Abstract
Background:
Interactions between physicians and the pharmaceutical industry have led to concerns about conflict of interest (COI), resulting in COI guidelines that suggest a threshold beyond which interactions may be considered unacceptable. Guidelines have also outlined the importance of public opinion on the topic. Consequently, we conducted a systematic review to determine the Canadian public's opinions of physician–pharmaceutical industry interactions.
Methods:
A systematic review of the standard health sciences literature as well as grey literature was conducted and a number of experts were contacted. Pre-determined eligibility criteria were used to identify appropriate studies. Meta-analysis of the study findings was not possible owing to the variety of methods of reporting outcomes, the types of interactions studied and the diversity of populations studied.
Results:
No studies on Canadian opinions were identified. Ten international studies (n=13,637), seven with patient groups and three with public citizens, were identified that examined opinions on aspects of awareness, acceptability, disclosure and perceived effects of physician–pharmaceutical industry interactions. Heterogeneity was observed in the awareness, acceptability and perceived effects of physician–pharmaceutical industry interactions; however, there appeared to be greater acceptability and fewer perceived effects with smaller, less costly interactions that directly benefit patients or a medical practice. Desire for disclosure of these interactions was consistent across studies.
Interpretation:
Research on the public's perception of physician–pharmaceutical industry interactions has been inadequate internationally and non-existent in Canada, and is urgently needed to help shape policies regarding potential conflict of interest.
Abstract
Contexte:
L'interaction entre les médecins et l'industrie pharmaceutique est source de préoccupation quant à la possibilité de conflits d'intérêts, ce qui a mené à des lignes directrices proposant un seuil au-delà duquel l'interaction pourrait être considérée inacceptable. Les lignes directrices font également voir l'importance de l'opinion publique sur le sujet. Nous avons donc mené une revue systématique pour déterminer quelle est l'opinion du public canadien sur l'interaction entre les médecins et l'industrie pharmaceutique.
Méthodologie:
Nous avons procédé à une revue systématique de la littérature scientifique et grise, et nous avons communiqué avec des spécialistes. Les études ont été choisies selon des critères d'admissibilité prédéterminés. Il a été impossible de procéder à une méta-analyse des conclusions des études étant donné la variété de méthodes pour la présentation des résultats, les types d'interaction considérés et la diversité des populations étudiées.
Résultats:
Nous n'avons trouvé aucune étude sur l'opinion des Canadiens. Nous avons repéré dix études internationales (N=13 637), dont sept portant sur des groupes de patients et trois sur des populations de citoyens, qui examinaient l'opinion au sujet de la prise de conscience, de l'acceptabilité, de la divulgation et des effets perçus en matière d'interaction entre les médecins et l'industrie pharmaceutique. Nous avons observé une hétérogénéité pour ce qui est de la prise de conscience, de l'acceptabilité et des effets perçus; cependant, il semble qu'il y a une plus grande acceptabilité et moins d'effets perçus pour ce qui est des interactions plus petites et moins coûteuses qui présentent un avantage direct pour les patients ou pour un cabinet médical. La divulgation de ces interactions est un souhait qui s'observe de façon constante dans toutes les études.
Interprétation:
À l'échelle internationale, la recherche sur la perception du public au sujet de l'interaction entre les médecins et l'industrie pharmaceutique reste inadéquate, alors qu'elle est absente au Canada. Il y a un besoin urgent pour ce type de recherche afin d'aider à orienter les politiques au sujet des conflits d'intérêts.
Interactions between physicians and the pharmaceutical industry are frequently documented and debated within the medical literature (Moynihan 2003; Higgins 2007; Blumenthal 2004; Lambert 2005). A recent study revealed that 94% of physicians have some form of interaction with the pharmaceutical industry (Moynihan 2003). In addition to the more common interactions such as pharmaceutical detailing, the exchange of drug samples and industry-sponsored meals, physicians are regularly solicited to participate in industry-funded research and attend industry-funded continuing medical education (CME). Furthermore, select groups of physicians are asked to lead industry-funded research, sit on advisory boards and deliver industry-developed presentations (Campbell et al. 2007; Ross et al. 2008; Kaiser Family Foundation 2002; Holmer 2001). While these interactions have resulted in important clinical benefits, such as the advancement of valuable treatments (Stossel 2005), a number of highly publicized adverse events have also occurred (Psaty and Kronmal 2008; Kondro 2004; Olivieri 2003). The effects of physician–pharmaceutical industry interactions on physician behaviour have been reviewed and suggest an impact on prescribing practices, professional behaviour and attitude towards interactions with the pharmaceutical industry (Wazana 2000). Consequently, the potential for negative effects has led to concerns from physicians, academics and regulatory boards regarding conflict of interest (COI), where COI is broadly defined as conditions that cause a physician's primary interest – patient welfare – to be adversely influenced by secondary powers (Holmes et al. 2004).
As a result of these frequent interactions, a number of regulatory and advisory bodies have issued COI guidelines suggesting a threshold beyond which physician– pharmaceutical industry interactions are considered unacceptable (CADTH 2006a; Canada's Research-Based Pharmaceutical Companies 2007; CMA 2007; RCPSC 2005; CFPC 2006). Professional colleges such as the Royal College of Physicians and Surgeons of Canada have included statements in their COI guidelines that suggest physicians reflect on what the public would think of a physician–pharmaceutical industry interaction when they are unsure whether it is appropriate (RCPSC 2005). The RCPSC believes that this is important because, according to its guidelines, “when physicians are seen or perceived to be in conflict of interest there is an inevitable erosion of public trust which is fundamental to our patients and society” (RCPSC 2005).
A number of previously published papers have articulated the same concerns, arguing that any physician–pharmaceutical industry interaction that leads the public to believe physicians are biased in their prescribing practices will affect the credibility of these physicians. Although the resulting biases may be unintentional, such public opinions could result in mistrust in physicians and in the greater healthcare system (Blumenthal 2004; Marco et al. 2006; Katz et al. 2003; Brennan and Mello 2007). However, it is only recently that the engagement of public opinion and public participation in health policy making has been recognized as important to ensure public trust and credibility of the healthcare system (CADTH 2006b; Ministry of Health and Long-Term Care 2006). Because public programs such as our medical and drug plans require the public's trust that physicians hold their patients' best interests paramount, we believe it is important to determine public opinions on this issue. Consequently, we undertook a systematic review of the literature with the primary goal of examining the Canadian public's perceptions on physician–pharmaceutical industry interactions. As a secondary question, we sought to understand the international public's opinions on this topic.
Methods
The literature search was oriented specifically to awareness, acceptability, desire for disclosure and perceived effects of physician–pharmaceutical industry interactions. The search was carried out in MEDLINE (1966 – April 2007), EMBASE (1980 – April 2007), Cochrane Database of Systematic Reviews (1966 – April 2007), Business Source Complete (1866 – April 2007) and ABI Inform Global (1971 – April 2007), using various combinations of the following terms: conflict of interest, drug industry, public opinion, physician, gift giving, medical ethics, drug manufacturer, pharmaceutical industry and marketing. Studies were limited to the English language, excluding letters and editorials.
In addition, the grey literature was searched using a general Internet search engine (Google.ca), as well as the online libraries of a number of relevant Internet sites (Healthyskepticism.org, Nofreelunch.org, Public Citizen Health Research group and Ipsos–Reid public polling). Finally, seven experts on the topic were contacted to make certain that no relevant studies were missed. Specific study characteristics were abstracted to determine those for inclusion, including (a) type of study – survey designs, focus groups or opinion polls, with either a random or convenience sampling method; (b) participants – adults, in the general public or patient groups. If public opinions were gathered alongside other groups' views, these were included if analyzed independently. Studies involving Canadian subjects were to be analyzed separately; (c) outcomes – outcome measurements of at least one of the following: (i) awareness of physician–pharmaceutical industry interactions; (ii) acceptability of interactions; (iii) disclosure of interactions; and (iv) effects of interactions.
Studies were initially examined for appropriateness of inclusion by one reviewer (JA), who was not blinded to study authorship. A data extraction sheet was developed and pilot-tested by the two data abstractors (JA and WW). Subsequently, two reviewers (JA and WW) independently extracted data from the studies. Data were collected on study design, sample characteristics and outcome measures. Disagreement among abstractors was resolved by consensus. A qualitative summary and meta-analysis of each type of outcome related to physician–pharmaceutical industry interactions was planned.
Results
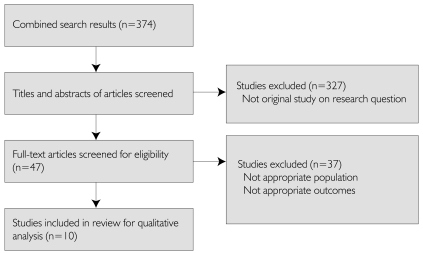
Three hundred and seventy-four studies were identified as potentially relevant. After review of the abstracts, 327 studies were discarded because they were not original studies on the proposed topic. Full text of the remaining 47 studies was retrieved for detailed evaluation, in which 37 studies were excluded because they did not meet inclusion criteria for participants or outcome measures. Ten studies met inclusion criteria for subsequent data extraction (Blake and Early 1995; Mainous et al. 1995; LaPuma et al. 1995; Gibbons et al. 1998; Eaton 2003; Wall Street Journal Online 2003; Kim et al. 2004; Hampson et al. 2006; Semin et al. 2006; Weinfurt et al. 2006). However, none of the 10 studies examined the primary study question regarding the Canadian public's opinions on physician–pharmaceutical industry interactions; instead, all had an international focus. As a result, only the secondary research question could be examined in this review. Figure 1 outlines the aforementioned process.
FIGURE 1.
Flow of information through the systematic review (PRISMA diagram)
Combined, the 10 studies surveyed a total of 13,637 participants (range of 139 to 5,478 participants per study). Seven studies used various survey designs (Blake and Early 1995; Mainous et al. 1995; LaPuma et al. 1995; Gibbons et al. 1998; Hampson et al. 2006; Semin et al. 2006; Kim et al. 2004), one study used focus groups (Weinfurt et al. 2006) and two used online Internet opinion polls (Eaton 2003; Wall Street Journal Online 2003) (Table 1). The studies examined various aspects of physician–pharmaceutical industry interactions, most notably awareness, acceptability, disclosure and perceived effects. The majority of studies focused on patient populations (Blake and Early 1995; LaPuma et al. 1995; Gibbons et al. 1998; Kim et al. 2004; Hampson et al. 2006; Semin et al. 2006; Weinfurt et al. 2006); however, one study used a random sample of adults (Mainous et al. 1995), while the studies with Internet opinion polls used a convenience sample of adults visiting their respective websites (Eaton 2003; Wall Street Journal Online 2003). None of the studies reported whether respondents were informed of the potential effects of physician–pharmaceutical industry interactions, or whether they were given any context regarding the interactions, prior to providing their opinions on the topic.
TABLE 1.
Studies investigating patient or public opinions on physician–pharmaceutical industry interactions
| Study, Year | Study design | Study site | Population (n) | Response rate | Interactions | Outcome measures |
|---|---|---|---|---|---|---|
| Blake & Early (1995) | Survey – Self-administered | Columbia, MO | Adult public and patients in two healthcare centres (486) | 83.1% | Gifts overall Small gifts Large gifts CME Drug samples Meals Social events |
Awareness of interactions Acceptability of interactions Effects of interactions |
| Mainous et al. (1995) | Survey – Telephone administered | Kentucky, Usa | Random sample of adults (649) | 55% | Office gifts Personal gifts |
Awareness of interactions Acceptability of interactions Effects of interactions |
| LaPuma et al. (1995) | Survey – Self-administered | Chicago, IL | Patients in healthcare centre (200) | 74% | Salary support Stock ownership Per-patient payment |
Acceptability of interactions Disclosure of interactions Effect of interactions |
| Gibbons et al. (1998) | Survey – Face-to-face interview | Washington, DC | Patients in two healthcare centres (196) | 96% at one centre; convenience sample at the other | Gifts overall Small gifts CME Drug samples Meals Travel |
Awareness of interactions Acceptability of interactions Effects of interactions |
| Eaton, for the British Medical Journal (2003) | Online opinion poll | Online visitors to BMJ website, international | Online adults (1,479) | Convenience sample | Gifts overall Meeting with PR |
Acceptability of interactions Disclosure of interactions |
| Wall Street Journal Online (2003) | Online opinion poll | Online members of Harris Interactive, USA | Online adult members (4,173) | Convenience sample | Meeting with PR CME |
Acceptability of interactions Effects of interactions |
| Kim et al. (2004) | Survey – Internet administered | Online members of Harris Interactive Chronic Illness Database, International | Patient members (5,478): – Coronary artery disease group (2,355) – Breast cancer group (1,006) – Depression group (2,117) |
86% | Personal income Researcher patent Researcher stocks Per capita payments |
Disclosure of interactions Effect of interactions |
| Hampson et al. (2006) | Survey – Face-to-face interview | Bethesda, MD Boston, MA Seattle, WA Denver, CO New Haven, CT |
Cancer trial patients in five healthcare centres (253) | 93% | Gifts overall Financial interactions Consulting Speaking fees Patent royalties Stock ownership |
Awareness of interactions Acceptability of interactions Disclosure of interactions Effect of interactions |
| Semin et al. (2006) | Survey – Face-to-face interview | Izmir Centrum, Turkey | Patients in 44 healthcare centres (584) | Not reported | Gifts overall Pharmaceutical promotion Small gifts Large gifts CME Drug samples Meals Travel |
Awareness of interactions Acceptability of interactions Effects of interactions |
| Weinfurt et al. (2006) | Focus group | Durham, NC New York, NY Chicago, IL |
16 focus groups of patients and adult public (139) | Not reported | Salary support Patent ownership Equity holdings Finder's fees Per capita payment |
Awareness of interactions Disclosure of interactions Effects of interactions |
CME = continuing medical education
PR = pharmaceutical representative
Meta-analysis of study results was not possible for a number of reasons, mainly because of the diversity in (a) reported outcomes, (b) types of physician–pharmaceutical industry interactions investigated and (c) populations sampled.
Awareness of physician–pharmaceutical industry interactions
Six of the 10 studies (Blake and Early 1995; Mainous et al. 1995; Gibbons et al. 1998; Hampson et al. 2006; Semin et al. 2006; Weinfurt et al. 2006) examined the public's awareness of various physician–pharmaceutical industry interactions (Table 2). Each of these studies examined simply whether a respondent was aware of an interaction occurring, rather than whether the respondent had actually seen it occur and whether the respondent thought the interaction was appropriate or not. In one study, nearly 83% of respondents were aware of pharmaceutical promotion in general (Semin et al. 2006), while another study revealed that 54% of respondents were aware of gifts given to physicians by the pharmaceutical industry (Gibbons et al. 1998). However, one study suggested that less than one-quarter of patients in research trials were aware of financial interactions in clinical trials (Hampson et al. 2006). Furthermore, another study using a focus group design suggested that a minority of participants were aware of potential financial interactions in clinical research (Weinfurt et al. 2006).
TABLE 2.
Awareness of physician–pharmaceutical industry interactions
| Percentage of respondents aware of physician–pharmaceutical industry interactions | |
|---|---|
| Aware (%) | |
| Gifts overall | 544 |
| Office gifts | 822 |
| Personal gifts | 322 |
| Pharmaceutical promotions | 82.79 |
| Small gifts – pen | 55.31 |
| Large gifts – coffee maker | 13.81 |
| CME – medical text | 34.61 |
| Samples – baby formula | 28.61 |
| Drug samples | 871 |
| Meals – dinner | 22.41 |
| Financial interactions in clinical trials | 238 |
Blake and Early, 1995 (self-administered survey, n=486);
Mainous et al. 1995 (telephone survey, n=649);
LaPuma et al. 1995 (self-administered survey, n=200);
Gibbons et al. 1998 (face-to-face survey, n=196);
Eaton 2003 (online poll, n=1,479);
Wall Street Journal Online 2003 (online poll, n=4,173);
Kim et al. 2004 (Internet-administered survey, n=5,478);
Hampson et al. 2006 (face-to-face survey, n=253);
Semin et al. 2006 (face-to-face survey, n=253);
Weinfurt et al. 2006 (focus group, n=139).
CME = continuing medical education
Awareness of specific physician–pharmaceutical industry interactions ranged considerably. One study suggested that respondents have a greater awareness of office gifts (82%) over personal gifts (32%) (Mainous et al. 1995). Another suggested that approximately half of the respondents were aware of small gifts such as pens (55.3%), although fewer respondents were aware of gifts such as coffee makers (13.8%) (Blake and Early 1995). In addition, 34.6% of respondents were aware of CME by way of a medical text (Blake and Early 1995), while 28.6% were aware of baby formula samples and nearly 90% were aware of drug samples (Blake and Early 1995). Furthermore, 22% of respondents were aware of dinners provided by a pharmaceutical company (Blake and Early 1995).
Acceptability of physician–pharmaceutical industry interactions
Eight studies (Blake and Early 1995; Mainous et al. 1995; LaPuma et al. 1995; Gibbons et al. 1998; Eaton 2003; Wall Street Journal Online 2003; Hampson et al. 2006; Semin et al. 2006) examined the acceptability of interactions between physicians and the pharmaceutical industry (Table 3). Five of these studies investigated the acceptability of specific interactions, while an additional two focused on the acceptability of financial interactions in clinical trials, and one looked at the acceptable monetary value of gifts. Considerable variation was observed in the acceptability of physician–pharmaceutical industry interactions. However, there was generally greater acceptability for smaller, less costly gifts, or interactions that directly benefited patients.
TABLE 3.
Acceptability of physician-pharmaceutical industry interactions
| Percentage of respondents in agreement with statements | |||
|---|---|---|---|
| Agree (%) | Do not agree (%) | Unsure (%) | |
| “Gifts are unethical” | 71.29 | 7.9 | 21.39 |
| “Physicians should stop receiving gifts” | 845 | 135 | 25 |
| Percentage of respondents that find specific interactions acceptable | |||
|---|---|---|---|
| Acceptable (%) | Not acceptable (%) | Unsure (%) | |
| Small gifts - pen | 67.31 | 17.51,194 | 13 |
| Small gifts - pocket knife | — | 384 | — |
| Small gifts - mug | — | 234 | — |
| Large gifts - coffee maker | 39.11 | 40.71 | 17.31 |
| CME - sponsorship | 726 | 116 | 186 |
| CME-medical text | 701 | 164, 16.91, 204 | 9.91 |
| CME-video | — | 184 | — |
| CME - conference expenses | 52.71 | 32.51 | 11.51 |
| Samples - baby formula | 41.41 | 44.21 | 10.91 |
| Drug samples | 82.11, 82.59 | 10.39, 7.61,224 | 7.29, 9.31 |
| Meals - lunch | — | 234 | — |
| Meals - dinner | 34.61 | 48.41, 474 | 14.61 |
| Social interactions - cocktail party | 40.51 | 43.41 | 131 |
| Social interactions - golf tournament | 40.31 | 41.61 | 14.61 |
| Social interactions - ice cream social | 55.61 | 281 | 12.81 |
| Travel | — | 594 | — |
| Percentage of respondents that find variable value of gifts acceptable | ||||
|---|---|---|---|---|
| Less than $25 (%) | $25–$1,000 (%) | No limit (%) | Unsure (%) | |
| Office gifts | 92 | 122 | 592 | 222 |
| Personal gifts | 322 | 142 | 332 | 202 |
| Percentage of respondents that find interactions with a pharmaceutical representative acceptable | ||||
|---|---|---|---|---|
| Should meet (%) | Should not meet (%) | Doctor's decision (%) | Unsure (%) | |
| Meeting with PR | 155, 216 | 86, 795 | 646 | 45, 76 |
| Percentage of respondents that find financial interactions in clinical trials acceptable | |||
|---|---|---|---|
| Permitted (%) | Permitted with limits (%) | Prohibit (%) | |
| Consulting | 828 | 58 | 138 |
| Speaking fees | 818 | 58 | 138 |
| Patent royalties | 708 | 58 | 238 |
| Stock ownership | 648 | 88 | 278 |
| Per patient payment | — | — | 563 |
Blake and Early, 1995 (self-administered survey, n=486);
Mainous et al. 1995 (telephone survey, n=649);
LaPuma et al. 1995 (self-administered survey, n=200);
Gibbons et al. 1998 (face-to-face survey, n=196);
Eaton 2003 (online poll, n=1,479);
Wall Street Journal Online 2003 (online poll, n=4,173);
Kim et al. 2004 (Internet-administered survey, n=5,478);
Hampson et al. 2006 (face-to-face survey, n=253);
Semin et al. 2006 (face-to-face survey, n=253);
Weinfurt et al. 2006 (focus group, n=139).
CME = continuing medical education
PR = pharmaceutical representative
Acceptability of Specific Interactions
The majority of respondents in two independent studies agreed with the blanket statements “gifts are unethical” (71.2%) (Semin et al. 2006) and “physicians should stop receiving gifts” (84%) (Eaton 2003). However, when specific interactions were cited, acceptability varied. A range of acceptability was found with regard to small gifts, such as pens, pocketknives and mugs. Few respondents found pens unacceptable (17.5%, Blake and Early 1995; 19%, Gibbons et al. 1998), and a minority of patients found various types of CME, such as medical texts, videos and conference expenses, unacceptable (16.9%–32.5%, Blake and Early 1995; 16%–20%, Gibbons et al. 1998). With regard to industry-sponsored meals, more respondents found dinners unacceptable (47%, Gibbons et al. 1998; 48.4%, Blake and Early 1995) than they did lunches (23%) (Gibbons et al. 1998). Finally, a range of acceptability was found for social interactions, such as cocktail parties, ice cream socials and participation in golf tournaments. Here, an ice cream social was found to be unacceptable by a minority of respondents (28.0%, Blake and Early 1995), while more respondents deemed a cocktail party (43.4%) and golf tournament (41.6%) unacceptable.
Acceptability of Financial Interactions in Clinical Trials
Two studies investigated the acceptability of financial interactions between physicians and the pharmaceutical industry specifically in clinical trials. Of these, one study (Hampson et al. 2006) of cancer patients examined the acceptability of consulting, speaking fees and patent royalties and stock ownership, with 13% to 27% of patients prohibiting the above interactions. A second study (LaPuma et al. 1995) examined the acceptance of per-patient payments from the pharmaceutical industry and reported that 56% of respondents found this interaction unacceptable.
Acceptability of Interaction with Pharmaceutical Representatives
Two online opinion polls examined the acceptability of interactions between physicians and pharmaceutical representatives (PRs) (Eaton 2003; Wall Street Journal Online 2003). The findings of the two studies varied considerably, with one opinion poll suggesting that 79% of respondents believed physicians should not meet with PRs (Eaton 2003). Conversely, nearly two-thirds of respondents (64%) in the second study believed that meeting with a PR should be a doctor's decision (Wall Street Journal Online 2003).
Acceptable Monetary Value of Gifts
One study (Mainous et al. 1995) investigated the acceptable monetary value of both office and personal gifts. Fifty-nine per cent of respondents thought that no monetary limit should be placed on office gifts. In regard to personal gifts, 32% thought they should be valued at less than $25, but a similar percentage of respondents (33%) thought that no limit was needed.
Disclosure of physician–pharmaceutical industry interactions
Five studies (LaPuma et al. 1995; Eaton 2003; Kim et al. 2004; Hampson et al. 2006; Weinfurt et al. 2006) investigated the disclosure of financial interactions between physicians and the pharmaceutical industry (Table 4). Combined, the studies investigated respondents' desire for disclosure of interactions, the importance of disclosing these interactions and whether disclosure was required for informed consent in clinical trials.
TABLE 4.
Disclosure of financial interactions in clinical trials
| Percentage of respondents that desire disclosure of financial interactions | ||||
|---|---|---|---|---|
| Yes (%) | Yes, if above certain monetary value (%) | No (%) | Unsure (%) | |
| Gifts overall | 318, 965 | 268 | 18 | 18 |
| Salary support | 813 | — | — | — |
| Stock ownership | 783 | — | — | — |
| Per-patient payment | 863 | — | — | — |
| Percentage of respondents that find disclosing financial interactions important | |||
|---|---|---|---|
| Extremely important (%)† | Somewhat important (%)† | Little/not very important (%)† | |
| Personal income | 58, 69, 567 | 24, 19, 267 | 17, 12, 187 |
| Researcher patent | 59, 64, 597 | 21, 21, 217 | 20, 15, 207 |
| Researcher stocks | 66, 72, 657 | 17, 15, 197 | 17, 13, 177 |
| Per capita payment | 50, 61, 467 | 27, 23, 267 | 23, 16, 287 |
| Percentage of respondents that require disclosure of financial interactions for informed consent | ||
|---|---|---|
| Yes (%)† | No (%)† | |
| Personal income | 68, 74, 647 | — |
| Researcher patent | 76, 82, 757 | — |
| Researcher stocks | 80, 85, 787 | — |
| Per capita payment | 70, 78, 647 | — |
Blake and Early, 1995 (self-administered survey, n=486);
Mainous et al. 1995 (telephone survey, n=649);
LaPuma et al. 1995 (self-administered survey, n=200);
Gibbons et al. 1998 (face-to-face survey, n=196);
Eaton 2003 (online poll, n=1,479);
Wall Street Journal Online 2003 (online poll, n=4,173);
Kim et al. 2004 (Internet-administered survey, n=5,478);
Hampson et al. 2006 (face-to-face survey, n=253);
Semin et al. 2006 (face-to-face survey, n=253);
Weinfurt et al. 2006 (focus group, n=139)
Results reported as coronary artery disease group (n=2,355), breast cancer group (n=1,006), depression group (n=2,117), respectively
Desire for Disclosure of Financial Interactions
All five of the studies examined respondents' desire for disclosure of financial interactions. An online opinion poll conducted by the British Medical Journal illustrated that 96% of visitors to its website wanted disclosure of interactions between physicians and the pharmaceutical industry (Eaton 2003). Three additional studies investigated patients' desire for disclosure in clinical trials. Seventy-eight per cent to 86% of patients at healthcare centres wanted doctors to disclose stock ownership, personal salary or per-patient fees from a sponsoring company prior to enrolment in a clinical trial (LaPuma et al. 1995). Furthermore, the majority of respondents in another study found the disclosure of interactions between physicians and the pharmaceutical industry “extremely important” (Kim et al. 2004). However, one study (Hampson et al. 2006) found that less than one-third of cancer patients (31%) wanted disclosure of researchers' financial interactions. Additionally, focus group data suggest that potential research participants varied considerably in their desire to know about such financial interactions as salary support, per capita payments, patent ownership and equity holdings (Weinfurt et al. 2006).
Disclosure of Financial Interactions Required for Informed Consent
One study (Kim et al. 2004) examined whether respondents believed disclosure of financial interactions was required for informed consent in clinical trials. The majority of respondents (64%–85%) believed that disclosure of personal salary, patent royalties, stock ownership and per capita payments was required for informed consent.
Perceived effects of physician–pharmaceutical industry interactions
Six studies (Blake and Early 1995; Mainous et al. 1995; Gibbons et al. 1998; Wall Street Journal Online 2003: Semin et al. 2006; Weinfurt et al. 2006) examined the perceived effects of various physician–pharmaceutical industry interactions (Table 5). Four outcomes were measured: the effect on the cost of healthcare, the quality of healthcare, prescribing practices and participation in a clinical trial.
TABLE 5.
Perceived effects of physician–pharmaceutical industry interactions
| Percentage of respondents that perceive effects on the cost of healthcare | ||||
|---|---|---|---|---|
| Increase cost (%) | No effect (%) | Decrease cost (%) | Unsure (%) | |
| Gifts overall | 334, 54.59, 642 | 231, 394, 10.39 | 3.11 | 284, 35.29 |
| Office gifts | 262 | 382 | 192 | 162 |
| Personal gifts | 422 | 302 | 142 | 142 |
| Percentage of respondents that perceive effects on quality of healthcare | ||||
|---|---|---|---|---|
| Negative effect (%) | No effect (%) | Positive effect (%) | Unsure (%) | |
| Office gifts | 132 | 612 | 142 | 122 |
| Personal gifts | 232 | 542 | 82 | 152 |
| Very worried (%) | Somewhat worried (%) | A little worried (%) | Not worried at all (%) | |
|---|---|---|---|---|
| Financial interactions | <18 | 68 | 118 | 808 |
| Percentage of respondents that perceive effects on prescribing practices | |||
|---|---|---|---|
| Influence (%) | Little/No influence (%) | Unsure (%) | |
| Gifts overall | 236, 29.19, 361, 701 | 24.51 | — |
| Small gifts – pen | 8.69, 314 | 76.69 | 14.89 |
| Small gifts – pocket knife | 284 | — | — |
| Small gifts – mug | 314 | — | — |
| Large gifts – medical device | 54.8, 68.39 | 10.9, 25.99 | 19.3, 20.89 |
| Large gifts – car seat cover | 35.39 | 46.19 | 18.69 |
| CME – medical text | 21.69, 384, 374 | 57.79 | 20.79 |
| CME – video | 384 | — | — |
| CME – conference expenses | 37.8, 51.29 | 21.2, 32.49 | 27.6, 29.89 |
| Drug sample | 424 | — | — |
| Meals – lunch | 294 | — | — |
| Meals – dinner | 484, 38.59 | 37.69 | 22.99 |
| Travel | 564, 64.29 | 12.59 | 23.39 |
| Percentage of respondents that would be inclined to participate in a research study following disclosure of physician–pharmaceutical interactions | ||||
|---|---|---|---|---|
| Less inclined (%)† | Depends on amount (%)† | Same as before (%)† | More inclined (%)† | |
| Personal income | 22, 31, 287 | 8, 13, 117 | 53, 46, 507 | 16, 10, 117 |
| Researcher patent | 26, 23, 317 | - | 62, 67, 607 | 12, 11, 97 |
| Researcher stocks | 37, 36, 407 | - | 55, 59, 547 | 8, 5, 67 |
| Per capita payment | 16, 23, 177 | - | 68, 65, 707 | 17, 12, 137 |
| Percentage of respondents that would participate in a clinical trial following disclosure of physician–pharmaceutical interactions | ||||
|---|---|---|---|---|
| Stop participation (%) | No effect on participation (%) | Encourage participation (%) | Other (%) | |
| Patent royalties | 148 | 708 | 78 | 98 |
| Percentage of respondents that would participate in a clinical trial following disclosure of physician–pharmaceutical interactions | ||||
|---|---|---|---|---|
| Stop participation (%) | No effect on participation (%) | Encourage participation (%) | Other (%) | |
| Stock ownership | 118 | 768 | 18 | 118 |
| Consulting | 128 | 758 | 68 | 78 |
| Speaking fees | 98 | 828 | 48 | 68 |
| Would not participate (%)† | Depends on amount (%)† | Still consider participation (%)† | Unsure (%)† | |
|---|---|---|---|---|
| Personal income | 12, 18, 187 | 9, 14, 127 | 79, 67, 707 | — |
| Researcher patent | 13, 11, 177 | — | 75, 72, 687 | 12, 17, 147 |
| Researcher stocks | 17, 14, 207 | — | 65, 61, 597 | 18, 26, 217 |
| Per capita payment | 7, 11, 97 | — | 83, 74, 807 | 10, 15, 117 |
| Percentage of respondents that believe financial interactions influence a physician to enrol patients in a clinical trial | ||
|---|---|---|
| Influence (%) | No Influence (%) | |
| Per-patient payments | 698 | - |
Blake and Early, 1995 (self-administered survey, n=486);
Mainous et al. 1995 (telephone survey, n=649);
LaPuma et al. 1995 (self-administered survey, n=200);
Gibbons et al. 1998 (face-to-face survey, n=196);
Eaton 2003 (online poll, n=1,479);
Wall Street Journal Online 2003 (online poll, n=4,173);
Kim et al. 2004 (Internet-administered survey, n=5,478);
Hampson et al. 2006 (face-to-face survey, n=253);
Semin et al. 2006 (face-to-face survey, n=253);
Weinfurt et al. 2006 (focus group, n=139).
Results reported as coronary artery disease group (n=2,355), breast cancer group (n=1,006), depression group (n=2,117), respectively CME = continuing medical education
Effects on the Cost of Healthcare
Two studies revealed that 33% (Gibbons et al. 1998) and 64% (Blake and Early 1995) of respondents believed that gifts to physicians increased the cost of healthcare. Additionally, one study suggested that 42% of respondents thought personal gifts increased healthcare costs, while only 22% thought the same of office gifts (LaPuma et al. 1995).
Effects on the Quality of Care
Three studies (LaPuma et al. 1995; Hampson et al. 2006; Weinfurt et al. 2006) investigated the perceived effects of gifts from the pharmaceutical industry on the quality of healthcare. In one study, 61% of respondents believed that the acceptance of office gifts had no effect on the quality of healthcare, while 54% thought the same of personal gifts (Mainous et al. 1995). A study of cancer patients in research trials revealed that the majority of respondents (80%) were “not worried at all” about financial interactions between physicians and the pharmaceutical industry (Hampson et al. 2006). Additionally, a study using focus groups noted that several patients thought financial interactions in clinical trials would make a physician do a better job (Weinfurt et al. 2006).
Effects on Prescribing Practices
Four studies (Blake and Early 1995; Gibbons et al. 1998; Wall Street Journal Online 2003; Semin et al. 2006) examined the effect of physician–pharmaceutical industry interactions on prescribing practices. Twenty-three per cent (Wall Street Journal Online 2003) and 70% (Blake and Early 1995) of respondents believed that gifts in general influenced physicians' prescribing. With regard to specific interactions, a minority of respondents believed that small gifts such as a pen, pocket knife or mug influenced prescribing practices (8.6%, Semin et al. 2006; 31%, Gibbons et al. 1998). A gift such as a car seat cover was thought to influence prescribing by 35.3% (Semin et al. 2006) of respondents, while 54.8%–68.3% (Semin et al. 2006) of respondents thought that a larger gift such as a medical device affected prescribing. CME, such as payment for conference expenses, a medical text and an educational video, were thought to influence prescribing by 21.6%–51.2% (Semin et al. 2006) of respondents, depending on the specific interaction. Furthermore, 42% of patients thought that drug samples influenced prescribing choice (Gibbons et al. 1998), and meals were thought to affect prescribing by 29%–48% (Gibbons et al. 1998) of respondents. Finally, the majority of respondents (56%, Gibbons et al. 1998; 64.2%, Semin et al. 2006) thought that sponsorship of travel would influence a physician's prescription choices.
Effects on Clinical Trial Participation
Three studies (LaPuma et al. 1995; Kim et al. 2004; Hampson et al. 2006) investigated whether the disclosure of various financial interactions would affect clinical trial participation. Two studies revealed that the majority of patients would still participate in a trial, given disclosure of financial interactions. Specifically, one study found that 70% to 82% of potential participants would still participate, depending on the financial interaction, such as patent royalties, stock ownership, consulting and speaking fees; however, the specific amount of financial interactions was not examined (Hampson et al. 2006). An additional study found similar results, where 59% to 83% of patients would still participate in a trial depending on the physician–pharmaceutical industry interaction, where personal income, researcher patent, researcher stocks and per capita payments were investigated (Kim et al. 2004). Finally, 69% of respondents believed that per-patient payments would influence physicians to enrol patients into a clinical trial (LaPuma et al. 1995).
Interpretation
No studies were found that examined Canadian opinions on the issue of physician interactions with the pharmaceutical industry. However, 10 studies were identified that examined perceptions of international respondents. All but three of these 10 studies exclusively investigated American perceptions, while one survey examined patient opinions in Turkey and two others examined opinions of international participants. Although findings of American opinions are often generalized to Canadians, factors such as differing healthcare systems, differing policies regarding direct-to-consumer advertising (DTCA) and possible differing attitudes towards private enterprises may cause differences in opinions regarding physician–pharmaceutical industry interactions between these two populations. In the US, full product drug advertisements are allowed and are heavily used, as opposed to the disease-oriented reminder advertisements that are permitted in Canada (Mintzes 2006; Wilkes et al. 2000). While many Canadians also see American advertisements, studies suggest that Americans have greater exposure to DTCA than their Canadian counterparts (Mintzes et al. 2002, 2003).
We found evidence of considerable variation in public awareness, acceptability and perceived effects of potential physician–pharmaceutical industry interactions. There appears to be greater acceptability and fewer perceived effects for smaller, less costly gifts that directly benefit patients or the medical practice. Conversely, desire for disclosure of these interactions was consistent among the majority of participants. As suggested previously, we also found some evidence of differences in the opinions of the public and those of physicians on physician–pharmaceutical industry interactions. One of the studies included in this review (Gibbons et al. 1998) reported that patients generally perceived pharmaceutical gifts to be less appropriate and more influential on prescribing than did those physicians who were surveyed.
Although 10 studies of physician–pharmaceutical industry interactions were identified in the review, research in the area is limited and fragmented: studies investigated public opinions of different types of physician–pharmaceutical industry interactions, using different populations in distinct settings. Furthermore, the majority of studies were individually narrow in scope, focusing on a specific type of interaction (for example, financial interactions). The few studies that have explored a range of potential physician–pharmaceutical interactions are now dated and may not capture the opinions of the current population, particularly given the negative media attention that the pharmaceutical industry has received in recent years (Psaty and Kronmal 2008; Puttagunta et al. 2002; Kondro 2004; Olivieri 2003). Additionally, the majority of studies examined the opinions of specific patient populations, rather than the general public. Patients with cancer, for example, may not be representative of the general public. It is likely that patients with cancer whose only access to a potentially effective chemotherapy is through entry in a clinical trial will be much less concerned about issues of conflict of interest than a healthy recent university graduate, for example.
Limitations
Although our review followed established methodologies, it had several limitations. No databases or journals were hand-searched for studies. Also, only one reviewer was responsible for determining whether studies met pre-defined inclusion criteria, and no quantitative analysis could be conducted – therefore, meta-analysis was impossible. Additionally, only the interactions between physicians and the pharmaceutical industry were examined in this review, although many healthcare professionals interact with the pharmaceutical industry on a regular basis. Finally, we did not consider the degree of sophistication of study participants and whether they recognized the association between interactions and potential adverse effects, or whether they were familiar with principles of conflict of interest – factors that have been considered in previous observational studies (Spingarn et al. 1996; Taylor and Bond 1991; Lurie et al. 1990; Avorn et al. 1982; Orlowski and Wateska 1992; Bowman and Pearle 1988).
Conclusion
Comprehensive research in this area should be undertaken to determine the opinions of the Canadian public on potential physician–pharmaceutical industry interactions in order to inform and direct policy regarding COI. This is important for four main reasons. First, private citizens are key stakeholders in the healthcare system, and therefore their opinions are important. Public trust in the largely publicly funded system is likely to be important to its ongoing efficiency. Second, the research may alert policy makers that the public needs further education on the consequences, both positive and negative, of physician–pharmaceutical industry interactions. Third, whatever is learned about these interactions is likely to be useful for further investigation of other health professions. Fourth, given the evidence found of varying opinions from other countries – divergence of opinion between the public and physicians – studying the Canadian context is essential to inform health professionals' current conflict-of-interest policies and guidelines.
Contributor Information
Janine Arkinson, Centre for Evaluation of Medicines, Hamilton, ON.
Anne Holbrook, Director, Division of Clinical Pharmacology & Therapeutics, McMaster University, Hamilton, ON.
Wojciech Wiercioch, Centre for Evaluation of Medicines, Hamilton, ON.
References
- Avorn J., Chen M., Hartley R. Scientific versus Commercial Sources of Influence on the Prescribing Behavior of Physicians. American Journal of Medicine. 1982;73(1):4–8. doi: 10.1016/0002-9343(82)90911-1. [DOI] [PubMed] [Google Scholar]
- Blake R.L., Jr, Early E.K. Patients' Attitudes about Gifts to Physicians from Pharmaceutical Companies. Journal of the American Board of Family Medicine. 1995;8(6):457–64. [PubMed] [Google Scholar]
- Blumenthal D. Doctors and Drug Companies. New England Journal of Medicine. 2004;351(18):1885–90. doi: 10.1056/NEJMhpr042734. [DOI] [PubMed] [Google Scholar]
- Bowman M.A., Pearle D.L. Changes in Drug Prescribing Patterns Related to Commercial Company Funding of Continuing Medical Education. Journal of Continuing Education in the Health Professions. 1988;8(1):13–20. doi: 10.1002/chp.4750080104. [DOI] [PubMed] [Google Scholar]
- Brennan T.A., Mello M.M. Sunshine Laws and the Pharmaceutical Industry. Journal of the American Medical Association. 2007;297(11):1255–57. doi: 10.1001/jama.297.11.1255. [DOI] [PubMed] [Google Scholar]
- Campbell E.G., Gruen R.L., Mountford J., Miller L.G., Cleary P.D., Blumenthal D. A National Survey of Physician–Industry Relationships. New England Journal of Medicine. 2007;356(17):1742–50. doi: 10.1056/NEJMsa064508. [DOI] [PubMed] [Google Scholar]
- Canada's Research-Based Pharmaceutical Companies. Principles & Integrity: Code of Conduct. Ottawa: Author; 2007. [Google Scholar]
- Canadian Agency for Drugs and Technologies in Health (CADTH) Conflict of Interest Guidelines for the Common Drug Review. Ottawa: Author; 2006a. [Google Scholar]
- Canadian Agency for Drugs and Technologies in Health (CADTH) Call for Public Members for Canadian Expert Drug Advisory Committee (CEDAC) and Compus Expert Review Committee (CERC) Ottawa: Author; 2006b. [Google Scholar]
- Canadian Medical Association (CMA) Guidelines for Interactions with Industry. Ottawa: Author; 2007. [Google Scholar]
- College of Family Physicians of Canada (CFPC) By-laws of the College of Family Physicians of Canada. Mississauga, ON: Author; 2006. [Google Scholar]
- Eaton L. Readers Want Transparency in Link between Doctors and Drug Firms. British Medical Journal. 2003;326(7403):1352. doi: 10.1136/bmj.326.7403.1352-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R.V., Landry F.J., Blouch D.L., Jones D.L., Williams F.K., Lucey C.R., Kroenke K. A Comparison of Physicians' and Patients' Attitudes toward Pharmaceutical Industry Gifts. Journal of General Internal Medicine. 1998;13(3):151–54. doi: 10.1046/j.1525-1497.1998.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson L.A., Agrawal M., Joffe S., Gross C.P., Verter J., Emanuel E.J. Patients' Views on Financial Conflicts of Interest in Cancer Research Trials. New England Journal of Medicine. 2006;355(22):2330–37. doi: 10.1056/NEJMsa064160. [DOI] [PubMed] [Google Scholar]
- Higgins S.P. Drug Representatives: Giving You Lunch or Stealing Your Soul? Dermatology Online Journal. 2007;13(4):5. [PubMed] [Google Scholar]
- Holmer A.F. Industry Strongly Supports Continuing Medical Education. Journal of the American Medical Association. 2001;285(15):2012–14. doi: 10.1001/jama.285.15.2012. [DOI] [PubMed] [Google Scholar]
- Holmes D.R., Firth B.G., James A., Winslow R., Hodgson P.K., Gamble G.L., Popp R.L., Harrington R.A. Conflict of Interest. American Heart Journal. 2004;147(2):228–37. doi: 10.1016/j.ahj.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Kaiser Family Foundation. National Survey of Physicians. Part II: Doctors and Prescription Drugs. Washington, DC: Author; 2002. [Google Scholar]
- Katz D., Caplan A.L., Merz J.F. All Gifts Large and Small. American Journal of Bioethics. 2003;3(3):39–46. doi: 10.1162/15265160360706552. [DOI] [PubMed] [Google Scholar]
- Kim S.Y., Millard R.W., Nisbet P., Cox C., Caine E.D. Potential Research Participants' Views Regarding Researcher and Institutional Financial Conflicts of Interest. Journal of Medical Ethics. 2004;30(1):73–79. doi: 10.1136/jme.2002.001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondro W. Lawsuits Mount in Wake of Rofecoxib (Vioxx) Withdrawal. Canadian Medical Association Journal. 2004;1335;171(11) doi: 10.1503/cmaj.1041692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert A. Comment on Doctors and Drug Companies. New England Journal of Medicine. 2005;352(7):733–34. doi: 10.1056/NEJM200502173520722. [DOI] [PubMed] [Google Scholar]
- LaPuma J., Stocking C.B., Rhoades W.D., Darling C.M., Ferner R.E., Neuberger J., VandenBurg M., Dews I., Tobias J.S. Financial Ties as Part of Informed Consent to Postmarketing Research: Attitudes of American Doctors and Patients. British Medical Journal. 1995;310(6995):1660–63. doi: 10.1136/bmj.310.6995.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie N., Rich E.C., Simpson D.R., Meyer J., Schiedermayer D.L., Goodman J.L., McKinney W.P. Pharmaceutical Representatives in Academic Medical Centers: Interaction with Faculty and Housestaff. Journal of General Internal Medicine. 1990;5(3):240–43. doi: 10.1007/BF02600542. [DOI] [PubMed] [Google Scholar]
- Mainous A.G., III, Hueston W.J., Rich E.C. Patients' Perceptions of Physician Acceptance of Gifts from the Pharmaceutical Industry. Archives of Family Medicine. 1995;4(4):335–39. doi: 10.1001/archfami.4.4.335. [DOI] [PubMed] [Google Scholar]
- Marco C.A., Moskop J.C., Solomon R.C., Geiderman J.M., Larkin G.L. Gifts to Physicians from the Pharmaceutical Industry: An Ethical Analysis. Annals of Emergency Medicine. 2006;48(5):513–21. doi: 10.1016/j.annemergmed.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Ministry of Health and Long-Term Care. Transparent Drug System for Patients Act 2006. 2006. Retrieved March 25, 2010. < http://www.health.gov.on.ca/english/public/legislation/drugs/hu_drugsact.html>.
- Mintzes B. Direct-to-Consumer Advertising of Prescription Drugs in Canada: What Are the Public Health Implications? Toronto: Health Council of Canada; 2006. [Google Scholar]
- Mintzes B., Barer M.L., Kravitz R.L., Bassett K., Lexchin J., Kazanijan A., Evans R.G., Pan R., Marion S.A. How Does Direct-to-Consumer Advertising (DTCA) Affect Prescribing? A Survey in Primary Care Environments with and without Legal DTCA. Canadian Medical Association Journal. 2003;169(5):405–12. [PMC free article] [PubMed] [Google Scholar]
- Mintzes B., Barer M.L., Kravitz R.L., Kazanijan A., Bassett K., Lexchin J., Evans R.G., Pan R., Marion S.A. Influence of Direct to Consumer Pharmaceutical Advertising and Patients' Requests on Prescribing Decisions: Two-Site Cross-Sectional Survey. British Medical Journal. 2002;324(7342):278–79. doi: 10.1136/bmj.324.7332.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan R. Who Pays for the Pizza? Redefining the Relationship between Doctors and Drug Companies. 1: Entanglement. British Medical Journal. 2003;326(7400):1189–92. doi: 10.1136/bmj.326.7400.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri N.F. Patients' Health or Company Profits? The Commercialization of Academic Research. Science and Engineering Ethics. 2003;9(1):29–41. doi: 10.1007/s11948-003-0017-x. [DOI] [PubMed] [Google Scholar]
- Orlowski J.P., Wateska L. The Effects of Pharmaceutical Firm Enticements on Physician Prescribing Patterns. There's No Such Thing as a Free Lunch. Chest. 1992;102(1):270–73. doi: 10.1378/chest.102.1.270. [DOI] [PubMed] [Google Scholar]
- Psaty B.M., Kronmal R.A. Reporting Mortality Findings in Trials of Refecoxib for Alzheimer Disease or Cognitive Impairment: A Case Study Based on Documents from Rofecoxib Litigation. Journal of the American Medical Association. 2008;299(15):1813–17. doi: 10.1001/jama.299.15.1813. [DOI] [PubMed] [Google Scholar]
- Puttagunta P.S., Caulfield T.A., Griener G. Conflict of Interest in Clinical Research: Direct Payment to the Investigators for Finding Human Subjects and Health Information. Health Law Review. 2002;10(2):30–32. [PubMed] [Google Scholar]
- Ross J.S., Hill K.P., Egilman D.S., Krumholz H.M. Guest Authorship and Ghostwriting in Publications Related to Rofecoxib. Journal of the American Medical Association. 2008;299(15):1800–12. doi: 10.1001/jama.299.15.1800. [DOI] [PubMed] [Google Scholar]
- Royal College of Physicians and Surgeons of Canada (RCPSC) Physicians and Industry – Conflicts of Interest. Ottawa: Author; 2005. [Google Scholar]
- Semin S., Guldal D., Ozcaker N., Mevsim V. What Patients Think about Promotional Activities of Pharmaceutical Companies in Turkey. Pharmacy World & Science. 2006;28(4):199–206. doi: 10.1007/s11096-006-9032-8. [DOI] [PubMed] [Google Scholar]
- Spingarn R.W., Berlin J.A., Strom B.L. When Pharmaceutical Manufacturers' Employees Present Grand Rounds, What Do Residents Remember? Academic Medicine. 1996;71(1):86–88. doi: 10.1097/00001888-199601000-00022. [DOI] [PubMed] [Google Scholar]
- Stossel T.P. Regulating Academic–Industrial Research Relationships – Solving Problems or Stifling Progress? New England Journal of Medicine. 2005;353(10):1060–65. doi: 10.1056/NEJMsb051758. [DOI] [PubMed] [Google Scholar]
- Taylor R.J., Bond C.M. Change in the Established Prescribing Habits of General Practitioners: An Analysis of Initial Prescriptions in General Practice. British Journal of General Practice. 1991;41(347):244–48. [PMC free article] [PubMed] [Google Scholar]
- Wall Street Journal Online. Americans Have Few Concerns about How Pharmaceutical Companies Market to Doctors. Poll conducted by Harris Interactive. 2003 [Google Scholar]
- Wazana A. Physicians and the Pharmaceutical Industry: Is a Gift Ever Just a Gift? Journal of the American Medical Association. 2000;283(3):373–80. doi: 10.1001/jama.283.3.373. [DOI] [PubMed] [Google Scholar]
- Weinfurt K.P., Frieman J.Y., Allsbrook J.S., Dinan M.A., Hall M.A., Sugarman J. Views of Potential Research Participants on Financial Conflicts of Interest: Barriers and Opportunities for Effective Disclosure. Journal of General Internal Medicine. 2006;21(9):901–06. doi: 10.1111/j.1525-1497.2006.00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes M.S., Bell R.A., Kravitz R.L. Direct-to-Consumer Prescription Drug Advertising: Trends, Impact, and Implications. Health Affairs (Millwood) 2000;19(2):110–28. doi: 10.1377/hlthaff.19.2.110. [DOI] [PubMed] [Google Scholar]