Abstract
We evaluated the immunocompetence of human T cells in humanized NOD-scid IL2r-γ-null (Hu—NSG) mice bearing a human thymic organoid, after multilinegage reconstitution with isogeneic human leukocytes. Delayed type hypersensitivity (DTH) response was assessed by a direct footpad challenge of the immunized hu-NSG host, or by transfer of splenocytes from immunized hu-NSG, along with antigen, into footpads of CB17 SCID mice [trans-vivo (tv) DTH]. Both methods revealed cellular immunity to tetanus toxoid (TT) or collagen type V (ColV). Immunohistochemical analysis of the swollen footpads revealed infiltration of human CD45+ cells, including CD3+ T cells, CD68+ macrophages and murine Ly6G+ neutrophils. We observed a significant correlation between % circulating human CD4+ cells and the direct DTH swelling response to TT. The tvDTH response to TT was inhibited by anti-IFNγ, while the tvDTH response to collagen V was inhibited by anti IL-17 antibody, mimicking the cytokine bias of adult human T cells to these antigens. Hu-NSG mice were also capable of mounting a B cell response (primarily IgM) to TT antigen. The activation of either Th1- or Th17 - dependent cellular immune response supports the utility of Hu-NSG mice as a surrogate model of allograft rejection and autoimmunity.
Keywords: Humanized mice, T cell response, Delayed Type Hypersensitivity, Trans-vivo Delayed Type Hypersensitivity, Th1 and Th17 response
Introduction
The utility of humanized mouse models in human immunobiology research depends on their ability to mimic a functional human immune system. Previous models have often fallen short due to the lack of strong HLA-restricted, T cell-mediated immune responses. The recently developed NOD/SCID/γc/KO (NSG) mouse, exhibits enhanced ability to support the engraftment of human hematopoietic stem cells (HSCs) [1–4]. The NSG mouse lacks mature lymphocytes, NK cells, and yet is relatively long-lived, permitting long-term studies of engraftment, infection, and immunity. Garcia [2, 5–6] and Yang [2, 5–6] have successfully generated humanized mice by transplanting human fetal liver HSCs into a NOD/SCID/γc/KO [NSG] mouse previously implanted with fetal thymic and liver tissues from the same donor. Both groups observed long-term, systemic human T cell homeostasis along with the systemic repopulation with human B cells, monocytes/macrophages, and dendritic cells (DC) in the recipient mice. The success of this model has been attributed to the generation of a human thymic organoid, derived from fetal tissue implanted underneath the kidney capsule, that permits the selection of developing T cells on autologous human Class I and Class II MHC antigen presenting molecules [2, 5–6]. T cells play a central role in the development of immune response. A crucial step in T-cell-mediated response is the recognition of antigen presenting cells and recruitment of antigen specific T cells to the respective target organ. The ability of humanized mice to mediate T cell–dependent responses has been modestly characterized in the NSG mice. The presence of cellular immune responses against Epstein-Barr virus (EBV) toxic shock syndrome toxin-1 (TSST-1).and HIV antigens has been documented by several groups [6–7]. Here we have address the immunocompetence of the engrafted human T cells by eliciting a delayed-type hypersensitivity (DTH) response to both Th-1 [Tetanus Toxoid/TT] and Th-17 [Collagen V/col(V)] antigens [8].
A DTH response is an in vivo index of cell-mediated immune response induced by antigen specific T memory cells. An antigen is introduced to the host in the priming phase, and then after re-challenge subcutaneously or intradermally, the DTH reaction is initiated when antigens are presented by antigen-presenting cells (APCs) to sensitized memory T cells in the draining lymph node and at the challenge site. T-cell activation elicits an influx of macrophages, monocytes, and lymphocytes at the site of antigen exposure resulting an induration and erythema between 24–48 hours post injection. A positive swelling reaction typically requires immunization 2–4 weeks prior to DTH testing; after the antigen challenge, a transient swelling usually peaks at 24–48 h. The DTH reaction is termed direct if the swelling response is recorded after subcutaneous or intradermal antigen injection into the primed individual. An alternative method for DTH testing involves the transfer of peripheral blood mononuclear cells plus antigen from the primed individual into the pinnae or footpads of naive mice. This indirect or “trans-vivo” (tv) DTH response is elicited by the localized human immune reaction and the recruitment and activation of murine PMNs, which produces a measurable swelling response within 24 hrs [9][26]. Minimally, the interaction of memory T cells with antigen-pulsed dendritic cells (DC) is sufficient to initiate a swelling response [26], while the interaction of Treg cells with DC is sufficient to prevent it [27]. Because of its exquisite sensitivity to detect antigen-specific regulatory and effector T cells, the tvDTH assay has proven useful for monitoring the immune status of clinical transplant recipients [12,17,18].
In humans the DTH response to a recall antigen such as TT involves the activation of MHC class II restricted CD4 T memory cells, as well as antigen presenting cells [APC] at the site of antigen injection. After encountering the antigen-loaded APC, a few antigen-specific T cells recognize their cognate MHC/peptide ligand, become activated, and secrete cytokines: IFN-γ and IL-2 for a Th-1 type response [10–11] and 1L-17 or IL-22 for Th-17 type responses [12]. The release of proinflammatory cytokines and chemokines secreted by the activated T cells, APC, and resident endothelial cells provide the amplification signals which recruit neutrophils, monocytes and more T cells, both antigen-specific and non-specific, to the site of inflammation. This leads to a second wave of amplification, disrupting the collagen bundles of the dermis 12–18 hours, and leading to a peak swelling response between 24–48 hrs after antigen challenge.
The most stringent test of a functional, well-integrated, cell mediated immune system is the direct DTH reaction. Although the DTH reaction in humans has been characterized histologically, the timing and key steps of the cellular interactions are only partially characterized in humans, limiting our mechanistic understanding. Rodent models have greatly expanded our knowledge of various cellular reactions; unfortunately, not all findings in animal studies are successfully translated to clinical settings. Immunological studies for transplantation have proven particularly difficult due in part to differences between murine and human immune systems. Hence there is a need for better predictive and reliable models to characterize the human immune responses to vaccines, autoantigens and in organ-transplant recipients, to donor alloantigens. The humanized NSG mouse model, which retains mouse innate immunity, but in which the adaptive immune system of the mouse has been replaced by a fetal human one, may be useful for investigating the mechanisms of human allograft rejection and to test the effect of novel immunosuppressive therapies. However this will work only to the extent that the human adaptive and mouse innate immune systems can collaborate effectively. In the present study we have demonstrated the ability of “humanized” NSG mice to mount both direct and indirect DTH responses to two different type of antigens, validating the use of this model for analysis of human T-dependent cell-mediated immunity.
Materials and Methods
Animals and human fetal tissues: Immunodeficient nonobese diabetic/severe combined immunodeficient (NOD/SCID/γ C/KO) mice were purchased from Jackson Labs (catalogue 005557) and used at 6 to 10 weeks of age.
Human fetal thymus and liver tissues of gestational age of 17 to 20 weeks were obtained from Advanced Bioscience Resource (Alameda, CA), and Albert Eistein Medical College ( New York, NY) . The recipient mice were conditioned with sublethal (2–3 Gy) whole-body irradiation and implanted with fetal Thy and Liv fragments under the recipient kidney capsule after irradiation. The mice also received an intravenous injection of purified CD34+isolated from the same fetal liver by the magnetic-activated cell sorter (MACS) separation system (Miltenyi Biotec, Auburn, CA). The purity of the injected CD34+ cells was at least 80–90%.
Levels of human hematopoietic cells in the mice that underwent transplantation were determined by multicolor flow cytometric (FCM) analysis using various combinations of the following antibodies: Antibodies used to detect specific human markers were as follows: pan CD45 (clone HI30); CD4 (clone RPA-T4 or OKT4); CD8α (clone RPA-T8); CD14 (clone M5E2); CD19 (clone HIB19); CD11c (clone S-HCL-3); CD123 (clone 9F5); pan HLA class I (clone W6/32); pan HLA class II (clone LN3); CD3 (clone SPVT-3b). Negative control antibodies were clone P3 (IgG1) and clone UPC10 (IgG2a). Antibodies were purchased from commercial sources directly conjugated to fluorescent dyes. For flow cytometric analysis, tissues (i.e. bone marrow, spleen, liver, blood, thymic organoid) were collected from mice 12–20 weeks after implantation of human cells. Single cell suspensions were prepared from solid tissues by gentle homogenization followed by filtration through a 0.45 µm strainer. Samples were subject to ACK lysis to remove red blood cells, or mononuclear cells were purified by density gradient centrifugation using Hisotopaque (Sigma). FACS analysis was performed on a FACScalibur (Becton Dickinson, Mountain View, CA). Dead cells were excluded from the analysis.
Histology
Tissues were fixed in 10% buffered formalin and embedded in paraffin (for hematoxylin and eosin [H&E] staining). Immunohistochemistry was performed on spleen, lymphnodes, thymic organoid, liver and footpad sections using polyclonal rabbit anti–human CD3 CD20, CD4, CD8, CD68 antibodies (DAKO). Sections were deparaffinized and rehydrated with water. Antigen retrieval was performed using rodent decloaker (Biocare Medical) with sections subsequently incubated with primary Abs: CD4, CD8, CD45 (Biocare Medical), CD20 & Ly6G (BD Pharmingen), CD3 & CD68 (Dako). Antibodies were detected using a mouse on mouse HRP-polymer kit (Biocare Medical) and visualized with DAB.
ELISA for human Immunoglobulins (IgM and IgG) quantification was performed using human IgM and human IgG enzyme-linked immunosorbent assay (ELISA) kits (Bethyl Laboratories, Montgomery, TX) according to the manufacturer‘s instructions . For the TT specific ELISA , microwells were coated with purified 10µg/ml TT(Sanofi-Aventis Pasteur) in PBS overnight at 4°C , then blocked with 2% bovine serum albumin for 1 hr, and washed 3× in PBS/.05%Tween-20. Serum samples were applied at varying dilutions to each well for 1 hr; after additional 3× wash in PBS/Tween, human IgM or IgG were detected using HRP-coupled isotype-specific secondary antibodies, 3× washes and addition of the chromogenic substrate o-phenylenediamine(0.55 mg/ml) and 0.1% H2O2 for 5–10 minutes, followed by 1 M H2SO4.
Tetanus/Diphtheria immunization
Mice were immunized with 1.5 limits of flocculation (lf) of pediatrics tetanus toxoid and diphtheria (TT/DT) vaccine (Adventis-Pasteur) subcutaneously in the inguinal pouch region.
Collagen V immunization
Mice were immunized with 100ug of bovine collagen V (col V) (Gift from Dr. David Wilkes) in Complete Fruend‘s Adjuvant (Sigma) subcutaneously in the inguinal pouch region. Two-three weeks post immunization mice were tested for a response to collagen V either directly (direct DTH assay) or splenocytes were collected and used in the trans-vivo DTH assay.
Direct DTH
Mice previously immunized to TT/DT and/or col V were monitored for a response by injecting .25lf TT/DT+PBS or 20ug of bovine col V+PBS subcutaneously into the right hind footpad. To control for swelling caused by the injection itself, PBS alone was injected into the left hind footpad. Changes in footpad swelling were measured 24 hours post injection using a dial-thickness gauge. Pre-injection measurements were subtracted from post-injection measurements to obtain specific swelling values. DTH reactivity is shown as the change in thickness, using units of 10−4 inches.
Trans-vivo DTH
For all TV- DTH assays, 4–8 × 106 splenocytes were injected into footpads of naïve CB17.SCID recipients along with co-injection inoculum. DTH reactivity was measured as the change in footpad thickness 24 hours post-injection over the pre-injection reading using a dial-thickness gauge and swelling is expressed in 10-4 inches. Co-injection inoculums include: PBS, 20µg donor antigen, 0.25lf TT/DT (Aventis-Pasteur Inc.) vaccine, 20µg col V,10µg anti-IFN-γ (BD Biosciences), 10µg anti-IL-1β (eBioscience), 10µg anti-TNF-α (eBiosciences), and 10µg anti-IL-17A (BD Biosciences)
Results
Multi-lineage human hematopoietic reconstitution NOD/SCID/γC/KO mice via implantation of human Thy/Liv fragments along with CD34+ cells
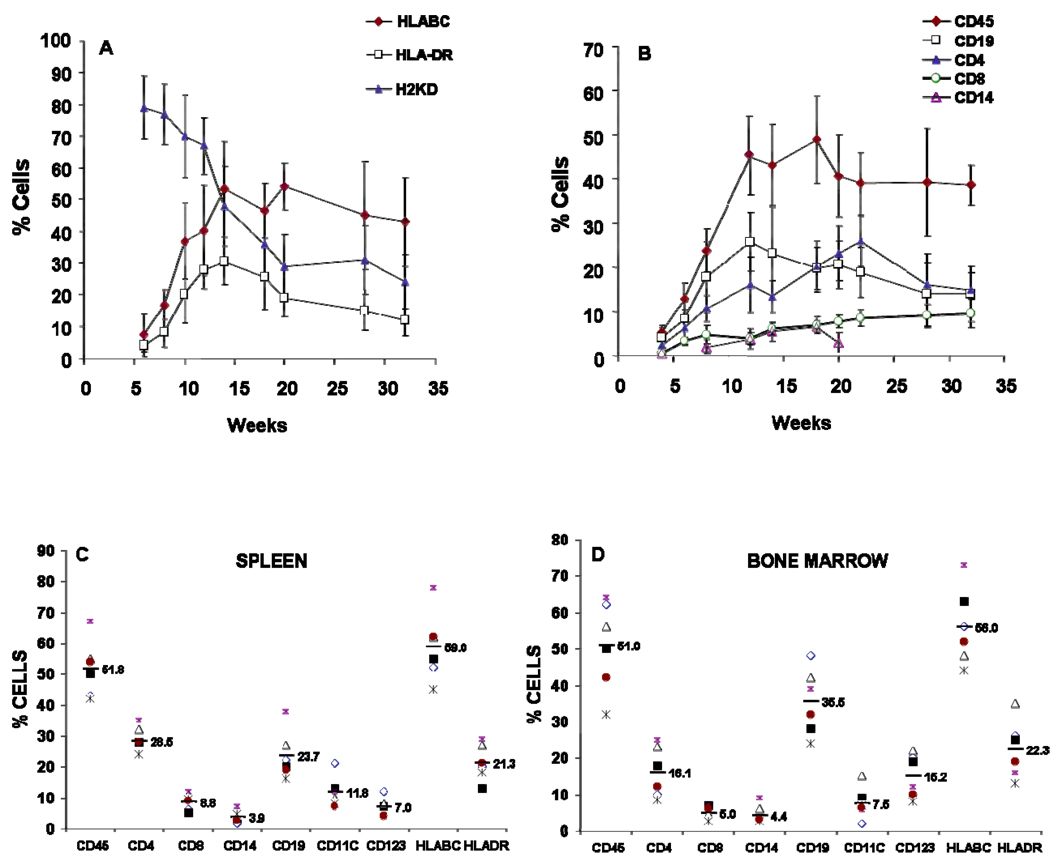
Humanized-mice were generated by implanting human fetal thymus and liver under the kidney capsule of 6–8 week old NSG mice, followed by injection of autologous CD34+ cells as described elsewhere (5). Typically we recovered sufficient cells and tissue to reconstitute 6–18 mice per human donor. Flow cytometry analysis was performed to detect the presence of the circulating human and murine cells in the peripheral blood of mice from 8–28 weeks post injection. The results (Figure 1A) showed an increase in the proportion of human cells in circulation (HLA-ABC+) from 7% at 6 weeks to >50% at 14–30 weeks. HLA class II+ (HLA-DR+) cells increased from 8 to 30% from 6–14 weeks, gradually declining to 15% at 30 weeks post engraftment. The human cell engraftment was accompanied by a decline in the levels of murine class I+ (H2-KdDd) expressing cells from 80% to 24% between weeks 6 and 32. A variable portion of mice, ≤5% of total, developed up to 90% human HLA-ABC+ circulating cells, along with signs of graft-versus host [GVH] disease [data not shown]; these mice were sacrificed after 22 weeks. These exceptionally high % HLA-ABC+ values are not included in Fig.1A.
Figure 1.
Quantification of engraftment in peripheral blood of NOD/SCID/γC/KO mice. Peripheral blood was collected from reconstituted mice at the indicated times after treatment and stained with anti-human specific antibodies for human pan HLABC, human Class II HLADR, Murine H2KD (A), human CD45 CD3 CD4 CD8 and CD19 (B). by flow cytometry. Each data point in Figures A and B represents the average n (`~30) ± SE values. Cells isolated from spleen (C) and bone marrow (D) from mice 12 week post implantation of the human tissues and were stained for the presence of human T cells (CD4, CD8), B cells (CD19), monocytes (CD14) and dendritic cells defined as lineage-negative and HLA-DR positive cells expressing either CD123 or CD11c. Data presented in C and D are obtained from six mice obtained from a single donor. FACS analysis was performed on a FACScalibur (Becton Dickinson, Mountain View, CA). ). FACS analysis was performed on a FACS calibur (Becton Dickinson, Mountain View, CA). Dead cells were excluded from the analysis.
The peripheral blood of the hu-NSG mice showed multi-lineage engraftment by human CD45+ cells (Figure 1B). B cells [CD19+] appeared rapidly and were the most abundant lymphocyte subset at 12 wks. T cells (CD4+ and CD8+) gradually overtook the B cells, surpassing them at 18 weeks (27% T vs. 20% B). We also observed a low level of circulating CD14+ monocytes (2–6%) and CD56+ NK cells (< 1%; data not shown) between 12 and 20 wks after reconstitution.
Evidence of muilti-lineage engraftment was also evident in the spleen (Figures 1C) and bone marrow (Figure 1D). Human CD45+ cells comprised around 50% of both spleen and bone marrow cells at 12 wks. Subset analysis of human CD45+ cells present in the marrow revealed the presence of CD34+ stem/progenitor cells [data not shown], CD14+ monocytes, CD19+ B cells and T cells (CD4+ and CD8+). B cells were relatively more abundant than T cells in the hu-NSG bone marrow at 12 weeks; the reverse (T>B) was true in spleen. Importantly, professional antigen presenting cells (APC), including CD14+ monocytes, and both myeloid (CD11c+) and plasmacytoid (CD123+) dendritic cells (DC) were present in both spleen and bone marrow. The relative proportions of pDCs and mDCs varied between spleen and bone marrow, with the highest proportion of pDC being found in the hu-NSG spleen.
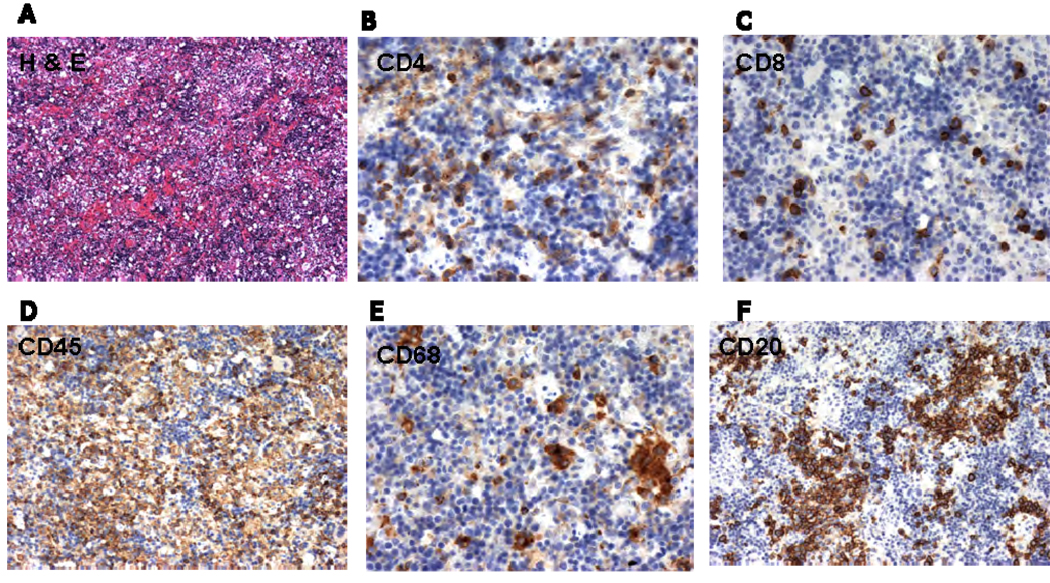
Representative immunohistochemical analyses depicting the histology of human cells in the spleen are shown in Figures 2A–2F. Note (Figure 2D) that human CD45+ leukocytes were interspersed with CD45neg (presumably murine) cells. CD4 (Figure 2B) and CD8 (Figure 2C) T cells are found scattered throughout the spleen, whereas phagocytic cells (CD68+) (Figure 2E) and B cells (Figure 2F) tended to be more clustered within the splenic pulp.
Figure 2.
Human reconstitution in the spleen was confirmed by Hematoxylin and Eosin staining (A) followed by Immunoperoxidase staining of human cells in sections of spleens 12 wk after engraftment. Presence of human, CD4 (B), CD8 (C), CD45 (D), CD68 (E), CD20 (F were observed in the spleen sections.
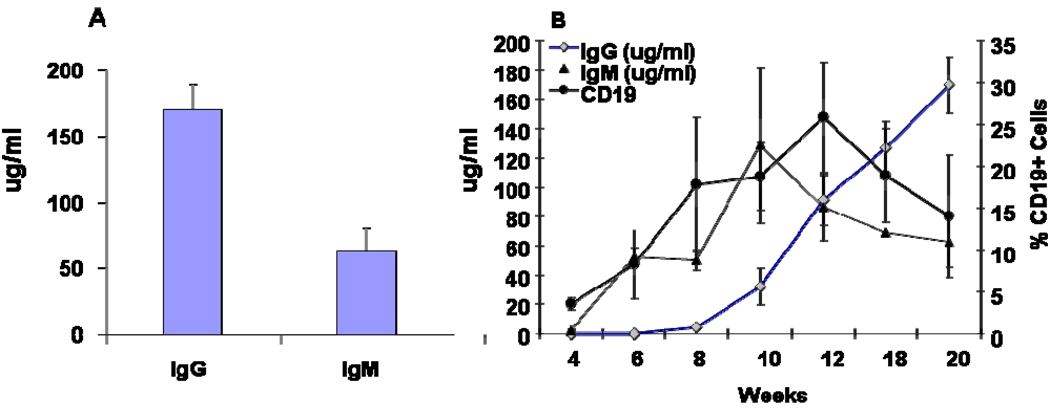
To determine whether engrafted human B cells were functional, we quantified the human immunoglobulins (Ig) in the serum of hu-NSG mice reconstituted with cells from several different fetal donors. At 12–14 weeks, the average serum levels of total human IgM and human IgG were 55 µg/ml and 165 µg/ml, respectively (Figure 3A). The timing of the appearance of the serum IgG and IgM in relation to circulating B cells was determined in a subset of mice at different time points from 4–24 weeks (Figure 3B). B cells (CD19+) appeared at 4 wks in peripheral blood, and was soon followed by the appearance of serum IgM at 6 weeks. Human IgG was not detectable until 8 weeks, and increased steadily through the observation period (to 20 weeks). To determine if antigen-specific B cell responses could be elicited, hu-NSG mice were immunized with tetanus toxoid (TT) in alum adjuvant at 12 weeks post engraftment and subsequently given a booster dose of the antigen by footpad injection [DTH assay] at 14 wks. Serum analysis of 2× immunized mice at 16 wks did not reveal anti-TT antibody [data not shown]. However at 24 wks, 10 wks after the TT booster injection, anti-TT human IgM was now detected in 3/3 hu-NSG mice(Table1).The amount was roughly 10% of the anti-TT IgM level found in an adult human serum, based on OD vs. serum titration curve analysis [data not shown] . Anti-TT IgG was found in 1/3 mice tested, at the lower limit of detection (Table1). Consistent with a long-lived B cell response to antigen, the sera of the same 3 hu-NSG mice contained human B cell activating factor (BAFF), a TNF family protein essential for B cell survival, at levels comparable to that found in a normal adult human serum (Table 1).
Figure 3.
Production of human immunoglobulin in NOD/SCID mice: Serum from mice from different groups was collected 12–14 weeks post engraftment and tested for the presence of human immunoglobulin IgG and IgM by ELISA (A). In a separate group of mice,(B) the serum was collected at different time points after human tissue/cell transplantation and the presence of human IgM and IgG were quantified by ELISA and correlated with the expression of CD19 positive circulating cells. Data are pooled from several different hu-NSG groups.
Table 1.
Serum anti-TT specific antibody in 24 week reconstituted hu-NSG mice, 10 weeks after priming and boosting with TT
| Hu-NSG mice | Adult HumanControl | |
|---|---|---|
| TT specific IgM (OD value) | 0.23, 0.34, 0.49 *1 | 0.83*2 |
| TT specific IgG (µg/ml) | 0.03, 0, 0 | 648.85 |
| BAFF in serum (pg/ml) | 48.2, 19.6, 31.8 | 67.0 |
Mouse serum was diluted at 1:100.
Human plasma was diluted at 1:160.
Analysis of cell types within the thymic organoid
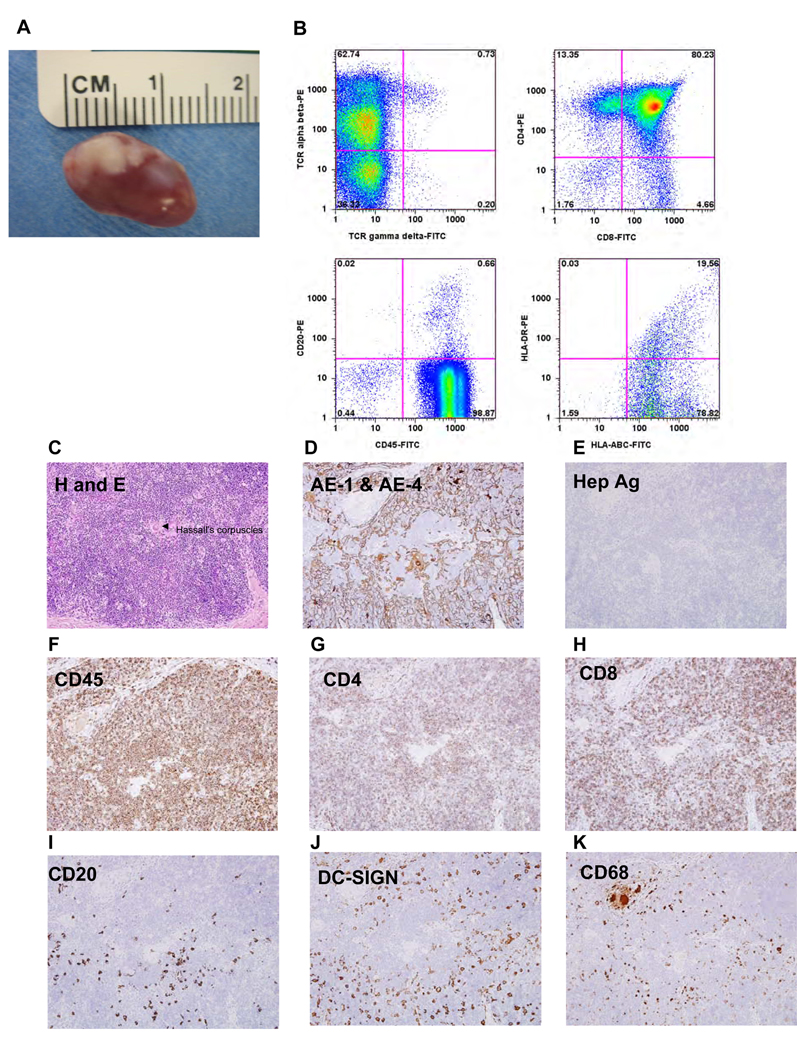
A thymic organoid was generated by co-injection of human thymus and liver fragments under the mouse kidney capsule. Figure 4A shows the gross appearance of the kidney at 12 weeks, the white area indicating the thymus organoid. Flow cytometric analysis of the thymic organoid revealed the presence of human MHC class I+ cells, some of which co-expressed class II HLA-DR (Figure 4B, lower right panel). The organoid also contained human T cells, the majority being immature CD4+/CD8+ double positive thymocytes (upper right panel , Fig. 4B) along with a low number of CD20+ B cells (Figure 4B, lower left). The majority of thymic cells were conventional TCRαβ+ T cells (>60% of total), a low % of which co-stained for TCRγδ; a few (≤1%) were purely TCRγδ + TCRαβ− (Figure 4B, upper left). When we analyzed the human thymic organoid (Figure 4C–4K) by immunohistochemistry, we found a clear separation of thymus from mouse kidney parenchyma and both a dense cortex and a sparsely populated medullary region, along with areas resembling the Hassal‘s corpuscles seen in human (but not murine) thymus [arrow, Fig 4C]. We observed CD4+ and CD8+ T cells in the cortex (Figures 4G, 4H) as well as some B cells (Figure 4I), DC-SIGN+ dendritic cells (Figure 4J), and CD68+ macrophages (Figure 4K). The DC-SIGN+ cells were confined to the cortical region; however, CD1a+ DC were present in the medulla (data not shown). Human CD45+ cells were observed in both the cortex and medullary regions (Figure 4F). The organoid contained thymic stromal protein AE-1/4 (Figure 4D) indicating abundant thymic epithelium; however the organoid was devoid of mature hepatocytes , as indicated by absence of hepatocyte surface antigen (Hep Ag) staining(Figure 4E).
Figure 4.
Analysis of human thymic organoid: Gross Macroscopic image of the human thymic organoid dissected from the kidney 12 weeks post engraftment (A). Representative flow cytometric data showing the presence of human MHC class I (HLABC), MHC class II (HLA-DR), T cells (CD4, CD8, TCR alpha beta, and B cells (CD19). Hematoxylin and eosin (H&E) of the thymic organoid revealing the presence of hassal‘s corpuscles (C) Immunohistochemical analysis of the thymic organoid using human specific antibodies. for thymic stromal antigen (AE-1/AE-4) (D), hepatocyte specific antigen (HSA) (E), CD45 (F) ,CD4 (G),CD8 (H), CD20 (I), DC-SIGN(J) and CD68 (H). All images are shown at a magnification of 200×.
Function of Human T cells assessed by delayed-type hypersensitivity (DTH)
Although human thymopoiesis can be achieved by transplantation of human CD34+ cord blood cells into neonatal BALB/c-RAG-2null or NOD/SCID null newborn mice, these models, which require human T cells to mature in the mouse thymus, have generated suboptimal human T-cell immunocompetence [13–15]. Because the autologous thymic organoid supplies all the immunogenetic components [for ex., HLA-A,B,C and DR antigens, Fig 4B] necessary for normal human T cell development, the adaptive immune system of the hu-NSG mice ought to provide a rigorous test to see if one could elicit an antigen-specific human T cell-mediated immune response in vivo.
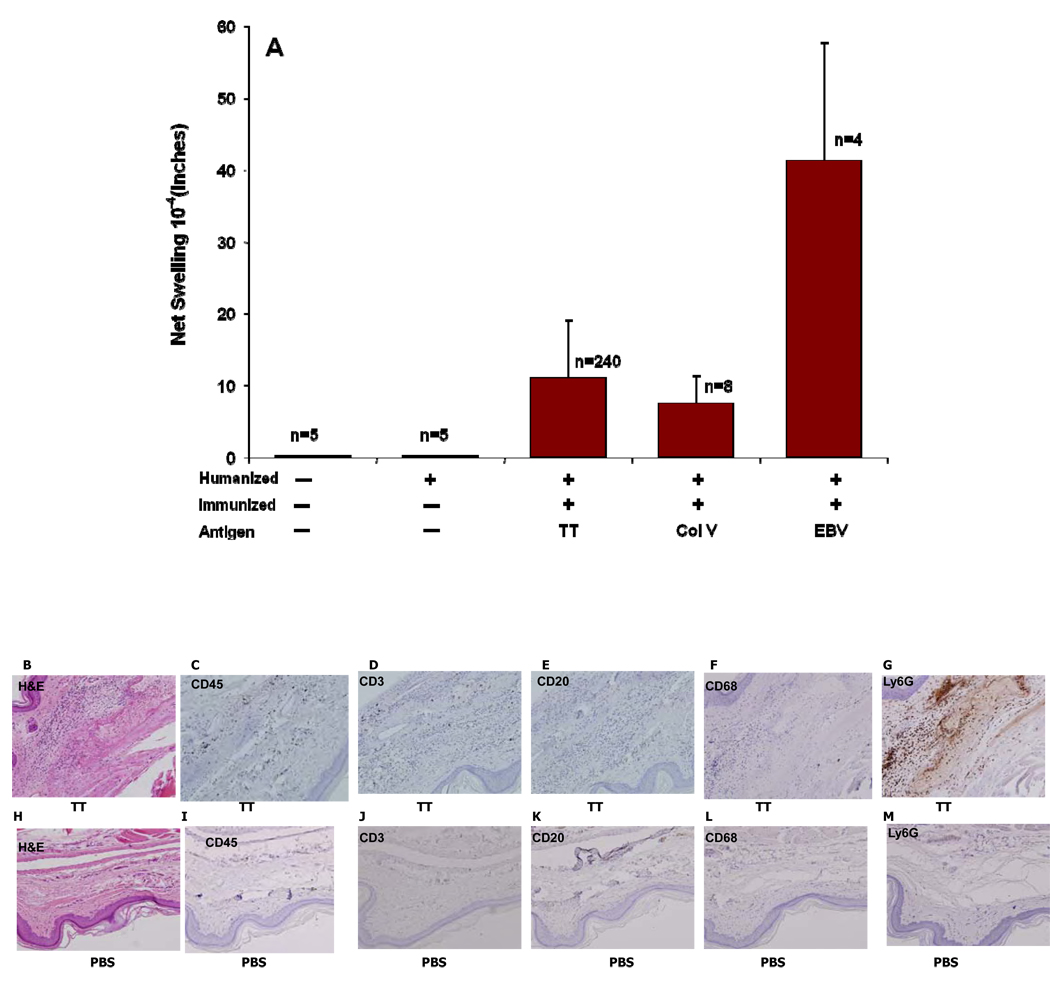
Twelve weeks after reconstitution, humanized NSG mice were either left untreated, or immunized with tetanus toxoid (TT) in alum [n=240 mice]; alternatively, some mice [n=8] were immunized with a “self” antigen, collagen type V (col V), administered in complete Freund‘s adjuvant. Two weeks later, the direct DTH reaction [16] was assessed by measuring change in footpad thickness 24 hrs after challenge with antigen alone, in the absence of adjuvant. As shown in Figure 5A, we observed a variable DTH response to each antigen. Control non-humanized mice, and humanized mice that were not immunized with the relevant antigen prior to challenge, had no swelling response at 24 hrs. after footpad challenge.
Figure 5.
DTH assay: The mice were immunized using 0.25 lf Tetanus Toxoid/Diphtheria Toxoid (TT/DT) vaccine in alum, Collagen V in CFA. Mice were tested for the response to the antigens in a direct DTH assay two weeks later. The swelling responses to the antigens after subtracting the change in the PBS injected are compiled in A. After recording the response, the footpads were harvested and stained during heamtoxylin and eosin (B, H) murine Ly6G antigens(G,M) and human specific CD45 (C,I), CD3 (D,J), CD68 (F,L), CD20(E,K). B–F represent the negative response to PBS and H to M represent the DTH response to TT antigen. All images are shown at a magnification of 200×.
By immunohistochemistry, we found increased recruitment of human CD45+ cells scattered in the subcutaneous tissue in the footpads injected with TT as compared to PBS control (compare Figure 5C vs. Figure 5I). CD3+ T cells were clearly present among the CD45+ human cells recruited to the TT challenge site (compare Fig 5D vs. 5J), as were CD68 macrophages (compare Fig 5F vs. 5L), whereas CD20+ B cells were absent (Fig 5E). Influx of murine PMNs (Ly6G+) cells was also observed at the site of injection with TT but not in the PBS control footpad (compare Fig 5G vs. 5M).
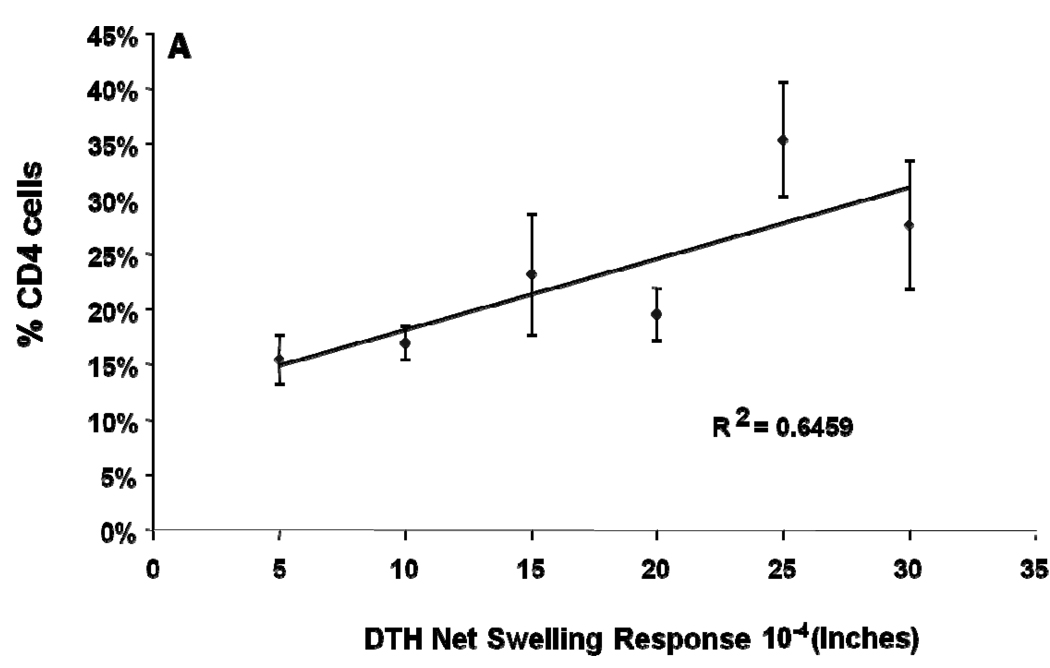
The results for anti-TT direct DTH shown in Figure 5A is based on 240 separate determinations with >30 different fetal HSC and thymus/liver donors. Table 2 ranks the anti-TT footpad swelling responses in 234 of the NSG mice in relation to subset composition of human leukocytes in peripheral blood. We found that while >75% of immunized hu-NSG mice had a DTH response to TT after a single immunization; the level of anti-TT DTH response was variable. However, there was a significant (r=0.64; p=0.04) correlation of direct DTH response to TT with the % of CD4+ T cells at the time of immunization (Table 1 and Figure 6). The % of circulating CD8, CD19, HLA-DR, and total CD45/HLA-ABC at 12 weeks post reconstitution did not correlate with the DTH response.
Table 2.
Mean PBMC composition [% of total cells] of hu-NSG mice immunized and challenged with TT, sorted according to footpad DTH assay swelling
| Number of Mice* |
Δ value of TT DTH (in ×10−4) |
%CD19 | %CD4 | % CD45 | %CD8 | %HLA-ABC | %HLA-DR |
|---|---|---|---|---|---|---|---|
| 53 | 0 | 18 | 16 | 42 | 6 | 36 | 17 |
| 29 | 5 | 23 | 15 | 35 | 4 | 35 | 18 |
| 78 | 10 | 19 | 17 | 38 | 5 | 37 | 21 |
| 12 | 15 | 23 | 23 | 52 | 6 | 35 | 10 |
| 49 | 20 | 20 | 20 | 42 | 6 | 36 | 15 |
| 3 | 25 | 6 | 35 | 50 | 15 | 55 | 8 |
| 10 | 30 | 9 | 28 | 40 | 9 | 47 | 12 |
Out of total of 234 hu-NSG mice tested for direct DTH response to TT
Figure 6.
DTH assay statistics: Data from Table 2 are plotted, showing the relationship between the percentage of circulating human CD4 T cells and response to TT challenge in the footpad at 12 weeks post engraftment. Linearity/r2 and p values are shown.
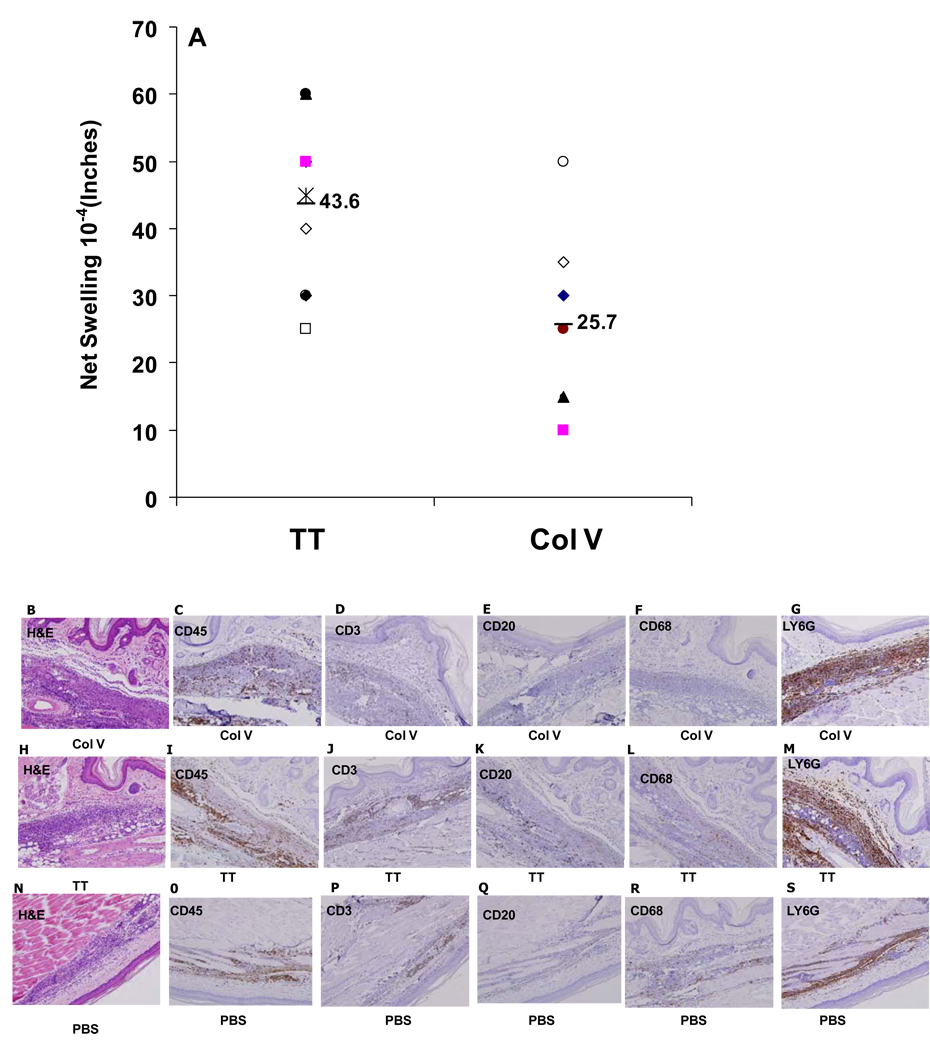
The direct DTH reaction requires antigen uptake by recirculating APC, followed by trafficking to lymphoid tissue, and trafficking of primed T cells to the challenge site, where accessory cell recruitment occurs. To determine the mechanisms involved in T cell-mediated immune function of the humanized- NSG mice, we used the trans-vivo DTH (tv-DTH) assay. This local adoptive transfer assay measures the ability of antigen-specific ‘primed’ T cells, when co-injected with antigen and APC, to elicit a recall response to antigen. It circumvents the requirement for T cell trafficking, since the contact between the T cells and antigen, presented by DCs and macrophages begin immediately after footpad injection in the subcutaneous tissue [17]. Splenocytes were harvested from two hu-NSG mice following immunization and rechallenge in the footpad. Co-injection of each hu-NSG splenocyte sample along with TT or Collagen V, induced a measurable swelling reaction in the adoptive host, CB17-SCID mouse footpad. Fig 7A shows the individual swelling responses of each hu-NSG mouse tested, as well as the mean tv-DTH responses to both antigens. The mean tv-DTH swelling responses to TT and col V were higher than the corresponding direct DTH response levels (compare Fig 7A vs. Fig. 5A). Immunohistochemical analysis of the tvDTH footpads revealed the presence of human CD45+ cells [Figures 7C, 7I and 70], including both CD3+ T cells [Figures 7D, 7J and 7P] and CD68+ macrophages Figures 7F, 7L and 7R] in TT-, col V-, or PBS control –challenged footpads. Not surprisingly, given the adoptive transfer of hu-NSG splenocytes, human CD20+ B cells [Fig. 7E, 7K ,7Q] and mouse Ly6G+ polymorphonuclear leukocytes(PMNs; Fig 7G,M, S)) were also seen. Note however that mouse PMN infiltration was far more intense in footpads of mice injected with TT or Col V antigen as compared with a control footpad injected with cells plus PBS only. In contrast to the restricted area within the subcutaneous tissue occupied by mouse PMNs in a control footpad [Fig 7S], mouse PMNs penetrated out of the subcutaneous fat layer and into the dermal and muscular layers in footpads co-injected with cells plus priming antigen (Figures 7G & 7M ).
Figure 7.
Tv DTH response in humanized mice: Splenocytes from humanized mice immunized with TT or Collagen V antigens were isolated and injected along with TT or Collagen V antigens in to the footpads CB17.SCID mice. The net DTH response was recorded after subtracting the PBS controls. After recording the response, the footpads were harvested and stained during heamtoxylin and Eosin (B, H, N) murine Ly6G antigens (G, M,S) human specific CD45 (C,I,O) CD3 (D, J,P) , CD20 (E,K,Q) and CD68 (F, L R) B,-G represent the Tv-DTH response to ColV, H to M represent the DTH response to TT antigen.N to S represent the DTH response to PBS. All images are shown at a magnification of 200×.
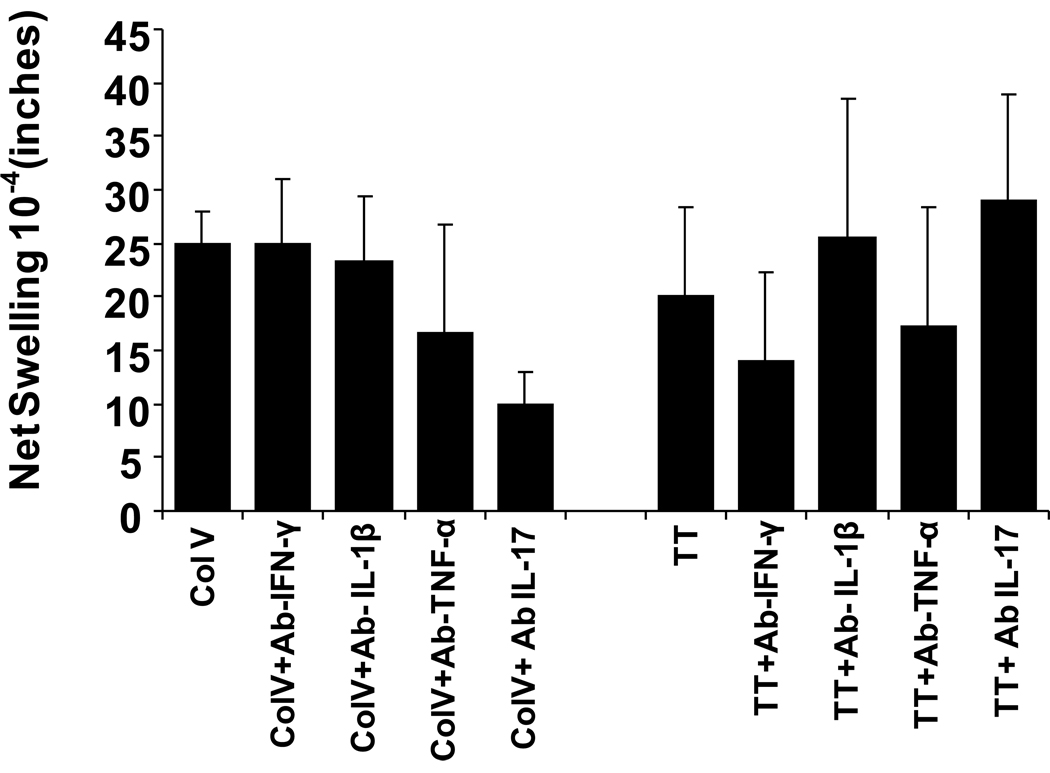
To identify the mechanism involved in the swelling reactions, we performed the tv-DTH assay in the presence of antibodies to specific human cytokines. As shown in Figure 8, the tv-DTH response to collagen V was markedly inhibited by antibody to IL-17, while antibody to IFNγ did not alter the swelling response. In contrast, the tv-DTH response to TT was not inhibited at all by antibodies to human IL-17 but was reduced by anti- human IFNγ antibody. These results were similar to the patterns of cytokine dependence seen in tvDTH responses to these same antigens with PBMC from lung transplant recipients [18].
Figure 8.
TV-DTH was performed by co-transfer of splenocytes pooled from n=4 humanized NOD/SCID mice, along with antigen used for priming, into the footpads of CB17SCID mice as described previously. Control values of swelling from transfer of hu-NSG splenocytes alone, w/o antigen, were subtracted to obtained net swelling values. The TV-DTH assay was performed using a mixture of cells , tetanus and diphtheria toxoid (TT/DT) or Collagen V, along with co injection of antibodies to human IFNγ, TNF-α, IL-1β , or IL-17. Values shown are mean ± SD of n=3 determinations.
Discussion
Complex biological processes require in vivo analysis, and important research advances have been obtained using mice as a model system for the study of many biological problems. However, mice are not humans, and the study of human immunobiology in vivo is limited by ethical and technical constraints. Humanized mice have been developed to overcome these limitations and have become an important research tool for systematic in vivo studies to address important questions relevant to human immunology. Recently, Lepus et al. [19] were able to generate a DTH reaction to KLH in the ear and footpad of antigen-primed NSG mice that were humanized at birth by intrahepatic injection of human CD34+ HSCs. While these results were encouraging, no quantitative analysis of the swelling reactions was reported. In the absence of a human thymic organoid to restrict T cell development to autologous HLA molecules, it is unlikely that such mice, whose T cells mature in a murine thymus, can develop a fully HLA-restricted T cell repertoire necessary to respond to human-restricted viruses[2, 5–6]. These mice in the present study had stable multi-lineage engraftment 10–12 weeks post engraftment and a well developed thymic organoid, complete with Hassal‘s corpuscles characteristic of human thymus. Despite the very early stage of development of the human immune system [still “pre-natal” in total age] the ability to generate antigen-specific humoral [IgM] and cellular [DTH] responses confirms the functionality of a “blended” immune system, consisting of a remnant population of approximately 40% murine, primarily innate immune leukocytes, and 60% T cells, B cells, myeloid, NK and dendritic cells of human origin. Importantly, the hu-NSG mice were able to mount cell-mediated immune responses to both a Th1-type (TT) and a Th17 type (col V) antigen. We have recently found that these antigens elicit similar Th1 and Th17-type polarized responses in both humans [18] and immunized C57BL/6 mice (M. Dart, A. David , and W. Burlingham , manuscript in preparation), suggesting that this polarization may be a fundamental property of the mammalian cellular immune response to these particular antigens. To the extent that innate immune mechanisms may direct the T cell response into a Th1 or Th17 pathway, the commonality between human and mouse DTH responses could explain why the response to TT and col V elicited a Th1 and Th17 response in the hu-NSG, even though the innate and adaptive arms of the immune system come from 2 different species.
The pattern of cytokine dependence of tv-DTH response to col V in the hu-NSG mice was similar to that seen in col V-sensitized humans and mice, with one interesting exception. In human tv-DTH using PBMC from col V-reactive lung transplant recipients and patients with idiopathic pulmonary fibrosis [12, 18] , besides a requirement for human IL17, there was a pronounced dependence of the swelling reaction upon human IL1β as well as TNFα. We concluded from analysis of the cellular requirements for this response that monocyte production of IL1β and TNFα was a critical downstream response to the IL17 produced by CD4 col V reactive Th-17 cells. The lack of IL1β dependence of the tv-DTH response in the col V- immunized NSG suggests that a) monocytes/macrophages in the spleen of the hu-NSG mice do not play any role in the Th-17 response as was seen with human PBMC [18]or b) that mouse , and not human monocytes/macrophages present in the hu-NSG spleen play this key accessory role.
As noted above, the direct DTH assay is a relatively high level test of the function of the human adaptive immune system. It also requires establishment and maintenance of a memory lymphocyte pool. Once naïve T cells recognize antigenic peptides in proper MHC [HLA] context, they will clonally expand and give rise to a population of T memory cells specific for the antigen. Upon rechallenge, it must mobilize these T cells along with accessory cells in response to antigen in the tissues. Without a human thymus organoid, T cells of neonatal NSG mice reconstituted with CD34+ human HSCs mediated a suboptimal DTH response to TNBS sensitization and ear challenge; exogenous recombinant human IL-7, a T cell survival factor, was required for optimal strength of response [20]. While we did not test for IL-7 levels in our model, the serum levels of human BAFF, which specifically promotes proliferation and survival of activated B cells [21] were near to that found in normal human serum [Table 1]. This may account for the ability to detect anti-TT IgM responses in hu-NSG mice 10 weeks after immunization [Fig 3.C]. The lack of antigen-specific IgG responses indicates that the hu-NSG mouse, at least at 24 weeks post-reconstitution, still lacks optimal conditions for germinal center formation and Ig class switch, despite adequate BAFF levels.
In summary, the response to a known Th-1 antigen, TT, both at the B cell[IgM] and T cell [direct DTH] level, and the response to a known Th-17 antigen, col(V), indicate that T helper cells educated in the autologous human thymus organoid are functional and provide an adequate level of immunocompetence for study of a wide range of human immunologic processes.
Acknowledgements
MERC foundation of Wisconsin Madison (Grant number 133HG98) awarded to Dr. Burlingham provided the major funding for this research. We also thank Dr. Debra Bloom for performing human BAFF analysis, and Drs. Wm Weidanz, Jenny Gumperz, Shannon Kenney, Clive Svensen, and Tim Kamp for their support in the development of the model
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7(2):118. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 2.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, Garcia JV. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12(11):1316. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 3.Larochelle A, Vormoor J, Lapidot T, Sher G, Furukawa T, Li Q, Shultz LD, Olivieri NF, Stamatoyannopoulos G, Dick JE. Engraftment of immune-deficient mice with primitive hematopoietic cells from beta-thalassemia and sickle cell anemia patients: implications for evaluating human gene therapy protocols. Hum Mol Genet. 1995;4(2):163. doi: 10.1093/hmg/4.2.163. [DOI] [PubMed] [Google Scholar]
- 4.Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, McKenna S, Mobraaten L, Rajan TV, Greiner DL, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154(1):180. [PubMed] [Google Scholar]
- 5.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108(2):487. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 6.Wege AK, Melkus MW, Denton PW, Estes JD, Garcia JV. Functional and phenotypic characterization of the humanized BLT mouse model. Curr Top Microbiol Immunol. 2008;324:149. doi: 10.1007/978-3-540-75647-7_10. [DOI] [PubMed] [Google Scholar]
- 7.Yajima M, Imadome KI, Nakagawa A, Watanabe S, Terashima K, Nakamura H, Ito M, Shimizu N, Honda M, Yamamoto N, Fujiwara S. A New Humanized Mouse Model of Epstein-Barr Virus Infection That Reproduces Persistent Infection, Lymphoproliferative Disorder, and Cell-Mediated and Humoral Immune Responses. J Infect Dis. 2008 doi: 10.1086/590502. [DOI] [PubMed] [Google Scholar]
- 8.Lopez M, Aguilera R, Perez C, Mendoza-Naranjo A, Pereda C, Ramirez M, Ferrada C, Aguillon JC, Salazar-Onfray F. The role of regulatory T lymphocytes in the induced immune response mediated by biological vaccines. Immunobiology. 2006;211(1–2):127. doi: 10.1016/j.imbio.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Burlingham WJ, Jankowska-Gan E. Mouse strain and injection site are crucial for detecting linked suppression in transplant recipients by trans-vivo DTH assay. Am J Transplant. 2007;7(2):466. doi: 10.1111/j.1600-6143.2006.01627.x. [DOI] [PubMed] [Google Scholar]
- 10.Laub R, Dorsch M, Meyer D, Ermann J, Hedrich HJ, Emmrich F. A multiple transgenic mouse model with a partially humanized activation pathway for helper T cell responses. J Immunol Methods. 2000;246(1–2):37. doi: 10.1016/s0022-1759(00)00288-x. [DOI] [PubMed] [Google Scholar]
- 11.Riemer AB, Klinger M, Wagner S, Bernhaus A, Mazzucchelli L, Pehamberger H, Scheiner O, Zielinski CC, Jensen-Jarolim E. Generation of Peptide mimics of the epitope recognized by trastuzumab on the oncogenic protein Her-2/neu. J Immunol. 2004;173(1):394. doi: 10.4049/jimmunol.173.1.394. [DOI] [PubMed] [Google Scholar]
- 12.Bobadilla JL, Love RB, Jankowska-Gan E, Xu Q, Haynes LD, Braun RK, Hayney MS, Munoz del Rio A, Meyer K, Greenspan DS, Torrealba J, Heidler KM, Cummings OW, Iwata T, Brand D, Presson R, Burlingham WJ, Wilkes DS. Th-17, monokines, collagen type V, and primary graft dysfunction in lung transplantation. Am J Respir Crit Care Med. 2008;177(6):660. doi: 10.1164/rccm.200612-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legrand N, Weijer K, Spits H. Experimental model for the study of the human immune system: production and monitoring of "human immune system" Rag2−/−gamma c−/− mice. Methods Mol Biol. 2008;415:65. doi: 10.1007/978-1-59745-570-1_4. [DOI] [PubMed] [Google Scholar]
- 14.Kuruvilla JG, Troyer RM, Devi S, Akkina R. Dengue virus infection and immune response in humanized RAG2(−/−)gamma(c)(−/−) (RAG-hu) mice. Virology. 2007;369(1):143. doi: 10.1016/j.virol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Berges BK, Wheat WH, Palmer BE, Connick E, Akkina R. HIV-1 infection and CD4 T cell depletion in the humanized Rag2−/−gamma c−/− (RAG-hu) mouse model. Retrovirology. 2006;3:76. doi: 10.1186/1742-4690-3-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black CA. Delayed type hypersensitivity: current theories with an historic perspective. Dermatol Online J. 1999;5(1):7. [PubMed] [Google Scholar]
- 17.VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, Chin T, Kusaka S, Geissler F, Pelletier RP, Orosz CG. Human allograft acceptance is associated with immune regulation. J Clin Invest. 2000;106(1):145. doi: 10.1172/JCI9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, Gopalakrishnan B, Cai J, Brand DD, Yoshida S, Cummings OW, Wilkes DS. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117(11):3498. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lepus CM, Gibson TF, Gerber SA, Kawikova I, Szczepanik M, Hossain J, Ablamunits V, Kirkiles-Smith N, Herold KC, Donis RO, Bothwell AL, Pober JS, Harding MJ. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/gammac−/−, Balb/c-Rag1−/−gammac−/−, and C.B-17-scid/bg immunodeficient mice. Hum Immunol. 2009;70(10):790. doi: 10.1016/j.humimm.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unsinger J, McDonough JS, Shultz LD, Ferguson TA, Hotchkiss RS. Sepsis-induced human lymphocyte apoptosis and cytokine production in "humanized" mice. J Leukoc Biol. 2009;86(2):219. doi: 10.1189/jlb.1008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt MR, Appel MC, Giassi LJ, Greiner DL, Shultz LD, Woodland RT. Human BLyS facilitates engraftment of human PBL derived B cells in immunodeficient mice. PLoS One. 2008;3(9):e3192. doi: 10.1371/journal.pone.0003192. [DOI] [PMC free article] [PubMed] [Google Scholar]