Abstract
Tumor Suppressor genes (TSGs) often locate at chromosomal regions with frequent deletions in tumors. Loss of 16q23 occurs frequently in multiple tumors, indicating the presence of critical TSGs at this locus, such as the well-studied WWOX. Herein we found that ADAMTS18, located next to WWOX, was significantly downregulated in multiple carcinoma cell lines. No deletion of ADAMTS18 was detected with multiplex differential DNA-PCR or high resolution 1-Mb array-based CGH analysis. Instead, methylation of the ADAMTS18 promoter CpG Island was frequently detected with methylation-specific PCR and bisulfite genome sequencing in multiple carcinoma cell lines and primary carcinomas, but not in any non-tumor cell line and normal epithelial tissue. Both pharmacological and genetic demethylation dramatically induced ADAMTS18 expression, indicating that CpG methylation directly contributes to the tumor-specific silencing of ADAMTS18. Ectopic ADAMTS18 expression leads to significant inhibition of both anchorage-dependent and -independent growth of carcinoma cells lacking the expression. Thus, through functional epigenetics, we identified ADAMTS18 as a novel functional tumor suppressor, being frequently inactivated epigenetically in multiple carcinomas.
Keywords: ADAMTS18, methylation, tumor suppressor gene, carcinoma, promoter
Introduction
Carcinogenesis is a multiple-step process involving the accumulation of multiple genetic and epigenetic changes that allows the clonal selection of progeny with growth and survival advantages (Hanahan and Weinberg, 2000; Ponder, 2001). These changes often cause the gain-of-function or activation of oncogenes and loss-of-function or inactivation of tumor suppressor genes (TSGs) (Jones and Baylin, 2002; Knudson, 2001). Oncogenes, once activated, can promote carcinogenesis by conferring certain selective growth advantages to the affected cell (Bishop, 1996). In contrast, TSGs have tumor inhibitory functions that need to be inactivated for tumor growth. TSGs inactivation is usually recessive since TSGs lose their functions only after the inactivation of both copies (Knudson, 2001). Inactivation of TSGs in human tumors is usually achieved through either the combination of one genetic change mainly chromosomal deletion, with another genetic or epigenetic change such as promoter methylation that silences gene transcription, or through epigenetic inactivation of both alleles (Jones and Baylin, 2002; Knudson, 2001). Frequently, TSGs identification begins with the detection of chromosomal deletions in tumor cells. While many oncogenes have been identified, much fewer TSGs were discovered, mainly due to difficulties in the detection and fine mapping of deletions. Comparative genomic hybridization (CGH), which complements the conventional cytogenetic techniques such as fluorescence in situ hybridization (FISH), was developed to screen for genome-wide chromosomal aberrations in tumors. CGH has facilitated TSG identification by providing more TSG loci for further genetic and functional analysis.
Through analyzing the CGH database of human tumors available online (http://amba.charite.de/~ksch/cghsuper/index.htm) and from literatures, we found that 16q23 was frequently deleted in a variety of tumors including tumors prevalent in our locality such as esophageal carcinoma (ESCC) and nasopharyngeal carcinoma (NPC) (Table 1), suggesting the presence of critical TSGs at this locus. Indeed, a well studied TSG - WWOX (WW domain containing oxidoreductase) is located within this region (Paige et al., 2001). In this study, we investigated whether other genes in this region could also function as TSGs. We found that ADAMTS18 (a disintegrin and metalloproteinase with thrombospondin motifs), located adjacent to WWOX, was significantly downregulated in multiple common tumors, as reported recently in breast cancer (Porter et al., 2004). ADAMTS18 belongs to the ADAMTS family of secreted proteases closely related to ADAM (a disintegrin and metalloproteinase-like, or adamalysin) proteinases, which are involved in ectodomain shedding or activation of diverse cell surface molecules including growth factors and adhesion receptors (Porter et al., 2005). Although the molecular functions of most ADAMTS proteins are unknown, some members, such as ADAMTS1, are known to contribute to the turn-over of extracellular matrix (ECM) and the progression of various diseases including cancers (Porter et al., 2005).
Table 1. Summary of 16q23 loss in human tumors (from CGH database and literatures).
| Tumor type | Number of cases |
Incidence of 16q23 loss |
Reference |
|---|---|---|---|
| Colorectal carcinoma | 87 | 10% | Online CGH database (http://amba.charite.de/~ksch/cghsuper/index.htm) |
| Brain tumor | 59 | 42% | |
| Ovarian carcinoma | 47 | 40% | |
| Breast carcinoma | 105 | 55% | |
| Lung carcinoma | 176 | 28% | |
| Head & neck squamous carcinoma | 171 | 18% | |
|
| |||
| Hepatocellular carcinoma | 48 | 63% | (Balsara et al., 2001) |
| Esophageal squamous cell carcinoma | 30 | 40% | (Riegman et al., 2001) |
| Gastric carcinoma | 24 | 50% | (Mori et al., 1999) |
| Nasopharyngeal carcinoma | 27 | 56% | (Lo et al., 2000) |
We further found that the tumor-specific downregulation of ADAMTS18 is mediated by promoter methylation rather than genetic deletion. Ectopic expression of ADAMTS18 in carcinoma cells lacking its expression led to dramatic inhibition of tumor cell growth, confirming that ADAMS18 is a novel functional tumor suppressor.
Results
Broad expression in normal tissues and frequent downregulation of ADAMTS18 in multiple carcinoma cell lines
TSGs are usually characterized by their downregulation during tumorigenesis. To assess whether ADAMTS18 was downregulated in tumors, we determined its expression in multiple normal tissues and a large collection of carcinoma cell lines with semi-quantitative RT-PCR. ADAMTS18 is highly expressed in most normal tissues examined (Figure 1a). In contrast, its expression was dramatically reduced or totally silenced in multiple carcinoma cell lines, including cell lines of esophagus, nasopharynx, stomach, colon, breast, lung and cervix; its expression remained high in all non-tumor epithelial cell lines including two normal mammary epithelial cell lines (HMEC and HMEpC) and three immortalized but non-transformed epithelial cell lines (nasopharyngeal, NP69 and esophageal, NE1 and NE3) (Figure 1b and summarized in Table 2). These results indicate that ADAMTS18 is frequently specifically downregulated in carcinoma cell lines.
Figure 1.
Downregulation of ADAMTS18 in tumor cell lines. (a) ADAMTS18 expression in human normal adult tissues was determined by semi-quantitative RT-PCR, with GAPDH as a control. (b) Downregulation of ADAMTS18 in tumor cell lines. Representative results are shown. Two normal mammary epithelial cell lines (HMEC and HMEpC) and three non-tumor aerodigestive epithelial cell lines (NE1, NE3, and NP69) were used as controls.
Table 2. Frequencies of ADAMTS18 CGI methylation in tumors and normal tissues studied.
| Origin | Cell line | Tissue | |
|---|---|---|---|
| Tumor | Esophageal squamous cell carcinoma (ESCC) |
88% (15/17) | 52% (24/46) |
| Nasopharyngeal carcinoma (NPC) |
100% (8/8) | 70% (30/43) | |
| Gastric carcinoma | 80% (8/10) | ||
| Hepatocellular carcinoma (HCC) |
38% (5/13) | 30% (6/20) | |
| Colon cancer | 100% (4/4) | ||
| Lung carcinoma | 60% (3/5) | ||
| Breast carcinoma (BrCa) | 78% (7/9) | 24% (5/21) | |
| Cervical carcinoma | 75% (3/4) | 63% (5/8) | |
| Prostate cancer | 67% (2/3) | ||
|
| |||
| Non-tumor | Surgical margin tissues of ESCC | 7% (3/46) | |
| Surgical non-tumor tissues of HCC | 0% (0/5) | ||
| Surgical margin tissues of breast carcinoma | 0% (0/7) | ||
ADAMTS18 downregulation is not due to genetic deletion
The downregulation of ADAMTS18 in carcinoma cell lines may be explained by genetic deletion, as it locates in the frequently deleted 16q23 locus. We thus carried out multiplex differential genomic DNA PCR to detect its homozygous deletion, using two independent primer pairs (Figure 2a). Surprisingly, no obvious deletion was detected in tumor cell lines with reduced or silenced expression (Figure 2b). This discrepancy to the previous CGH results of 16q23 deletion could be explained by the fact that conventional CGH detects chromosomal deletions with a limited resolution of ~10 Mb. Thus, we further analyzed our high-resolution array-based CGH (aCGH) data obtained with the Sanger Institute 1-Mb human whole genome arrays (3040 BAC clones). Consistent with previous conventional CGH results (Table 1), we did detect a hemizygous 16q23.1 deletion (~2 Mb) in 3 out of 15 carcinoma cell lines examined (10 esophageal and 5 nasopharyngeal carcinoma) (Figure 2c, Ying & Tao, in preparation). However, ADAMTS18 itself actually locates just outside this deletion in all cell lines with this deletion (Figure 2c). Taken together, these results indicate that other mechanisms should be responsible for ADAMTS18 downregulation in tumors.
Figure 2.
ADAMTS18 is not deleted in tumor cell lines. (a) Schematic graph shows the positions of primers for RT-PCR (F/R) and genomic DNA-PCR (F/GR and GF/R2). (b) The abundance of ADAMTS18 relative to GAPDH was determined by multiplex differential genomic DNA-PCR. PBMC, NP69 and NE3 were used as normal controls. +: with expression, −: without expression. (c) Representative results of 1-Mb aCGH (Sanger whole-genome BAC array) in NPC and ESCC cell lines, showing that ADAMTS18 is located just outside a hemizygous deletion at 16q23.1. Cytoband of 16q is shown, with normalized log2 aCGH signal ratios (−1 to 1) plotted. Each black dot represents a single BAC clone. The ADAMTS18 locus with nearby regions is shown as in Ensemble Human Contigview (http://www.ensembl.org/). The right panel shows candidate genes located at this region. The far right panel indicates the genomic structure of ADAMTS18 with its transcription direction and all the exons. Coding exons are filled in black.
Methylation of the ADAMTS18 CGI contributes to its downregulation in carcinoma cell lines
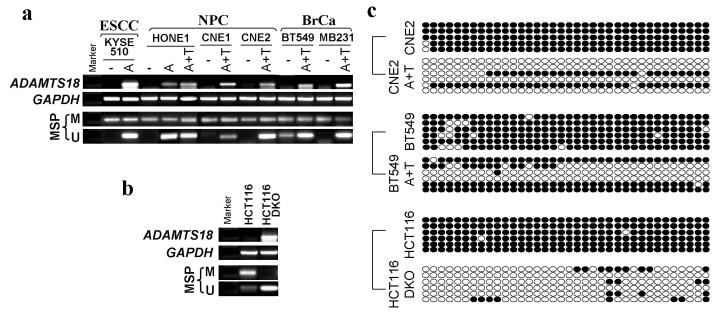
TSGs can also be frequently inactivated through epigenetic mechanisms, mainly methylation of promoter CpG Islands (CGI) that are generally unmethylated in normal tissues (Jones and Baylin, 2002). A typical CGI was found near the ADAMTS18 exon 1 by CpG Island Searcher (http://ccnt.hsc.usc.edu/cpgislands2), using the following criteria: GC content >55%, Obs CpG/Exp CpG >0.65, and length ~500 bp (Figure 3a), indicating that ADAMTS18 is vulnerable to methylation-mediated silencing. We thus analyzed the methylation status of the ADAMTS18 CGI with methylation-specific PCR (MSP) in a number of carcinoma cell lines. As expected, the ADAMTS18 CGI was methylated in all cell lines with silenced or reduced expression (Figure 3b and Table 2). One of the 3 nude mice-passaged NPC tumors (C15) also had methylation, with another one (C17) weakly methylated. In contrast, no methylation was detected in all 5 non-tumor epithelial cell lines (Figure 3b), indicating that ADAMTS18 CGI methylation is tumor-specific.
Figure 3.
Methylation of the ADAMTS18 CGI in tumor cells. (a) Schematic structure of the ADAMTS18 CGI, with the exons 1 and 2, MSP primer sites and BGS region indicated. Each short vertical line represents one CpG site. The methylation status of the ADAMTS18 CGI was analyzed by MSP (b) and BGS (c). +: with expression, ±: weak expression, −: no expression. M: methylated; U: unmethylated. For BGS, each circle indicates one CpG site and circles filled in black represent methylated CpG sites. One row of circles represents an individual allele of the ADAMTS18 CGI analyzed.
We further examined the methylation status of the ADAMTS18 CGI by high resolution bisulfite genome sequencing (BGS) analysis of 36 CpG sites within the CGI, including those CpG sites analyzed by MSP. Nearly all CpG sites examined were methylated in tumor cell lines while only scattered methylated CpG sites could be detected in non-tumor cell lines (Figure 3c), confirming the tumor-specific methylation of ADAMTS18 CGI.
Activation of ADAMTS18 expression by pharmacologic and genetic demethylation
To further determine whether CGI methylation directly mediates ADAMTS18 silencing, we compared the ADAMTS18 expression levels in tumor cells before and after treatment with the DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine (Aza), together with or without histone deacetylase inhibitor Trichostatin A (TSA). ADAMTS18 expression was significantly induced after drug treatment even with Aza alone (Figure 4a). Meanwhile, both MSP and BGS showed that the CGI was dramatically demethylated in the presence of drug (Figure 4a and c), revealing a direct link between ADAMTS18 silencing and CpG methylation. Similar result was observed in the colorectal cancer cell line HCT116 with double knock-out of two major DNA methyltransferases DNMT1 and DNMT3B that function together to maintain cellular DNA methylation (Figure 4b and c). These results confirm that ADAMTS18 downregulation is directly mediated by CGI methylation.
Figure 4.
Restorations of ADAMTS18 expression by demethylation. (a) Pharmacological demethylation of the ADAMTS18 CGI by Aza (A) and TSA (T) induced its expression. ADAMTS18 expression before and after drug treatment was determined by RT-PCR (upper panel), and demethylation was confirmed by MSP (lower two panels) and BGS (c). (b-c) Genetic demethylation of the ADAMTS18 CGI also activated its expression. ADAMTS18 expression in HCT116 cells and HCT116 cells with double knock-out of DNMT1 and DNMT3B (DKO) (b, upper panel) and demethylation of its CGI (b, lower two panels) are shown.
Methylation of the ADAMTS18 CGI in primary carcinomas
We further analyzed the ADAMTS18 methylation status in a large collection of primary carcinoma samples. Methylation was detected in a variety of tumors but seldom in the corresponding non-tumor tissues (Figure 5 and Table 2), highlighting the importance of tumor-specific ADAMTS18 methylation in tumorigenesis.
Figure 5.
Methylation of the ADAMTS18 CGI in primary tumors as analyzed by MSP. M: methylated; U: unmethylated. Representative results are shown. T: tumors; N: paired non-tumor tissues.
Ectopic ADAMTS18 expression inhibits tumor cell growth
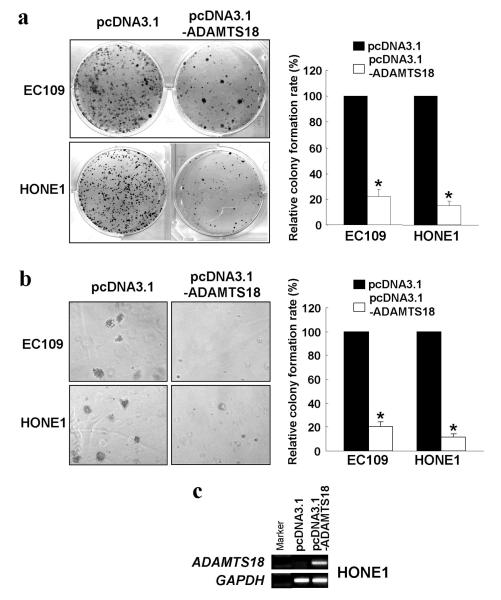
The tumor-suppressor function of ADAMTS18 was investigated by a gain-of-function approach with monolayer colony formation and soft agar assays. Mammalian expression vector pcDNA3.1 containing ADAMTS18 full-length open reading frame was introduced into two carcinoma cell lines with reduced ADAMTS18 expression, HONE1 (nasopharyngeal) and EC109 (esophageal), and the numbers of surviving colonies after transfection and G418 selection were counted. After ADAMTS18 re-expression, the colony numbers of both cell lines were significantly reduced in both monolayer and soft agar culture (p<0.01) (Figure 6 a, b and c), indicating that ADAMTS18 suppresses both anchorage-dependent and -independent growth of tumor cells.
Figure 6.
Ectopic expression of ADAMTS18 inhibits tumor cell growth. The effect of ectopic ADAMTS18 expression on tumor cell growth was investigated by monolayer colony formation assay (a) and soft agar assay (b). Quantitative analyses of colony numbers are shown in the right panel as values of mean ± standard deviation. P values were calculated using student's t-test. The asterisk indicates statistical significant difference (p<0.01). The expression level of ADAMTS18 in the transfected HONE1 cells was examined by RT-PCR (c).
Discussion
We report here that ADAMTS18 is downregulated in multiple carcinomas through promoter methylation rather than genetic deletion. Ectopic expression of ADAMTS18 in carcinoma cells lacking its expression leads to dramatic growth inhibition. Thus, our results indicate that ADAMS18 is a new functional tumor suppressor epigenetically silenced in tumors.
Limited numbers of TSGs have been identified due to technical difficulties in precisely defining chromosomal deletions. CGH has greatly facilitated TSG identification through the genome-wide screening of gene copy number changes in tumors, although it still has a limited resolution of ~10 Mb. High-resolution aCGH can identify micro-deletions which have not been able to be detected previously (Pinkel et al., 1998). We used a 3040-BAC clone based CGH-array (Sanger Institute whole-genome array) with a resolution of ~1Mb to detect genetic changes in common tumors in our locality (Ying et al., 2006). Our results are largely consistent with previous findings with conventional CGH. However, we detected significantly more micro-deletions with much better resolution, thus greatly reducing the number of candidate genes for further functional validation and novel TSG identification.
On the other side, however, TSGs are more frequently inactivated through epigenetic mechanisms or a combination of genetic and epigenetic aberrations, than through biallelic genetic inactivation (Jones and Baylin, 2002). Here, we identified a new candidate TSG, ADAMTS18, silenced in multiple carcinomas by CGI methylation rather than genetic deletion, although it is located within a chromosomal region with frequent deletion detected by conventional CGH analyses in various tumors. ADAMTS18 belongs to a family of secreted proteases with 19 members, closely related to the ADAM proteinases that are involved in ectodomain shedding of diverse cell surface molecules. Unlike the mammalian ADAMs that are, with few exceptions, transmembrane proteins, the ADAMTSs are secreted proteins. Some ADAMTSs can bind to the extra-cellular matrix (ECM), while the substrates of other orphan ADAMTSs remain unknown. A few ADAMTSs, including ADAMTS1, 3, 5, 8, 9, 10 and 18, have been reported to be downregulated in some tumors including breast cancer (Porter et al., 2004; Masui et al., 2001), indicating important roles of ADAMTSs in tumorigenesis. Tumor-specific promoter methylation has also been reported to contribute to the downregulation of ADAMTS1, 8 and 9 in tumors (Dunn et al., 2004; Lind et al., 2006; Lo et al., 2007).
Similar to other ADAMTSs, ADAMTS18 contains multiple domains, including a metalloproteinase catalytic domain with a reprolysin-type zinc-binding motif, a disintegrin-like domain, and a central and 5 C-terminal TS (thrombospondin type 1) repeat domains (Porter et al., 2005; Cal et al., 2002). However, no substrate or binding partner of ADAMTS18 has been identified and thus the molecular mechanism of ADAMTS18 functioning as a tumor suppressor remains unknown. Some ADAMTSs, including ADAMTS1 and ADAMTS8, were proven to have potent anti-angiogenesis function, possibly through the interaction of their TS repeat domains with the membrane protein CD36 on endothelial cells (Porter et al., 2005). However, ADAMTS18 lacks the conserved motif in TS repeat domains present in ADAMTS1 and ADAMTS8 that is supposed to be important for their anti-angiogenesis functions. Furthermore, as shown in this study, ADAMTS18 directly suppresses both anchorage-dependent and -independent growth of tumor cells (Figure 5), indicating that ADAMTS18 could function directly to inhibit tumor cell growth without the dependence on anti-angiogenesis. Many ADAMTSs, probably including ADAMTS18, play important roles in the turn-over of ECM through the cleavage of major components of ECM such as aggrecan (Porter et al., 2005). The turn-over of ECM may thus provide a non-permissive environment for tumor growth, through the production of growth suppressive molecules or the degradation of growth promoting components. It is also possible that ADAMTS18 may disrupt the activation of growth factor receptors on the plasma membrane of tumor cells by the cleavage of these receptors or their co-receptors. In addition to its metalloproteinase catalytic domain, ADAMTS18 comprises several protein-protein interaction domains such as the disintegrin-like domain and TS repeat domains. Through these domains, ADAMTS18 may bind directly to growth promoting or inhibitory molecules and modulate their effects on tumor cells. In parallel, ADAMTS1 has been shown to bind to vascular endothelial growth factor (VEGF) and prevent VEGF-promoted proliferation of endothelial cells (Luque et al., 2003). Proteomic screening of its binding partners and substrates would provide more information on how ADAMTS18 functions as a tumor suppressor.
Although we found that promoter methylation frequently silences ADAMTS18, other mechanisms might also be involved in inactivating ADAMTS18 in tumors. For instance, the ADAMTS18 promoter was unmethylated in several cell lines with no expression (Figure 1b and 3b), indicating that some repressors or histone remodeling might contribute to this transcriptional silencing. On the other hand, genetic mutations may also inactivate ADAMTS18. A very recent comprehensive mutation study reported 2 missense mutations (R382K and K455T, both within the metalloproteinase catalytic domain) of ADAMTS18 in 2/11 colon tumors (Sjoblom et al., 2006). However, the biologic implication of these mutations in tumorigenesis remains to be further investigated.
Multiple studies have shown that aberrant CGI methylation can be used as a sensitive marker for cancer diagnosis and prognosis prediction (Nakata et al., 2006; Baylin and Herman, 2000; Gius et al., 2005; Cottrell and Laird, 2003). Large scale analysis with more tumor samples is needed to assess whether ADAMTS18 promoter methylation can be used as a new biomarker for cancer diagnosis and prognosis prediction.
Materials and Methods
Cell lines and tissue DNA/RNA samples
Multiple carcinoma cell lines were used (Chan et al., 2007; Seng et al., 2007; Qiu et al., 2004; Ying et al., 2005), including esophageal (EC1, EC18, EC109, HKESC1, HKESC2, HKESC3, SLMT1, KYSE30, KYSE70, KYSE140, KYSE150, KYSE180, KYSE270, KYSE410, KYSE450, KYSE510 and KYSE520), nasopharyngeal (NPC) (C666-1, CNE1, CNE2, HK1, HONE1, HNE1, 6-10B and BM1), hepatocellular (HepG2, huH1, huH4, huH6, huH7, Hep3B, SNU387, SNU398, SNU423, SNU449, SNU475, Mahlavu and PLC/PRF/5), lung (A427, A549, H1299, H358 and H292), gastric (Kato III, YCC1,YCC2, YCC3, YCC6, YCC7, YCC9, YCC10, YCC11 and YCC16), colon (HCT116, HT-29, LoVo and SW480), breast (BT549, MB231, MB468, MCF-7, SK-BR-3, T47D, ZR-75-1, YCC-B1 and YCC-B3), cervical (HeLa, CaSki, C33A and SiHa) and prostate (DU145, LNCap, PC3). Three nude mice-passaged undifferentiated NPC tumors derived from North Africans (C15, C17, C18) were also studied (Busson et al., 1988). Two human mammary epithelial cell lines, HMEC (Cambrex, Cat #CC-2251) and HMEpC (Applied Biosystems, Cat #CA-830-05a) and three immortalized but non-transformed epithelial cell lines (esophageal - NE1 and NE3, nasopharyngeal - NP69) (Deng et al., 2004) were used as controls. DNA and RNA samples from various primary carcinomas have been described previously (Qiu et al., 2004; Ying et al., 2005; Zhou et al., 2005; Seng et al., 2007). Cells were treated with Aza (Sigma, St Louis, MO, USA) (10 μmol/L for 3 days) or Aza together with TSA (100 ng/mL for an additional day) as described previously (Ying et al., 2005).
Construction of the ADAMTS18 expressing vector
Part of the ADAMTS18 ORF (1-942 bp) was amplified by PCR using the high-fidelity Accuprimer Taq polymerase (Invitrogen). The PCR product was cloned into the pCR4-TOPO vector (Invitrogen). After sequence verification, the insert was sub-cloned using NheI and EcoRI restriction sites into the neomycin resistant mammalian expression vector pcDNA3.1 (Invitrogen). The resulting construct was then ligated with the rest of the ADAMTS18 ORF cut from pBluescriptR-ADAMTS18 (purchased from RZPD, Germany) using EcoRI and XbaI sites to generate the ADAMTS18 full-length cDNA expressing vector.
Semi-quantitative Reverse Transcription (RT)-PCR and multiplex differential DNA-PCR
Genomic DNA and total RNA were extracted using Tri Reagent (Ying et al., 2005). RNA was reverse-transcribed using the MuLV reverse transcriptase (GeneAmp RNA PCR kit, Applied Biosystems). PCR was performed as previously described (Tao et al., 2002; Chan et al., 2007). Primers used were ADAMTS18F (F): 5′-tagccagtgacagcagcag, ADAMTS18R (R): 5′-ctaagtgcagttcctgtcca; ADAMTS18GF (GF): 5′-ctgctctccagctttggttt, ADAMTS18GR (GR): 5′-tttatgtgacttgcagctcg and ADMTS18R2 (R2): 5′-gctgaggtaatggcgagatg. F/R (194-bp product) was used for RT-PCR, while F/GR (249-bp product) and GF/R2 (265-bp product) were used for multiplex differential genomic DNA-PCR (Qiu et al., 2004). RT-PCR was performed for 37 cycles (annealing temperature 55°C) using the AmpliTaq Gold (Applied Biosystem) with DMSO (5% final concentration). Multiplex differential genomic DNA-PCR was performed for 35 cycles (annealing temperature 58°C) using AmpliTaq Gold, using 0.1 μg of DNA per 12.5 μl PCR reaction.
Array-CGH (aCGH)
Whole-genome arrays (~1-Mb resolution with 3,040 BAC/PAC clones) were provided by the Wellcome Trust Sanger Institute, UK. ACGH was performed and analyzed as previously described (Ying et al., 2006). Briefly, sample DNA (600 ng) was labeled with Cy5-dCTP (Amersham Pharmacia), while reference DNA of normal PBMCs from healthy Chinese donors was labeled with Cy3-dCTP. Hybridized slides were scanned using an Axon 4000B scanner (Axon Instruments Inc., Union City, CA) and analyzed with the GenePixPro 4.0 image analysis software.
Methylation-specific PCR (MSP) and Bisulfite Genomic Sequencing (BGS)
Bisulfite modification of DNA, MSP and BGS were carried out as described (Tao et al., 2002; Tao et al., 1999). For MSP, methylation-specific primers (annealing temperature 60°C, 40 cycles) were: ADAMTS18m1: 5′-ttgtagttcggtaggttcgc (forward) and ADAMTS18m2: 5′-actccaaataaaaaccgccg (reverse); and unmethylation-specific primers (annealing temperature 60°C, 38 cycles) were: ADAMTS18u1: 5′-aaattgtagtttggtaggtttgt (forward) and ADAMTS18u2: 5′-caactccaaataaaaaccacca (reverse). MSP primers were confirmed previously for not amplifying any unbisulfited DNA and thus specific. For BGS, bisulfite-treated DNA was amplified using BGS primers (annealing temperature 60°C, 40 cycles), ADAMTS18BGS1: 5′-gttttagtttYggtttagggagtt and ADAMTS18BGS2: 5′-aacRcactccataatcaaatac, and cloned into pCR4-TOPO vector (Invitrogen). At least 5 colonies were randomly chosen for sequencing.
Colony formation and soft agar assays
Colony formation and soft agar assays were performed as previously described to evaluate tumor cell growth in vitro (Jin et al., 2006; Ying et al., 2006). Cells were cultured overnight in a 12-well plate (1.0 × 105/well) and transfected with pcDNA3.1 or the ADAMTS18-expressing vector using FuGENE 6 (Roche). 48 hours later, the transfectants were replated in triplicate and cultured for 10-15 days in complete RPMI1640 medium containing G418 (400 μg/ml), without (colony formation assay) or with 0.33% agar (soft agar assay). For colony formation assays, surviving colonies were stained with Gentian Violet after methanol fixation and visible colonies (≥ 50 cells) were counted. For soft agar assays, visible colonies were counted under microscopy. The experiments were performed three times independently.
Acknowledgements
This project was supported by a Michael and Betty Kadoorie Cancer Genetics Research Program (MBKCGRP) grant to QT. We thank Drs. Bert Vogelstein, George Tsao, (Dolly Huang), Kaitai Yao, Ya Cao, and Shuen-Kuei Liao for some cell lines.
References
- Balsara BR, Pei J, De RA, Simon D, Tosolini A, Lu YY, et al. Human hepatocellular carcinoma is characterized by a highly consistent pattern of genomic imbalances, including frequent loss of 16q23.1-24.1. Genes Chromosomes. Cancer. 2001;30:245–253. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1083>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16:168–174. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- Bishop JM. The discovery of proto-oncogenes. FASEB J. 1996;10:362–364. doi: 10.1096/fasebj.10.2.8641572. [DOI] [PubMed] [Google Scholar]
- Busson P, Ganem G, Flores P, Mugneret F, Clausse B, Caillou B, et al. Establishment and characterization of three transplantable EBV-containing nasopharyngeal carcinomas. Int. J. Cancer. 1988;42:599–606. doi: 10.1002/ijc.2910420422. [DOI] [PubMed] [Google Scholar]
- Cal S, Obaya AJ, Llamazares M, Garabaya C, Quesada V, Lopez-Otin C. Cloning, expression analysis, and structural characterization of seven novel human ADAMTSs, a family of metalloproteinases with disintegrin and thrombospondin-1 domains. Gene. 2002;283:49–62. doi: 10.1016/s0378-1119(01)00861-7. [DOI] [PubMed] [Google Scholar]
- Chan SL, Cui Y, van HA, Li H, Srivastava G, Jin H, et al. The tumor suppressor Wnt inhibitory factor 1 is frequently methylated in nasopharyngeal and esophageal carcinomas. Lab Invest. 2007 doi: 10.1038/labinvest.3700547. doi:10.1038. [DOI] [PubMed] [Google Scholar]
- Cottrell SE, Laird PW. Sensitive detection of DNA methylation. Ann. N. Y. Acad. Sci. 2003;983:120–130. doi: 10.1111/j.1749-6632.2003.tb05967.x. [DOI] [PubMed] [Google Scholar]
- Deng W, Tsao SW, Guan XY, Lucas JN, Si HX, Leung CS, et al. Distinct profiles of critically short telomeres are a key determinant of different chromosome aberrations in immortalized human cells: whole-genome evidence from multiple cell lines. Oncogene. 2004;23:9090–9101. doi: 10.1038/sj.onc.1208119. [DOI] [PubMed] [Google Scholar]
- Dunn JR, Panutsopulos D, Shaw MW, Heighway J, Dormer R, Salmo EN, et al. METH-2 silencing and promoter hypermethylation in NSCLC. Br. J. Cancer. 2004;91:1149–1154. doi: 10.1038/sj.bjc.6602107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gius D, Bradbury CM, Sun L, Awwad RT, Huang L, Smart DD, et al. The epigenome as a molecular marker and target. Cancer. 2005;104:1789–1793. doi: 10.1002/cncr.21395. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Jin H, Sperka T, Herrlich P, Morrison H. Tumorigenic transformation by CPI-17 through inhibition of a merlin phosphatase. Nature. 2006;442:576–579. doi: 10.1038/nature04856. [DOI] [PubMed] [Google Scholar]
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- Knudson AG. Two genetic hits (more or less) to cancer. Nat. Rev. Cancer. 2001;1:157–162. doi: 10.1038/35101031. [DOI] [PubMed] [Google Scholar]
- Lind GE, Kleivi K, Meling GI, Teixeira MR, Thiis-Evensen E, Rognum TO, et al. ADAMTS1, CRABP1, and NR3C1 identified as epigenetically deregulated genes in colorectal tumorigenesis. Cell Oncol. 2006;28:259–272. doi: 10.1155/2006/949506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo KW, Teo PM, Hui AB, To KF, Tsang YS, Chan SY, et al. High resolution allelotype of microdissected primary nasopharyngeal carcinoma. Cancer Res. 2000;60:3348–3353. [PubMed] [Google Scholar]
- Lo PH, Leung AC, Kwok CY, Cheung WS, Ko JM, Yang LC, et al. Identification of a tumor suppressive critical region mapping to 3p14.2 in esophageal squamous cell carcinoma and studies of a candidate tumor suppressor gene, ADAMTS9. Oncogene. 2007;26:148–157. doi: 10.1038/sj.onc.1209767. [DOI] [PubMed] [Google Scholar]
- Luque A, Carpizo DR, Iruela-Arispe ML. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J. Biol. Chem. 2003;278:23656–23665. doi: 10.1074/jbc.M212964200. [DOI] [PubMed] [Google Scholar]
- Masui T, Hosotani R, Tsuji S, Miyamoto Y, Yasuda S, Ida J, et al. Expression of METH-1 and METH-2 in pancreatic cancer. Clin. Cancer Res. 2001;7:3437–3443. [PubMed] [Google Scholar]
- Mori Y, Matsunaga M, Abe T, Fukushige S, Miura K, Sunamura M, et al. Chromosome band 16q24 is frequently deleted in human gastric cancer. Br. J. Cancer. 1999;80:556–562. doi: 10.1038/sj.bjc.6690391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata S, Sugio K, Uramoto H, Oyama T, Hanagiri T, Morita M, et al. The methylation status and protein expression of CDH1, p16(INK4A), and fragile histidine triad in nonsmall cell lung carcinoma: epigenetic silencing, clinical features, and prognostic significance. Cancer. 2006;106:2190–2199. doi: 10.1002/cncr.21870. [DOI] [PubMed] [Google Scholar]
- Paige AJ, Taylor KJ, Taylor C, Hillier SG, Farrington S, Scott D, et al. WWOX: a candidate tumor suppressor gene involved in multiple tumor types. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11417–11422. doi: 10.1073/pnas.191175898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkel D, Segraves R, Sudar D, Clark S, Poole I, Kowbel D, et al. High resolution analysis of DNA copy number variation using comparative genomic hybridization to microarrays. Nat. Genet. 1998;20:207–211. doi: 10.1038/2524. [DOI] [PubMed] [Google Scholar]
- Ponder BA. Cancer genetics. Nature. 2001;411:336–341. doi: 10.1038/35077207. [DOI] [PubMed] [Google Scholar]
- Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem. J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter S, Scott SD, Sassoon EM, Williams MR, Jones JL, Girling AC, et al. Dysregulated expression of adamalysin-thrombospondin genes in human breast carcinoma. Clin. Cancer Res. 2004;10:2429–2440. doi: 10.1158/1078-0432.ccr-0398-3. [DOI] [PubMed] [Google Scholar]
- Qiu GH, Tan LK, Loh KS, Lim CY, Srivastava G, Tsai ST, et al. The candidate tumor suppressor gene BLU, located at the commonly deleted region 3p21.3, is an E2F-regulated, stress-responsive gene and inactivated by both epigenetic and genetic mechanisms in nasopharyngeal carcinoma. Oncogene. 2004;23:4793–4806. doi: 10.1038/sj.onc.1207632. [DOI] [PubMed] [Google Scholar]
- Riegman PH, Vissers KJ, Alers JC, Geelen E, Hop WC, Tilanus HW, et al. Genomic alterations in malignant transformation of Barrett's esophagus. Cancer Res. 2001;61:3164–3170. [PubMed] [Google Scholar]
- Seng TJ, Low JS, Li H, Cui Y, Goh HK, Wong ML, et al. The major 8p22 tumor suppressor DLC1 is frequently silenced by methylation in both endemic and sporadic nasopharyngeal, esophageal, and cervical carcinomas, and inhibits tumor cell colony formation. Oncogene. 2007;26:934–944. doi: 10.1038/sj.onc.1209839. [DOI] [PubMed] [Google Scholar]
- Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- Tao Q, Huang H, Geiman TM, Lim CY, Fu L, Qiu GH, et al. Defective de novo methylation of viral and cellular DNA sequences in ICF syndrome cells. Hum. Mol. Genet. 2002;11:2091–2102. doi: 10.1093/hmg/11.18.2091. [DOI] [PubMed] [Google Scholar]
- Tao Q, Swinnen LJ, Yang J, Srivastava G, Robertson KD, Ambinder RF. Methylation status of the Epstein-Barr virus major latent promoter C in iatrogenic B cell lymphoproliferative disease. Application of PCR-based analysis. Am. J. Pathol. 1999;155:619–625. doi: 10.1016/S0002-9440(10)65157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying J, Li H, Seng TJ, Langford C, Srivastava G, Tsao SW, et al. Functional epigenetics identifies a protocadherin PCDH10 as a candidate tumor suppressor for nasopharyngeal, esophageal and multiple other carcinomas with frequent methylation. Oncogene. 2006;25:1070–1080. doi: 10.1038/sj.onc.1209154. [DOI] [PubMed] [Google Scholar]
- Ying J, Srivastava G, Hsieh WS, Gao Z, Murray P, Liao SK, et al. The stress-responsive gene GADD45G is a functional tumor suppressor, with its response to environmental stresses frequently disrupted epigenetically in multiple tumors. Clin. Cancer Res. 2005;11:6442–6449. doi: 10.1158/1078-0432.CCR-05-0267. [DOI] [PubMed] [Google Scholar]
- Zhou L, Jiang W, Ren C, Yin Z, Feng X, Liu W, et al. Frequent hypermethylation of RASSF1A and TSLC1, and high viral load of Epstein-Barr Virus DNA in nasopharyngeal carcinoma and matched tumor-adjacent tissues. Neoplasia. 2005;7:809–815. doi: 10.1593/neo.05217. [DOI] [PMC free article] [PubMed] [Google Scholar]