Abstract
Objective
Previous positron emission tomography (PET) imaging studies have demonstrated that cocaine dependence is associated with a decrease in dopamine type 2 and 3 (D2/D3) receptor binding in cocaine-dependent individuals relative to healthy comparison subjects. However, given the nature of PET imaging, it is possible that the measured decrease in radiotracer binding results from an increase in baseline dopamine levels. The purpose of this study was to measure D2/D3 receptors following acute dopamine depletion in cocaine-dependent volunteers relative to healthy comparison subjects.
Method
Cocaine-dependent volunteers (N=15) and healthy matched comparison subjects (N=15) were scanned using PET, with the dopamine receptor radiotracer [11C]raclopride, at baseline and again following acute depletion of endogenous dopamine via alpha-methyl-para-tyrosine (AMPT) administration. Changes in radiotracer binding were measured in the subdivisions of the striatum (caudate, putamen, and ventral striatum) in addition to the striatum as a whole.
Results
Findings revealed that cocaine-dependent volunteers exhibited lower levels of endogenous dopamine relative to comparison subjects, which was measured as an increase in [11C]raclopride binding following AMPT administration. The increase in [11C]raclopride binding in the striatum was 11.1% (SD=4.4%) in healthy comparison subjects and 5.7% (SD=5.9%) in cocaine-dependent volunteers. Similar differences were seen in the subdivisions of the striatum.
Conclusions
The decrease in striatal D2/D3 receptors associated with cocaine dependence cannot be attributed to higher levels of endogenous dopamine.
Positron emission tomography (PET) and radiotracers selective for the dopamine type 2 and 3 receptors (D2/D3) can be used to measure differences in receptor binding in the human brain. Using this technology and the radiotracer [11C]raclopride, previous studies have reported that cocaine dependence is associated with a decrease in D2/D3 binding in cocaine-dependent individuals relative to healthy comparison subjects (1, 2).
However, PET radioligands do not image receptors occupied by endogenous levels of neurotransmitters, and previous studies have shown that acute depletion of endogenous dopamine increases radiotracer binding. The reduction in endogenous dopamine increases the percentage of receptors available to bind to the radiotracer by reducing the pool of receptors occupied by dopamine. A paradigm has been developed to acutely deplete dopamine using the drug alpha-methyl-para-tyrosine (AMPT), which inhibits tyrosine hydroxylase and reduces endogenous levels of dopamine in the brain (3). Based on this paradigm, AMPT has been used in both PET and single photon emission computed tomography (SPECT) studies to image the percent of D2/D3 receptors occupied by endogenous dopamine, and occupancies ranging from 9% to 28% have been reported in comparison subjects (3–6).
Only one previous study, conducted by Abi-Dargham et al. (4), has measured levels of endogenous dopamine at the D2/D3 receptors in a psychiatric population, which showed that schizophrenia patients exhibited a higher level of dopamine relative to comparison subjects. Abi-Dargham et al. demonstrated that AMPT administration resulted in a 19% increase in [123I]iodobenzamide binding in subjects with schizophrenia relative to only a 9% increase in matched healthy comparison subjects. Importantly, no difference in D2/D3 receptor binding potential was seen between these two groups prior to dopamine depletion. However, after AMPT administration, schizophrenia subjects were found to have higher binding potential (11%) relative to comparison subjects. Thus, the study demonstrated that between-group differences in D2/D3 receptor binding potential can be masked by differences in the level of endogenous dopamine occupying the D2/D3 receptors.
In light of the finding by Abi-Dargham et al., it is imperative to consider the possibility that the decrease in [11C]raclopride binding potential seen in cocaine-dependent subjects could result from higher levels of endogenous dopamine. Previous studies have demonstrated that subjects with cocaine dependence have a 12% lower [11C]raclopride binding potential on average relative to matched comparison subjects (1, 2). However, it is possible that differences in the levels of endogenous dopamine may have contributed to this observation. Since the radioligand is administered at tracer doses, differences in the occupancy of D2/D3 receptors by endogenous dopamine between subjects would translate directly into differences in apparent binding potential of the same relative magnitude.
In order to test the possible contribution of receptor occupancy by endogenous dopamine to the observed difference in [11C]raclopride binding potential between cocaine-dependent subjects and comparison subjects, we used [11C]raclopride to measure D2/D3 receptors before and after acute dopamine depletion with AMPT in a group of cocaine-dependent subjects (N=15) and matched healthy comparison subjects (N=15).
Method
The present study was approved by the New York State Psychiatric Institute Institutional Review Board, and all participants provided written informed consent. The cocaine-dependent volunteers were 25 to 45 years old and medically healthy and fulfilled DSM-IV criteria for cocaine dependence, with no other current axis I diagnosis. In addition, they were not seeking treatment but were informed that a referral for treatment was available. Healthy comparison subjects were between the ages of 25 and 45 years old and had no current or past DSM-IV axis I disorder.
Fifteen cocaine-dependent subjects (13 men/two women; mean age: 39 years [SD=6 years]) and 15 healthy comparison subjects (13 men/two women; mean age: 39 years [SD=5 years], p= 0.8) were enrolled in the study. Subjects were matched for 1) ethnicity (healthy comparison subjects: African American [N=13], Caucasian [N=2]; cocaine-dependent subjects: African American [N=13], Caucasian [N=1], Hispanic [N=1]) and 2) cigarette smoking (healthy comparison subjects smoked 10 cigarettes per day [SD=4] and included three nonsmokers; cocaine-dependent subjects smoked 11 cigarettes per day [SD=5] and included two non-smokers). Cocaine-dependent subjects reported smoking crack cocaine an average of 13.4 years (SD=6.4 years) and spending $196 (SD=$73) weekly over the past 6 months.
All subjects were scanned using PET, with the dopamine receptor radiotracer [11C]raclopride, at baseline and again following AMPT administration. Subjects were admitted to the Irving Center for Clinical Research for the administration of AMPT. Cocaine-dependent subjects were admitted 14 days prior to the baseline PET and underwent random urine toxicology tests to confirm cocaine abstinence. Healthy comparison subjects were admitted prior to the first dose of AMPT. The dose of AMPT was administered using a sliding scale, adjusted to a participant's weight, as follows: 59–75 kg=1,000 mg per dose; 76–92 kg=1,250 mg per dose; 93–115 kg=500 mg per dose. Eight doses of AMPT were administered every 6 hours beginning 49 hours prior to the second PET (post-AMPT) scan, with the last dose administered 1 hour prior to the second scan. Since AMPT can produce crystalluria, all subjects 1) received sodium bicarbonate, 1,300 mg daily, 2) were required to drink 4 liters of water every 24 hours, and 3) underwent daily urinalysis of AMPT administration. A frequent side effect of AMPT is extrapyramidal symptoms. These symptoms were assessed daily using the Extrapyramidal Symptom Rating Scale (7), a scale designed to detect drug-induced movement disorders.
PET Scans
[11C]Raclopride was administered as a bolus with constant infusion, and PET scans were acquired using ECAT EXACT HR+ (Siemens/CTI, Knoxville, Tenn.) in a three-dimensional mode of eight 5-minute-duration frames (obtained 40 to 80 minutes after the initial [11C]raclopride bolus), as previously described (8). A 10-minute transmission scan was obtained prior to acquisition of the emission data. All participants underwent two PET scans with [11C]raclopride (one at baseline and one following AMPT administration). Four venous samples were analyzed to obtain the plasma concentration of nonmetabolized [11C]raclopride (μCi/ml), as described in a previous report (8). A plasma sample for analysis of homovanillic acid levels was obtained for each scan 20 minutes prior to injection and assayed by gas chromatography as previously described (4).
Receptor availability of D2/D3 was estimated for [11C]raclopride nondisplaceable binding potential (BPND), which was defined using the following equation (regional tissue distribution volume=VT [ml/cm3]; region of interest=ROI; cerebellum=CER; fND=free fraction in nonspecific distribution brain volume; Bmax= concentration of D2/D3 receptors [nmol per gram of tissue]; KD= inverse of the affinity of the radiotracer for the receptor [see reference 9 for details]):
The cerebellum was used as the reference region. The regional tissue distribution volume for the cerebellum was measured for each condition in order to assess the effect of AMPT on nonspecific binding between groups, as described elsewhere (8). The free fraction of [11C]raclopride in the plasma was compared across conditions and groups.
The percent occupancy of D2/D3 receptors as a result of endogenous dopamine was calculated as the percent change in nondisplaceable binding potential (%ΔBPND), using the following equation:
Region of Interest Analysis
Image analysis was performed using MEDx (Sensor Systems, Inc., Sterling, Va.). Each subject underwent magnetic resonance imaging (MRI), acquired using a GE Signa EXCITE 3T/94-cm scanner (GE Medical Systems, Milwaukee). Regions of interest were drawn from each subject's MRI, and both motion correction and PET-MRI registration were performed as described elsewhere (8, 9). The striatum was divided into the caudate, putamen, and ventral striatum as described in a previous report (10). Briefly, the ventral striatum was identified using set landmarks, and the caudate and putamen were further subdivided along their rostral-caudal axes using the anterior commissure. The following regions of interest were derived: ventral striatum, which includes the nucleus accumbens and ventral portions of the caudate and putamen; precommissural dorsal caudate; precommissural dorsal putamen; postcommissural caudate; and postcommissural putamen. This method of subdividing the striatum was developed to reflect the functional input to the striatum in several ways. First, the ventral striatum receives input from the limbic brain regions. Second, the pre- and postcommissural caudate and precommissural putamen largely receive input from the associative cortex. Third, the postcommissural putamen is largely involved in sensorimotor processing (see reference 10 for details). Activity from the right and left regions were averaged together, and a weighted average (weighted by subregion volume) was used to derive non-displaceable binding potential for the striatum as a whole.
Statistical Analysis
Group demographic comparisons were performed with unpaired t tests. Differences in [11C]raclopride nondisplaceable binding potential and percent change in nondisplaceable binding potential between cocaine-dependent and healthy comparison subjects were analyzed using a repeated-measures analysis of variance (ANOVA), with the region of interest as the repeated measure and diagnostic group as the cofactor (SPSS Statistics, Chicago). The Huynh-Feldt correction was used in the event of violations of sphericity assumptions.
Results
The total dose of AMPT was 120.7 mg/kg (SD=9.2) for the comparison group and 121.8 mg/kg (SD=5.4) for the cocaine-dependent group (p=0.7). Using the Extrapyramidal Symptom Rating Scale, we found that only three of the 15 cocaine-dependent subjects experienced extrapyramidal symptoms relative to seven comparison subjects. All extrapyramidal symptoms were experienced on day 2 of AMPT administration (none were reported on day 1 of AMPT administration) and included tremor, rigidity, and akathisia.
PET Scan
PET scan parameters are shown in Table 1. No significant difference was seen between groups or between conditions for injected dose, specific activity, volume of distribution of the cerebellum, or plasma free fraction of [11C]raclopride. Homovanillic acid levels decreased in both groups in response to AMPT (baseline condition: healthy comparison subjects, 15.4 ng/ml [SD=2.1]; cocaine-dependent subjects: 15.1 ng/ml [SD=5.0], p=0.8; AMPT condition: healthy comparison subjects, 5.0 ng/ml [SD=2.0]; cocaine-dependent subjects, 5.8 ng/ml [SD= 1.2], p=0.2). Thus, the percent of decrease in homovanillic acid was 65.2% (SD=8%) in comparison subjects and 65.5% (SD=15%) in cocaine-dependent subjects (p=0.5). The volumes of the regions of interest did not differ between the two groups (all p values >0.4).
TABLE 1. Parameters of [11C]Raclopride Scans at Baseline and Following AMPT Administration.
| Scan Parameter | Treatment Condition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | AMPT | |||||||||
| Healthy Comparison Subjects (N=15) | Cocaine-Dependent Subjects (N=15) | Unpaired t test (p) | Healthy Comparison Subjects (N=15) | Cocaine-Dependent Subjects (N=15) | Unpaired t test (p) | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Injected dose (mCi) | 8.0 | 1.0 | 8.0 | 1.4 | 0.97 | 7.5 | 1.2 | 8.1 | 0.8 | 0.10 |
| Specific activity (Ci/mmol) | 1,533 | 140 | 1,477 | 160 | 0.32 | 1,470 | 194 | 1,532 | 72 | 0.30 |
| Regional tissue distribution volume of the cerebellum (ml/g) | 0.37 | 0.12 | 0.36 | 0.19 | 0.90 | 0.37 | 0.16 | 0.35 | 0.20 | 0.82 |
| Percent free fraction | 4.0 | 0.5 | 4.2 | 0.8 | 0.50 | 4.5 | 0.6 | 4.4 | 1.5 | 0.82 |
In the baseline condition (pre-AMPT administration), cocaine-dependent subjects exhibited significantly lower [11C]raclopride nondisplaceable binding potential relative to comparison subjects (repeated-measures ANOVA: region factor, p<0.001; group factor, p=0.02; group-by-region interaction: p=0.04), as shown in Table 2. Similarly, in the AMPT condition, cocaine-dependent subjects had lower nondisplaceable binding potential relative to comparison subjects (repeated-measures ANOVA: region factor, p<0.001; group factor, p=0.002; group-by-region interaction, p=0.001), also shown in Table 2. Examination of the individual regions showed a significant decrease in non-displaceable binding potential in cocaine-dependent subjects in each region (for both conditions), with the exception of the posterior caudate. Notably, cocaine-dependent subjects exhibited a greater decrease in D2/D3 receptor nondisplaceable binding potential following AMPT administration relative to comparison subjects.
TABLE 2. [11C]Raclopride Nondisplaceable Binding Potential at Baseline and Following AMPT Administration.
| Striatal Subdivision | Treatment Condition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | AMPT | |||||||||
| Healthy Comparison Subjects (N=15) | Cocaine-Dependent Subjects (N=15) | Unpaired t test (p) | Healthy Comparison Subjects (N=15) | Cocaine-Dependent Subjects (N=15) | Unpaired t test (p) | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Ventral striatum | 2.12 | 0.26 | 1.88 | 0.29 | 0.02 | 2.37 | 0.26 | 1.96 | 0.38 | 0.002 |
| Precommissural dorsal caudate | 2.25 | 0.16 | 2.01 | 0.34 | 0.02 | 2.49 | 0.19 | 2.11 | 0.40 | 0.002 |
| Precommissural dorsal putamen | 2.62 | 0.26 | 2.39 | 0.31 | 0.04 | 2.93 | 0.30 | 2.51 | 0.36 | 0.002 |
| Postcommissural caudate | 1.34 | 0.15 | 1.31 | 0.26 | 0.67 | 1.54 | 0.20 | 1.45 | 0.30 | 0.33 |
| Postcommissural putamen | 2.59 | 0.22 | 2.35 | 0.34 | 0.03 | 2.96 | 0.25 | 2.54 | 0.42 | 0.002 |
| Striatum | 2.30 | 0.18 | 2.08 | 0.28 | 0.01 | 2.59 | 0.20 | 2.22 | 0.35 | 0.001 |
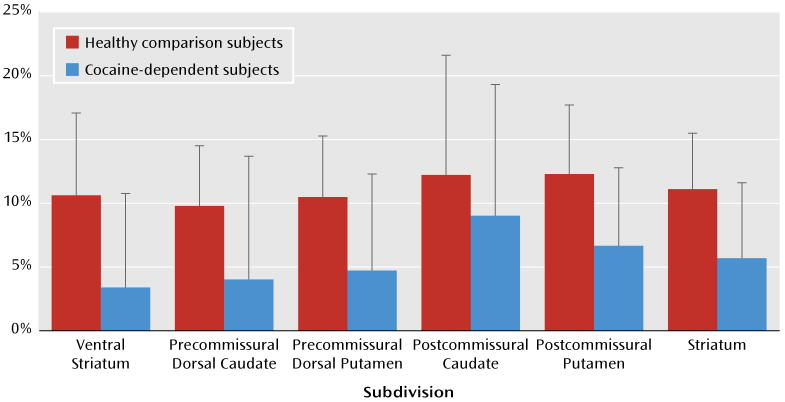
Cocaine dependence was associated with less change in D2/D3 receptors (percent change in nondisplaceable binding potential) following AMPT administration, as shown in Table 3 (repeated-measures ANOVA: region factor, p=0.1; group factor, p=0.006; group-by-region interaction, p=0.5). This percent change in nondisplaceable binding potential was significant in each region, with the exception of the posterior caudate (Figure 1).
TABLE 3. Estimated Occupancy of D2/D3 Receptors Measured as AMPT-Induced Change in [11C]Raclopride Nondisplaceable Binding Potential.
| Striatal Subdivision | Increase in Nondisplaceable Binding Potential (%) | ||||
|---|---|---|---|---|---|
| Healthy Comparison Subjects (N=15) | Cocaine-Dependent Subjects (N=15) | Unpaired t test (p) | |||
| Mean | SD | Mean | SD | ||
| Ventral striatum | 10.6 | 6.5 | 3.4 | 7.4 | 0.007 |
| Precommissural dorsal caudate | 9.8 | 4.7 | 4.0 | 9.7 | 0.04 |
| Precommissural dorsal putamen | 10.5 | 4.8 | 4.7 | 7.6 | 0.02 |
| Postcommissural caudate | 12.2 | 9.4 | 9.0 | 10.3 | 0.43 |
| Postcommissural putamen | 12.3 | 5.4 | 6.7 | 6.1 | 0.01 |
| Striatum | 11.1 | 4.4 | 5.7 | 5.9 | 0.009 |
FIGURE 1. Percent Change in [11C]Raclopride Nondisplaceable Binding Potential for Cocaine-Dependent and Healthy Comparison Subjects Following AMPT Administrationa.
a The percent change is significant in each region, with the exception of the posterior caudate.
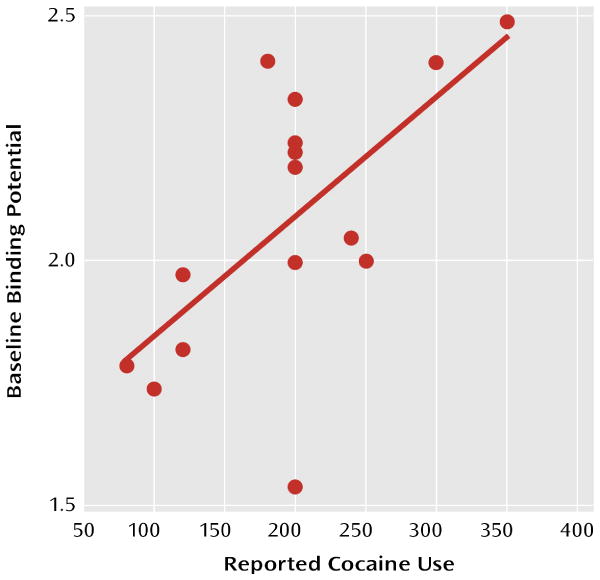
A significant correlation was seen between the amount of reported cocaine use and D2/D3 receptor nondisplaceable binding potential in the striatum for both the baseline (r=0.64, p=0.01 [Figure 2]) and AMPT (r=0.54, p=0.04) conditions. No relationship was seen between percent change in nondisplaceable binding potential and cocaine use, and no relationship was seen between striatal nondisplaceable binding potential and years of cocaine use.
FIGURE 2. Correlation Between Baseline Values of [11C]Raclopride Nondisplaceable Binding Potential and the Amount of Cocaine Use (money spent per week) in Cocaine-Dependent Subjectsa.
a r=0.64, p=0.01.
A post hoc comparison of the comparison subjects who experienced extrapyramidal symptoms versus those who did not showed no significant difference in percent change in nondisplaceable binding potential or percent change in plasma homovanillic acid between the two groups of comparison subjects, but this analysis was limited by the small number of subjects per group.
Discussion
The results of the present study demonstrate that cocaine-dependent subjects have lower levels of endogenous dopamine relative to healthy comparison subjects. Thus, the decrease in baseline D2/D3 receptor binding potential (nondisplaceable binding potential) seen in the cocaine-dependent subjects cannot be attributed to differences in the percentage of D2/D3 receptors occupied by dopamine, and the endogenous dopamine levels may have masked even greater differences between cocaine-dependent and healthy comparison subjects than those differences observed previously.
We assumed that the AMPT-induced increase in D2/D3 receptor binding potential resulted from a reduction in the percentage of receptors bound to endogenous dopamine rather than upregulation of D2/D3 receptors in the setting of dopamine depletion. This assumption was based on previous studies of rodents (3, 11, 12), which showed that acute dopamine depletion with reserpine, 6-hydroxydopamine, or AMPT did not produce D2/D3 receptor upregulation in the short-term. Notably, the investigators using AMPT (3) designed their experiment in rodents to emulate their analysis of human subjects, and high-dose AMPT (400 mg/kg per day) was administered in the rodents for the same time period used for the human subjects. Compared with saline-treated animals, no difference was seen in D2/D3 receptor Bmax, indicating that receptor upregulation did not occur. However, based on the literature published to date, it is unknown if there are differences in receptor externalization between humans and rodents. In addition, although this dosing regimen is expected to result in a 70%–80% depletion of striatal dopamine (4), the exact degree of depletion is not known in the absence of animal studies.
The lower levels of endogenous dopamine seen in the present study are consistent with some, although not all, preclinical studies of rodents. Previous studies (13–29) have shown either no change or a decrease in the levels of endogenous dopamine in rodents following chronic exposure to cocaine. As outlined in Table 4, these studies are almost evenly split between those showing a decrease in baseline levels of dopamine in cocaine-treated rats relative to comparison rats and those showing no difference in this measure. The methods between these studies vary in terms of the amount of cocaine administered, method of cocaine administration (self-administered, investigator-administered, or yoked-administered), and duration of cocaine administration. In general, the studies showing a decrease in endogenous dopamine used a higher dose of cocaine for longer periods of time. In addition, more of these studies used cocaine self-administration, which may be a more accurate reflection of the pattern of cocaine use in humans.
TABLE 4. Previously Reported Levels of Baseline Dopamine in Cocaine-Treated Rodents.
| Study | Brain Tissue | Treatment Administration |
|---|---|---|
| Wilson et al. (13) | Striatum | Average use: 9.25 mg/kg in divided doses for at least 28 days (self-administered) |
| Akimoto et al. (14) | Striatum | 20 mg/kg per day for 14 days (investigator-administered) |
| Segal and Kuczenski (15) | Caudate, nucleus accumbens | 10 mg/kg per day for 4 days (investigator-administered) |
| Kalivas and Duffy (16) | Striatum | 15 mg/kg per day for 4 days (investigator-administered) |
| Kalivas and Duffy (17) | Nucleus accumbens | 15 or 30 mg/kg per day for 7 days (investigator-administered) |
| Parsons et al. (18) | Nucleus accumbens | 20 mg/kg per day for 10 days (investigator-administered) |
| Chefer and Shippenberg (19) | Nucleus accumbens | 20 mg/kg per day for 5 days (investigator-administered) |
| Zapata et al. (20) | Nucleus accumbens | 0.5 mg/kg infusion for 90 minutes per 5 days (yoked- and self-administered) |
| Zhang et al. (21) | Nucleus accumbens, caudate | 10 mg/kg per day for 7 days (investigator-administered) |
| Wilson et al. (22) | Nucleus accumbens | Average use: 90 mg/kg in divided doses for at least 21 days (self-administered) |
| Pettit et al. (23) | Nucleus accumbens | 10 mg/kg per day for 5 days; 20 mg/kg per day for 25 days (investigator-administered) |
| Mateo et al. (24) | Nucleus accumbens | Following a fixed ratio schedule for 5 days; four discrete trials; 24 hours/day for 10 days (self-administered; average cocaine use: 750–800 mg/kg in 10 days) |
| Gerrits et al. (25) | Nucleus accumbens | 30 μg infusion per 3 hours or maximum of 60 infusions per 5 days (self-administered) |
| Maisonnueve et al. (26) | Striatum (ventral and dorsal) | 3 × 10–15 mg/kg for 13 days (investigator-administered) |
| Weiss et al. (27) | Nucleus accumbens | Unlimited access intravenous doses (self-administered) |
| Rossetti et al. (28) | Ventral striatum | 15 mg/kg per 2 days for 15 days (investigator-administered) |
| Robertson et al. (29) | Ventral striatum | 30 mg/kg per day for 18 days (investigator-administered) |
In the present study, cocaine dependence was associated with a lower AMPT-induced change in [11C]raclopride binding in the striatum, measured as a whole, and in each of the striatal subdivisions, with the exception of the posterior caudate. There was no significant difference in the baseline measures of D2/D3 receptor binding potential in this brain region between cocaine-dependent and comparison subjects. This finding is consistent with our previous study (2), which also showed no difference in baseline measures of D2/D3 receptor binding potential in the posterior caudate in a separate cohort of cocaine-dependent and comparison subjects. Together, these data suggest that the posterior caudate is spared in cocaine dependence, both in terms of baseline D2/D3 receptor binding potential and levels of endogenous dopamine, although the rationale for this is not clear. The caudate posterior to the anterior commissure has been rarely investigated in animal studies, and thus it is unknown if there is some inherent difference in this brain region that would cause it to be spared. Moreover, to our knowledge, no other imaging investigator group has compared D2/D3 receptor binding potential between cocaine-dependent and comparison subjects in this brain region, and it will be important for this finding to be replicated.
Although, to the best of our knowledge, this is the first report to measure levels of endogenous dopamine in cocaine-dependent subjects, several previous studies have used PET or SPECT and AMPT to estimate endogenous dopamine levels in healthy comparison subjects. Laruelle et al. (3) used [123I]iodobenzamide and AMPT (8 g over a 48-hour period), which produced a 28% (SD=16%) increase in binding potential and a 70% (SD=12%) decrease in homovanillic acid levels in nine healthy subjects. Abi-Dargham et al. (4) used this same method to assess the occupancy of D2/D3 receptors by endogenous dopamine in schizophrenia patients relative to healthy comparison subjects. AMPT-induced dopamine depletion significantly increased D2/D3 receptor availability by 19% (SD=11%) in patients with schizophrenia relative to 9% (SD=7%) in healthy comparison subjects. Verhoeff et al. (5, 6) conducted two studies using [11C]raclopride to measure levels of endogenous dopamine in healthy comparison subjects. The first study reported that AMPT (4.5 g over a 25-hour period) increased [11C]raclopride binding by 18.5% (SD= 3.0%) in the striatum and produced a 71% (SD=11%) decrease in plasma homovanillic acid levels in six healthy comparison subjects (5). In their second study, six comparison subjects were imaged with [11C]raclopride, and AMPT administration (5.35 g over a 29-hour period) resulted in a significant increase in binding potential (13.3% [SD=5.9%]) in the striatum and decreased homovanillic acid levels (62% [SD=17%]) (6).
As a result of limited scanner resolution, these previous studies imaged the striatum as a whole and could not separate the signal among the caudate, putamen, and ventral striatum. More recently, studies using the PET radiotracer [18F]fallypride, which also labels the D2/D3 receptor, and AMPT have measured endogenous dopamine in the subdivisions of the striatum using a high-resolution PET scanner. Riccardi et al. (30) reported that AMPT (71.4 mg/kg) increased binding potential by 8.8% in the caudate, 11.2% in the putamen, and 10.6% in the ventral striatum in healthy subjects. However, a subsequent study (31) showed no effect of a lower dose of AMPT (3 g/70 kg per day for 44 hours) on [18F]fallypride binding in healthy comparison subjects.
Thus, our results in healthy comparison subjects are comparable with those previously reported in the literature, which demonstrate that approximately 10%–20% of D2/D3 receptors are occupied by endogenous dopamine. In addition, we observed no difference among the subdivisions with respect to the percent of D2/D3 receptors occupied by endogenous dopamine in either cocaine-dependent subjects or healthy comparison subjects. This finding in the healthy comparison group is consistent with that of Riccardi et al. (30), who did not report a difference in the percent of occupied D2/D3 receptors among the caudate, putamen, and ventral striatum in healthy subjects. However, Kegeles et al. (32) recently reported that, in schizophrenia, the percent of AMPT-induced increase in [11C]raclopride differed among the striatal subregions and was higher in the anterior caudate compared with the other subdivisions. In the present study, we observed no difference in the AMPT-induced change in [11C]raclopride binding among the striatal subregions in the cocaine-dependent subjects, suggesting that the levels of endogenous dopamine are fairly uniform throughout the striatum.
It is important to note that previous studies of cocaine dependence have also shown a reduction in presynaptic dopamine release in response to a psychostimulant, measured as a decrease in [11C]raclopride binding (1, 33). The increase in synaptic dopamine following psychostimulant administration results in a decrease in [11C]raclopride binding, presumably as a result of competition between dopamine and the radiotracer for the receptors, although the mechanism is likely more complex than competition alone (34, 35). In cocaine-dependent subjects, there is a blunting of psychostimulant-induced radiotracer displacement relative to comparison subjects, which is generally interpreted as a reduction in presynaptic dopamine release. However, it is possible that an increase in the percent of D2/D3 receptors occupied by dopamine could produce a blunting of psychostimulant-induced radiotracer displacement. If more receptors are occupied by dopamine in the baseline condition, then fewer receptors would be available to bind to the surge of dopamine produced by the stimulant challenge. In this scenario, it is possible that cocaine dependence is associated with normal or even elevated presynaptic dopamine release (sensitization), but this phenomenon is masked by a high percentage of D2/D3 receptors occupied by dopamine. However, the results of the present study suggest that the blunted stimulant-induced [11C]raclopride displacement cannot be ascribed to the percent of D2/D3 receptors occupied by endogenous dopamine. Although there may still be other issues that affect this measure of synaptic dopamine (36), our study demonstrates that an excess of baseline dopamine in cocaine-dependent volunteers is not likely a factor.
Interestingly, only three cocaine-dependent subjects in the present study experienced extrapyramidal symptoms, measured with the Extrapyramidal Symptom Rating Scale, relative to seven healthy comparison subjects. This was unexpected, given that previous studies (37–39) have suggested that cocaine abuse increases the risk of extrapyramidal side effects in patients treated with high-potency neuroleptics. However, the cocaine-dependent subjects exhibited less change in endogenous dopamine following AMPT administration, which suggests that the relative change in endogenous dopamine, rather than the absolute levels, may contribute to the development of extrapyramidal symptoms.
In summary, the results of the present study indicate that cocaine dependence is associated with a decrease in the levels of striatal dopamine. Taken in the context of previous studies, these results contribute to a series of findings that provide consistent evidence that cocaine dependence is associated with a decrease in dopamine transmission in the striatum. Imaging studies have shown that cocaine dependence is associated with a reduction in D2/D3 receptors, stimulant-induced presynaptic dopamine release, reduced striatal dopamine synthesis, and—now—a reduction in endogenous dopamine (1, 2, 33, 40).
Acknowledgments
Supported by grants P50-DA 09236 and 1 UL1 RR024156-03.
Research for this study was made possible by the Irving Center for Clinical Research, where the participants were admitted.
The authors thank Lawrence Kegeles and Anissa Abi-Dargham for assistance with implementing the AMPT administration paradigm. The authors also thank Daria Orlowska and Stephanie Cooke for technical assistance.
Footnotes
Dr. Narendran has received contracts for scientific research from GlaxoSmithKline. Dr. Slifstein is a consultant for GlaxoSmithKline and Amgen. Dr. Kleber is a consultant for Abbott Laboratories, Alkermes Pharmaceuticals, Cephalon, Johnson and Johnson, Purdue Pharma, and Reckett Benckiser; he has served on the scientific advisory boards of the Grunenthal Group and U.S. World Medical; and he has served as a speaker for Johnson and Johnson. Drs. Martinez, Broft, Kumar, Liu, and Van Heertum and Ms. Greene report no competing interests.
References
- 1.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 2.Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankle WG, Cooper T, Kleber HD, Fischman MW, Laruelle M. Cocaine dependence and D2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- 3.Laruelle M, D'Souza CD, Baldwin RM, Abi-Dargham A, Kanes SJ, Fingado CL, Seibyl JP, Zoghbi SS, Bowers MB, Jatlow P, Charney DS, Innis RB. Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology. 1997;17:162–174. doi: 10.1016/S0893-133X(97)00043-2. [DOI] [PubMed] [Google Scholar]
- 4.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verhoeff NP, Kapur S, Hussey D, Lee M, Christensen B, Papatheodorou G, Zipursky RB. A simple method to measure baseline occupancy of neostriatal dopamine D2 receptors by dopamine in vivo in healthy subjects. Neuropsychopharmacology. 2001;25:213–223. doi: 10.1016/S0893-133X(01)00231-7. [DOI] [PubMed] [Google Scholar]
- 6.Verhoeff NP, Hussey D, Lee M, Tauscher J, Papatheodorou G, Wilson AA, Houle S, Kapur S. Dopamine depletion results in increased neostriatal D2, but not D1, receptor binding in humans. Mol Psychiatry. 2002;7:233, 322–328. doi: 10.1038/sj.mp.4001062. [DOI] [PubMed] [Google Scholar]
- 7.Chouinard G, Margolese HC. Manual for the Extrapyramidal Symptom Rating Scale (ESRS) Schizophr Res. 2005;76:247–265. doi: 10.1016/j.schres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with PET, I: accuracy and precision of D2 parameter measurements in the ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Slifstein M, Laruelle M. Models and methods for derivation of in vivo neuroreceptor parameters with PET and SPECT reversible radiotracers. Nucl Med Biol. 2001;28:595–608. doi: 10.1016/s0969-8051(01)00214-1. [DOI] [PubMed] [Google Scholar]
- 10.Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography, part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 11.Ross SB, Jackson DM. Kinetic properties of the accumulation of 3H-raclopride in the mouse brain in vivo. Naunyn Schmiede-bergs Arch Pharmacol. 1989;340:6–12. doi: 10.1007/BF00169199. [DOI] [PubMed] [Google Scholar]
- 12.Hume SP, Opacka-Juffry J, Myers R, Ahier RG, Ashworth S, Brooks DJ, Lammertsma AA. Effect of L-dopa and 6-hydroxy-dopamine lesioning on [11C]raclopride binding in rat striatum, quantified using PET. Synapse. 1995;21:45–53. doi: 10.1002/syn.890210107. [DOI] [PubMed] [Google Scholar]
- 13.Wilson JM, Nobrega JN, Corrigall WA, Coen KM, Shannak K, Kish SJ. Amygdala dopamine levels are markedly elevated after self-but not passive-administration of cocaine. Brain Res. 1994;668:39–45. doi: 10.1016/0006-8993(94)90508-8. [DOI] [PubMed] [Google Scholar]
- 14.Akimoto K, Hamamura T, Otsuki S. Subchronic cocaine treatment enhances cocaine-induced dopamine efflux, studied by in vivo intracerebral dialysis. Brain Res. 1989;490:339–344. doi: 10.1016/0006-8993(89)90251-5. [DOI] [PubMed] [Google Scholar]
- 15.Segal DS, Kuczenski R. In vivo microdialysis reveals a diminished amphetamine-induced DA response corresponding to behavioral sensitization produced by repeated amphetamine pretreatment. Brain Res. 1992;571:330–337. doi: 10.1016/0006-8993(92)90672-v. [DOI] [PubMed] [Google Scholar]
- 16.Kalivas P, Duffy P. The effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse. 1990;5:48–58. doi: 10.1002/syn.890050104. [DOI] [PubMed] [Google Scholar]
- 17.Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I: dopamine axon terminals. J Neurosci. 1993;13:276–284. doi: 10.1523/JNEUROSCI.13-01-00276.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsons LH, Smith AD, Justice JB., Jr Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse. 1991;9:60–65. doi: 10.1002/syn.890090109. [DOI] [PubMed] [Google Scholar]
- 19.Chefer VI, Shippenberg TS. Changes in basal and cocaine-evoked extracellular dopamine uptake and release in the rat nucleus accumbens during early abstinence from cocaine: quantitative determination under transient conditions. Neuroscience. 2002;112:907–919. doi: 10.1016/s0306-4522(02)00099-4. [DOI] [PubMed] [Google Scholar]
- 20.Zapata A, Chefer VI, Ator R, Shippenberg TS, Rocha BA. Behavioural sensitization and enhanced dopamine response in the nucleus accumbens after intravenous cocaine self-administration in mice. Eur J Neurosci. 2003;17:590–596. doi: 10.1046/j.1460-9568.2003.02491.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Loonam TM, Noailles PA, Angulo JA. Comparison of cocaine- and methamphetamine-evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions. Ann N Y Acad Sci. 2001;937:93–120. doi: 10.1111/j.1749-6632.2001.tb03560.x. [DOI] [PubMed] [Google Scholar]
- 22.Wilson JM, Nobrega JN, Carroll ME, Niznik HB, Shannak K, Lac ST, Pristupa ZB, Dixon LM, Kish SJ. Heterogeneous subregional binding patterns of H-3-WIN 35,428 and H-3-GBR 12,935 are differentially regulated by chronic cocaine self-administration. J Neurosci. 1994;14(5 part 2):2966–2979. doi: 10.1523/JNEUROSCI.14-05-02966.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pettit HO, Pan HT, Parsons LH, Justice JBJ. Extracellular concentration of cocaine and dopamine are enhanced during chronic cocaine administration. J Neurochem. 1990;55:798–804. doi: 10.1111/j.1471-4159.1990.tb04562.x. [DOI] [PubMed] [Google Scholar]
- 24.Mateo Y, Lack CM, Morgan D, Roberts DC, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology. 2005;30:1455–1463. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- 25.Gerrits MA, Petromilli P, Westenberg HG, Di Chiara G, van Ree JM. Decrease in basal dopamine levels in the nucleus accumbens shell during daily drug-seeking behaviour in rats. Brain Res. 2002;924:141–150. doi: 10.1016/s0006-8993(01)03105-5. [DOI] [PubMed] [Google Scholar]
- 26.Maisonneuve IM, Ho A, Kreek MJ. Chronic administration of a cocaine “binge” alters basal extracellular levels in male rats: an in vivo microdialysis study. J Pharmacol Exp Ther. 1995;272:652–657. [PubMed] [Google Scholar]
- 27.Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 1992;593:314–318. doi: 10.1016/0006-8993(92)91327-b. [DOI] [PubMed] [Google Scholar]
- 28.Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- 29.Robertson MW, Leslie CA, Bennett JP., Jr Apparent synaptic dopamine deficiency induced by withdrawal from chronic cocaine treatment. Brain Res. 1991;538:337–339. doi: 10.1016/0006-8993(91)90451-z. [DOI] [PubMed] [Google Scholar]
- 30.Riccardi P, Baldwin R, Salomon R, Anderson S, Ansari MS, Li R, Dawant B, Bauernfeind A, Schmidt D, Kessler R. Estimation of baseline dopamine D2 receptor occupancy in striatum and extrastriatal regions in humans with positron emission tomography with [18F]fallypride. Biol Psychiatry. 2008;63:241–244. doi: 10.1016/j.biopsych.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Cropley VL, Innis RB, Nathan PJ, Brown AK, Sangare JL, Lerner A, Ryu YH, Sprague KE, Pike VW, Fujita M. Small effect of dopamine release and no effect of dopamine depletion on [18F]fallypride binding in healthy humans. Synapse. 2008;62:399–408. doi: 10.1002/syn.20506. [DOI] [PubMed] [Google Scholar]
- 32.Kegeles L, Frankle W, Gil R, Narendran R, Slifstein M, Hwang DR, Cangiano C, Haber S, Abi-Dargham A, Laruelle M. Schizophrenia is associated with increased synaptic dopamine in associative rather than limbic regions of the striatum: implications for mechanisms of action of antipsychotic drugs. Arch Gen Psychiatry. in press. [Google Scholar]
- 33.Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- 34.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Logan J, Fowler JS, Dewey SL, Volkow ND, Gatley SJ. A consideration of the dopamine D2 receptor monomer-dimer equilibrium and the anomalous binding properties of the dopamine D2 receptor ligand, n-methyl spiperone. J Neural Transm. 2001;108:279–286. doi: 10.1007/s007020170073. [DOI] [PubMed] [Google Scholar]
- 36.Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62:851–869. doi: 10.1002/syn.20566. [DOI] [PubMed] [Google Scholar]
- 37.van Harten PN, van Trier JC, Horwitz EH, Matroos GE, Hoek HW. Cocaine as a risk factor for neuroleptic-induced acute dystonia. J Clin Psychiatry. 1998;59:128–130. doi: 10.4088/jcp.v59n0307. [DOI] [PubMed] [Google Scholar]
- 38.Catalano G, Catalano MC, Rodriguez R. Dystonia associated with crack cocaine use. South Med J. 1997;90:1050–1052. doi: 10.1097/00007611-199710000-00017. [DOI] [PubMed] [Google Scholar]
- 39.Maat A, Fouwels A, de Haan L. Cocaine is a major risk factor for antipsychotic induced akathisia, parkinsonism and dyskinesia. Psychopharmacol Bull. 2008;41:5–10. [PubMed] [Google Scholar]
- 40.Wu JC, Bell K, Najafi A, Widmark C, Keator D, Tang C, Klein E, Bunney BG, Fallon J, Bunney WE. Decreasing striatal 6-FDOPA uptake with increasing duration of cocaine withdrawal. Neuropsychopharmacology. 1997;17:402–409. doi: 10.1016/S0893-133X(97)00089-4. [DOI] [PubMed] [Google Scholar]