uses, a process that poses further risk of contamination of the water. These boreholes were considered to be under the highest risk of the groundwater sources.
As for the stream sites, three streams each with two sites – upstream and downstream sampling points – were surveyed. The stream sites US1, US2 and US3 were located upstream in low-density population catchments which do not receive discharge of sewage effluent; these were categorized as low influenced sites while the corresponding stream sites DS1, DS2 and DS3 were located downstream in highly populated areas whereby effluent channels join the respective streams. These were categorized as high influenced sites.
Physicochemical parameters
Dissolved oxygen (DO), temperature (TEMP), electrical conductivity (EC) and salinity (SAL) were measured in situ with YSI 85/10 FT meter (Yellow Springs, Ohio) while pH was measured using Hydrus 100 meter (FB 50101, UK). Probes were calibrated at 25°C before sampling and the calibration was verified upon returning from the field. Membrane electrode method (4500-O G) was used to measure five days’ biochemical oxygen demand (BOD5). Total suspended solids (TSS) were assessed using the gravimetric technique (2540D). For determination of nitrates plus nitrites (NO3− + NO2−), a defined volume of sample water was filtered through premuffled glass fibre filters (GF/C; Whatman, Springfield Mill, England), and the filtrate was analysed using calorimetric method (4500-NO3−F). To determine water hardness (HARD), titration method (3500-Ca-D) was used. Chlorides (CHL) measurement was done using calorimetric method (4500-Cl−-C). All analytical methods were applied as described in APHA (1995).
Sampling and analysis of SFB in soil, faeces and sewage
Single samples of faeces were obtained from 15 healthy adults, 10 dogs feeding on leftovers from human diet, 10 pigs feeding frequently on cereal products and occasionally on leftovers from human diet, 10 hens feeding on varieties of cereal products, 10 goats and 10 cattle feeding solely on different plant materials from the farm. Each single sample of faeces from the respective individual was obtained from its own site. Soil samples were obtained from ten sites selected at random in areas with and without settlements. A soil auger was used to obtain soil sample core of the top 10 cm soil layer from ten randomly selected spots within a 50 m radius of the sampled site (Byamukama et al. 2005). Soil and faecal samples were separately put into 200 ml sterile wide-mouthed jars using a sterile spoon. For each site, a separate sterile spoon was used to avoid cross contamination of the samples from different sites. Samples were then transported to the laboratory via cold storage for analysis within 8 h of taking the first sample. Aliquots from each of the ten soil spots were homogeneously mixed in the laboratory to form a composite soil sample for the respective site.
Twelve sewage samples were obtained from the Dar es Salaam University sewage channel that receives all the sewage from the university accommodation with approximately 6300 students (data from University Student Accommodation Bureau, USAB). Additionally, 15 polluted water samples were collected from 15 stream sites. The streams receive faecal materials from the nearby settlements. Tap water was collected randomly from ten different points in the city (Figure 1). Sampling was done according to Standard Methods (APHA 1995) using sterile glass bottles. Samples were transported to the laboratory in an iced cooler and analysed within 8 h of collection.
For each faecal and soil sample, 1 g was added to 100 ml sterile demineralized water, hand shaken and sonicated for 1 min in a Bransonic PC-650 (Branson Ultrasonics Corp, Danbury, Conn.) and allowed to stand for 1 h to allow particles to settle (Byamukama et al. 2005). Demineralized water rather than a buffer solution was used in order to mimic bacterial extraction conditions by possible rainwater influence, and a sonication treatment resulted to optimal dissociation of faecal bacteria from soil and faecal samples. The supernatants, sewage, polluted water and tap water samples were either diluted or whole volumes (10−4 ml–100 ml) were filtered through sterile cellulose nitrate membrane filters (Millipore type HAWG 047 SI, 0.45 μm pore size, 47 mm diameter), and thereafter placed on Human Bifid Sorbitol Agar (HBSA) (Mara & Oragui 1983). The plates were inverted and incubated at 37°C for 48 h in an anaerobic jar containing Anaerocult A anaerobic system (Merck, Darmstadt, Germany).
Enumeration of SFB was undertaken according to the previous studies using HBSA medium (Mara & Oragui 1983; Long et al. 2003). Deep-yellow, domed and mucoid colonies resulting from the fermentation of sorbitol were scored as presumptive SFB. Well-isolated presumptive SFB were randomly selected from HBSA agar plate and subjected to Gram staining followed by microscopic observation of individual cells exhibiting typical SFB cell morphology. A total of 83% of 286 colonies on HBSA had cells with characteristic Gram positive, Y- and/or spatulated shapes in the configurations of bacterial strings, which are typical of SFB strains (Jagals & Grabow 1996; Long et al. 2003). The enumeration of SFB occurred simultaneously with background bacteria as a result of poor selectivity of the medium. SFB were derived out of background bacteria using characteristic SFB colony morphology (dome shape, mucoid) and colour (deep yellow). Possible SFB growth inhibition by excessive numbers of background bacteria was tested using environmental isolates of SFB and background bacteria in differing ratios of cell abundances of each of the two growing both as single and mixed cultures. In this test, up to a ratio of 1: 102 SFB to background bacteria no inhibition effect could be determined. However, in ratios 1: 103 and above SFB could not be detected.
Sampling and microbiological analysis of borehole and river waters
Water sampling was done according to Standard Methods (APHA 1995) using 1-litre sterile glass bottles. The water samples were transported to the laboratory for analysis under conditions similar to those described above for soil and faecal samples. A range of volumes (0.0001–300 ml) of water samples was filtered and tested for SFB as described above while for total coliforms (TC), faecal coliforms (FC), Escherichia coli and Clostridium perfringens spores (CP) were tested according to Byamukama et al. (2005).
Survival of SFB and coliforms in water
Three replicate water samples were taken from each category of sites (BL3, BH2, OP2, US2, DS2) according to Standard Methods (APHA 1995) using 1-litre cotton-wool-stoppered glass bottles. Water samples were transported to the laboratory at room temperature protected from solar radiation. At the laboratory, samples were shaken vigorously and initial (T0) concentrations (CFU/100 ml) of SFB and coliform (TC, FC, E. coli) from each sample were determined before incubating them at room temperature. The concentrations in each sample were again determined at 24, 48, 72, 96 and 120 h (T24 to T120) after shaking the bottles with the samples vigorously. Culturable concentrations of SFB and coliform (TC, FC, E. coli) were obtained by membrane filtration as described above.
DATA ANALYSIS
Statistical tests were performed using SPSS for Windows, version 11.0 (SPSS Inc., Chicago, Illinois). Percentage non-parametric coefficient of variation (CV) was calculated as CV = (p75–p25)/p50 × 100, where p75, p25, p50 are 75th and 25th percentiles and the median. Cluster analysis based on physicochemical data, z-score standardization, squared Euclidean distance and within-group average linkages were selected in order to evaluate for relationship between sites. Other distance and linkage algorithms (data not shown) were also applied and gave comparable results for the given physicochemical data set. Non-parametric Spearman rank was used to analyse correlations in the given data set. Statistical significance was set at a probability of p < 0.05 for all the tests applying a Bonferroni correction for multiple testing.
Ability of faecal indicators to distinguish different levels of faecal pollution was tested accordingly to the established approach described in Byamukama et al. (2005). Briefly, the following steps were performed: category I – pooling faecal indicator data sets from borehole water as belonging to corresponding groups of contamination (i.e. pooled lowrisk boreholes (BL), pooled medium-risk boreholes (BH), pooled high-risk boreholes (OP), c.f. study site description) and analysing them for statistical differences (i.e. BL vs. BH, BH vs. OP, BL vs. OP); category II – comparing BL, BH, OP data sets to the pooled data as recovered from all the investigated river locations (S) (i.e. BL vs. S, BH vs. S, OP vs. S); and finally, category III – pooling and comparing of data from upstream (US) and downstream sites (DS). The extent of differences in indicator levels between the established category sites BL, BH, OP, US, DS, S was determined by using the bacteriological concentration ratios of the medians (BCRM) in accordance with the previous established work (Byamukama et al. 2005). Statistical significance of detected differences between compared groups was checked using the non-parametric Mann Whitney Test (c.f. Table 5).
Table 5.
Discrimination capacity of SFB, coliforms and C. perfringens spores (CP) for habitats differing in presumptive faecal pollution levels (7 tested pairs)*
| Fecal indicators | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | FC | E. coli | CP | SFB | |||||||
| Compared category |
Compared Pairs | +/−† | log10 BCRM‡ | +/− | log10 BCRM | +/− | log10 BCRM | +/− | log10 BCRM | +/− | log10 BCRM |
| I | BL–BH | − | 0.7 | − | 0.4 | − | 0.6 | − | 0.4 | − | np |
| BL–OP | + | 3.7 | − | 2.8 | + | 3.1 | + | 2.7 | (+) | np | |
| BH–OP | + | 3.0 | + | 2.4 | + | 2.5 | + | 2.4 | (+) | np | |
| II | BL–S | + | 5.6 | + | 4.9 | + | 5.1 | + | 4.4 | (+) | np |
| BH–S | + | 4.9 | + | 4.4 | + | 4.5 | + | 4.0 | (+) | np | |
| OP–S | + | 1.9 | + | 2.0 | + | 2.0 | + | 1.7 | + | 1.8 | |
| III | US–DS | − | 0.3 | − | 0.5 | − | 0.7 | + | 0.6 | + | 1.4 |
Abbreviations: For BL, BH, OP, US, DS, TC, FC, CP, SFB see Tables 2 and 3; S, pooled stream data set including US and DS.
Mann–Whitney test for pairwise difference, + indicates p < 0.05 while—indicates p > 0.05 and corrected for multiple tests using Bonferroni correction.
log10 BCRM = log10 of the bacteriological concentration ratio of the medians; np = calculation was not possible as SFB were not detectable in BL and BH and thus no algorithm could be applied. However, from a practical point of view a significant difference between not detectable and detected concentrations exists (+). n = 6 pooled sites × 18 to 36 samples per pooled site.
First-order die-off kinetics was assumed to be a reasonable model for the microcosm die-off experiments; die-off coefficients were computed from the slope of the regression line obtained from the ln-transformed data set. SFB concentrations from various potential pollution sources were compared after unit conversion from gram and ml to cubic centimetre following standard conversions assuming 1 ml equals 1 cm3, and 1 cm3 ≈ 1 g, respectively.
RESULTS
Sources of sorbitol-fermenting Bifidobacteria (SFB) in the tropical environment
SFB could be isolated from human faeces, sewage and water polluted with sewage but not from any of the other animal faeces, tap water and soil samples (Table 1). The concentration of SFB was highest in human faeces with a median of log10 11.4 cfu per 100 cm3. Observed median concentrations of SFB in sewage and water polluted with sewage were log10 6.8 cfu per 100 cm3 and log10 3.5 – cfu per 100 cm3, respectively. As compared to the concentration range of SFB from human faeces (approx. log10 3 of variation), the observed concentration range of SFB was far increased for sewage and polluted water samples – as related to the kind of sewage, stage of treatment and the extent/age of faecal pollution. The isolation of SFB on HBSA agar was paralleled by the growth of potentially interfering background bacteria (Table 1). Assuming a relative detection threshold of ≥ 1 SFB colony within 100 bacterial background colonies for the used HBSA (see Methods section for details), median detection limits (MDL) and their ranges could be estimated for all the investigated types of samples (Table 1).
Table 1.
Occurrence and abundance (cfu/100 cm3) of sorbitol-fermenting Bifidobacteria (SFB), associated background and detection limits from respectively investigated environmental sources
| Log cfu per 1 cm3 faeces or per 100 cm3 water/wastewater | ||||||||
|---|---|---|---|---|---|---|---|---|
| Samples | SFB | Background bacteria | DL† | |||||
| Source | N* | Positive (%) | Median | Range | Median | Range | MDL‡ | RDL§ |
| Human faeces | 15 | 100 | 9.4 | 8.1–10.4 | 11.4 | 9.9–12.7 | – | – |
| Sewage | 12 | 100 | 6.8 | 3.4–7.9 | 8.8 | 4.4–10.2 | – | – |
| Polluted water | 15 | 100 | 3.5 | 2.8–5.6 | 5.5 | 4.4–8.8 | – | – |
| Pig faeces | 10 | 0 | nd¶ | nd | 5.5 | 5.2–5.7 | <3.5 | <3.2–3.7 |
| Cattle faeces | 10 | 0 | nd | nd | 4.8 | 4.3–5.2 | <2.8 | <2.3–2.9 |
| Hen faeces | 10 | 0 | nd | nd | 6.8 | 6.0–7.4 | <4.8 | <4.0–4.9 |
| Goat faeces | 10 | 0 | nd | nd | 5.6 | 5.4–6.7 | <3.6 | <3.4–4.7 |
| Dog faeces | 10 | 0 | nd | nd | 4.2 | 3.3–4.9 | <2.0 | <1.3–2.9 |
| Soil | 10 | 0 | nd | nd | 4.8 | 3.9–5.6 | <2.8 | <1.9–3.1 |
| Tap water | 10 | 0 | nd | nd | 1.0 | 0.8–1.4 | <0.1 | <0.6 |
Number of replicates (N).
Estimated detection limit (DL) of HBSA, assuming a detection threshold of ≥ 1 SFB colony within 100 colonies of background bacteria (c.f. materials and methods).
Median detection limit (MDL).
Range of detection limit (RDL).
SFB were not detectable above the given detection limits (nd).
Physicochemical characterization of the investigated aquatic habitats (water source types)
The physicochemical characteristics of the sampling sites are shown in Table 2. The physicochemical parameters varied greatly between stream and groundwater; correlation analysis (Table 4) revealed high and significant positive correlations between EC, SAL, CHL and HARD (p < 0.05) and between BOD5 and TSS (p < 0.05). Groundwater had significantly higher levels of chloride, hardness, electrical conductivity, NO3− + NO2− and salinity compared to stream water (p < 0.01). There were also observable significant differences (p < 0.05) in physicochemical parameters between sites (i.e. selected water source types). The noted differences in the physicochemical characteristics between water source types were reflected by cluster analysis based on coefficients of similarity measured by squared Euclidean Distance (Figure 2). Generally, cluster analysis divided the sites into two large clusters (I and II) based on groundwater and stream physicochemical data. Cluster I consisted of groundwater sites while cluster II contained stream sites. Site OP1, however, was exceptional; it clustered with stream water sites. Grouping was confirmed by principal component analysis (data not shown). The analysis indicated that the investigated stream and groundwater habitats represent unique physicochemical habitats. It should be mentioned that the physicochemical characterization is a valuable basis facilitating the correct interpretation of results as recovered from the further microbiological studies.
Table 2.
Physicochemical parameters of the various water type categories
| Water types |
Temp (°C) | pH | DO (mg/l) | TSS (mg/l) | BOD5 (mg/l) | Chloride (mg/l) | Salinity (mg/l) | Hardness (mg/l CaCO3) |
Electrical conductivity (μS/cm) |
Nitrate plus nitrite (mg/l) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | R | M | R | M | R | M | R | M | R | M | R | M | R | M | R | M | R | M | R | |
| BL | 28.3 | 25.7–33.6 | 6.2 | 5.8–7.5 | 2.9 | 1.1–8.4 | 2 | 0.1–34 | 5.6 | 0.1–17 | 486 | 290–850 | 1.1 | 0.5–30 | 392 | 213–1,800 | 2,400 | 1,100–5,900 | 19 | 1.2–92 |
| BH | 29.6 | 27.8–32.7 | 6.8 | 6.5–7.1 | 2.1 | 1.3–7.3 | 1.8 | 0.2–42 | 6.1 | 1.0–18 | 348 | 220–590 | 1.0 | 0.4–1.3 | 336 | 170–660 | 2,100 | 790–2,800 | 39 | 9.2–75 |
| OP | 28.3 | 25.8–29.3 | 6.4 | 6.1–6.9 | 2.5 | 0.5–8.1 | 16 | 3.6–720 | 35.9 | 23–48 | 249 | 8.7–390 | 0.7 | 0.1–1.3 | 119 | 9.6–310 | 1,500 | 150–2,700 | 22 | 2.8–100 |
| US | 27.8 | 25.0–33.6 | 7.7 | 7.1–8.3 | 4.7 | 1.7–8.9 | 102 | 6.8–4,800 | 86.9 | 33–200 | 37 | 17.8–320 | 0.2 | 0.1–0.8 | 122 | 39–220 | 450 | 210–1,700 | 7 | 0.2–16 |
| DS | 27.4 | 24.9–29.0 | 7.6 | 7.3–7.9 | 1.1 | 0.8–8.6 | 161 | 14.2–3,600 | 109.6 | 78–180 | 126 | 21.9–360 | 0.4 | 0.1–1.1 | 113 | 30–270 | 810 | 220–2,200 | 12 | 4.8–31 |
Abbreviations: M, Median; R, Range; BL, Pooled low-risk boreholes; BH, Pooled medium-risk boreholes; OP, Pooled high-risk boreholes; US, Pooled upstream sites and DS, Pooled downstream sites; Temp, Water temperature; DO, Dissolved oxygen; TSS, Total suspended solids; BOD5, Five days’ biological oxygen demand; n = 5 habitats × 15–18 single samples.
Table 4.
Spearman correlation coefficient (r) for faecal indicators and physicochemical parameters of ground- and surface waters*
| TC | FC | E. coli | CP | SFB | TEMP | DO | EC | pH | SAL | BOD5 | TSS | CHL | HARD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | 1.00 | |||||||||||||
| FC | 0.96 | 1.00 | ||||||||||||
| E. coli | 0.86 | 0.90 | 1.00 | |||||||||||
| CP | 0.92 | 0.94 | 0.86 | 1.00 | ||||||||||
| SFB | 0.86 | 0.87 | 0.81 | 0.84 | 1.00 | |||||||||
| TEMP | −0.40 | −0.47 | −0.41 | −0.46 | −0.42 | 1.00 | ||||||||
| DO | − 0.09 | − 0.08 | − 0.14 | − 0.17 | − 0.15 | − 0.01 | 1.00 | |||||||
| EC | −0.62 | −0.59 | −0.58 | −0.63 | −0.62 | 0.35 | 0.12 | 1.00 | ||||||
| pH | 0.64 | 0.64 | 0.62 | 0.62 | 0.61 | − 0.24 | 0.01 | −0.48 | 1.00 | |||||
| SAL | −0.61 | −0.58 | −0.58 | −0.62 | −0.61 | 0.32 | 0.16 | 0.99 | −0.48 | 1.00 | ||||
| BOD5 | 0.88 | 0.87 | 0.76 | 0.84 | 0.81 | − 0.28 | 0.02 | −0.55 | 0.63 | −0.54 | 1.00 | |||
| TSS | 0.73 | 0.72 | 0.64 | 0.69 | 0.69 | −0.37 | 0.05 | −0.61 | 0.48 | −0.59 | 0.79 | 1.00 | ||
| CHL | −0.67 | −0.66 | −0.69 | −0.71 | −0.65 | 0.35 | 0.19 | 0.85 | −0.44 | 0.84 | −0.63 | −0.63 | 1.00 | |
| HARD | −0.57 | −0.53 | −0.54 | −0.59 | −0.54 | 0.16 | 0.15 | 0.79 | − 0.29 | 0.80 | −0.56 | −0.66 | 0.85 | 1.00 |
| NO3− + NO2− | −0.49 | −0.50 | −0.45 | −0.55 | −0.47 | 0.42 | − 0.16 | 0.42 | −0.52 | 0.40 | −0.51 | −0.45 | 0.43 | 0.32 |
Correlation significant on p < 0.05 (Bonferroni corrected for multiple testing). All values are significant except those italic. See Tables 2 and 3 for TC, FC, CP, SFB, BOD5, DO, TEMP and TSS abbreviations. EC, Electrical conductivity; SAL, Salinity; CHL, Chlorides; HARD, Hardness; n = 75–90 samples per parameter.
Figure 2.
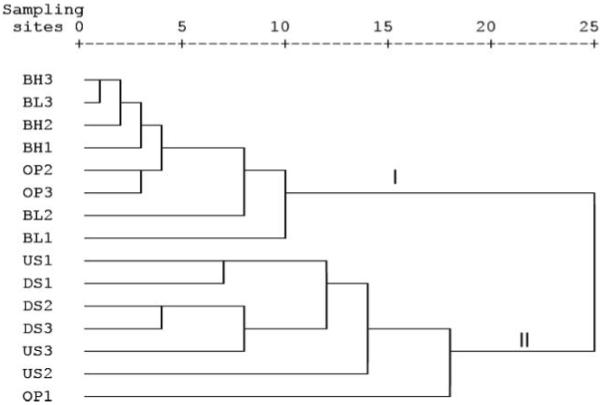
Grouping of sampling sites by cluster analysis using within-group average linkage, square Euclidean distance and physicochemical data set. Cluster I = Groundwater sites, Cluster II = Stream sites, n = 75-90 single samples per parameter. For abbreviations see Figure 1.
Occurrence of SFB in the investigated water source types
SFB were not detected in either BL or BH sites (Table 3). However, SFB were detected in samples from OP sites with pit latrines and septic tanks in the vicinity, US sites receiving minor faecal material from low-density settlements and DS sites that received sewage effluent from heavily populated areas. SFB levels ranged from not detectable to log10 5.0 cfu, not detectable to log10 5.5 cfu and log10 4.7 cfu to log10 6.8 cfu per 100 ml for OP, US and DS sites, respectively (Table 3). In each investigated stream, US sites had significantly lower SFB levels compared to DS sites (p < 0.05, n = 18).
Table 3.
Levels of faecal bacterial indicators in various water type categories
| Fecal indicator concentration (log cfu/100 ml) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | FC | E. coli | CP | SFB | ||||||||||||||||
| Sampling sites |
PS (%) | M | R | CV (%) | PS (%) | M | R | CV (%) | PS (%) | M | R | CV (%) | PS (%) | M | R | CV (%) | PS (%) | M | R | CV (%) |
| BL | 100 | 2.1 | 1.3–3.5 | 38 | 100 | 1.9 | 1–2.5 | 37 | 72 | 1.2 | 0.0–1.9 | 102 | 61 | 1.0 | 0.0–2.5 | 177 | 0 | nd* | nd* | nd |
| BH | 100 | 2.7 | 1.3–3.8 | 31 | 100 | 2.3 | 1–2.9 | 23 | 88 | 1.8 | 0.0–2.7 | 54 | 66 | 1.3 | 0.0–2.9 | 184 | 0 | nd† | nd† | nd |
| OP | 100 | 5.8 | 4.5–6.1 | 9 | 100 | 4.7 | 3.8–5.5 | 14 | 83 | 4.3 | 0.0–5.3 | 23 | 100 | 3.7 | 2.5–4.3 | 23 | 72 | 3.2 | 0.0–5.0 | 126 |
| US | 100 | 7.5 | 6.5–7.9 | 7 | 100 | 6.4 | 4.9–7.3 | 7 | 94 | 6.0 | 0.0–6.7 | 12 | 100 | 5.1 | 3.0–5.8 | 11 | 88 | 4.4 | 0.0–5.5 | 23 |
| DS | 100 | 7.8 | 7.0–8.6 | 8 | 100 | 6.9 | 6.1–7.7 | 9 | 100 | 6.7 | 5.8–7.2 | 6 | 100 | 5.6 | 5.0–6.7 | 7 | 100 | 5.8 | 4.7–6.8 | 23 |
SFB were not detectable (nd) above the median detection limit of log 0.28 cfu/100 ml (range log 0.1–1.7 cfu/100 ml).
SFB were not detectable (nd) above the median detection limit of log 0.60 cfu/100 ml (range log 0.1–1.5 cfu/100 ml).
Abbreviations: For BL, BH, OP, US and DS see Table 2, TC, Total coliform; FC, Faecal coliform; CP, Clostridium perfringens; SFB, Sorbitol-fermenting Bifidobacteria; PS, Percentage positive samples; M, Median; R, Range; CV, Percentage non-parametric-based coefficient of variations for the pooled data set ((p75–p25)/p50 × 100 where p75 and p25 are 75 and 25 percentiles, respectively, while p50 is a median); n = 5 habitats × 18 single samples
Enumeration of SFB on HBSA agar for the water samples occurred simultaneously with background bacteria. In each sampling site, the concentrations of background bacteria were higher than those of SFB. The median percentage of SFB to total bacteria grown on HBSA at OP was somewhat lower (2.0%) compared to 3.9% and 4.3% for DS and US sites. When experimental tests for possible growth inhibition by excessive numbers of background bacteria on SFB were done, up to a ratio of at least 1: 100 (SFB to background bacteria), no inhibition could be detected. In ratios ≥ 1: 103, SFB colonies could not be observed, as the plate was overgrown by background bacteria. These experimental results are in agreement to the lowest detectable percentages of SFB to background bacteria enumerated in the investigated water habitats (i.e. 0.9%).
Survival of SFB and coliforms in surface and groundwater microcosms
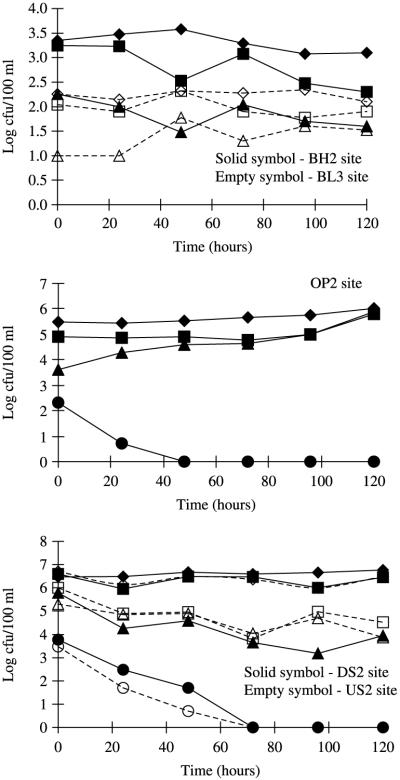
The survival of SFB and coliforms (i.e. E. coli, FC, TC) in the stream and groundwater microcosms incubated at room temperature is given in Figure 3. All coliform fractions in the examined water samples showed an extended survival (>120 h) or even regrowth (e.g. E. coli/FC in OP2 microcosms, p < 0.05). In contrast, SFB declined steadily and T90 values (i.e. time needed for one log10 reduction estimated from the assumed first-order die-off kinetics) were in the range of 0.6, 0.9 and 1.7 days for OP2, US2 and DS2, respectively. This is equivalent to a decay rate of −0.016 h−1 for OP2, −0.028 h−1 for US2 and −0.033 h−1 for DS2.
Figure 3.
Survival of sorbitol-fermenting Bifidobacteria in comparison to coliform bacteria (E. coli, FC, TC) in microcosms using water from stream- and groundwater habitats incubated at room temperature for 120 h. Values plotted are the mean of three samples per faecal indicator and site. SFB were not detected in samples from site BL3 and BH2 throughout the sampling period and during the survival determination. For BL3, BH2, OP2, US2 and DS2 abbreviations see Figure 1; ◆, Total coliform (TC) (BH2, OP2, DS2 sites); ■, Fecal coliform (FC) (BH2, OP2, DS2 sites); ▲, E. coli (BH2, OP2, DS2 sites); ●, Sorbitol-fermenting Bifidobacteria (SFB) (OP2, DS2 sites); ◇, TC (BL3, US2 sites); □, FC (BL3, US2 sites); △, E. coli (BL3, US2 sites) and ○, SFB (US2 sites).
Discrimination capacity of SFB, coliforms and C. perfringens spores at the investigated aquatic habitats
For the pooled data set, all the investigated faecal indicators (SFB, E. coli, FC, TC, C. perfringens spores) revealed high to very high correlations (0.81–0.96, p < 0.05) amongst each other (Table 4), including high correlations between SFB and the remaining indicators (r = 0.81–0.87; p < 0.05). Furthermore, all indicators reflected moderate to high positive correlations to BOD5 and TSS (r = 0.64–0.88; p < 0.05) and low to moderate negative correlations to TEMP, EC, SAL, CHL, HARD and NO3− + NO2− (r = −0.40 to − 0.71; p < 0.05) (Table 4). It should be mentioned that increased correlations between the indicators and parameters of the pooled data set were expected due to the selected faecal pollution gradient – a methodical prerequirement for the possibility to compare differently polluted habitat types (c.f. methods for habitat selection, statistics, faecal pollution gradient approach): category I comparisons – as supported by the log10 BCRM values (ranging from 0.4 to 3.7) and the Mann–Whitney test, SFB, TC, E. coli and C. perfringens spores showed a good ability (2 out of 3 compared pairs) in distinguishing data sets between pooled low-risk borehole samples (BL) vs. pooled high-risk borehole samples (OP) and pooled medium-risk borehole samples (BH) vs. pooled high-risk borehole samples (OP) (Table 5). However, it was not possible to distinguish pooled low-risk borehole samples (BL) from pooled medium-risk borehole samples (BH) with any of the applied bacterial indicators, as differences in indicator levels were too small (i.e. log10 BCRM ranging from 0.4 to 0.7); category II comparisons – all the applied indicators could distinguish indicator levels found in any of the borehole water sample categories (BL, BH, OP) vs. the pooled stream data set (S) (Table 5), revealing median concentration differences up to very high values (i.e. log10 BCRM ranging from 1.7 to 5.6); category III comparisons – in contrast to the comparisons including borehole water sources (category I, II), significant differences for the indicators in the ability to discriminate upstream (US) vs. downstream sources (DS) were evident. Increasing log10 BCRM ratios of 0.3, 0.5, 0.7, 0.6 and 1.4 for TC, FC, E. coli, CP and SFB, respectively, were observed. Only SFB and C. perfringens spores were able to discriminate, in a statistically significant way (p < 0.05), US vs. DS sources as influenced by faecal pollution caused by the respective settlements (Table 5).
DISCUSSION
Human faeces are the primary source of SFB in the considered Tanzanian tropical environment under the given method and detection limit (Table 1). The concentrations of SFB determined in human faeces in this study (geometric mean log 9.4 cfu/cm3) are in remarkable accordance with that of temperate regions (geometric mean log 9.8 cfu/cm3) (Mara & Oragui 1983) and only slightly differ from those reported in other tropical regions (geometric mean log 8.1 cfu/cm3, Zimbabwe; geometric mean log 9.1 cfu/cm3, Nigeria) (Mara & Oragui 1985) using the HBSA method. The observed slight difference with data from the tropical study could be due to diet differences, as SFB require rigorous nutrients for their survival/growth (Sinton et al. 1998; Nebra et al. 2003). Dilution and physical processes (Rhodes & Kator 1999; Nebra et al. 2003) as well as injury due to the presence of oxygen or its derivatives (Mara & Oragui 1983) could have influenced the numbers of SFB observed in sewage and sewage polluted waters, as well as due to the fact that the medium can only isolate bifidobacteria that are able to ferment sorbitol – in this case B. adolescentis and B. breve – which occur only in human faeces (Mara & Oragui 1983).
The densities of SFB reported for surface water types in this study are comparable to that reported by Resnick & Levin (1981) for river samples collected near the outfall and much higher than those reported in tropical rain-forest watershed in Puerto Rico (Carrillo et al. 1985) and in freshwater streams and rivers in Zimbabwe and Nigeria (Mara & Oragui 1985). In general, the high values of SFB were observed at downstream sites (c.f. DS, Table 3) during the rainy month (March, 2005), the time when the majority of the inhabitants were emptying their septic tanks into the streams as a means of avoiding the costs of transporting the septic contents to the municipal treatment plant. The absence of SFB in non-sewage impacted aquatic habitats and isolation only in the sites contaminated with human faecal materials is a strong indication that SFB are not part of the natural microflora and do not regrow or accumulate in the environment. This feature was further supported by the fact that SFB was not isolated from intensively sampled soil. According to the performed microcosm experiments (c.f. Figure 3), SFB were unable to multiply in both stream and groundwater under room temperature.
In comparison with coliform indicators (E. coli, FC, TC) and C. perfringens spores, SFB was the only indicator absent in the sites free from human faecal contaminations throughout the study period. It was also the only indicator that did not persist in the studied microcosms. Such characteristics obviously enabled SFB to discriminate the studied upstream (US) vs. downstream (DS) sites with differing faecal pollution from anthropogenic sources (i.e. sewage influents from settlements, c.f. habitat selection criteria in methods section) better than the other faecal indicators (Table 5). It should be mentioned that also C. perfringens spores, closely followed by E. coli, were able to distinguish US vs. DS sites in a statistically significant way (Table 5). However, the median concentration differences in indicator levels between US and DS (c.f. Table 5) were 5- to 6-fold higher for SFB (log10 BCRM 1.4) as compared to CP (log10 BCRM 0.6) or E. coli (log10 BCRM 0.7). Our observations are thus in agreement with previous studies that recommended the use of Bifidobacteria such as SFB for surface water monitoring after observing extended survival and regrowth of coliforms in the warm tropical climate (Evison & James 1975; Carrillo et al. 1985; Hazen & Toranzos 1990).
In contrast to the performed surface water comparisons (US vs. DS), almost all the investigated bacterial indicators showed good discrimination ability between category I comparisons (within groundwater source types) and category II comparisons (river vs. groundwater sources) (c.f. Table 5). Remarkably, in five out of seven comparisons, TC revealed the highest median differences (log10 BCRM up to 5.6) in comparison with the rest of the studied setup. For example, immediate bacterial surface influence from river water to well water would be most sensitively detected by TC rather than by E. coli, C. perfringens spores or SFB. However, it has to be emphasized that TC are unlikely linked to specific faecal pollution but rather to a general surface influence event in the investigated well habitats (i.e. associated with soil, sediment and sewage influence from the surface).
The numbers of SFB declined very rapidly in the conducted microcosm experiments revealing a range of T90 values from 0.6 to 1.7 days. This indicates SFB as an indicator of very recent human faecal contamination in surface and groundwater. A single strong faecal pollution event, for example contamination of stagnant water resources, can – at a maximum – only be detected for a few days. Many of the existing bacterial, viral and protozoan waterborne pathogens as well as indicators show far higher persistence as compared to the persistence of SFB in the aquatic habitat. For example, for groundwater it was recently reviewed that coliphages, poliovirus, echovirus and Salmonella spp. show average T90 values of approximately 2.5 weeks. Hepatitis A and Coxsackievirus even revealed higher average T90 values of 4 to 7 weeks in groundwater (John & Rose 2005). Thus it becomes very clear that SFB cannot replace standard faecal indicators (showing longer environmental persistence) for routine water quality investigations. In contrast, SFB is likely an excellent parameter in order to specifically complement routine investigations when human faecal pollution has to be localized or verified. For example, temporary complementation of investigation programs with SFB can help to decide whether human influence is likely when questionable results from routine indicators exist. However, low persistence of SFB in the environment has to be considered when selecting appropriate investigational sampling designs and intervals.
The findings of this study show that SFB validated in temperate regions under field conditions (Jagals et al. 1995; Jagals & Grabow 1996; Rhodes & Kator 1999; Lynch et al. 2002; Long et al. 2003) could also be applied in tropical lowland areas such as Dar es Salaam, Tanzania, as an indicator of very recent and specific human faecal contamination. However, the routine use seems currently not practical since the additional efforts and requirements for SFB investigations might not be affordable for a standard water quality laboratory in a country like Tanzania. First, because of the more rapid die-off as compared to other indicators the diagnostic time frame to detect relevant faecal pollution is shorter. Modification of the sampling intervals and locations – depending on the respective environment – is thus likely needed in order to avoid increased rates of false negative results. Second, HBSA designed for detection of SFB is vulnerable to background bacteria. Such bacteria tend to be higher in numbers than SFB on the plate, especially in heavily polluted waters such as the samples from downstream sites in this study. The fact that SFB could not be recovered at the ratio of ≥ 1: 103 (SFB to background bacteria) was evidence that background bacteria may overgrow or inhibit the growth of SFB. Practically, they can complicate enumeration of the SFB colonies in the respective method by the existence of false positives, a scenario that could explain the 17% false positives obtained during the confirmation experiment. Thus, there is a need to improve the existing method in order to make it more applicable and also cheaper for its routine use.
On the basis of our results, the main source of SFB in the studied urban tropical lowland environment is human faeces. SFB does not occur in soil uncontaminated with recent human faecal material nor does it regrow in river and groundwater habitats. As a result of the rapid die-off, SFB exhibit a specific value as an indicator of very recent human faecal pollution (diagnostic time frame hours to days) in aquatic habitats in this tropical region of eastern Africa. It is clear that this low persistence has to be considered when selecting an appropriate investigation design in order to avoid false negative results (e.g. in case faecal associated pathogens are available but not SFB due to rapid die-off). However, according to the performed faecal discrimination analysis, SFB showed better indication values for different levels of faecal pollution for surface water (stream water) than coliform fractions or C. perfringens spores. Taken together, SFB can be considered an excellent parameter for situations when recent human faecal pollution has specifically to be detected in the tropical aquatic environment. Because of its exclusive faecal origin and human specificity it seems also worthwhile to include SFB in future risk evaluation studies at tropical water resources in order to evaluate under which situations risks of infection may be indicated.
ACKNOWLEDGEMENTS
This study was performed within the framework of the Austrian Development Cooperation (ADC Project 612-00) within the M.Sc. Programme ‘Environmental Sciences, Specialization Limnology & Wetland Ecosystems’ through the Institute for Limnology, Austrian Academy of Sciences (OEAW) in co-operation with UNESCO-IHE, Institute for Water Education, Delft, The Netherlands. This study was also supported by the FWF (Austrian Science Foundation) translational research project L414-B03 granted to AHF. We thank the technicians of the Molecular Biology and Biotechnology Department, University of Dar es Salaam, Tanzania, for sampling assistance.
Contributor Information
Douglas Mushi, Department of Biological Sciences, Sokoine University, P.O. Box 3038, Morogoro, Tanzania.
Denis Byamukama, Department of Biochemistry, Makerere University, P.O. Box 7062, Kampala, Uganda.
Amelia K. Kivaisi, Department of Molecular Biology and Biotechnology, University of Dar es Salaam, P.O. Box 35060, Dar es Salaam, Tanzania
Robert L. Mach, Institute of Chemical Engineering, Research Area Applied Biochemistry and Gene Technology, Research Group Environmental Microbiology and Molecular Ecology, Vienna University of Technology, Getreidemarkt 9/166-5-2, A-1060, Vienna, Austria
Andreas H. Farnleitner, Institute of Chemical Engineering, Research Area Applied Biochemistry and Gene Technology, Research Group Environmental Microbiology and Molecular Ecology, Vienna University of Technology, Getreidemarkt 9/166-5-2, A-1060, Vienna, Austria, Tel.: +43 1 58801 17256, a.farnleitner@aon.at
REFERENCES
- APHA . Standard Methods for the Examination of Water and Wastewater. 19th edition American Public Health Association/American Water Works Association/Water Environmental Federation; Washington, DC: 1995. [Google Scholar]
- Bonjoch E, Balleste E, Blanch AR. Multiplex PCR with 16S rRNA Gene-targeted primers of Bifidobacterium spp. to identify sources of fecal pollution. Appl. Environ. Microbiol. 2004;70(5):3171–3175. doi: 10.1128/AEM.70.5.3171-3175.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byamukama D, Mach RL, Kansiime F, Manafi M, Farnleitner AH. Discrimination efficacy of fecal pollution detection in different aquatic habitats of a high altitude tropical country using presumptive coliform, Escherichia coli and Clostridium perfringens spores. Appl. Environ. Microbiol. 2005;71(1):65–71. doi: 10.1128/AEM.71.1.65-71.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo M, Estrada E, Hazen TC. Survival and enumeration of the fecal indicators Bifidobacteria adolescentis and Escherichia coli in a tropical rain forest watershed. Appl. Environ. Microbiol. 1985;50:468–476. doi: 10.1128/aem.50.2.468-476.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmarais TR, Solo-Gabriele HM, Palmer CJ. Influence of soil on fecal indicator organisms in a tidally influenced subtropical environment. Appl. Environ. Microbiol. 2002;68:1165–1172. doi: 10.1128/AEM.68.3.1165-1172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasar BS. Some factors associated with geographical variations in the intestinal microflora. In: Skinner FA, Carr JG, editors. The Normal Microbial Flora of Man. Academic Press; London: 1974. pp. 187–196. [PubMed] [Google Scholar]
- Evison LM, James A. Bifidobacterium as an indicator of human fecal pollution in water. Prog. Water Technol. 1975;7:57–66. [Google Scholar]
- Hazen TC, Toranzos GA. Tropical source water. In: McFeters GA, editor. Drinking Water Microbiology, Progress and Recent Developments. Springer-Verlag; Berlin: 1990. pp. 32–53. [Google Scholar]
- Jagals P, Grabow WOK. An evaluation of sorbitol-fermenting Bifidobacteria as specific indicators of human fecal pollution of environmental water. Water SA. 1996;22:235–238. [Google Scholar]
- Jagals P, Grabow WOK, De Villiers CJ. Evaluation of indicators for assessment of human and animal fecal pollution of surface run-off. Water Sci. Technol. 1995;31(5–6):235–241. [Google Scholar]
- Jimenez L, Muniz I, Toranzos GA, Hazen TC. Survival and activity of Salmonella typhimurium and Escherichia coli in tropical freshwater. J. Appl. Bacteriol. 1989;67:61–69. doi: 10.1111/j.1365-2672.1989.tb04955.x. [DOI] [PubMed] [Google Scholar]
- John DE, Rose JB. Review of factors affecting microbial survival in groundwater. Environ. Sci. Technol. 2005;39:7345–7356. doi: 10.1021/es047995w. [DOI] [PubMed] [Google Scholar]
- Long SC, Shafer E, Arango C, Siraco D. Evaluation of three source tracking indicator organisms for watershed management. J. Water Suppl. Res. Technol. 2003;52(8):565–575. [Google Scholar]
- Lynch PA, Gilpin BJ, Sinton LW, Savill MG. The detection of Bifidobacterium adolescentis by colony hybridization as an indicator of human fecal pollution. J. Appl. Microbiol. 2002;92:526–533. doi: 10.1046/j.1365-2672.2002.01557.x. [DOI] [PubMed] [Google Scholar]
- Mara DD, Oragui JI. Sorbitol-fermenting Bifidobacteria as specific indicators of human fecal pollution. J. Appl. Bacteriol. 1983;55:349–357. doi: 10.1111/j.1365-2672.1983.tb01331.x. [DOI] [PubMed] [Google Scholar]
- Mara DD, Oragui JI. Bacteriological methods for distinguishing between human and animal fecal pollution of water: results of fieldwork in Nigeria and Zimbabwe. Bull. World Health Organ. 1985;63(4):773–783. [PMC free article] [PubMed] [Google Scholar]
- Nebra Y, Bonjoch X, Blanch AR. Use of Bifidobacterium dentium as an indicator of the origin of fecal water pollution. Appl. Environ. Microbiol. 2003;69(5):2651–2656. doi: 10.1128/AEM.69.5.2651-2656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov F, Orskov I. Enterobacteriaceae. In: Broude AI, editor. Medical Microbiology and Infectious Diseases. W.B. Saunders Co; Philadelphia, PA: 1981. pp. 340–352. [Google Scholar]
- Parveen S, Portier KM, Robinson K, Edmiston L, Tamplin ML. Discriminant analysis of ribotype profiles of Escherichia coli for differentiating human and nonhuman sources of fecal pollution. Appl. Environ. Microbiol. 1999;65(7):3142–3147. doi: 10.1128/aem.65.7.3142-3147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Rosas N, Hazen TC. In situ survival of Vibrio cholerae and Escherichia coli in a tropical rain forest watershed. Appl. Environ. Microbiol. 1989;55(2):495–499. doi: 10.1128/aem.55.2.495-499.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reischer GH, Haider JM, Sommer R, Stadler H, Keiblinger KM, Hornek R, Zerobin W, Mach RL, Farnleitner AH. Quantitative microbial faecal source tracking with sampling guided by hydrological catchment dynamics. J. Environ. Microbiol. 2008;10:2598–2608. doi: 10.1111/j.1462-2920.2008.01682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick IG, Levin MA. Assessment of Bifidobacteria as indicators of human fecal pollution. Appl. Environ. Microbiol. 1981;42(3):433–438. doi: 10.1128/aem.42.3.433-438.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes MW, Kator H. Sorbitol-fermenting Bifidobacteria as indicators of diffuse human fecal pollution in estuarine watersheds. J. Appl. Microbiol. 1999;87:528–535. doi: 10.1046/j.1365-2672.1999.00845.x. [DOI] [PubMed] [Google Scholar]
- Rivera SC, Hazen TC, Toranzos GA. Isolation of fecal coliforms from pristine sites in a tropical rainforest. Appl. Environ. Microbiol. 1988;54:513–517. doi: 10.1128/aem.54.2.513-517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TM, Rose JB, Jenkins TM, Farrah SR, Lukasik J. Microbial source tracking: current methodology and future directions. Appl. Environ. Microbiol. 2002;68(12):5796–5803. doi: 10.1128/AEM.68.12.5796-5803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinton LW, Finlay RK, Hannah DJ. Distinguishing human from animal faecal contamination in water: a review. NZ J. Mar. Freshwater Res. 1998;32:323–348. [Google Scholar]
- Solo-Gabriele HM, Wolfert MA, Desmarais TR, Palmer CJ. Sources of Escherichia coli in a coastal subtropical environment. Appl. Environ. Microbiol. 2000;66:230–237. doi: 10.1128/aem.66.1.230-237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toranzos GA, McFeters GA. Detection of indicator microorganisms in environmental freshwaters. In: Hurst CJ, Knudsen GR, McInerney MJ, Stetzenbach LD, Walter MV, editors. Manual of Environmental Microbiology. American Society of Microbiology; Washington, DC: 1997. pp. 184–194. [Google Scholar]
- WHO . Guidelines for Drinking Water Quality: Surveillance and Control of Community Supplies. 2nd edition Vol. 3. World Health Organization; Geneva, Switzerland: 1997. [Google Scholar]
- Wright RC. The survival patterns of selected fecal bacteria in tropical fresh waters. Epidemiol. Infect. 1989;103:603–611. doi: 10.1017/s0950268800031009. [DOI] [PMC free article] [PubMed] [Google Scholar]