Abstract
Cadherins are a family of transmembrane glycoproteins that mediate cell-to-cell adhesion. A change in cadherin type in cells, i.e., cadherin switching, induces changes in the character of the cell. Recent studies of the developing rat adenohypophysis found that primordial cells co-expressed E- and N-cadherins, but that hormone-producing cells lost E-cadherin and ultimately possessed only N-cadherin. In the present study, we examined the roles of cadherin switching in cytogenesis of anterior pituitary cells by observing prolactin mRNA and protein expression in lactotrophs that were transformed with an E-cadherin expression vector. In hormone-producing cells that were transfected with a pIRES2-ZsGreen1 plasmid with a full-length E-cadherin cDNA (rE-cad-IZ) insert in primary culture, we detected E- and N-cadherins on plasma membrane and E-cadherin in cytoplasm. In these rE-cad-IZ-transfected cells, in situ hybridization revealed prolactin mRNA signals that were at a level identical to that in control cells, while prolactin protein was barely detectable using immunocytochemistry. The mean signal intensity of prolactin protein in rE-cad-IZ-transfected cells was approximately one fourth that in intact cells and in null-IZ-transfected cells (P<0.01). These results suggest that the expression of E-cadherin does not affect prolactin mRNA transcription; rather, it reduces prolactin protein content, presumably by affecting trafficking of secretory granules.
Keywords: anterior pituitary, E-cadherin, prolactin, transfection, hormone production
I. Introduction
Cadherins are a large family of transmembrane glycoproteins that mediate specific cell-to-cell adhesion in a Ca2+-dependent manner [23]. There are many types of cadherin, including E-cadherin, which is expressed mainly in epithelial tissues, and N-cadherin, which is expressed in neural tissues [23]. Each cadherin associates in a homophilic manner to promote specific cell-to-cell adhesion [11]. Recently, it has been suggested that cadherin initiates signal transduction through catenins, which bind to the cadherin cytoplasmic domain [11, 15, 19].
It has been reported that the cadherin type in cells changes during neural tube formation, optic cap formation, and emigration of neural crest cells [17, 20, 26]. This change is referred as cadherin switching [25]. Many studies have shown that cadherin switching alters the character of cells. One representative example of this phenomenon is the epithelial-to-mesenchymal transition of cells, in which the cadherin type switches from E- to N-cadherin [4, 25].
In the pituitary gland, it was reported that hormone-producing cells express N-cadherin [8, 24]. In addition, Kikuchi et al. reported that cells in the adenohypophyseal placode co-expressed both E- and N-cadherin, and that hormone-producing cells lost expression of E-cadherin during cell differentiation [9, 10]. In addition, there appeared to be a close relation between cadherin switching and the onset of hormone synthesis or accumulation [9, 10]. These findings suggest that an investigation of the experimental expression of E-cadherin in mature hormone-producing cells might increase our understanding of the roles of cadherin switching in the cytogenesis of anterior pituitary cells.
Hormone secretion in the anterior pituitary gland is regulated by a variety of humoral factors, i.e., by hypothalamic hormones, gonadal hormones, thyroid hormones, and certain growth factors [3, 14]. It is generally known that prolactin secretion is negatively regulated by dopamine in vivo. However, under in vitro conditions, prolactin secretion increases in the absence of stimulation, as it is no longer strongly inhibited by dopamine [1]. Using in situ hybridization and immunocytochemical techniques, we utilized lactotrophs to examine whether forced E-cadherin expression by gene transfection affects prolactin mRNA expression and prolactin production.
II. Materials and Methods
Animals
Male Sprague-Dawley rats (Japan SLC, Shizuoka, Japan) and male transgenic S100b-GFP rats [6] that express green fluorescent protein under the promoter control of the S100 beta protein gene, a marker of folliculo-stellate cells, were used (age, 8–10 weeks; weight, 250–300 g). The S100b-GFP rats were bred in our laboratory. All rats were given ad libitum access to food and water and kept under a light cycle of 12 hr light and 12 hr darkness. All animals were treated in accordance with the Guidelines for Animal Experimentation of Jichi Medical University, which are based on the NIH Guidelines for the Care and Use of Laboratory Animals.
Primary culture
Rats were perfused with Ca2+- and Mg2+-free (CMF) Hanks’ solution through the left ventricle and bled from the right atrium under deep Nembutal anesthesia. Anterior pituitary glands were excised and cells were dispersed as described in Kikuchi et al. [8]. Dispersed cells were resuspended in Medium 199 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Sigma Aldrich, St. Louis, MO, USA), 100 u/ml penicillin, and 100 µg/ml streptomycin (Invitrogen), plated on 8-well glass chamber slides (0.8 cm2/well; Nalge Nunc International, Rochester, NY, USA) at a density of 1×105 cells/400 µl/cm2, and cultured at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Transfection
Complimentary DNA was synthesized using the total RNA fraction from anterior pituitaries of male SD rats, and PCR was performed to amplify the DNA encoding the entire rat E-cadherin (NM 031334) using specific oligonucleotide primers with extension of the XhoI and KpnI sites to the 3' terminals (forward, 5'-GCT CGA GTG TTT GCT CGG CGT TTG C-3'; reverse, 5'-CCA TGG ATC CAC ACA GGA ACG ACT C-3'). After purification, the specific PCR product was digested by XhoI (Takara, Shiga, Japan) and KpnI (Takara), ligated with pIRES2-ZsGreen1 plasmid (Clontech, Mountain View, CA, USA), and cloned. The recombinant plasmid is referred to as rE-cad-IZ.
Anterior pituitary cells were cultured for 2 days as described above and then transfected with the rE-cad-IZ plasmid using Lipofectamine 2000 (Invitrogen), as per the instructions provided by the manufacturer. As a control, a pIRES2-ZsGreen1 plasmid with null insert (null-IZ) was used. Cells were cultured for 48 hr after the transfection procedure and utilized for analysis.
Immunocytochemistry
Culture cells were rinsed with Hanks’ solution and fixed with 4% paraformaldehyde in 25 mM phosphate buffer (pH 7.4) for 30 min at room temperature. After immersion in PBS containing 2% normal goat serum for 30 min at room temperature, cells were incubated in PBS with monoclonal antibody against E-cadherin (dilution of 1:1000, BD Biosciences, San Jose, CA, USA) and polyclonal antibody against N-cadherin (dilution of 1:100, IBL, Gunma, Japan) overnight at 30°C. After washing with PBS, cells were incubated in PBS with anti-rabbit IgG conjugated with Alexa 568 (Invitrogen) and anti-mouse IgG conjugated with Alexa 633 (Invitrogen) for 30 min at 30°C. Cells were observed through a confocal laser microscope (FV1000; Olympus, Tokyo, Japan).
In situ hybridization
In situ hybridization was performed as described in Fujiwara et al. [5]. The complementary DNA fragment for the rat prolactin mRNA (NM 012629) was amplified from rat cDNA by PCR using specific oligonucleotide primers (forward, 5'-TGC AGA TGA GAA AGC AGT GG-3'; reverse, 5'-TTC AGG ATA GGC CTG GCT AA-3'). The amplified cDNA fragment was ligated into the pGEM-T easy vector (Promega, Madison, WI, USA) and cloned. Antisense and sense digoxigenin (DIG)-labeled cRNA probes were synthesized using the Roche DIG RNA labeling kit (Roche Diagnostics, Penzberg, Germany).
Primary culture cells were treated with 4% paraformaldehyde in 25 mM phosphate buffer (pH 7.4) for 15 min, with 0.125 µg/ml proteinase K (Invitrogen) for 1 min, and with 4% paraformaldehyde for 10 min at room temperature. Hybridization was performed in a solution containing 50% formamide, 10% dextran sulfate (Wako Pure Chemical Industries, Osaka, Japan), 3× saline-sodium citrate, 120 mM phosphate buffer (pH 7.4), 1× Denhardt’s solution (Nacalai Tesque, Kyoto, Japan), 125 µg/ml tRNA (Invitrogen), and 100 µg/ml sonicated sperm DNA (Invitrogen) overnight at 45°C. After hybridization, the RNA probe was washed in 2× SSC with 50% formamide at 45°C, and incubated in PBS with monoclonal antibody against digoxigenin (Roche) overnight at 4°C. After washing with PBS, cells were incubated in PBS with anti-rabbit prolactin antibody (Chemicon International, Temecula, CA, USA) for 90 min at 30°C, then in PBS with anti-rabbit IgG conjugated with Alexa 568 (Invitrogen) and anti-mouse IgG conjugated with Alexa 633 (Invitrogen) for 30 min at 30°C. Cells were observed through a confocal laser microscope (FV1000; Olympus, Tokyo, Japan). MetaMorph software (Molecular Devices, Downingtown, PA, USA) was used to quantify signal intensities on immunocytochemistry and in situ hybridization.
III. Results
Forced expression of E-cadherin in hormone-producing cells in the anterior pituitary of adult rats
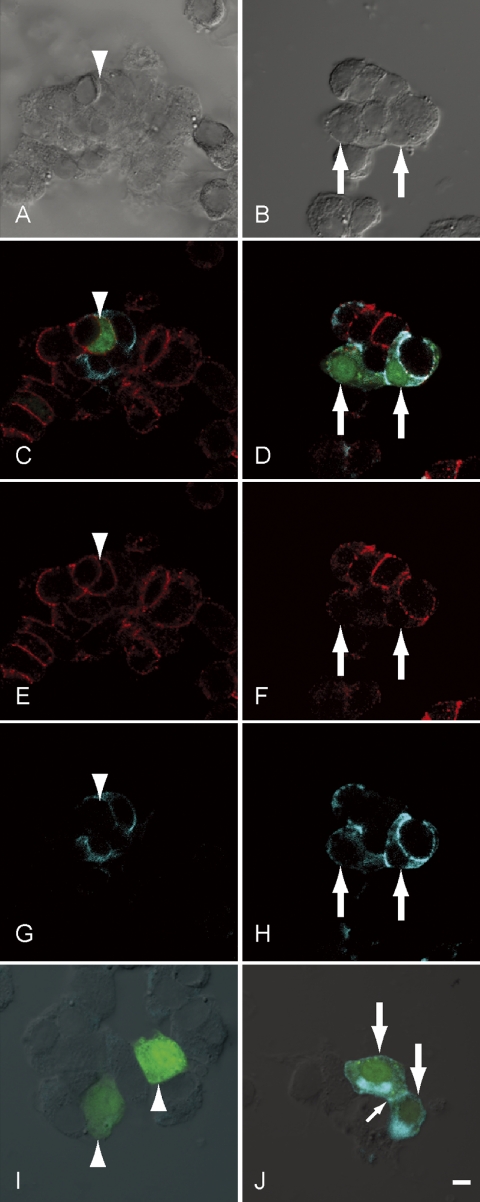
Transfected cells were identified with fluorescence derived from the ZsGreen1 plasmid, as shown in Figure 1 (indicated by arrows and arrowheads). The transfection rate was usually 2 to 3% and no difference in the rate was observed among cell types. The transfected cells did not differ in shape on differential interference contrast images (Fig. 1A and 1B). We detected N-cadherin on the plasma membrane (Fig. 1D and 1F) and E-cadherin both on the plasma membrane and in the cytoplasm of all cells transfected with the rE-cad-IZ plasmid (indicated by arrows in Fig. 1D, 1F, and 1H). In contrast, we detected only N-cadherin on the plasma membrane of cells transfected with the null-IZ plasmid (indicated by arrowheads in Fig. 1C, 1E, and 1G). These results are not sufficient to confirm that E-cadherin was successfully induced in hormone-producing cells, because rE-cad-IZ transfection of non-hormone-producing folliculo-stellate cells, which express both E- and N-cadherin [8], may yield similar results. Thus, to confirm forced E-cadherin expression in hormone-producing cells, we transfected purified hormone-producing cells with rE-cad-IZ. Hormone-producing cells were purified from transgenic S100b-GFP rat anterior pituitary cells [6] by means of fluorescence-activated cell sorting. Transfected hormone-producing cells did not differ in cell shape on differential interference contrast images (Fig. 1I and 1J). E-cadherin was detected on the plasma membrane of rE-cad-IZ-transfected cells (indicated by the small arrow in Fig. 1J) and in the cytoplasm of hormone-producing cells. However, we did not detect E-cadherin in null-IZ-transfected hormone-producing cells (Fig. 1I).
Fig. 1.
Immunohistochemistry of E-cadherin and N-cadherin in transformed anterior pituitary cells. Anterior pituitary cells were transfected with pIRES2-ZsGreen1 plasmid with a null-insert, null-IZ (A, C, E and G, in the identical field), and the same plasmid with cDNA for the entire region of rat E-cadherin, rE-cad-IZ (B, D, F and H, in the identical field). A and B: Differential interference contrast images of transformed cells. C and D: Merged images of immunocytochemistry for E-cadherin (light blue), N-cadherin (red), and fluorescence from ZsGreen1 (green). E and F: Immunoreaction of N-cadherin. G and H: Immunoreaction of E-cadherin. Immunocytochemistry for E-cadherin and a phase-contrast image of transfected hormone-producing cells sorted by fluorescence-activated cell sorting are also shown in I and J. I: Purified hormone-producing cells were transfected with null-IZ. J: Purified hormone-producing cells were transfected with rE-cad-IZ. The differential interference contrast image is merged. The differential interference contrast image is merged. Arrows and arrowheads indicate cells transfected with rE-cad-IZ and null-IZ, respectively. A small arrow shows the boundary of cells transfected with rE-cad-IZ. Bar=5 µm.
Prolactin immunoreactivities and mRNA signals in hormone-producing cells expressing E-cadherin
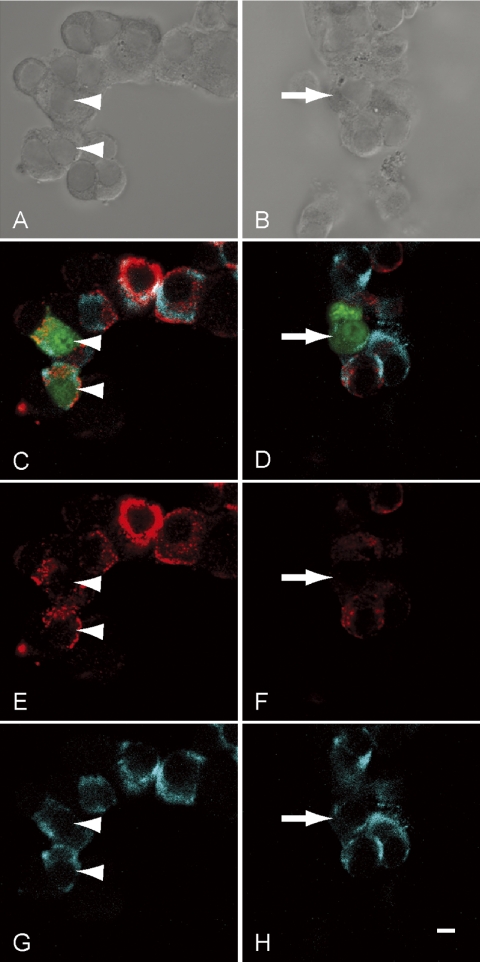
We detected prolactin immunoreactivity and mRNA signals in primary culture by means of immunocytochemical and in situ hybridization techniques. In intact cells, obvious prolactin immunoreactivity (Fig. 2C, 2D, 2E, and 2F) and mRNA signals (Fig. 2C, 2D, 2G and 2H) were obvious. In null-IZ-transfected cells (indicated by the arrowheads in Fig. 2), prolactin immunoreactivity and mRNA signals (Fig. 2C, 2E and 2G) were identical to those detected in intact cells. In contrast, in rE-cad-IZ-transfected cells (indicated by the arrows in Fig. 2), prolactin mRNA signals (Fig. 2D and 2H) were detected, but prolactin immunoreactivity (Fig. 2C and 2F) was barely detectable.
Fig. 2.
In situ hybridization and immunocytochemistry of transfected anterior pituitary cells. Anterior pituitary cells in primary culture were transfected with null-IZ (A, C, E and G, in the identical field) and with rE-cad-IZ (B, D, F and H, in the identical field). A and B: Differential interference contrast images of transformed cells. C and D: Merged images of immunoreaction of prolactin protein (red), in situ hybridization signal for prolactin mRNA (light blue), and fluorescence from ZsGreen1 (C and D). E and F: Immunoreaction of prolactin. G and H: In situ hybridization signals for prolactin mRNA. Arrows and arrowheads show cells transfected with rE-cad-IZ and null-IZ, respectively. Bar=5 µm.
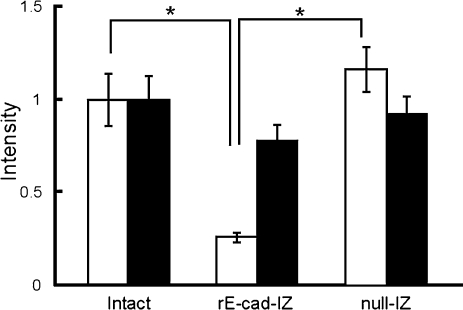
To confirm that prolactin immunoreactivity was suppressed in rE-cad-IZ-transfected cells, we classified prolactin mRNA signal intensities and prolactin immunoreactivity. As shown in the Table, all cells in the intact group and null-IZ-transfected group displayed strong immunoreactivity. In the rE-cad-IZ-transfected group, there was no evidence of strong immunoreactivity, i.e., all cells showed weak or no immunoreactivity (Table 1). Next, we used MetaMorph software to quantify the fluorescence intensity on immunocytochemistry and in situ hybridization (Fig. 3). The mean signal intensity of prolactin protein in rE-cad-IZ-transfected cells was approximately 25% of that of intact cells and null-IZ-transfected cells. In contrast, the mean signal intensity of prolactin mRNA signals in rE-cad-IZ-transfected cells did not differ from that of intact cells and null-IZ-transfected cells.
Table 1.
Classification of prolactin immunoreactivity in prolactin mRNA-positive cells
| + | ± | − | |
|---|---|---|---|
| intact | 30 | 0 | 0 |
| rE-cad-IZ | 0 | 28 | 2 |
| null-IZ | 30 | 0 | 0 |
Thirty prolactin mRNA-positive cells were categorized into 3 groups by prolactin immunoreactivity: +, strong immunoreactivity; ±, weak immunoreactivity; −, undetectable.
Fig. 3.
Fluorescence intensities of immunocytochemistry for prolactin protein in prolactin mRNA-positive cells. Fluorescence intensities were quantified from intact rE-cad-IZ-transfected cells and null-IZ-transfected cells. Each group consisted of 30 prolactin mRNA-positive cells. The fluorescence intensities are expressed as the ratio to the mean value of the intact group. Open and solid columns represent the fluorescence intensity of prolactin immunoreactivity and prolactin mRNA, respectively. Data are mean±S.E.
*: statistically significant (P<0.01 by the Bonferroni test).
IV. Discussion
This is the first report to show forced expression of E-cadherin in rat anterior pituitary cells and its effects on hormone production. Using immunocytochemical techniques, we detected exogenous E-cadherin on hormone-producing cells. Exogenous E-cadherin and endogenous N-cadherin were successfully detected on the plasma membrane of hormone-producing cells that were transfected with rE-cad-IZ (Fig. 1D, 1F, 1H, and 1J). In contrast, we detected only N-cadherin in null-IZ-transfected cells (Fig. 1C, 1E, and 1G). These results suggest that E-cadherin derived from a transferred gene can be transported to the plasma membrane in hormone-producing cells.
The level of prolactin mRNA expression in rE-cad-IZ-transfected cells appeared similar to that in untransfected cells and null-IZ-transfected cells (Figs. 2 and 3). However, the immunoreactivity of prolactin in rE-cad-IZ-transfected cells was significantly lower than in untransfected cells and null-IZ-transfected cells (Figs. 2 and 3). These results show that exogenous E-cadherin does not affect prolactin mRNA transcription; rather, it decreases the protein content of prolactin. These conflicting results indicate that the reduction in prolactin protein results from inhibition of prolactin protein synthesis, acceleration of prolactin release, or enhancement of prolactin digestion in the rE-cad-IZ-transfected lactotrophs. It is unlikely that E-cadherin expression inhibited translation, which leaves two possibilities.
Firstly, digestion of secretory granules in E-cadherin-expressing lactotrophs may have been accelerated. It has been reported that nascent E-cadherin binds to catenin complex to expedite transport from the Golgi network to the plasma membrane [13]. In addition, it was reported that E-cadherin that had not bound to catenin complex was not transported to the plasma membrane, but was instead degraded in lysosomes [16]. As shown in Figure 1, exogenous E-cadherin was detected in cytoplasm, which suggests that the catenin complex present was insufficient for the amount of E-cadherin that had been overexpressed. If E-cadherin is located on hormone secretory granules, as is the case in pancreatic beta cells [2], the granules with E-cadherin not bound to catenin would be transported to lysosomes for degradation. Although this process may indeed explain the decreased prolactin content in transfected lactotrophs, we believe the next hypothesis is more likely.
One of the most important effects of E-cadherin expression on cell phenotype appears on cytoskeletal fibers. Cytoskeletal fibers are anchored to E-cadherin on the plasma membrane through catenin [19] and the amount of E-cadherin is reported to possess a close relation with the amount of cytoskeletal fibers [22]. In pancreatic beta cells, it has been shown that destabilization of F-actin affects insulin secretion both positively [18] and negatively [12]. E-cadherin is also reported to enhance the activity of motor proteins of the myosin family [7, 22]. Thus, factitious expression of E-cadherin would alter cytoskeletal structure of lactotrophs, and the change could accelerate trafficking of secretory granules, which may be another reason for the decreased prolactin content in transfected lactotrophs.
Many studies have reported changes in gene expression after cadherin switching [4, 25]. However, the differences in downstream signal transduction between E- and N-cadherin have yet to be clarified. Seidel et al. reported that a type of p120 catenin, which binds to the intracellular domain of each cadherin, differs between E- and N-cadherin [21]. Unfortunately, at present it is difficult to describe the molecular mechanisms that cause the changes in gene expression which underlie cadherin switching. The present study has shown that forced E-cadherin expression in lactotrophs of adult rats significantly reduced prolactin content. We are inclined to conclude that this result is linked to the observation that E-cadherin expression is lost in hormone-producing cells at the onset of hormone production in the developing adenohypophysis [9].
V. Acknowledgments
We wish to thank Professor K. Inoue (Saitama University, Japan) for supplying the transgenic S100b-GFP rat, and Y. Furukawa (Jichi Medical University, Japan) for technical support in fluorescence-activated cell sorting.
This work was partly supported by a Grant-in-Aid for Scientific Research (C) (19590194) and (21570067) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and by promotional funds from the Keirin Race of the JKA.
VI. Reference
- 1.Ben-Jonathan N., Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr. Rev. 2001;22:724–763. doi: 10.1210/edrv.22.6.0451. [DOI] [PubMed] [Google Scholar]
- 2.Bosco D., Rouiller D. G., Halban P. A. Differential expression of E-cadherin at the surface of rat beta-cells as a marker of functional heterogeneity. J. Endocrinol. 2007;194:21–29. doi: 10.1677/JOE-06-0169. [DOI] [PubMed] [Google Scholar]
- 3.Denef C. Paracrinicity: the story of 30 years of cellular pituitary crosstalk. J. Neuroendocrinol. 2008;20:1–70. doi: 10.1111/j.1365-2826.2007.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eastham A. M., Spencer H., Soncin F., Ritson S., Merry C. L., Stern P. L., Ward C. M. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007;67:11254–11262. doi: 10.1158/0008-5472.CAN-07-2253. [DOI] [PubMed] [Google Scholar]
- 5.Fujiwara K., Maekawa F., Kikuchi M., Takigami S., Yada T., Yashiro T. Expression of retinaldehyde dehydrogenase (RALDH)2 and RALDH3 but not RALDH1 in the developing anterior pituitary glands of rats. Cell Tissue Res. 2007;328:129–135. doi: 10.1007/s00441-006-0345-7. [DOI] [PubMed] [Google Scholar]
- 6.Itakura E., Odaira K., Yokoyama K., Osuna M., Hara T., Inoue K. Generation of transgenic rats expressing green fluorescent protein in S-100beta-producing pituitary folliculo-stellate cells and brain astrocytes. Endocrinology. 2007;148:1518–1523. doi: 10.1210/en.2006-1390. [DOI] [PubMed] [Google Scholar]
- 7.Ivanov A. I., Hunt D., Utech M., Nusrat A., Parkos C. A. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol. Biol. Cell. 2005;16:2636–2650. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kikuchi M., Yatabe M., Fujiwara K., Takigami S., Sakamoto A., Soji T., Yashiro T. Distinctive localization of N- and E-cadherins in rat anterior pituitary gland. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2006;288:1183–1189. doi: 10.1002/ar.a.20384. [DOI] [PubMed] [Google Scholar]
- 9.Kikuchi M., Yatabe M., Kouki T., Fujiwara K., Takigami S., Sakamoto A., Yashiro T. Changes in E- and N-cadherin expression in developing rat adenohypophysis. Anat. Rec. (Hoboken) 2007;290:486–490. doi: 10.1002/ar.20516. [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi M., Yatabe M., Fujiwara K., Horiguchi K., Kusumoto K., Kouki T., Sakamoto A., Yashiro T. Spatio-temporal relation between cadherin switching and cytogenesis of hormone-producing cells in the developing rat adenohypophysis. Anat. Sci. Int. 2009;84:155–160. doi: 10.1007/s12565-009-0020-7. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs E. M., Ali R. G., McCormack A. J., Yap A. S. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J. Biol. Chem. 2002;277:6708–6718. doi: 10.1074/jbc.M109640200. [DOI] [PubMed] [Google Scholar]
- 12.Li G., Rungger-Brandle E., Just I., Jonas J. C., Aktories K., Wollheim C. B. Effect of disruption of actin filaments by Clostridium botulinum C2 toxin on insulin secretion in HIT-T15 cells and pancreatic islets. Mol. Biol. Cell. 1994;5:1199–1213. doi: 10.1091/mbc.5.11.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lock J. G., Stow J. L. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol. Biol. Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuno A., Nagashima T., Katakami H., Sanno N., Teramoto A., Takekoshi S., Osamura R. Y., Kirino T., Lloyd R. V. Production of pituitary hormone by human pituitary adenoma is under autocrine and paracrine regulation of hypothalamic hormones secreted from adenoma cells. Acta Histochem. Cytochem. 2003;36:415–420. [Google Scholar]
- 15.Mclachlan R. W., Kraemer A., Helwani F. M., Kovacs E. M., Yap A. S. E-cadherin adhesion activates c-Src signaling at cell-cell contacts. Mol. Biol. Cell. 2007;18:3214–3223. doi: 10.1091/mbc.E06-12-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyashita Y., Ozawa M. A dileucine motif in its cytoplasmic domain directs beta-catenin-uncoupled E-cadherin to the lysosome. J. Cell Sci. 2007;120:4395–4406. doi: 10.1242/jcs.03489. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa S., Takeichi M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development. 1998;125:2963–2971. doi: 10.1242/dev.125.15.2963. [DOI] [PubMed] [Google Scholar]
- 18.Orci L., Gabbay K. H., Malaisse W. J. Pancreatic beta-cell web: its possible role in insulin secretion. Science. 1972;175:1128–1130. doi: 10.1126/science.175.4026.1128. [DOI] [PubMed] [Google Scholar]
- 19.Potter E., Bergwitz C., Brabant G. The cadherin-catenin system: implications for growth and differentiation of endocrine tissues. Endocr. Rev. 1999;20:207–239. doi: 10.1210/edrv.20.2.0362. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto A., Murata K., Suzuki H., Yatabe M., Kikuchi M. Immunohistochemical observation of co-expression of E- and N-cadherins in rat organogenesis. Acta Histochem. Cytochem. 2008;41:143–147. doi: 10.1267/ahc.08026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seidel B., Braeg S., Adler G., Wedlich D., Menke A. E- and N-cadherin differ with respect to their associated p120ctn isoforms and their ability to suppress invasive growth in pancreatic cancer cells. Oncogene. 2004;23:5532–5542. doi: 10.1038/sj.onc.1207718. [DOI] [PubMed] [Google Scholar]
- 22.Stehbens S. J., Paterson A. D., Crampton M. S., Shewan A. M., Ferguson C., Akhmanova A., Parton R. G., Yap A. S. Dynamic microtubules regulate the local concentration of E-cadherin at cell-cell contacts. J. Cell Sci. 2006;119:1801–1811. doi: 10.1242/jcs.02903. [DOI] [PubMed] [Google Scholar]
- 23.Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu. Rev. Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- 24.Tsuchiya B., Sato Y., Kameya T., Okayasu I., Mukai K. Differential expression of N-cadherin and E-cadherin in normal human tissues. Arch. Histol. Cytol. 2006;69:135–145. doi: 10.1679/aohc.69.135. [DOI] [PubMed] [Google Scholar]
- 25.Wheelock M. J., Shintani Y., Maeda M., Fukumoto Y., Johnson K. R. Cadherin switching. J. Cell Sci. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 26.Xu L., Overbeek P. A., Reneker L. W. Systematic analysis of E-, N- and P-cadherin expression in mouse eye development. Exp. Eye Res. 2002;74:753–760. doi: 10.1006/exer.2002.1175. [DOI] [PubMed] [Google Scholar]