Abstract
Objectives
To determine if exogenous S-adenosyl-l-methionine (AdoMet), a commonly used nutritional supplement, increases the level of plasma homocysteine (Hcy), a potential cardiovascular risk factor, in healthy human subjects.
Design
Double-blind, placebo-controlled, randomized clinical trial.
Setting
Mayo Clinic, Rochester, Minnesota.
Subjects
Fifty-two (52) healthy human volunteers.
Intervention
Subjects received placebo or AdoMet (800 mg per day) for 4 weeks. Hcy levels were measured before and after administration of AdoMet or placebo.
Outcome Measures
The primary outcome measure was change in Hcy level. Secondary outcome measures included an interim Hcy determination (at 2 weeks) and changes in levels of high-sensitivity C-reactive protein (hsCRP), lipids, and alanine aminotransferase.
Results
There was no statistically significant change in Hcy between groups. Similarly, no statistically significant differences in change in Hcy or hsCRP levels were observed at 2 or 4 weeks. There was a small but statistically significant increase (p < 0.04) in alanine aminotransferase at week 2 and a statistically significant decrease (p < 0.04) in total cholesterol in the AdoMet group compared with the placebo group.
Conclusions
AdoMet at a daily dose of 800 mg for 4 weeks does not appear to significantly affect Hcy levels in the blood.
Introduction
The Centers for Disease Control and Prevention (CDC) estimated that 30% of adults use dietary supplements regularly1 and spend $1.3 billion to $1.7 billion annually on these supplements.2 In a large population-based survey conducted by the CDC involving more than 31,000 participants, 36% of the adults reported using some form of complementary and alternative medicine (CAM) therapy in the preceding year.3 S-adenosyl-l-methionine (AdoMet or SAM-e) has been available as a dietary supplement in the United States since 1999. AdoMet has been studied for use in the treatment of depression, liver cirrhosis, cholestasis, degenerative joint disease, fibromyalgia, and neurologic disorders.4–6 AdoMet is metabolized to S-adenosyl-l-homocysteine (AdoHcy) in methylation reactions. AdoHcy can then be hydrolyzed by AdoHcy hydrolase to homocysteine (Hcy) and adenine (Fig. 1). This metabolic pathway raises the possibility that individuals who take supplemental doses of AdoMet could develop elevated levels of Hcy, a potentially unfavorable outcome. To determine the effect of the dietary supplement AdoMet on plasma Hcy levels, we conducted a double-blind, placebo-controlled, randomized clinical trial.
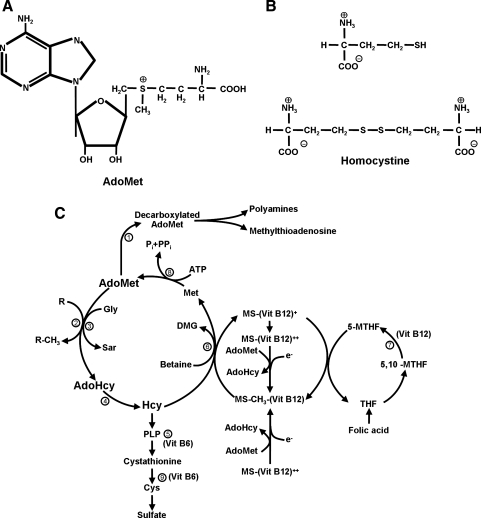
FIG. 1. (A).
The structure of S-adenosyl-l-methionine (AdoMet). (B) Homocysteine (Hcy) and homocystine. Hcy is a monomer, and homocystine is an Hcy dimer. (C) Metabolic pathways of AdoMet and Hcy. The circled numbers correspond to enzymatic reactions: 1, AdoMet decarboxylase; 2, AdoMet-dependent; MT, methyltransferase; 3, Gly-N-MT; 4, S-adenosyl-l-homocysteine (AdoHcy) hydrolase; 5, cystathione β-synthase; 6, betaine Hcy MT; 7, MTHF reductase; 8, AdoMet synthetase; 9, cystathione γ-lase. The AdoMet, AdoHcy, and Hcy pathway is emphasized in bold. ATP, adenosine triphosphate; Cys, cysteine; DMG, dimethyl-glycine; Gly, glycine; Met, methionine; MS, methyltetrahydrofolate-Hcy MT; MTHF, methyltetrahydrofolate; Pi, inorganic phosphate; PPi, inorganic diphosphate; PLP, pyridoxal phosphate; Sar, sarcosine; THF, tetrahydrofolate.
Subjects and Methods
Study subjects
Between June 2005 and April 2006, healthy human volunteers were screened for inclusion and exclusion criteria at Mayo Clinic in Rochester, Minnesota. Included as study subjects were volunteers able to provide informed consent and complete the study. Excluded were volunteers younger than 18 years or older than 65 years; pregnant women or women actively trying to conceive; nursing mothers; volunteers with bipolar disorder or panic disorder; and smokers. Exclusion medications that could alter Hcy levels included antilipid medications (e.g., statins, gemfibrozil, ezetimibe, niacin); antidepressant medications; androgens (e.g., testosterone); anti-epileptics; azuridine; carbamazepine; clioquinol; cyclosporine; fibrates; hydrochlorothiazide; levodopa; metformin; methotrexate; nicotinic acid; nitrous oxide; phenytoin; omeprazole; estrogen (subjects taking estrogen-containing oral contraceptive pills were not excluded); penicillamine; triamterene; and trimethoprim. Potential volunteers taking any of these medications were excluded from study participation.
Study design and sample size
We performed a double-blind, placebo-controlled, randomized clinical trial. Subjects were randomly assigned to receive placebo or AdoMet. The effect size was defined as the expected mean difference divided by the standard deviation (SD) of differences. With a sample size of 52 subjects, that is, 26 subjects in each of the 2 study groups (placebo and AdoMet), we could detect an effect size of 0.92 or larger between the changes in measurements from baseline to follow-up between the 2 groups with more than 90% power and an α level of 0.05, assuming a 2-sided, 2-sample t test is appropriate. This is considered a large effect size.7 Assuming an Hcy level of 10 μmol/L and an SD of difference to be 2 μmol/L, we would have more than 90% power to detect a change of 1.83 μmol/L using a 2-sided, 2-sample t test.
Randomization of subjects was done according to a random number table. Subject allocation was blinded to the researchers and was conducted by a centralized pharmacy. The study design was approved by the Mayo Clinic Institutional Review Board. Written informed consent was obtained from all subjects. Subjects participating in the study received a small remuneration for their participation.
AdoMet source
AdoMet was obtained from Pharmacist's Ultimate Health (St Paul, MN) and was tested independently by Source Naturals (Scotts Valley, CA) (manufacturer lot no. 4516, threshold lot no. B128014). For each tablet with a specified content of 400 mg of AdoMet, the analysis revealed 413 mg of AdoMet (103.25%). Placebo tablets, identical in appearance to AdoMet, were analyzed by Supplement & Nutrition Technologies, Inc. (Chandler, AZ). Each placebo tablet contained 662 mg of cantab (sugar), 400 mg of whey, 1.6 mg of riboflavin, 24.4 mg of stearic acid, and 12 mg of magnesium stearate. An AdoMet dose of 800 mg per day was used. This dose has been well tolerated in previous clinical trials.8–13
Laboratory studies
Data collection included determination of baseline complete blood cell count, creatinine, lipids (total cholesterol, triglycerides, and high-density lipoprotein [HDL]), alanine aminotransferase (ALT), Hcy, high-sensitivity C-reactive protein (hsCRP), folate, vitamin B6, and vitamin B12. ALT and Hcy were evaluated at 2 and 4 weeks. Additionally, lipid levels and hsCRP were rechecked at 4 weeks. Values were obtained from fasting participants. The Mayo Medical Laboratories' Hcy assay uses liquid chromatography electron spray tandem mass spectrometry.14 Inter- and intra-assay coefficients of variation for this assay were 2.9% to 5.9% and 3.6% to 5.3%, respectively, at mean Hcy concentrations of 3.9, 22.7, and 52.8 μmol/L.14 All the laboratory studies were conducted at the Mayo Clinic.
Statistical analysis
Subjects were followed for 4 weeks with laboratory studies at baseline and at weeks 2 and 4. We calculated the differences for laboratory studies between the baseline and week 2 or week 4 measurements in the individual patient. If the subject was unavailable for follow-up, the laboratory test result remained at whatever the last cycle was. The median and range values (minimum, maximum) on laboratory studies were compared between the 2 groups using the 2-sided Wilcoxon rank sum test because the assumption of normal distribution might be violated. Mean ± SD was compared using 2-sample t test, otherwise. To account for the measurements at baseline, similar analyses were done using the percent change in measurements from baseline to week 2 or week 4 in place of actual differences: (baseline – week 2)/baseline × 100% or (baseline – week 4)/baseline × 100%. Secondary end points of interest included changes in hsCRP, lipid, and ALT levels between baseline and week 2 and week 4 measurements. Adverse events and other categorical subject demographic variables were compared using the Fisher exact test or the Pearson chi square test. All analyses were based on the total 52 subjects whom we intended to treat. Any p values less than 0.05 were considered statistically significant.
Results
The study group comprised 41 women (78.9%) and 11 men (21.1%). The mean age was 35.3 years (range, 18–60 years) (Fig. 2). There were no statistically significant differences between baseline subject characteristics or baseline laboratory studies in the placebo and AdoMet groups (Table 1).Adverse events were minor and were not significantly different between the placebo and AdoMet groups (Table 2).
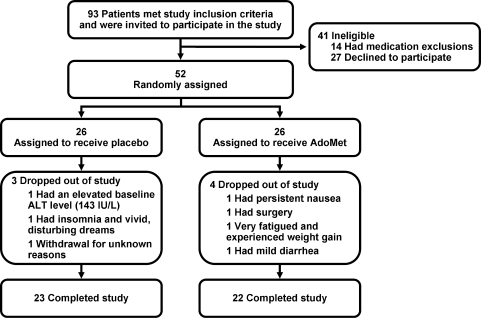
FIG. 2.
Flow diagram of clinical trial. AdoMet, S-adenosyl-l-methionine; ALT, alanine aminotransferase.
Table 1.
Subject Characteristics
| Placebo (n = 26) | AdoMet (n = 26) | Total (n = 52) | p | |
|---|---|---|---|---|
| Sex | >0.99 | |||
| Male | 5 (19.2%) | 6 (23.1%) | 11 (21.1%) | |
| Female | 21 (80.8%) | 20 (76.9%) | 41 (78.9%) | |
| Race | 0.33 | |||
| White | 15 (57.7%) | 18 (69.2%) | 33 (63.5%) | |
| Other | 0 | 1 (3.9%) | 1 (1.9%) | |
| Unknown or did not disclose | 11 (42.3%) | 7 (26.9%) | 18 (34.6%) | |
| Age, mean ± SD, years | 35.0 ± 12.0 | 35.7 ± 11.7 | 35.3 ± 11.8 | 0.83 |
p-values: Fisher exact test values (sex), Pearson chi square test values (race), and 2-sample t test (age).
AdoMet, S-adenosyl-l-methionine; SD, standard deviation.
Table 2.
Adverse Events
| Placebo (n = 26) | AdoMet (n = 26) | Total (n = 52) | p | |
|---|---|---|---|---|
| Bloating or flatulence | 4 (15.4%) | 1 (3.9%) | 5 (9.6%) | 0.35 |
| Abdominal cramps/pain | 1 (3.9%) | 2 (7.7%) | 3 (5.8%) | >0.99 |
| Nausea | 0 | 2 (7.7%) | 2 (3.9%) | 0.49 |
| Headache/migraine | 0 | 2 (7.7%) | 2 (3.9%) | 0.49 |
| Fatigue | 1 (3.9%) | 1 (3.9%) | 2 (3.9%) | >0.99 |
| Diarrhea | 0 | 1 (3.9%) | 1 (1.9%) | >0.99 |
| Anorexia | 0 | 1 (3.9%) | 1 (1.9%) | >0.99 |
| Constipation | 0 | 1 (3.9%) | 1 (1.9%) | >0.99 |
| Weight gain | 0 | 1 (3.9%) | 1 (1.9%) | >0.99 |
| Dry mouth | 0 | 1 (3.9%) | 1 (1.9%) | >0.99 |
| Insomnia | 1 (3.9%) | 0 | 1 (1.9%) | >0.99 |
| Urinary frequency | 1 (3.9%) | 0 | 1 (1.9%) | >0.99 |
p-values are Fisher exact test values.
AdoMet, S-adenosyl-l-methionine.
The primary endpoint, change in Hcy at 4 weeks, was not significantly different between the 2 groups. Likewise, there were no statistically significant differences in change in Hcy or hsCRP at 2 or 4 weeks (Table 3).This was true for absolute differences, relative percent changes, and log transformations of Hcy and HDL, and when the data were analyzed in a non–intention-to-treat manner, or only on the subjects who completed the study (23 subjects in the placebo group and 22 subjects in the AdoMet group). There was a small increase in ALT at 2 weeks in the AdoMet group compared with the placebo group (p < 0.035). There was a small, but statistically significant, decrease (p < 0.04) in total cholesterol in the AdoMet group compared with the placebo group. Interestingly, the subject with the highest baseline Hcy level (13.1 μmol/L, just above the normal limit) had a decline in Hcy during AdoMet treatment to 12.3 μmol/L (2 weeks) and 10.7 μmol/L (4 weeks). This individual had a baseline creatinine of 1.5mg/dL. Elevated creatinine is associated with increased Hcy levels.
Table 3.
Laboratory Study Results Expressed as Median (Minimum, Maximum) Values
| Variable | Placebo (n = 26) | AdoMet (n = 26) | p |
|---|---|---|---|
| Hcy, μmol/L | |||
| Baseline | 7.5 (4.9, 10.5) | 8.3 (4.5, 13.1) | 0.18 |
| Week 2 | 6.9 (5.5, 9.8) | 8.8 (4.9, 12.4) | 0.26 |
| Week 4 | 7.5 (5.6, 10.5) | 8.2 (4.4, 12.2) | 0.32 |
| Baseline – week 2 | 0.0 (−2.3, 2.1) | 0.1 (−2.5, 3.3) | 0.94 |
| Baseline – week 4 | 0.0 (−2.3, 2.1) | 0.0 (−2.0, 2.9) | 0.53 |
| (Baseline – week 2)/baseline × 100% | 0.5 (−30.7, 23.3) | 1.3 (−40.4, 29.2) | 0.82 |
| (Baseline – week 4)/baseline × 100% | 0.0 (−30.7, 21.4) | 0.0 (−28.2, 23.4) | 0.39 |
| ALT, IU/L | |||
| Baseline | 21.0 (13.0, 143.0) | 22.0 (14.0, 45.0) | 0.91 |
| Week 2 | 20.5 (10.0, 143.0) | 25.0 (0.0, 44.0) | 0.29 |
| Week 4 | 23.0 (10.0, 143.0) | 23.5 (0.0, 47.0) | 0.76 |
| Baseline – week 2 | 1.0 (−20.0, 12.0) | −1.0 (−23.0, 17.0) | 0.03 |
| Baseline – week 4 | 0.0 (−8.0, 13.0) | 0.0 (−13.0, 16.0) | 0.54 |
| (Baseline – week 2)/baseline × 100% | 6.1 (−37.0, 33.3) | −3.9 (−109.5, 100.0) | 0.03 |
| (Baseline – week 4)/baseline × 100% | 0.0 (−38.1, 27.3) | 0.0 (−42.9, 100.0) | 0.62 |
| HsCRP, mg/dL | |||
| Baseline | 0.1 (0.0, 0.5) | 0.0 (0.0, 0.9) | 0.20 |
| Week 4 | 0.1 (0.0, 0.6) | 0.1 (0.0, 1.2) | 0.78 |
| Baseline – week 4 | 0.0 (−0.3, 0.2) | 0.0 (−1.1, 0.7) | 0.69 |
| (Baseline – week 4)/baseline × 100% | 0.0 (−157.0, 85.8) | 0.0 (−4122.0, 75.4) | 0.74 |
| Total cholesterol, mg/dL | |||
| Baseline | 185.5 (98.0, 267.0) | 182.5 (111.0, 238.0) | 0.99 |
| Week 4 | 198.5 (108.0, 267.0) | 181.0 (121.0, 234.0) | 0.20 |
| Baseline – week 4 | 0.0 (−64.0, 38.0) | 2.5 (−32.0, 43.0) | 0.04 |
| (Baseline – week 4)/baseline × 100% | 0.0 (−65.3, 26.0) | 1.3 (−17.6, 24.2) | 0.05 |
| HDL, mg/dL | |||
| Baseline | 52.5 (26.0, 75.0) | 51.0 (35.0, 112.0) | 0.58 |
| Week 4 | 55.5 (30.0, 73.0) | 47.5 (26.0, 109.0) | 0.76 |
| Baseline – week 4 | −0.5 (−13.0, 18.0) | 1.5 (−8.0, 18.0) | 0.09 |
| (Baseline – week 4)/baseline × 100% | −0.8 (−50.0, 32.1) | 2.4 (−17.5, 29.5) | 0.12 |
| Triglycerides, mg/dL | |||
| Baseline | 102.5 (42.0, 161.0) | 89.5 (46.0, 143.0) | 0.71 |
| Week 4 | 104.5 (39.0, 217.0) | 88.5 (38.0, 168.0) | 0.28 |
| Baseline – week 4 | −0.5 (−124.0, 68.0) | 1.0 (−32.0, 31.0) | 0.37 |
| (Baseline – week 4)/baseline × 100% | −0.5 (−133.3, 56.2) | 0.8 (−38.9, 36.7) | 0.33 |
p-values are Wilcoxon rank sum values.
AdoMet, S-adenosyl-l-methionine; ALT, alanine aminotransferase; Hcy, homocysteine; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein.
Discussion
AdoMet is a frequently used dietary supplement. It is important to study potential toxicities and cardiovascular risk factors from supplement use. Our results indicated no change in Hcy levels. Loehrer and colleagues studied the effect of oral AdoMet (400 mg) on plasma 5-methyltetrahydrofolate (5-MTHF), AdoHcy, Hcy, and methionine levels in 14 healthy human subjects over a 24-hour period.8 They found a transient increase in AdoMet plasma levels from (mean ± standard error) 38.0 ± 13.4 to 361.8 ± 66.4 nmol/L (p < 0.001), which returned to baseline values with a half-life of 1.7 ± 0.3 hours. AdoHcy and 5-MTHF showed significant transient increases from 29.9 ± 3.7 to 51.7 ± 7.1 nmol/L, and from 25.1 ± 2.5 to 36.2 ± 3.5nmol/L, respectively (p < 0.001). Hcy and methionine did not change significantly during the 24-hour study period. AdoMet did not inhibit MTHF reductase, the 5-MTHF–forming enzyme, but rather caused a transient increase in 5-MTHF, a key cofactor in Hcy metabolism, which they suggested could result in lower Hcy levels. The authors hypothesized that “lack of change of homocysteine concentrations after AdoMet administration could be explained by sufficient capacity of homocysteine handling.”8
Gören et al. published their results of AdoMet administration on Hcy levels.15 In that study, an open-label, single-arm design, 15 healthy subjects were enrolled. Subjects received oral AdoMet for 4 weeks titrated up to 1,600 mg per day. AdoMet levels became significantly elevated from baseline: baseline, 0.75 ± 0.12nmol/mL; week 2, 0.79 ± 0.13nmol/mL (p < 0.01); and week 4, 0.78 ± 0.12nmol/mL (p = 0.002). However, no patients developed increased Hcy levels with AdoMet treatment at week 4 (7.29 ± 1.91 μmol/L; p = 0.11). At the week 2 Hcy assessment, there was an increase in Hcy from the baseline level of 6.93 ± 1.52 μmol/L to 7.58 ± 2.10 μmol/L, which was of borderline statistical significance (P = 0.05). One subject with a family history of mania developed a manic reaction. No other psychiatric changes were noted. No patients discontinued AdoMet because of adverse reactions, which were primarily mild gastrointestinal tract symptoms.
To our knowledge, our study is the first placebo-controlled, double-blind, randomized trial of the effect of a 4-week administration of AdoMet on Hcy levels in human subjects. AdoMet was well tolerated in this study with adverse effects similar to those of placebo. There was no significant change in ALT levels, consistent with the prior use of AdoMet in the treatment of liver diseases.16–19 We found no statistically significant changes in Hcy levels, which suggests that the AdoMet/AdoHcy/Hcy system has the capacity to process increased exogenous AdoMet without a substrate (AdoMet) mass action effect increase in product (Hcy level). This observation agrees with the short-term data of Loehrer et al.8 and the 4-week study by Gören et al.15 Table 4 compares the effect of exogenous AdoMet administration on Hcy in these 2 studies and in our study (Table 4).AdoMet dosing at 800 mg per day is approximately 2 mmol of AdoMet per day. This is comparable to methionine-loading studies using roughly 50 mmol of methionine. Methionine loading (oral or intravenous bolus of methionine followed by blood or urine testing for Hcy) has been used as a diagnostic tool to evaluate the integrity of Hcy metabolism. It is possible that higher AdoMet doses beyond those routinely used in humans could perturb the Hcy system and result in changes in Hcy level.
Table 4.
Comparison of Studies on the Effect of S-Adenosyl-l-Methionine (AdoMet) on Homocysteine (Hcy) Levels in Humans
| |
Study characteristics |
Plasma Hcy, μmol/L |
|||||
|---|---|---|---|---|---|---|---|
| Reference | N | AdoMet dose | Duration | Baseline | 24 h | 2 wk | 4 wk |
| Loehrer et al, 19978 | 14 | 400 mg | 1 dose | 8.2 | 6.6a | ND | ND |
| Gören et al, 200415 | 15 | 1,600 mg/d | 4 wk | 6.93 | ND | 7.58 | 7.29 |
| Present study | 52 | 800 mg/d | 4 wk | ||||
| Placebo | 7.5 | ND | 7.3 | 7.7 | |||
| AdoMet | 8.5 | ND | 8.3 | 8.3 | |||
Maximally decreased level seen over 24 hours.
ND, not determined.
Several factors may limit the generalizability of our results. We did not measure in vivo AdoMet levels. Gören et al. found small but statistically significant increases in AdoMet levels with exogenous AdoMet administration.15 Since our study subjects had normal Hcy levels at baseline, we do not know the effect of exogenous AdoMet on subjects with above-normal Hcy levels. It is possible that there would be no effect on Hcy levels (as in this study); a decrease in Hcy levels by increasing the active form of folate (5-MTHF); or an increase in Hcy by a mass action effect of the substrate (AdoMet). Although our trial was larger than prior studies, the individual Hcy variations in a larger cohort were not accounted for and genetic factors that may change Hcy levels were not tested. Measuring plasma Hcy level may not determine tissue levels of Hcy. Also, changes in Hcy may take place over a longer period than that studied or be associated with conditions such as impaired kidney function. As the cosubstrate for methylation reactions, AdoMet may be of interest in epigenetic studies of DNA methylation and in studies of hypomethylating agents in cancer. Unfortunately, we did not measure the effect of AdoMet on the DNA methylation of specific genes or on global DNA methylation patterns.
Our study is strengthened by the study design (randomized, placebo-controlled, double-blind), a priori power calculations, a larger study group than prior studies, and measurement of other factors of interest (hsCRP, lipids, ALT). However, other studies have shown that an elevated blood Hcy level is a risk factor for atherothrombotic vascular disease.20,21 Elevated Hcy has been associated with increased risk of death from cardiovascular causes,22,23 coronary heart disease,23,24 carotid atherosclerosis,25 stroke,23,26 and deep vein thrombosis.27 Also, Hcy-lowering therapy has been found to improve outcomes after percutaneous coronary intervention.28–32 However, since our trial was conducted, the results of recent studies of Hcy-lowering therapy to prevent cardiovascular events have been negative.33,34 Some epidemiologic studies have associated Hcy as a risk factor for dementia, while a recent study did not find a benefit to Hcy-lowering therapy on cognitive decline.35 In vitro studies have demonstrated that exogenous AdoMet can restore normal gene expression and decrease β-amyloid production through gene methylation in neuroblastoma cells grown in folate- and vitamin B12–deprived media.36 Therefore, the lack of increase in Hcy and a potential benefit of AdoMet in dementia suggest additional clinical trials could be contemplated in humans. The role of AdoMet in epigenetic changes in other diseases, such as cancer, is an area for future research.
In conclusion, AdoMet seems well tolerated, and in a dose of 800 mg per day for 4 weeks does not appear to significantly affect plasma Hcy levels. Future clinical trials of AdoMet should monitor Hcy levels with extended use of AdoMet to confirm its safety with long-term use.
Footnotes
Presented in abstract form at the American Society of Hematology 48th Annual Meeting and Exposition, Orlando, Florida, December 9–12, 2006. Portions of this manuscript have been published in abstract form in Blood 2006;108(11 part 1):428a.
Acknowledgments
This study was supported by intramural funds from the Division of General Internal Medicine, Mayo Clinic, Rochester, Minnesota (Clinicaltrials.gov number, NCT00284011).
We are indebted to Richard Weinshilboum, M.D., from the Department of Molecular Pharmacology and Experimental Therapeutics, Mayo Clinic, for his thoughtful review of the manuscript.
Disclosure Statement
No competing financial interests exist.
References
- 1.Balluz LS. Kieszak SM. Philen RM. Mulinare J. Vitamin and mineral supplement use in the United States: Results from the third National Health and Nutrition Examination Survey. Arch Fam Med. 2000;9:258–262. doi: 10.1001/archfami.9.3.258. Erratum in Arch Fam Med 2000;9:652. [DOI] [PubMed] [Google Scholar]
- 2.Ervin RB. Wright JD. Kennedy-Stephenson J. Use of dietary supplements in the United States, 1988–94. Vital Health Stat 11. 1999;244(i–iii):1–14. [PubMed] [Google Scholar]
- 3.Barnes PM. Powell-Griner E. McFann K. Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data. 2004;343:1–19. [PubMed] [Google Scholar]
- 4.Jacobsen S. Danneskiold-Samsøe B. Andersen RB. Oral S-adenosylmethionine in primary fibromyalgia: Double-blind clinical evaluation. Scand J Rheumatol. 1991;20:294–302. doi: 10.3109/03009749109096803. [DOI] [PubMed] [Google Scholar]
- 5.S-Adenosyl-l-Methionine for Treatment of Depression, Osteoarthritis, and Liver Disease. Summary, Evidence Report/Technology Assessment: Number 64. Publication No. 02-E033. Rockville, MD: Agency for Healthcare Research and Quality; 2002. [Google Scholar]
- 6.Bottiglieri T. S-Adenosyl-l-methionine (SAMe): From the bench to the bedside: molecular basis of a pleiotrophic molecule. Am J Clin Nutr. 2002;76:1151S–1157S. doi: 10.1093/ajcn/76/5.1151S. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. Statistical power and analysis for the behavioral sciences, Rev. ed. New York: Academic Press; 1977. [Google Scholar]
- 8.Loehrer FM. Schwab R. Angst CP. Haefeli WE. Fowler B. Influence of oral S-adenosylmethionine on plasma 5-methyltetrahydrofolate, S-adenosylhomocysteine, homo-cysteine and methionine in healthy humans. J Pharmacol Exp Ther. 1997;282:845–850. [PubMed] [Google Scholar]
- 9.Fetrow CW. Avila JR. Efficacy of the dietary supplement S-adenosyl-l-methionine. Ann Pharmacother. 2001;35:1414–1425. doi: 10.1345/aph.1Z443. [DOI] [PubMed] [Google Scholar]
- 10.SAMe for depression. Med Lett Drugs Ther. 1999;41:107–108. [PubMed] [Google Scholar]
- 11.Mischoulon D. Fava M. Role of S-adenosyl-l-methionine in the treatment of depression: A review of the evidence. Am J Clin Nutr. 2002;76:1158S–1161S. doi: 10.1093/ajcn/76/5.1158S. [DOI] [PubMed] [Google Scholar]
- 12.Friedel HA. Goa KL. Benfield P. S-adenosyl-l-methionine: A review of its pharmacological properties and therapeutic potential in liver dysfunction and affective disorders in relation to its physiological role in cell metabolism. Drugs. 1989;38:389–416. doi: 10.2165/00003495-198938030-00004. [DOI] [PubMed] [Google Scholar]
- 13.Lipinski JF. Cohen BM. Frankenburg FT, et al. Open trial of S-adenosylmethionine for treatment of depression. Am J Psychiatry. 1984;141:448–450. doi: 10.1176/ajp.141.3.448. [DOI] [PubMed] [Google Scholar]
- 14.Magera MJ. Lacey JM. Casetta B. Rinaldo P. Method for the determination of total homocysteine in plasma and urine by stable isotope dilution and electrospray tandem mass spectrometry. Clin Chem. 1999;45:1517–1522. [PubMed] [Google Scholar]
- 15.Gören JL. Stoll AL. Damico KE. Sarmiento IA. Cohen BM. Bioavailability and lack of toxicity of S-adenosyl-l-methionine (SAMe) in humans. Pharmacotherapy. 2004;24:1501–1507. doi: 10.1592/phco.24.16.1501.50943. [DOI] [PubMed] [Google Scholar]
- 16.Chawla RK. Lewis FW. Kutner MH. Bate DM. Roy RG. Rudman D. Plasma cysteine, cystine, and glutathione in cirrhosis. Gastroenterology. 1984;87:770–776. [PubMed] [Google Scholar]
- 17.Williams R. Lieber CS. The role of SAMe in the treatment of liver disease. Drugs. 1990;40(Suppl 3):S1–S2. doi: 10.2165/00003495-199000403-00002. [DOI] [PubMed] [Google Scholar]
- 18.Almasio P. Bortolini M. Pagliaro L. Coltorti M. Role of S-adenosyl-l-methionine in the treatment of intrahepatic cholestasis. Drugs. 1990;40(Suppl 3):S111–S123. doi: 10.2165/00003495-199000403-00011. [DOI] [PubMed] [Google Scholar]
- 19.Milkiewicz P. Mills CO. Roma MG. Ahmed-Choudhury J. Elias E. Coleman R. Tauroursodeoxycholate and S-adenosyl-l-methionine exert an additive ameliorating effect on taurolithocholate-induced cholestasis: A study in isolated rat hepatocyte couplets. Hepatology. 1999;29:471–476. doi: 10.1002/hep.510290215. [DOI] [PubMed] [Google Scholar]
- 20.Boushey CJ. Beresford SA. Omenn GS. Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease: Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 21.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: A meta-analysis. JAMA. 2002;288:2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 22.Bots ML. Launer LJ. Lindemans J. Hofman A. Grobbee DE. Homocysteine, atherosclerosis and prevalent cardiovascular disease in the elderly: The Rotterdam Study. J Intern Med. 1997;242:339–347. doi: 10.1046/j.1365-2796.1997.00239.x. [DOI] [PubMed] [Google Scholar]
- 23.Malinow MR. Bostom AG. Krauss RM. Homocyst(e)ine, diet, and cardiovascular diseases: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation. 1999;99:178–182. doi: 10.1161/01.cir.99.1.178. [DOI] [PubMed] [Google Scholar]
- 24.Stampfer MJ. Malinow MR. Willett WC, et al. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA. 1992;268:877–881. [PubMed] [Google Scholar]
- 25.Selhub J. Jacques PF. Bostom AG, et al. Association between plasma homocysteine concentrations and extracranial carotid-artery stenosis. N Engl J Med. 1995;332:286–291. doi: 10.1056/NEJM199502023320502. [DOI] [PubMed] [Google Scholar]
- 26.Perry IJ. Refsum H. Morris RW. Ebrahim SB. Ueland PM. Shaper AG. Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet. 1995;346:1395–1398. doi: 10.1016/s0140-6736(95)92407-8. [DOI] [PubMed] [Google Scholar]
- 27.den Heijer M. Koster T. Blom HJ, et al. Hyperhomocysteinemia as a risk factor for deep-vein thrombosis. N Engl J Med. 1996;334:759–762. doi: 10.1056/NEJM199603213341203. [DOI] [PubMed] [Google Scholar]
- 28.Schnyder G. Roffi M. Pin R, et al. Decreased rate of coronary restenosis after lowering of plasma homocysteine levels. N Engl J Med. 2001;345:1593–1600. doi: 10.1056/NEJMoa011364. [DOI] [PubMed] [Google Scholar]
- 29.Schnyder G. Pin R. Roffi M. Flammer Y. Hess OM. Association of plasma homocysteine with the number of major coronary arteries severely narrowed. Am J Cardiol. 2001;88:1027–1030. doi: 10.1016/s0002-9149(01)01983-x. [DOI] [PubMed] [Google Scholar]
- 30.Schnyder G. Roffi M. Flammer Y. Pin R. Hess OM. Association of plasma homocysteine with restenosis after percutaneous coronary angioplasty. Eur Heart J. 2002;23:726–733. doi: 10.1053/euhj.2001.2962. [DOI] [PubMed] [Google Scholar]
- 31.Schnyder G. Roffi M. Flammer Y. Pin R. Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss Heart study: A randomized controlled trial. JAMA. 2002;288:973–979. doi: 10.1001/jama.288.8.973. [DOI] [PubMed] [Google Scholar]
- 32.Schnyder G. Roffi M. Flammer Y, et al. Effect of homocysteine-lowering therapy on restenosis after percutaneous coronary intervention for narrowings in small coronary arteries. Am J Cardiol. 2003;91:1265–1269. doi: 10.1016/s0002-9149(03)00281-9. [DOI] [PubMed] [Google Scholar]
- 33.Lonn E. Yusuf S. Arnold MJ, et al. Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. doi: 10.1056/NEJMoa060900. Erratum in N Engl J Med 2006;355:746. [DOI] [PubMed] [Google Scholar]
- 34.Bønaa KH. Njølstad I. Ueland PM, et al. NORVIT Trial Investigators. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 35.McMahon JA. Green TJ. Skeaff CM. Knight RG. Mann JI. Williams SM. A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med. 2006;354:2764–2772. doi: 10.1056/NEJMoa054025. [DOI] [PubMed] [Google Scholar]
- 36.Fuso A. Seminara L. Cavallaro RA. D'Anselmi F. Scarpa S. S-adenosylmethionine/homocysteine cycle alterations modify DNA methylation status with consequent deregulation of PS1 and BACE and beta-amyloid production. Mol Cell Neurosci. 2005;28:195–204. doi: 10.1016/j.mcn.2004.09.007. Erratum in Mol Cell Neurosci 2006;32:419. [DOI] [PubMed] [Google Scholar]