Abstract
Objective
UDP-glucuronosyltransferase (UGT) 2B7 was recently identified as the main enzyme mediating efavirenz N-glucuronidation. In this study we determined whether selected UGT2B7 polymorphisms could be used to enhance the prediction of efavirenz plasma concentrations from CYP2B6 and CYP2A6 genotypes.
Methods
Mid-dose efavirenz plasma concentrations were determined in 94 HIV-infected Ghanaian patients at 2–8 weeks of antiretroviral therapy. CYP2B6 and CYP2A6 genotypes had been previously reported. UGT2B7 exon 2 SNPs c.735A>G (UGT2B7*1c; rs28365062) and c.802C>T (H268Y; UGT2B7*2; rs7439366) were determined by direct sequencing with UGT2B7*1a defined as the reference allele. Relationships between efavirenz plasma concentrations, demographic variables and genotypes were evaluated by univariate and multivariate statistical approaches.
Results
The mean (±SD) mid-dose efavirenz plasma concentration was 3218 (±3905) ng/mL with coefficient of variation of 121%. Independent predictors of efavirenz concentration included CYP2B6 c.516TT genotype (4,030 ng/mL increase; 95% CI, 2,882–5,505 ng/mL, P<0.001), UGT2B7*1a carrier status (475 ng/mL increase; 95% CI, 138–899 ng/mL, P=0.004) and CYP2A6*9 and/or *17 carrier status (372 ng/mL increase; 95% CI, 74–742 ng/mL, P=0.013). Overall, CYP2B6 c.516TT genotype, UGT2B7*1a carrier status and CYP2A6*9 or *17 carrier status accounted for 45.2%, 10.1%, and 8.6% of the total variance, respectively.
Conclusions
Our findings demonstrate independent effects of CYP2A6 and UGT2B7 genetic variation on efavirenz disposition beyond that of the CYP2B6 polymorphisms. The development and testing of a pharmacogenetic algorithm for estimating the appropriate dose of efavirenz should incorporate genotypic data from both the oxidative and glucuronidation pathways.
Keywords: CYP2B6, CYP2A6, UGT2B7, genetic polymorphisms, efavirenz concentration
Introduction
Efavirenz is an essential component of first-line antiretroviral regimen for HIV-infected patients [1, 2], as well as the preferred third drug in patients with tuberculosis (TB) coinfection requiring rifampin-containing therapy [3, 4]. The fixed-dose of 600 mg/day for adults is associated with significant inter-individual variability in plasma concentrations as well as clinical effects [5–7]. The variability in plasma concentrations appears to be even wider during co-administration with rifampin-containing TB therapy [8, 9]. Mid-dose or trough efavirenz plasma concentrations below 1000 ng/mL has been associated with increased risk of virologic failure [6, 7, 10], while concentrations above 4000 ng/mL have been associated with risk of central nervous system side effects [6, 7]. Although, this therapeutic range has not been validated by other investigators [11], the well-known substantial inter-individual variability (>100% coefficient of variation) in efavirenz plasma concentrations after fixed standard dosing has the potential to place some individuals at risk of supra- or sub-therapeutic concentrations.
Efavirenz is oxidized primarily by hepatic CYP2B6 to form 8-hydroxy and 7-hydroxy efavirenz, with minor contributions from CYP3A4/5 and CYP2A6 [12, 13]. The CYP2B6 gene is highly polymorphic and subject to pronounced inter-individual variability in expression and function [14]. It has also been shown that CYP2A6 genetic variation may account for some of the unexplained variability in efavirenz plasma concentrations [15, 16]. Efavirenz also undergoes direct conjugation to form an N-glucuronide [13, 17]. UDP-glucuronosyltransferase (UGT) 2B7 was recently identified as the main UGT isoform responsible for the efavirenz glucuronidation [18]. In previous studies, we investigated the pharmacogenetics of efavirenz including effects of CYP2B6 and CYP2A6 polymorphisms in two cohorts of HIV-infected patients [16, 19]. In this study, we have performed additional exploratory analyses to investigate the potential utility of UGT2B7 genotyping as a means to improve the prediction of efavirenz plasma concentrations over that of the CYP2B6 and CYP2A6 genotypes alone.
METHODS
Study patients
The study included 94 HIV-infected Ghanaian patients who were enrolled between January 2004 and December 2007. The inclusion and exclusion criteria are previously published [16, 19]. HIV-infected patients with or without new TB coinfection, aged at least 18 years old, antiretroviral naïve and CD4 count ≤ 250 cell/μL were prospectively enrolled. Fifty-six patients (60%) had TB coinfection and 48 patients (51%) were receiving rifampin-containing therapy at pharmacokinetic sampling. All patients received efavirenz at 600 mg daily dose plus two nucleoside reverse transcriptase inhibitors. The studies were reviewed and approved by the Institutional Review Boards of the appropriate institutions. A signed informed consent was obtained from all patients prior to enrollment.
Pharmacokinetic sampling
Mid-dose blood samples obtained at weeks 4 and 8 of therapy in one group of 66 patients were included in these analyses. The mean efavirenz concentration at these two timed points was used in the final analysis. Up to the time of pharmacokinetic sampling, adherence as assessed by patient self-reports, pharmacy refill records and pill count was excellent in all except one patient who was not included in the analysis. Mid-dose sampling is frequently used in clinical studies of efavirenz disposition for patient convenience since the drug is invariably taken at bedtime to minimize central nervous system side effects during the day [6, 10]. In the second group of 28 patients, blood samples including a 12-hour sample after observed dosing were obtained at two weeks of antiretroviral therapy. Data from the two studies were combined in order to enhance the statistical power of the analyses. Multivariate analyses (described below) confirmed that the identified genotype associations were independent of the data source (cohort 1 or 2) thereby validating merging of the data sets.
Pharmacokinetic analysis
Efavirenz plasma concentrations were measured using a validated high-performance liquid chromatography (HPLC)/UV method [20]. The laboratory is CLIA certified, and participates in quarterly national and international external proficiency testing.
CYP2B6, CYP2A6 and UGT2B7 genotyping
Subjects were genotyped for CYP2B6 c.516G>T (Q172H, rs3745274), c.983T>C (I328T, rs28399499), CYP2A6 *9B, (g.1836G>T, rs8192726), and CYP2A6 *17 (g.5065G>A, c.1093G>A, M365V, rs28399454) as previously described [16]. Genotypes for the UGT2B7 exon 2 SNPs c.802C>T (H268Y; UGT2B7*2; rs7439366) and c.735A>G (UGT2B7*1c; rs28365062) were determined by genomic PCR amplification and sequencing as previously described with minor modifications [21, 22]. In addition to UGT2B7 c.802C>T (H268Y; UGT2B7*2; rs7439366) being nonsynonymous (H268Y), c.735A>G (UGT2B7*1c; rs28365062) was chosen for this analysis because they allowed discrimination of the 3 most common UGT2B7 alleles that have been identified to date [23, 24]. UGT2B7*1a (reference), UGT2B7*2, and UGT2B7*1c alleles were inferred from the SNP genotype data according to the UGT Allele Nomenclature Committee recommendation (http://www.ugtalleles.ulaval.ca).
Statistical analysis
Statistical analyses were performed using Sigmaplot 11 software (Systat, San Jose, CA). Univariate analyses of effects of patient demographics and enzyme genotypes on efavirenz mid-dose concentrations were assessed by Mann-Whitney rank sum test or by linear regression. A forward stepwise multiple linear regression analysis was used construct a predictive model using patient demographic factors and genotypes as independent variables and log10 efavirenz concentrations as the dependent variable. Efavirenz concentration data was log-transformed to achieve data normality for the multiple regression analysis. A P value < 0.05 was considered significant.
Results
Study population
Of 94 patients, the mean (±SD) age was 39 (±8) years, body weight was 54 (±11) kilograms, and body mass index (BMI) was 19.3 (±3.9). Forty-four patients (47%) were female. The mean (±SD) mid-dose efavirenz plasma concentration was 3218 (±3905) ng/mL with coefficient of variation of 121%. Twenty-one patients (11 receiving rifampin) and nine patients (5 receiving rifampin) had efavirenz concentrations over 4000 ng/mL and under 1000 ng/mL, respectively.
Predictors of efavirenz concentration identified by univariate analysis
In the univariate analysis, only CYP2B6 516TT genotype as well as CYP2A6*9, CYP2A6*9 and/or *17, UGT2B7*1a and *2 carrier status were significantly associated with altered efavirenz plasma concentration (Table 1).
Table 1.
Predictors of mid-dose efavirenz plasma concentration assessed by univariate analysis
| Variable | Efavirenz concentration (ng/mL) |
P value | |
|---|---|---|---|
| Median | IQR | ||
| Agea | --- | --- | 0.719 |
| Weighta | --- | --- | 0.199 |
| BMIa | --- | --- | 0.055 |
| Gender | 0.542 | ||
| F (n = 44) | 1641 | 1252 – 2980 | |
| M (n = 50) | 1752 | 1281 – 4058 | |
| History of alcohol use | 0.045 | ||
| No (n = 67) | 1597 | 1239 – 2714 | |
| Yes (n = 27) | 2325 | 1441 – 5481 | |
| Concurrent rifampin | 0.266 | ||
| No (n = 48) | 1582 | 1272 – 2731 | |
| Yes (n = 46) | 2493 | 1283 – 4281 | |
| CYP2B6 c.516 TT genotype | < 0.001 | ||
| GG/GT (n = 76) | 1528 | 1138 – 2161 | |
| TT (n = 18) | 7568 | 5092 – 10726 | |
| CYP2B6 c.983 TC genotype | 0.346 | ||
| TT (n = 86) | 1681 | 1262 – 3397 | |
| TC (n = 8) | 2515 | 1795 – 3271 | |
| CYP2A6*9 | 0.028 | ||
| Non-carrier (n = 83) | 1597 | 1216 – 3013 | |
| Carrier (n = 11) | 3102 | 1820 – 4521 | |
| CYP2A6*17 | 0.509 | ||
| Non-carrier (n = 71) | 1685 | 1216 – 3097 | |
| Carrier (n = 23) | 1950 | 1347 – 4129 | |
| CYP2A6*9 and/or *17 | 0.044 | ||
| Non-carrier (n = 62) | 1560 | 1126 – 2993 | |
| Carrier (n = 32) | 2162 | 1460 – 4244 | |
| UGT2B7*1a | 0.021 | ||
| Non-carrier (n = 64)b | 1408 | 1111 – 2140 | |
| Carrier (n = 23)b | 1925 | 1421 – 4332 | |
| UGT2B7*1c | 0.310 | ||
| Non-carrier (n = 64)b | 1773 | 1004 – 3645 | |
| Carrier (n = 23)b | 1766 | 1393 – 3250 | |
| UGT2B7*2 | 0.020 | ||
| Non-carrier (n = 40)b | 2306 | 1436 – 5903 | |
| Carrier (n = 47)b | 1562 | 1240 – 2334 | |
Relationship between continuous variables and log10 mid-dose efavirenz plasma concentration were assessed by linear regression. All other variables were assessed by Student’s t-test.
PCR amplification of UGT2B7 exon 2 was not successful in 7 of 94 patients.
Independent predictors of efavirenz concentration identified by multiple linear regression
A forward stepwise multiple linear regression analysis was then performed to identify independent predictors of efavirenz concentration and estimate the contribution of each factor to pharmacokinetic variability. CYP2B6 c.516TT genotype was the first variable to enter the model accounting for a 4,030 ng/mL increase (95% CI, 2,882–5,505 ng/mL, P<0.001) in efavirenz concentration. UGT2B7*1a carrier status was the second variable to enter the model associated with a 475 ng/mL (95% CI, 138–899 ng/mL, P=0.004) increase in efavirenz concentration. CYP2A6*9 and/or *17 carrier status was the last variable to enter the model associated with 372 ng/mL increase (95% CI, 74–742 ng/mL, P=0.013). Other factors examined including CYP2B6 516GG genotype, CYP2B6 983TC genotype, CYP2B6*9 carrier status, CYP2B6*17 carrier status, UGT2B7*1c carrier status, UGT2B7*2 carrier status, rifampin co-administration, age, sex, alcohol use, body weight and BMI were not significantly associated with log10 efavirenz concentration. The final linear regression model was:
Where [EFV] is efavirenz concentration (in ng/mL), CYP2B6 c.516TT genotype (0=GG/GT, 1=TT), UGT2B7*1a carrier status (0=*1a non-carrier, 1=*1a carrier), CYP2A6*9 or *17 (0=*9 or *17 non-carrier, 1=*9 or *17 carrier). The coefficient of determination (R2) from the regression analysis was 0.652 (P<0.001) indicating that 65.2% of the total variance was explained by the model. CYP2B6 c.516TT genotype, UGT2B7*1a carrier status and CYP2A6*9 or *17 carrier status and accounted for 45.2%, 10.1%, and 8.6% of the total variance, respectively.
Gene-gene interactions
It has been proposed that the alternate pathways for efavirenz metabolism (mediated by CYP2A6 or UGT2B7) might only be of importance in individuals with impaired CYP2B6 metabolism [15] (i.e. those with CYP2B6 c.516TT genotype). Consequently we also assessed the possibility of such gene-gene interactions by inclusion of several gene-gene interaction terms (CYP2B6 c.516TT genotype x UGT2B7*1a carrier status and CYP2B6 c.516TT genotype x CYP2A6*9 or *17 variant status) in our multiple linear regression model. However, we could not detect any statistically significant interactions between CYP2B6 c.516TT genotype and UGT2B7*1a carrier status (P=0.325), or between CYP2B6 c.516TT genotype and CYP2A6*9 or *17 variant status (P=0.605) using this approach.
Discussion
The results of this study identify UGT2B7 genetic polymorphism (specifically the UGT2B7*1a allele) as an additional independent predictor of efavirenz plasma concentration beyond that provided by the well studied CYP2B6 c.516G>T polymorphism [25] and the recently identified contribution of several CYP2A6 variants [15, 16]. Importantly, our findings provide the first in vivo evidence supporting a role for UGT2B7 in the metabolism of efavirenz by showing a significant relationship between efavirenz plasma concentrations and UGT2B7 genetic variation.
Consistent with previous reports [19, 26–30], the CYP2B6 516G>T SNP was by far the key determinant of inter-individual variability in efavirenz plasma concentrations in our cohorts, and the effect was observed irrespective of rifampin co-administration. Based on multiple linear regression analysis the CYP2B6 516G>T SNP accounted for as much as 45% of the observed variability in efavirenz concentrations with the UGT2B7*1a allele accounting for a further 10% of variability, and the CYP2A6 slow metabolizing variants explaining another 9% of variability. The resultant regression model also indicates that the presence of the CYP2B6 516TT genotype is associated with an average 345% higher efavirenz concentration with smaller but statistically significant effects from the UGT2B7*1a allele (41% higher efavirenz concentrations) and CYP2A6 slow metabolizing variants (32% higher efavirenz concentrations). The current discoveries of genetic variants associated with altered efavirenz levels in the alternate pathways of efavirenz metabolism have the potential to improve the ability to identify patients who could be treated with effectively reduced or increase efavirenz dose through predictive genetic testing.
We observed over 120% variability in the mid-dose efavirenz plasma concentration in our patients with 32% of them having concentrations outside the presumed therapeutic range. Although the utility of pharmacogenetic data to predict treatment failure with efavirenz is not well studied, CYP2B6 c.516TT genotype has been associated with a higher frequency of CNS side effects [27, 31]. Severe CNS toxicities associated with supra-therapeutic efavirenz concentrations has also been reported in individual with CYP2B6 516TT genotype, most of whom benefited from dose reduction to 200 mg daily, while others required discontinuation of efavirenz [32–34]. In contrast, higher efavirenz doses up to 1600 mg daily were required to achieve desired plasma concentrations, as well as virologic suppression in two patients with no identifiable slow-metabolizing phenotype mutation who were also treated rifampin [35]. Taken together, there may be a role for tailored dosing in some patients and this can be improved by pharmacogenetic prediction of individual’s likelihood to have concentrations outside the therapeutic range.
The main limitation of our study is the somewhat limited number of CYP2B6, CYP2A6 and UGT2B7 SNPs studied as rarer functional SNPs might impact efavirenz clearance. We also did not evaluate the possible contribution of CYP3A4/5 genetic variation to efavirenz concentrations. However, the multivariate modeling suggested that over 60% of the variability in efavirenz concentrations in our population was explained by our genetic data. Antiretroviral therapy is currently a life-long undertaken and optimization of drug regimens will reduce the chances of undesired outcomes such as toxicities or virologic failure. In African populations, CYP2B6 516TT genotype is common and a priori dose reduction based on genetic testing has been proposed to reduce cost and minimize toxicities [28]. Accurate identification of outliers who would benefit from efavirenz dose adjustment at the population level would require a strategy that includes maximized prediction of drug exposure based clinical and genetic factors using a combination of genetic factors [36]. Our findings demonstrate independent effects of CYP2A6, and UGT2B7 genetic variation on efavirenz disposition beyond that due to CYP2B6 polymorphisms. The development and testing of a pharmacogenetic algorithm for estimating the appropriate dose of efavirenz should incorporate genotypic data from both the oxidative and glucuronidation pathways.
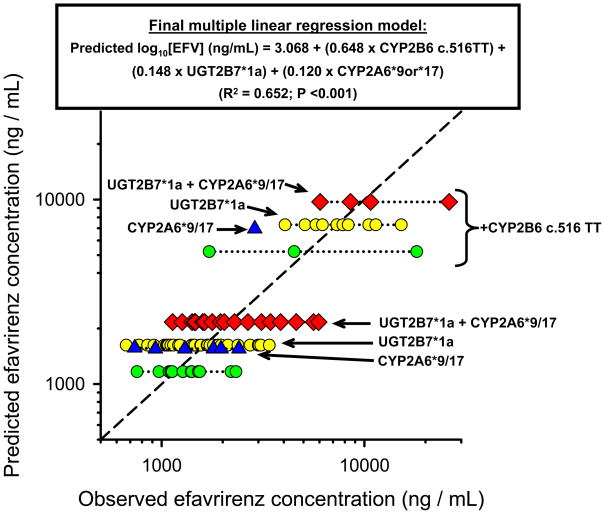
Fig. 1.
Scatter plot showing the relationship between pharmacogenetic-predicted efavirenz mid-dose plasma concentrations (y-axis) and observed concentration (x-axis) in 94 HIV-infected patients. Log10 efavirenz mid-dose plasma concentrations predictions for each subject were made based on their genotype carrier status (CYP2B6 c.516TT, CYP2A6*9 or *17 and/or UGT2B7*1a as indicated by arrows) using the pharmacogenetic algorithm derived by multiple linear regression analysis (model and associated goodness of fit statistics are shown at the top of graph). Log10 efavirenz concentration units are back-transformed into linear units for presentation in the plot.
Acknowledgments
We thank the study participants, the study coordinators, Adjoa Obo-Akwa and Esther Manche as well as the study nurse, Janet May Ayi of Korle Bu Teaching Hospital. This research was funded in part by a 2004 developmental grant from the Lifespan/Tufts/Brown Center for AIDS Research Grant Number (P30AI042853), NIH K23 developmental award (NIH K23 AI071760) to A. Kwara and an ACRiA grant from the Doris Duke Foundation to Dr Lartey. Dr Court was supported by grant R01GM061834 from the National Institute of General Medical Sciences (NIGMS), National Institutes of Health (Bethesda, MD). The University of North Carolina at Chapel Hill, Center for AIDS research #9P30 AI50410, Clinical Pharmacology and Analytical Chemistry Laboratory (CPACL) performed the efavirenz concentrations. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations.
Sources of support: This work was supported in part by Centers for AIDS Research at Lifespan/Brown/Tufts University (P30AI42853), NIH K23 Developmental award (AI071760) from NIAID and grant R01GM061834 from NIGMS.
Footnotes
Authors’ contributions
Study concept and design: Kwara, Lartey, Court.
Conduct of study and acquisition of data: Lartey, Sagoe, Kenu, Kwara, Court.
Analysis and interpretation of data and drafting of manuscript: Kwara, Court
Critical revision of the manuscript for important intellectual content: All authors.
Review of the manuscript: All authors.
References
- 1.DHHS. US Department of Health and Human Services (DHHS) Panel on Antiretroviral Guideline for Adults and Adolescents. [Last accessed July 10, 2009];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2008 November 3; Available at http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 2.Hammer SM, Eron JJ, Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 3.CDC. [Last accessed July 10, 2009];Managing drug interactions in the treatment of HIV-related tuberculosis. [online] 2007 Available from URL: http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm.
- 4.Pozniak AL, Miller RF, Lipman MC, Freedman AR, Ormerod LP, Johnson MA, et al. BHIVA treatment guidelines for tuberculosis (TB)/HIV infection 2005. HIV Med. 2005;6 (Suppl 2):62–83. doi: 10.1111/j.1468-1293.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 5.Csajka C, Marzolini C, Fattinger K, Decosterd LA, Fellay J, Telenti A, et al. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin Pharmacol Ther. 2003;73:20–30. doi: 10.1067/mcp.2003.22. [DOI] [PubMed] [Google Scholar]
- 6.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–75. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 7.Stahle L, Moberg L, Svensson JO, Sonnerborg A. Efavirenz plasma concentrations in HIV-infected patients: inter- and intraindividual variability and clinical effects. Ther Drug Monit. 2004;26:267–270. doi: 10.1097/00007691-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Friedland G, Khoo S, Jack C, Lalloo U. Administration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIV. J Antimicrob Chemother. 2006;58:1299–1302. doi: 10.1093/jac/dkl399. [DOI] [PubMed] [Google Scholar]
- 9.Matteelli A, Regazzi M, Villani P, De Iaco G, Cusato M, Carvalho AC, et al. Multiple-dose pharmacokinetics of efavirenz with and without the use of rifampicin in HIV-positive patients. Curr HIV Res. 2007;5:349–353. doi: 10.2174/157016207780636588. [DOI] [PubMed] [Google Scholar]
- 10.Langmann P, Weissbrich B, Desch S, Vath T, Schirmer D, Zilly M, Klinker H. Efavirenz plasma levels for the prediction of treatment failure in heavily pretreated HIV-1 infected patients. Eur J Med Res. 2002;7:309–314. [PubMed] [Google Scholar]
- 11.Leth FV, Kappelhoff BS, Johnson D, Losso MH, Boron-Kaczmarska A, Saag MS, et al. Pharmacokinetic parameters of nevirapine and efavirenz in relation to antiretroviral efficacy. AIDS Res Hum Retroviruses. 2006;22:232–239. doi: 10.1089/aid.2006.22.232. [DOI] [PubMed] [Google Scholar]
- 12.Desta Z, Saussele T, Ward B, Blievernicht J, Li L, Klein K, et al. Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics. 2007;8:547–558. doi: 10.2217/14622416.8.6.547. [DOI] [PubMed] [Google Scholar]
- 13.Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther. 2003;306:287–300. doi: 10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]
- 14.Lang T, Klein K, Fischer J, Nussler AK, Neuhaus P, Hofmann U, et al. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics. 2001;11:399–415. doi: 10.1097/00008571-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 15.di Iulio J, Fayet A, Arab-Alameddine M, Rotger M, Lubomirov R, Cavassini M, et al. In vivo analysis of efavirenz metabolism in individuals with impaired CYP2A6 function. Pharmacogenet Genomics. 2009;19:300–309. doi: 10.1097/FPC.0b013e328328d577. [DOI] [PubMed] [Google Scholar]
- 16.Kwara A, Lartey M, Sagoe KW, Rzek NL, Court MH. CYP2B6 (c. 516G-->T) and CYP2A6 (*9B and/or *17) polymorphisms are independent predictors of efavirenz plasma concentrations in HIV-infected patients. Br J Clin Pharmacol. 2009;67:427–436. doi: 10.1111/j.1365-2125.2009.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mutlib AE, Chen H, Nemeth GA, Markwalder JA, Seitz SP, Gan LS, Christ DD. Identification and characterization of efavirenz metabolites by liquid chromatography/mass spectrometry and high field NMR: species differences in the metabolism of efavirenz. Drug Metab Dispos. 1999;27:1319–1333. [PubMed] [Google Scholar]
- 18.Belanger AS, Caron P, Harvey M, Zimmerman PA, Mehlotra RK, Guillemette C. Glucuronidation of the antiretroviral drug efavirenz (EFV) by UGT2B7 and an in vitro investigation of drug-drug interaction with zidovudine (AZT) Drug Metab Dispos. 2009 doi: 10.1124/dmd.109.027706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwara A, Lartey M, Sagoe KW, Xexemeku F, Kenu E, Oliver-Commey J, et al. Pharmacokinetics of efavirenz when co-administered with rifampin in TB/HIV co-infected patients: pharmacogenetic effect of CYP2B6 variation. J Clin Pharmacol. 2008;48:1032–1040. doi: 10.1177/0091270008321790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rezk NL, Crutchley RD, Yeh RF, Kashuba AD. Full validation of an analytical method for the HIV-protease inhibitor atazanavir in combination with 8 other antiretroviral agents and its applicability to therapeutic drug monitoring. Ther Drug Monit. 2006;28:517–525. doi: 10.1097/00007691-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Court MH, Krishnaswamy S, Hao Q, Duan SX, Patten CJ, Von Moltke LL, Greenblatt DJ. Evaluation of 3′-azido-3′-deoxythymidine, morphine, and codeine as probe substrates for UDP-glucuronosyltransferase 2B7 (UGT2B7) in human liver microsomes: specificity and influence of the UGT2B7*2 polymorphism. Drug Metab Dispos. 2003;31:1125–1133. doi: 10.1124/dmd.31.9.1125. [DOI] [PubMed] [Google Scholar]
- 22.Kwara A. Interindividual variability in pharmacokinetics of generic nucleoside reverse transcriptase inhibitors in TB/HIV-Coinfected Ghanaian patients; UGT2B7* 1c is associated with facter zidovudine clearance and glucuronidation. J of Clin Pharmacol. doi: 10.1177/0091270009338482. published on July 23, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holthe M, Rakvag TN, Klepstad P, Idle JR, Kaasa S, Krokan HE, Skorpen F. Sequence variations in the UDP-glucuronosyltransferase 2B7 (UGT2B7) gene: identification of 10 novel single nucleotide polymorphisms (SNPs) and analysis of their relevance to morphine glucuronidation in cancer patients. Pharmacogenomics J. 2003;3:17–26. doi: 10.1038/sj.tpj.6500139. [DOI] [PubMed] [Google Scholar]
- 24.Saeki M, Saito Y, Jinno H, Tanaka-Kagawa T, Ohno A, Ozawa S, et al. Single nucleotide polymorphisms and haplotype frequencies of UGT2B4 and UGT2B7 in a Japanese population. Drug Metab Dispos. 2004;32:1048–1054. [PubMed] [Google Scholar]
- 25.Telenti A, Zanger UM. Pharmacogenetics of anti-HIV drugs. Annu Rev Pharmacol Toxicol. 2008;48:227–256. doi: 10.1146/annurev.pharmtox.48.113006.094753. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandran G, Hemanth Kumar AK, Rajasekaran S, Kumar P, Ramesh K, Anitha S, et al. CYP2B6 G516T polymorphism but not rifampin coadministration influences steady-state pharmacokinetics of efavirenz in human immunodeficiency virus-infected patients in South India. Antimicrob Agents Chemother. 2009;53:863–868. doi: 10.1128/AAC.00899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas DW, Ribaudo HJ, Kim RB, Tierney C, Wilkinson GR, Gulick RM, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18:2391–2400. [PubMed] [Google Scholar]
- 28.Nyakutira C, Roshammar D, Chigutsa E, Chonzi P, Ashton M, Nhachi C, Masimirembwa C. High prevalence of the CYP2B6 516G-->T(*6) variant and effect on the population pharmacokinetics of efavirenz in HIV/AIDS outpatients in Zimbabwe. Eur J Clin Pharmacol. 2008;64:357–365. doi: 10.1007/s00228-007-0412-3. [DOI] [PubMed] [Google Scholar]
- 29.Rotger M, Tegude H, Colombo S, Cavassini M, Furrer H, Decosterd L, et al. Predictive value of known and novel alleles of CYP2B6 for efavirenz plasma concentrations in HIV-infected individuals. Clin Pharmacol Ther. 2007;81:557–566. doi: 10.1038/sj.clpt.6100072. [DOI] [PubMed] [Google Scholar]
- 30.Tsuchiya K, Gatanaga H, Tachikawa N, Teruya K, Kikuchi Y, Yoshino M, et al. Homozygous CYP2B6 *6 (Q172H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz-containing regimens. Biochem Biophys Res Commun. 2004;319:1322–1326. doi: 10.1016/j.bbrc.2004.05.116. [DOI] [PubMed] [Google Scholar]
- 31.Rotger M, Colombo S, Furrer H, Bleiber G, Buclin T, Lee BL, et al. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics. 2005;15:1–5. doi: 10.1097/01213011-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Gatanaga H, Hayashida T, Tsuchiya K, Yoshino M, Kuwahara T, Tsukada H, et al. Successful efavirenz dose reduction in HIV type 1-infected individuals with cytochrome P450 2B6 *6 and *26. Clin Infect Dis. 2007;45:1230–1237. doi: 10.1086/522175. [DOI] [PubMed] [Google Scholar]
- 33.Gatanaga H, Oka S. Successful genotype-tailored treatment with small-dose efavirenz. AIDS. 2009;23:433–434. doi: 10.1097/QAD.0b013e32831940e3. [DOI] [PubMed] [Google Scholar]
- 34.van Luin M, Brouwer AM, van der Ven A, de Lange W, van Schaik RH, Burger DM. Efavirenz dose reduction to 200 mg once daily in a patient treated with rifampicin. AIDS. 2009;23:742–744. doi: 10.1097/QAD.0b013e32832914a3. [DOI] [PubMed] [Google Scholar]
- 35.Cabrera SE, Cordero M, Iglesias A, Valverde MP, Dominguez-Gil A, Garcia MJ. Efavirenz-rifampicin interaction: therapeutic drug monitoring to efavirenz dosage optimization in HIV/TBC patients. AIDS. 2008;22:2549–2551. doi: 10.1097/QAD.0b013e3283189c07. [DOI] [PubMed] [Google Scholar]
- 36.Arab-Alameddine M, Di Iulio J, Buclin T, Rotger M, Lubomirov R, Cavassini M, et al. Pharmacogenetics-based population pharmacokinetic analysis of efavirenz in HIV-1-infected individuals. Clin Pharmacol Ther. 2009;85:485–494. doi: 10.1038/clpt.2008.271. [DOI] [PubMed] [Google Scholar]