Abstract
ICAT (Inhibitor of β-catenin and T cell factor) inhibits the interaction between β-catenin and TCF/LEF transcription factor and serves as a negative regulator of Wnt signaling. In a subset of ICAT knockout mice, significant delay in the ureteric bud branching and renal agenesis are observed. In order to examine the process of this developmental defect, molecular changes were analyzed in fetal ICAT–/– kidneys with a focus on Wnt-signaling associated factors. The protein level of active β-catenin was elevated in ICAT–/– kidneys. DNA microarray and immunohistochemical analyses revealed that the expression of a Wnt target gene Pitx-2 was enhanced in ICAT–/– kidneys. There was no genotypic difference in the expression level of another Wnt target gene, c-Ret. These results suggest that the enhancement of Pitx-2 expression induced by activated Wnt signaling leads to delays in ureteric bud branching and subsequent renal agenesis. In the ICAT–/– kidneys which developed to E18.5 without any apparent defect, renal glomeruli, convoluted tubules and collecting ducts were decreased in density and showed abnormal structure. ICAT may be required for various developmental stages during renal development.
Keywords: ICAT, Wnt signal, Pitx-2, kidney, ureteric bud
I. Introduction
There are two intracellular pools of β-catenin, one associated with cadherins at the cell surface and a soluble one in the cytoplasm, whose state and concentration are critical for Wnt signaling. In the absence of a Wnt signal, β-catenin in the cytoplasmic pool is phosphorylated by GSK-3β, and then ubiquitinated and degraded by the proteasome pathway. Upon Wnt signaling, β-catenin is stabilized by its dephosphorylation. As a consequence, dephosphorylated “active” β-catenin can access the nucleus where it interacts with members of the TCF family of transcription factors to modulate the expression of defined targets [14, 15]. ICAT (Inhibitor of β-catenin and T cell factor) inhibits β-catenin binding to TCF/LEF transcription factor and functions as a negative regulator of Wnt signaling [8, 16]. In our previous study, we reported that homozygotes (ICAT−/−) of ICAT knockout mice exhibit enhanced Wnt signaling and various severe morphological abnormalities in the craniofacial bones as well as either bilateral or unilateral renal agenesis. Craniofacial abnormalities were found in about one half and renal agenesis was found in 13% of ICAT−/− mice [10]. Developmental observation showed a retardation in ureteric branching in ICAT−/− mice at the embryonic day (E) 12.5; in ICAT−/− mice, 20% of kidneys showed T-shaped (2 tips) ureteric bud showing only one bifurcation, which was never seen in ICAT+/+ or ICAT+/− kidneys. Moreover, many apoptotic cells were seen in the non-aggregated metanephric mesenchyme in ICAT−/− kidneys [2].
Recent gene-targeting studies have identified a number of genes whose disruption results in renal agenesis. Many of these gene products are involved in the outgrowth of the ureteric bud from the Wolffian duct and/or invasion of the bud into the metanephric blastema. Gdnf, Wt1, Pax2, Six1, Sall1 and Eya1 expressed in the metanephric blastema and Gfra1, c-Ret, Lim1, Emx2 and Pax2 expressed in the ureteric bud are required for these developmental processes; targeted disruption of any one of these genes disrupts the ureteric bud invasion and induces renal agenesis. In the next stage, Wnt 4 is required for the tubulogenesis in the metanephros and its deficiency also causes renal agenesis or hypoplasia. Under the regulation by these essential factors, many downstream gene products, which directly modulate cellular behavior, are involved in various processes of renal development such as the branching morphogenesis of ureteric epithelium, the differentiation of functional cell types, and so on [13].
Since the deficiency of ICAT protein caused retardation of the ureteric bud branching and increased apoptosis, some of above mentioned factors involved in kidney development are expected to be functionally affected in ICAT−/− kidneys. Considering that targeted disruption of ICAT gene would result in the activation of Wnt signaling, the identification of the Wnt target genes which were up-regulated in ICAT−/− kidneys would help to understand the mechanism for renal agenesis. In order to identify the gene, we applied DNA microarray analysis and immunohistochemistry and compared the gene expression at E12.5 between normal ICAT+/+ kidneys and ICAT−/− kidneys which showed developmental retardation with T-shaped ureteric bud.
In addition to the identification of the Wnt target genes, the expression patterns of β-catenin were examined in ICAT−/− kidneys in order to assess whether there were any additional effects of ICAT gene disruption on the Wnt signaling pathway other than the effect on the complex formation between β-catenin and TCF transcription factor.
II. Materials and Methods
Animals
Generation and characterization of the ICAT knockout mouse have been described in our previous article [10]. ICAT+/+, ICAT+/− and ICAT−/− mice at the E12.5, E13.5 and E18.5 were used. In this study, animals were cared for according to the guidelines for animal study of Fujita Health University.
Antibodies
Polyclonal anti-Pitx-2 antibody was purchased from Sigma and was used at 1:100 dilution. Monoclonal anti-pan cytokeratin antibody was purchased from Sigma and was used with 1:50 dilution. Monoclonal anti-active-β-catenin antibody was purchased from Upstate and was used with 1:100 dilution. Monoclonal anti β-catenin antibody which recognizes total β-catenin regardless of phosphorylation status was purchased from BD Transduction and was used with 1:50 dilution. Polyclonal anti-c-Ret antibody was purchased from IBL and was used with 1:20 dilution. Alexa Fluor 488 anti-rabbit IgG antibody and Alexa Fluor 568 anti-mouse IgG antibody were purchased from Molecular Probes and both of them were used with 1:200 dilution.
Whole-mount observation of metanephroi
Metanephric kidneys were dissected out from ICAT+/− and ICAT−/− mice at E13.5, fixed by 4% paraformaldehyde/PBS, replaced to PBS, and observed using a stereoscopic microscope (MZFL III, Leica).
DNA microarray analysis
Metanephric kidneys were dissected out from 4 ICAT+/+ mice and 10 ICAT−/− mice at E12.5 and stored in RNA later (Qiagen) at −20°C. RNA extraction, reverse transcription and synthesis of labeled cRNA with Cyanin 5 or Cyanin 3 were performed according to manufacturer’s instructions. Samples were hybridized to Whole Mouse Genome oligo DNA microarray (Agilent) overnight and spot values were measured with microarray scanner (Agilent). GeneSpring GX 7.3.1 (Agilent) was used for the analysis.
Immunofluorescence
Metanephric kidneys were removed at E12.5, fixed by 4% paraformaldehyde/PBS, washed in 10% sucrose/PBS and frozen. Frozen sections of 8 µm were prepared with a cryostat (CM1850, Leica) and were treated sequentially with 0.1% Triton-X 100 (Sigma) for 20 min, L.A.B. Solution (Polysciences) for 10 min, and 5% normal goat serum for 30 min at room temperature. They were then incubated in the diluted first antibody solution overnight at 4°C and washed in PBS. The sections were then incubated in the secondary antibody solution containing DAPI (Doujin Kagaku) for 30 min at room temperature and washed in PBS. The sections were enclosed by mounting agent (ProLong Gold antifade reagent, Molecular Probes). The immunostained sections were observed and photographed with a fluorescence microscope (Axiovert 200M, Carl Zeiss). Signal intensity by each antibody was measured using Axiovision. Three ureteric epithelial areas of 1000–5000 µm2 and three aggregated mesenchymal areas of 3000–6000 µm2 were encompassed in the photographed image and the mean value of fluorescent intensity within each area was measured. Statistical difference between ICAT+/+ and ICAT−/− intensities was evaluated by F-test and Student’s t-test.
Whole-mount immunofluorescence
Metanephric kidneys were removed at E12.5, fixed by 4% paraformaldehyde/PBS and stored in 100% methanol at −20°C. After rehydration with PBS, they were blocked in 5% normal goat serum/PBST (0.1% Tween 20/PBS) for 60 min at 37°C and incubated in the diluted first antibody solution overnight at 4°C. After PBST washing, they were incubated with the secondary antibody solution for 3 hr at room temperature. After PBST and PBS washing, samples were mounted in glycerin and observed by a fluorescence microscope. Signal intensity was measured using Axiovision. Three areas of 8000–13000 µm2 each containing a ureteric tip were encompassed in the photographed image and the mean value of fluorescent intensity within each area was measured. Statistical difference between ICAT+/+ and ICAT−/− intensities was evaluated by F-test and Student’s t-test.
HE staining
Metanephric kidneys were removed at E18.5 and fixed by 4% paraformaldehyde/PBS. After dehydration and clearing by xylene, they were embedded in paraffin. Sections of 5 µm thickness were stained by hematoxylin and eosin (HE). HE-stained sections were observed by a light microscope (BX50, Olympus) and photographed. The number of renal glomeruli was counted using three frontal sections (ventral and dorsal sections which cross the cortex and a centered section which crosses the cortex and medulla) from each kidney. The density index was then calculated as the ratio of the total number of glomeruli in three sections to the total metanephric area of the three sections. Mean values of histological data of ICAT+/+, ICAT+/− and ICAT−/− mice were compared by ANOVA followed by Fisher’s PLSD post hoc test.
III. Results
Delayed branching of the ureteric bud in ICAT knockout mice
As heterozygous (ICAT+/−) mice showed the same phenotype as wild type (ICAT+/+) mice, we used the wild type and the heterozygous mice as the control in this study. The number of tips of ureteric bud in kidneys of ICAT+/− mice at E12.5 was 5.93±1.09 (mean±standard deviation), whereas that of ICAT−/− mice was 4.49±1.14 (p<0.001), revealing that the number of ureteric bud branching in ICAT−/− mice was significantly low (Fig. 1A, B; [2]). In particular a T-shaped ureteric bud with two tips, which was never seen in ICAT+/+ mice at E12.5, was observed in 22.2% of the kidneys of ICAT−/− mice (Fig. 1B; [2]). Therefore, we speculated that the delayed ureteric bud branching in ICAT−/− kidneys led to apoptosis of non-aggregated mesenchymal cells around the ureteric bud, and that half of the ICAT−/− kidneys with T-shaped ureteric bud may not develop further, resulting in renal deficiency [2]. However, since the size of the kidneys (long axis) in ICAT−/− mice was statistically not reduced at E13.5 (data not shown), the effect of apoptosis may not appear until later stages.
Fig. 1.
Delay of the ureteric bud branching in ICAT−/− mice. (A, B) Whole mount immunostaining for cytokeratin showing ureteric bud branching at E12.5. A: ICAT+/− kidney. A ureteric bud normally branches making 7 tips. B: ICAT−/− kidney. T-shaped ureteric bud after first branching with abnormally dilated tips. An arrow indicates Wolffian duct. Bar=200 µm.
Increased expression of active β-catenin in kidneys of ICAT knockout mice
Before the identification of the Wnt target genes, we checked for any additional effect of ICAT gene disruption on the Wnt signaling pathway by examining the expression of dephosphorylated “active” β-catenin in ICAT knockout mice using immunofluorescence. In kidneys of ICAT+/+ mice, the active β-catenin was localized in the apical part of the cytoplasm and at the cell-cell adhesion site of the epithelial cells in the ureteric bud, while the expression in mesenchymal cells was faint (Fig. 2C). In the kidneys of ICAT−/− mice, strong expression of active β-catenin was found in the cytoplasm of aggregated mesenchymal cells surrounding the ureteric bud (Fig. 2D), as well as in the apical part of the cytoplasm and the cell-cell adhesion sites of epithelial cells in ureteric bud. The signal intensities of active β-catenin in the ureteric epithelium and the aggregated mesenchyme of ICAT−/− kidneys were 1.17-fold (P=0.016) and 1.23-fold (P=0.018) higher than those of ICAT+/+ kidneys, respectively. On the other hand, the immunoreactivity by another antibody which recognizes total β-catenin regardless of phosphorylation status was statistically unchanged in ICAT−/− kidneys (data not shown). These results show that the cytoplasmic amount of active β-catenin was increased in ICAT−/− kidneys.
Fig. 2.
Double immunofluorescence staining for active β-catenin and Pitx-2 in kidneys at E12.5. (A, C, E, G) ICAT+/+ kidney. Dotted circles indicate a renal vesicle. (B, D, F, H) ICAT−/− kidney. (C, D) Active β-catenin. Note the elevated expression of active β-catenin in accumulated mesenchymal cells around the ureteric bud epithelium in the ICAT−/− kidney. (E, F) Pitx-2. Pitx-2 expression is very low in the ICAT+/+ kidney but is substantial in the ICAT−/− kidney. (G, H) Nuclear staining with DAPI. Bar=20 µm.
High expression of Pitx-2 gene in kidneys of ICAT knockout mice
Gene expression patterns in kidneys of ICAT+/+ mice and ICAT−/− mice at E12.5 were compared by DNA microarray analysis to identify the Wnt target gene which is activated in kidneys of ICAT−/− mice. Fourteen kinds of gene were up-regulated in ICAT−/− kidneys in which the ICAT−/−/ICAT+/+ ratio was more than 10. On the other hand, 20 kinds of gene were down-regulated in ICAT−/− kidneys with their ratios less than 0.1 (Table 1). Among these 34 kinds of gene, Pitx-2 (paired-like homeodomain transcription factor 2) that was reported by Kioussi et al. [4] was found in the Gene Ontology category of Wnt target genes (biological process categories) (http://www. geneontology.org).
Table 1.
Gene expression which notably changed in kidneys of E12.5 ICAT –/– mice
| Increased genes | ||||
|---|---|---|---|---|
| No. | ICAT–/–/ICAT+/+ ratio | Common name | Description or Product | GO biological process; molecular function |
| 1 | 24.38 | p8, B8Ag, CFAg, Caga, MRP8, CP-10 | S100 calcium binding protein A8 | chemotaxis |
| 2 | 22.33 | p14, Cagb, GAGB, L1Ag, BEE14, MRP14 | S100 calcium binding protein A9 | actin cytoskeleton reorganization |
| 3 | 21.17 | 3930401C23 | T-box 4 | angiogenesis |
| 4 | 21.05 | BC117090 | stefin A1-like protein | cysteine protease inhibitor activity |
| 5 | 20.96 | AK089257 | predicted gene, EG433016 | cysteine protease inhibitor activity |
| 6 | 17.6 | Brx1, Ptx2, Rieg | paired-like homeodomain transcription factor 2 | Wnt receptor signaling pathway |
| 7 | 17.07 | BC117090 | stefin A1-like protein | cysteine protease inhibitor activity |
| 8 | 16.46 | Bft, Potx, Ptx1, P-OTX | paired-like homeodomain transcription factor 1 | cartilage development |
| 9 | 14.51 | islet 2 | insulin related protein 2 | multicellular organismal development |
| 10 | 14.14 | 9930020N01Rik | ankyrin repeat and kinase domain containing 1 | protein amino acid phosphorylation |
| 11 | 13.56 | Cnlp, MCLP, CAP18, Cramp, FALL39 | cathelicidin antimicrobial peptide | defense response |
| 12 | 12.11 | Tbx5 | T-box 5 | emdryonic arm morphogenesis |
| 13 | 11.5 | MGC107339 | tetratricopeptide repeat domain 9B | protein folding |
| 14 | 10.44 | AK016846 | Mus musculus adult male testis cDNA | |
| Decreased genes | ||||
|---|---|---|---|---|
| No. | ICAT–/–/ICAT+/+ ratio | Common name | Description or Product | GO biological process; molecular function |
| 1 | 0.0991 | AI503486, mkIAA0666, E130308H01 | dishevelled associated activator of morphogenesis 1 | actin cytoskeleton organization and biogenesis |
| 2 | 0.0928 | TLL, Tlx, frc, Mtl1, MTll, XTLL, fierce | nuclear receptor subfamily 2, group E, member 1 | anterior commissure morphogenesis |
| 3 | 0.0908 | AK038959 | Mus musculus adult male hypothalamus cDNA | |
| 4 | 0.0873 | 2010321J07Rik | UDP glucuronosyltransferase 2 family, polypeptide A3 | metabolic process |
| 5 | 0.0849 | Cpd2, Caps2 | Ca2+-dependent activator protein for secretion 2 | exocytosis |
| 6 | 0.0839 | T2R4, T2R07, mGR06, Tas2r7, mt2r43, STC5-1 | taste receptor, type 2, member 107 | detection of chemical stimulus |
| 7 | 0.0809 | 1110014K05Rik | late cornified envelope 1C | |
| 8 | 0.074 | MOR245-19P | olfactory receptor 1307 | G-protein coupled receptor protein signaling pathway |
| 9 | 0.0598 | AGT1, XAT2, AGT-1 | aspartate/glutamate transporter 1 | amino acid transport |
| 10 | 0.0504 | MOR211-1 | olfactory receptor 1502 | G-protein coupled receptor protein signaling pathway |
| 11 | 0.0455 | AK017313 | RIKEN cDNA 5430416B10 gene | |
| 12 | 0.0423 | AK028476 | RIKEN cDNA A330078L11 gene | |
| 13 | 0.0353 | EC-SOD | superoxide dismutase 3, extracellular | response to hypoxia |
| 14 | 0.0336 | Tac2r | tachykinin receptor 2 | G-protein coupled receptor protein signaling pathway |
| 15 | 0.0321 | Tsap6, pHyde | STEAP family member 3 | apoptosis, cell cycle |
| 16 | 0.0299 | RP23-281P6.1 | hypothetical protein LOC66328 | metabolic process |
| 17 | 0.029 | PTG, Ppp1r5 | protein phosphatase 1, regulatory (inhibitor) subunit 3C | carbohydrate metabolic process |
| 18 | 0.0233 | NAP022465-001 | ||
| 19 | 0.02 | Rslcan-7, mszf59-2 | zinc finger protein 458 | reguration of transcription, DNA-dependent |
| 20 | 0.016 | NM_177220 | Mus musculus RIKEN cDNA A630031M04 gene | integral to membrane |
High expression of Pitx-2 protein in kidneys of ICAT knockout mice
In order to confirm the high expression of Pitx-2 mRNA in ICAT−/− kidneys detected by DNA microarray analysis, expression of Pitx-2 protein at E12.5 was examined by immunofluorescence in the kidneys of ICAT+/+ mice presenting multiple-branched ureteric buds and in those of ICAT−/− mice presenting T-shaped ureteric buds. Although the intensity of Pitx-2 signal in ICAT+/+ kidneys was very low, it was substantial in ICAT−/− kidneys (Fig. 2E, F). The signal intensities of ICAT−/− ureteric epithelium and aggregated mesenchyme were 1.39-fold (P=0.0048) and 1.38-fold (P=0.0089) higher than those of ICAT+/+, respectively. Therefore, it was shown that the expression of Pitx-2 in ICAT−/− kidneys was enhanced both in mRNA and protein level. From these results, we speculated that the enhanced expression of Pitx-2 caused delayed ureteric bud branching and subsequent arrest of renal development.
Expression of c-Ret in kidneys of ICAT knockout mice
C-Ret is a tyrosine kinase receptor for Gdnf and is essential for ureteric bud branching [11]. Although c-Ret is one of the Wnt target genes [18], our DNA microarray analysis did not detect any change in the genetic expression level of c-Ret (data not shown).
The expression pattern of c-Ret protein was then examined in ICAT+/+ and ICAT−/− kidneys at E12.5 by immunofluorescence. In this experiment, we used whole-mount samples and counterstained them with anti-cytokeratin antibody to compare the expression pattern of c-Ret to the whole shape of ureteric bud in the kidney. Expression of c-Ret was observed at each tip of ureteric bud in ICAT+/+ kidneys in accordance with the expression of cytokeratin (Fig. 3A, D, G, arrows 1–6). A similar expression pattern of c-Ret and cytokeratin was observed also in ICAT−/− kidneys (Fig. 3B, E, H, arrows 7–8). Levels of immunoreactivity of c-Ret at the tips of ureteric bud in ICAT+/+ and ICAT−/− kidneys were comparable and showed no statistically significant difference (Fig. 3D, E, data not shown).
Fig. 3.
Whole mount double immunofluorescence staining for c-Ret and cytokeratin at E12.5. Arrows indicate tips of the ureteric bud. (A, D, G) ICAT+/+ kidney. (B, E, H) ICAT−/− kidney. (C, F, I) ICAT+/+ kidney (negative control). F: Anti c-Ret antibody is replaced by the same concentration of normal rabbit IgG. I: Anti cytokeratin antibody is replaced by the same concentration of normal mouse IgG. (D, E) c-Ret. c-Ret is mainly localized in the branched ureteric bud tips. The intensity of the c-Ret immunoreactivities was identical in ICAT+/+ and ICAT−/−. (G, H) Cytokeratin. Cytokeratin is localized in the ureteric bud epithelium. In the ICAT+/+ kidney, the ureteric bud has six branched tips (G, arrows 1–6) while in the ICAT−/− kidney the ureteric bud has only two branched tips (H, arrows 7, 8). Dotted circles indicate the outline of the kidney. Asterisks (B, E, H) indicate Wolffian duct. Bar=200 µm.
From these results, we concluded that there was no difference in the expression level of c-Ret mRNA or c-Ret protein between ICAT+/+ and ICAT−/−kidneys.
Histological abnormalities in kidneys of ICAT knockout mice at E18.5
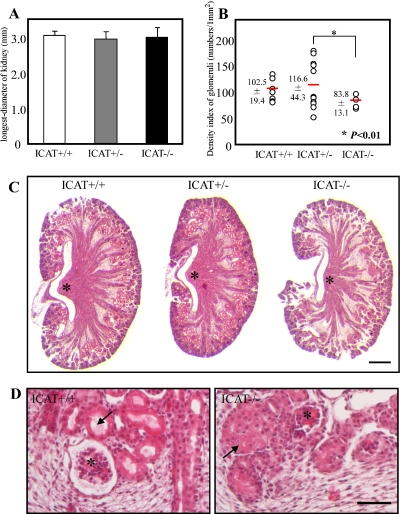
All of the ICAT+/− kidneys and almost 90% of the ICAT−/− kidneys develop without any obvious defect [2]. It is an interesting question whether there are any differences between the ICAT+/+ kidneys and the ICAT+/− kidneys or the ICAT−/− kidneys which escaped developmental arrest. Thus, we investigated the histology of the kidney which had developed grossly normally at E18.5. The longest axis of kidney was 3.1±0.14 mm in ICAT+/+ (n=18), 2.94±0.23 mm in ICAT+/− (n=38) and 2.99±0.30 mm in ICAT−/− (n=45) mice, without significant difference between genotypes (P=0.293, Fig. 4A). In HE-stained sections, however, ICAT−/− kidneys showed significant histological features compared with ICAT+/+ and ICAT+/− kidneys (Fig. 4C). The collecting ducts and Henle’s loops were dense in the medulla of ICAT+/+ and ICAT+/− kidneys, while they were relatively sparse in ICAT−/− kidneys. Though renal papillae protruded into calyces in ICAT+/+ and ICAT+/− kidneys, the protrusion of renal papillae was barely seen in ICAT−/− kidneys (asterisks in Fig. 4C). In the cortex, density of convoluted tubules was low (Fig. 4C), diameter of each tubule was narrow and its lumen was collapsed in ICAT−/− kidneys (arrows in Fig. 4D). Subsequently, the density indices of renal glomeruli were compared. The density index was 102.5±19.41 in ICAT+/+ (n=8), 116.6±44.25 in ICAT+/− (n=12) and 83.8±13.08 in ICAT−/− (n=10), indicating that the density of glomeruli in ICAT−/− kidneys was significantly reduced as compared with ICAT+/− kidneys (Fig. 4B, p<0.01). There was no significant difference between ICAT+/+ and ICAT+/− kidneys (P=0.173) and between ICAT+/+ and ICAT−/− kidneys (P=0.082). Observation of the innermost glomeruli revealed that Bowman’s capsule is not dilated in ICAT−/− kidney (asterisks in Fig. 4D). The abundance ratio of glomeruli whose Bowman’s space were dilated was 34.6% (ICAT+/+), 33.5% (ICAT+/−) and 16.3% (ICAT−/−), respectively, and was statistically reduced in ICAT−/− kidneys (P=0.0092).
Fig. 4.
Renal histology at E18.5. A: Length of the longest axis of the kidney. There is no significant difference among the three genotypes. B: Density index of glomeruli. Glomeruli in the ICAT−/− kidneys are significantly reduced (*P<0.01, red lines show the mean values). C: HE-stained sections crossing the longest axis of the kidney. There is no obvious difference between the ICAT+/+ and ICAT+/− kidneys. In ICAT−/− kidneys, the collecting ducts and Henle’s loops in the medulla are sparse, and the renal papillae (asterisks) less protruded into the renal calyces. Bar=200 µm. D: Magnified views of the boundary between the cortex (upper) and medulla (lower) of ICAT+/+ (left) and ICAT−/− (right) kidneys. Asterisks indicate innermost glomeruli and arrows indicate convoluted tubules. The lumen of the convoluted tubules seems to collapse and Bowman’s capsule is not dilated in ICAT−/− kidney. Bar=50 µm.
IV. Discussion
Effect of ICAT deficiency on the cytosolic level of active β-catenin
Enhancement of Wnt signaling by ICAT gene disruption was confirmed using ICAT−/− brains in our previous study [10]. However, the possibility that additional steps in Wnt signaling pathway other than the interaction between β-catenin and TCF might be affected by ICAT deficiency had yet to be examined. In the present study, we obtained a result showing that the amount of active β-catenin was increased in the developing kidneys of ICAT−/− mice. Increase of active β-catenin without a change in the total cytosolic β-catenin level suggests an increased reception of Wnt signals and the subsequent inactivation of GSK-3β in the tissue [15]. This change may be caused secondarily by ICAT deficiency, but further mechanistic studies are needed. Therefore, the up-regulation of a Wnt target gene, Pitx-2 in ICAT−/− kidneys, which we found in this study, would be the result of two kinds of activation in Wnt signaling: a direct enhancement of β-catenin/TCF complex formation and an indirect increased input of Wnt signaling into the cell.
High expression of Wnt target gene Pitx-2 in ICAT knockout mice
Pitx (pituitary homeobox)-2 is a bicoid-type transcription factor which was originally characterized as a factor involved with autosomal-dominant disorder, Rieger syndrome [12]. Afterward, Pitx-2 has been shown to be important for the left-right axis formation [9]. In organogenesis, Pitx-2 is required for the development of the pituitary, eyes, teeth, palate, heart, hindlimb bud growth and formation of hindlimbs [7].
Based on our assumption that the elevated expression of Pitx-2 causes the deficiency in ureteric bud branching, the effect would be mediated by changes in expression level of Pitx-2-target gene(s). Pitx-2 has been reported to be expressed in the adult mouse kidney [3], but its target gene in the kidney is unknown. In other organs, for example, expression of Wnt11 and BMP4, both of which are involved in the early kidney development [17], has been reported to be regulated by Pitx-2 [5, 19]. Although our microarray analysis revealed many genes whose expression levels were up- or down-regulated in ICAT−/− kidney, the putative Pitx-2 target genes, including Wnt11 or Bmp4, were not listed. Up- or down-regulation of gene expression detected in the present microarray analysis would, therefore, be caused by a yet unidentified genetic cascade downstream of Pitx-2. An alternative possibility, which we cannot rule out at the present, is that the gene targeting of ICAT modulated the expression of yet unidentified Wnt target genes. In addition, another question about the result of our microarray analysis, is whether gene(s) actually involved in the renal deficiency in ICAT−/− mice are listed or not, because the roles of the listed genes in renal development are still unknown. Further elucidation of the cascade and identification of downstream gene whose expression change is responsible for the renal deficiency would provide us with novel insights into the molecular mechanism of renal development.
Stable expression of c-Ret in ICAT knockout mice
C-Ret is expressed in the epithelium of the ureteric bud tip and functions as a receptor for glial-derived neurotrophic factor (Gdnf). C-Ret/Gdnf signal regulates the ureteric branching positively through reciprocal cooperation with Wnt11 [6]. Aside from the functional interaction with Wnt11, Zheng et al. has reported that c-Ret was a target gene of canonical Wnt signaling in PC12 cells [18]. However, our present study did not detect any changes of c-Ret expression in ICAT−/− kidneys either in mRNA level or in protein level. This discrepancy may be due to cell specificity, as Zheng et al. reported that the enhancement of c-Ret expression by Wnt signaling is cell-type specific [18] and that the factors responsible for this specificity have yet to be determined. On the other hand, a recent study by Clarke et al. [1] has found that Pax2 and Pax8, both of which are expressed in developing kidney, can bind to the promoter region of c-Ret gene and enhance its expression directly. In developing kidney, therefore, the expression of c-Ret may be regulated mainly by Pax transcription factors and the activation of Wnt signaling in ICAT−/− kidneys would not have influenced the expression level of c-Ret. Accordingly, Gdnf/c-Ret signaling is considered not to be related with either the delayed ureteric bud branching or the subsequent renal agenesis in ICAT−/− mice.
Histological abnormalities in the kidney of the ICAT knockout mouse that escaped developmental arrest
The incidence rate of the renal agenesis (including both bilateral and unilateral defects) in ICAT−/− mice is 13% [2], and nearly 90% of ICAT−/− metanephroi grow up to be permanent kidneys without developmental arrest. In ICAT−/− kidneys that have survived without developmental arrest, however, the densities of glomeruli, renal tubules and collecting ducts were reduced as compared with those in ICAT+/+ and ICAT+/− kidneys and their structures were abnormal. Although we have not examined the renal functions in ICAT−/− mice, the renal tubules and Bowman’s capsule without enough dilation may indicate insufficiency in urine production. It would be intriguing to study ICAT functions in the kidney from its early organogenesis to late functional developmental processes.
In conclusion, we examined the changes of gene expression in developing ICAT−/− kidneys and found that the expression of the canonical Wnt target gene, Pitx-2, was increased. Increased expression of Pitx-2 might have led to the developmental defect in ICAT−/− kidneys through an unidentified genetic cascade downstream of Pitx-2.
V. Acknowledgements
We wish to thank Dr. R. Nomura for helpful discussions, and K. Yanagisawa, Y. Takeuchi and K. Hikita for technical support. This study was supported in part by a Grant-in-Aid for Scientific Research (C) 17590179 to Y. H. and a Grant-in-Aid for Scientific Research (B) 16390051 to T. S. from the Japan Society for the Promotion of Science, and grants from the Fujita Health University Research Fund to Y. H. and T. S.
VI. References
- 1.Clarke J. C., Patel S. R., Raymond R. M., Jr., Andrew S., Robinson B. G., Dressler G. R., Brophy P. D. Regulation of c-Ret in the developing kidney is responsive to Pax2 gene dosage. Hum. Mol. Genet. 2006;15:3420–3428. doi: 10.1093/hmg/ddl418. [DOI] [PubMed] [Google Scholar]
- 2.Hasegawa Y., Satoh K., Iizuka-Kogo A., Shimomura A., Nomura R., Akiyama T., Senda T. Loss of ICAT gene function leads to arrest of ureteric bud branching and renal agenesis. Biochem. Biophys. Res. Commun. 2007;362:988–994. doi: 10.1016/j.bbrc.2007.08.085. [DOI] [PubMed] [Google Scholar]
- 3.Hjalt T. A., Amendt B. A., Murray J. C. PITX2 regulates procollagen lysyl hydroxylase (PLOD) gene expression: implications for the pathology of Rieger syndrome. J. Cell Biol. 2001;152:545–552. doi: 10.1083/jcb.152.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kioussi C., Briata P., Baek S. H., Rose D. W., Hamblet N. S., Herman T., Ohgi K. A., Lin C., Gleiberman A., Wang J., Brault V., Ruiz-Lozano P., Nguyen H. D., Kemler R., Glass C. K., Wynshaw-Boris A., Rosenfeld M. G. Identification of a Wnt/Dvl/beta-catenin --> Pitx2 pathway mediating cell-type-specific proliferation during development. Cell. 2002;111:673–685. doi: 10.1016/s0092-8674(02)01084-x. [DOI] [PubMed] [Google Scholar]
- 5.Liu W., Selever J., Lu M. F., Martin J. F. Genetic dissection of Pitx2 in craniofacial development uncovers new functions in branchial arch morphogenesis, late aspects of tooth morphogenesis and cell migration. Development. 2003;130:6375–6385. doi: 10.1242/dev.00849. [DOI] [PubMed] [Google Scholar]
- 6.Majumdar A., Vainio S., Kispert A., McMahon J., McMahon A. P. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development. 2003;130:3175–3185. doi: 10.1242/dev.00520. [DOI] [PubMed] [Google Scholar]
- 7.Marcil A., Dumontier E., Chamberland M., Camper S. A., Drouin J. Pitx1 and Pitx2 are required for development of hindlimb buds. Development. 2003;130:45–55. doi: 10.1242/dev.00192. [DOI] [PubMed] [Google Scholar]
- 8.Mosimann C., Hausmann G., Basler K. Beta-catenin hits chromatin: regulation of Wnt target gene activation. Nat. Rev. Mol. Cell Biol. 2009;10:276–286. doi: 10.1038/nrm2654. [DOI] [PubMed] [Google Scholar]
- 9.Ryan A. K., Blumberg B., Rodriguez-Esteban C., Yonei-Tamura S., Tamura K., Tsukui T., de la Pena J., Sabbagh W., Greenwald J., Choe S., Norris D. P., Robertson E. J., Evans R. M., Rosenfeld M. G., Izpisua Belmonte J. C. Pitx2 determines left-right asymmetry of internal organs in vertebrates. Nature. 1998;394:545–551. doi: 10.1038/29004. [DOI] [PubMed] [Google Scholar]
- 10.Satoh K., Kasai M., Ishidao T., Tago K., Ohwada S., Hasegawa Y., Senda T., Takada S., Nada S., Nakamura T., Akiyama T. Anteriorization of neural fate by inhibitor of beta-catenin and T cell factor (ICAT), a negative regulator of Wnt signaling. Proc. Natl. Acad. Sci. U S A. 2004;101:8017–8021. doi: 10.1073/pnas.0401733101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuchardt A., D’Agati V., Larsson-Blomberg L., Costantini F., Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 12.Semina E. V., Reiter R., Leysens N. J., Alward W. L., Small K. W., Datson N. A., Siegel-Bartelt J., Bierke-Nelson D., Bitoun P., Zabel B. U., Carey J. C., Murray J. C. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat. Genet. 1996;14:392–399. doi: 10.1038/ng1296-392. [DOI] [PubMed] [Google Scholar]
- 13.Shah M. M., Sampogna R. V., Sakurai H., Bush K. T., Nigam S. K. Branching morphogenesis and kidney disease. Development. 2004;131:1449–1462. doi: 10.1242/dev.01089. [DOI] [PubMed] [Google Scholar]
- 14.Sharpe C., Lawrence N., Martinez Arias A. Wnt signalling: a theme with nuclear variations. Bioessays. 2001;23:311–318. doi: 10.1002/bies.1045. [DOI] [PubMed] [Google Scholar]
- 15.Staal F. J., Noort Mv., Strous G. J., Clevers H. C. Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Rep. 2002;3:63–68. doi: 10.1093/embo-reports/kvf002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tago K., Nakamura T., Nishita M., Hyodo J., Nagai S., Murata Y., Adachi S., Ohwada S., Morishita Y., Shibuya H., Akiyama T. Inhibition of Wnt signaling by ICAT, a novel beta-catenin-interacting protein. Genes Dev. 2000;14:1741–1749. [PMC free article] [PubMed] [Google Scholar]
- 17.Yu J., McMahon A. P., Valerius M. T. Recent genetic studies of mouse kidney development. Curr. Opin. Genet. Dev. 2004;14:550–557. doi: 10.1016/j.gde.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Zheng S., Ramachandran B., Haigh J. R., Palos T. P., Steger K., Howard B. D. The induction of ret by Wnt-1 in PC12 cells is atypically dependent on continual Wnt-1 expression. Oncogene. 1996;12:555–562. [PubMed] [Google Scholar]
- 19.Zhou W., Lin L., Majumdar A., Li X., Zhang X., Liu W., Etheridge L., Shi Y., Martin J., Van de Ven W., Kaartinen V., Wynshaw-Boris A., McMahon A. P., Rosenfeld M. G., Evans S. M. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nat. Genet. 2007;39:1225–1234. doi: 10.1038/ng2112. [DOI] [PMC free article] [PubMed] [Google Scholar]