Abstract
Objective:
Dominant, left anteromedial temporal lobe resection (AMTLR) for seizure control carries risks to verbal episodic memory and visual object naming. Consistent with traditional thinking, verbal memory decline is considered a consequence of hippocampal removal and naming decline has been attributed to lateral temporal resection. Interestingly, recent findings suggest a potential relation between visual naming and hippocampal integrity, which is consistent with studies that link the hippocampus with higher level visual processing. Historically, naming has been evaluated using visual object naming tasks; however, naming can also be assessed using auditory verbal descriptions. Recent cortical stimulation studies have shown a neuroanatomic distinction between visual naming and auditory description naming. We speculated that unlike visual naming, the hippocampus is not involved in auditory naming, and hypothesized that left AMTLR would not result in auditory naming decline, despite visual naming and verbal memory decline.
Methods:
In this cohort study, we tested auditory naming, visual naming, and verbal memory in 25 left medial temporal lobe epilepsy (MTLE) and 20 right MTLE patients pre-AMTLR and 1 year post-AMTLR.
Results:
Left AMTLR patients declined in visual naming and verbal memory, with no decline in auditory naming. Right AMTLR patients exhibited no decline.
Conclusions:
Results suggest that left anteromedial temporal lobe resection presents a greater risk to visual naming than auditory naming in patients with left medial temporal lobe epilepsy.
GLOSSARY
- AMTLR
= anteromedial temporal lobe resection;
- ANOVA
= analysis of variance;
- ANT
= Auditory Naming Test;
- BNT
= Boston Naming Test;
- CVLT
= California Verbal Learning Test;
- HS
= hippocampal sclerosis;
- MTLE
= medial temporal lobe epilepsy;
- RT
= response time;
- TOT
= tip-of-the-tongue;
- VNT
= Visual Naming Test.
Left anteromedial temporal lobe resection (AMTLR) for seizure control is associated with naming and verbal memory decline.1 Historically, naming has been assessed using visual confrontation naming tasks. Recent studies have also employed auditory description naming (“auditory naming”), which requires naming based on orally presented descriptions.2,3 Using neurostimulation mapping, we found anterior temporal stimulation more likely disrupts auditory naming, whereas posterior temporal stimulation typically disrupts both auditory and visual naming.4 Similar to visual naming,5,6 removing auditory naming sites results in naming decline.7
The posterior location of visual naming sites8,9 might appear puzzling given consistent reports of postoperative visual naming decline. However, some studies suggest that visual naming decline following left AMTLR might actually be related to hippocampal resection.10–13 This is consistent with investigations describing a close link between the hippocampus and higher level visual processing.14 To our knowledge, there are no analogous reports regarding higher level auditory processing and the hippocampus; thus, auditory-based cognitive processing might not be as closely associated with the hippocampus. That auditory naming sites tend to cluster in anterior temporal cortex might suggest that AMTLR may be, in particular, detrimental to auditory naming. However, in patients with longstanding left medial TLE (MTLE), auditory naming sites tended to be shifted posteriorly, approximately 4.5 cm from the temporal pole, and consequently are less likely to have auditory naming sites within the anterior 3.5 cm removed with AMTLR.15 We therefore hypothesized that auditory naming would not decline following left AMTLR, despite decline in visual naming and verbal memory.
To test this, we examined preoperative and postoperative auditory naming, visual naming, and verbal memory in left language-dominant patients with left or right MTLE. We included right MTLE patients and verbal memory testing to ensure groups would demonstrate well-established, postoperative changes.
METHODS
Subjects.
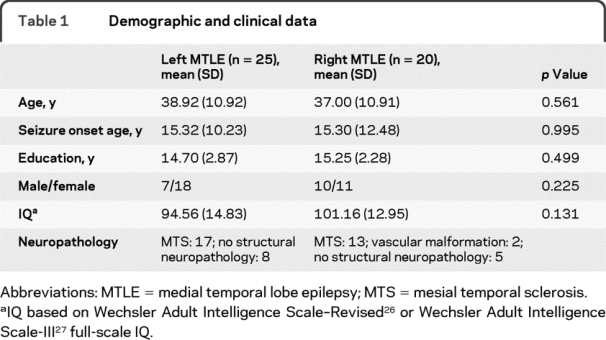
Participants were 45 consecutive unilateral MTLE patients at Columbia University Medical Center (25 left, 20 right) who met inclusion criteria, completed preoperative and postoperative auditory and visual naming tests, and underwent unilateral AMTLR between February 2000 and February 2008. For diagnosis, patients had a medial temporal lesion (n = 2) or unilateral hippocampal sclerosis (HS) on histopathologic analysis and seizures consistent with MTLE from video-EEG (n = 30). In the absence of HS, medial seizure onset was determined via unilateral depth and grid implantation (n = 13). Patients with multifocal brain abnormalities or dual pathology were excluded. Patients were native English speakers, or learned English before age 5 and were fully educated in English. Patients were also required to have unambiguous evidence of left hemisphere language dominance. This was determined by intracarotid amobarbital testing16 in all patients, with the exception of one right-handed, right MTLE patient, with neuropsychological and postictal testing17 consistent with left hemisphere language dominance. Demographic and clinical information are presented in table 1. There were no significant group differences in age, education, IQ, or gender.
Table 1 Demographic and clinical data
Standard protocol approvals, registrations and consents.
This study was approved by the Institutional Review Board at Columbia University Medical Center. Written informed consent was obtained from all patients participating in this study.
Preoperative and postoperative testing.
In this cohort study, patients completed 3 naming instruments: 50-item Auditory Naming Test (ANT),2 50-item Visual Naming Test (VNT),2 and 60-item Boston Naming Test (BNT).18 The ANT requires naming orally described items; the VNT and BNT require naming line-drawn objects (VNT and BNT items are distinct). ANT and VNT instructions emphasize rapid responding, with a 20-second time limit, and normative data exist for number correct, response time (RT), and tip-of-the-tongue (TOT, defined as number of correct responses 2–20 seconds post stimulus presentation, or only following phonemic cueing). BNT allows 20 seconds to name stimuli, with normative data for only number correct. We calculated an additional measure comprised of items not named within 20 seconds, yet subsequently named following phonemic cueing (BNTcue). The VNT differs from the BNT in word frequency of picture names; the VNT contains familiar, mid- to high-frequency items whereas the BNT contains several low-frequency items (e.g., protractor), confounding naming with vocabulary demands.
Two scores from the California Verbal Learning Test (CVLT),19 short-delay free recall and long-delay free recall, assessed episodic memory. CVLT short-delay measures the total number of words recalled following 5 consecutive presentations of a 16-word list and a single presentation of a distractor list. Long-delay recall measures the number of items recalled following a 20-minute delay. Patients also completed a 7-point Likert-type scale rating frequency of word-finding difficulty.
Patients were tested preoperatively and approximately 1 year postoperatively (left AMTLR: mean = 15.0 months, SD = 6.2; right AMTLR: mean = 20.1, SD = 16.5), with no group differences (p = 0.18).
Surgical procedure.
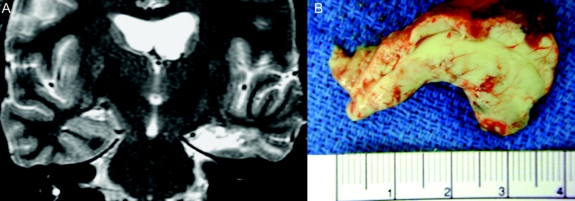
Patients underwent AMTLR, as described by Spencer et al.20 This includes resection of up to 3.5 cm of the anterior, middle, and inferior temporal gyri, removal of the uncus/amygdala (without extending superior to the level of the anterior choroid fissure), and radical resection of the hippocampus (including the tail, posterior to the crossing of the choroid plexus over the hippocampus) and adjacent parahippocampal gyrus. Hippocampal resection was performed en bloc, with a 3.5- to 4-cm-length specimen (specimen measured without “straightening”). Resection size varied only in so far as the size of the hippocampus varies among patients. All patients had postoperative MRI scans confirming hippocampal removal (figure 1).
Figure 1 Anteromedial temporal lobe resection from a representative study patient
(A) Postoperative MRI scan at the level of the body of the hippocampus. Resected structures include hippocampus, parahippocampal gyrus, and a portion of the fusiform gyrus. (B) Hippocampal specimen with small amount of adjacent brain tissue (mainly parahippocampal gyrus). Note that it measures 3.5 cm in its natural configuration, and that it includes the hippocampal tail.
Statistical analyses.
Independent sample t tests and χ2 analyses were performed to assess group differences in age, education, IQ, and gender. Group differences in naming and memory scores were assessed via multivariate analysis of variance (ANOVA). McNemar symmetry χ2 examined the relative proportion of patients who declined in auditory vs visual naming. Pearson correlations assessed the relation between naming and memory scores. For post hoc analysis of individual patients, we operationally defined meaningful decline as pre–postoperative performance reduction ≥1.5 SD, consistent with recently used methods.21
RESULTS
Results of 1-way, multivariate ANOVA comparing preoperative auditory and visual naming scores (table 2) between right and left groups revealed poorer naming performance among left MTLE patients for ANT number correct, RT and TOT, VNT RT, BNT, and BNTcue (all p < 0.05; VNT TOT: p = 0.06). Groups showed no significant difference for VNT number correct.
Table 2 Preoperative and postoperative auditory and visual naming scores and results of repeated measures multivariate analysis of variance
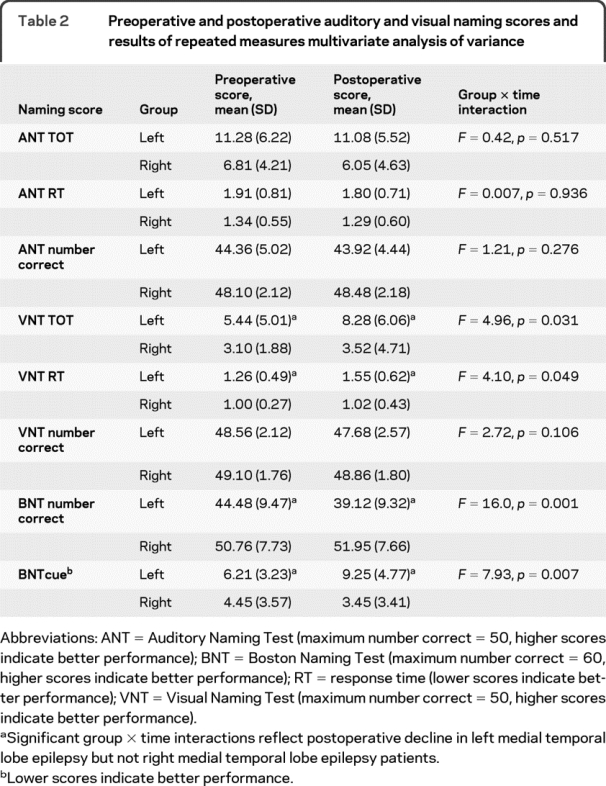
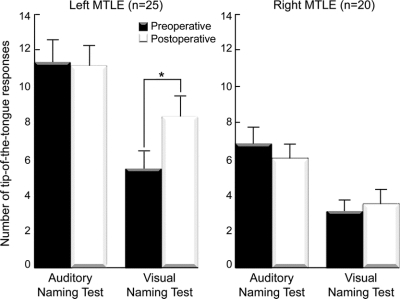
With respect to postoperative change, results of repeated measures, multivariate 2-way (group by time) ANOVA revealed main effects of group for all naming scores (all p < 0.01) except ANT and VNT number correct (both p > 0.09). A main effect of time was evident only for BNT (p = 0.02). Most importantly, main effects were modified by significant group by time interactions for VN RT, VN TOT, BNT, and BNTcue, reflecting postoperative decline in visual naming, but not auditory naming in the left MTLE group (table 2, figure 2). There were no interactions for VN number correct or any auditory naming scores (all p > 0.30).
Figure 2 Preoperative and postoperative naming performance
Higher scores indicate poorer performance. Error bars indicate SEM. *p < 0.05.
Given the left MTLE group naming findings, we examined the proportion of patients who declined in auditory and visual naming. Patients were classified as having declined if any ANT, VNT, or BNT scores declined postoperatively ≥1.5 SD (excluding BNTcue due to absent normative data). Among left MTLE patients, 6 (24%) declined in auditory naming, only 1 of which was auditory naming exclusive, whereas 16 (64%) declined on visual naming, 10 of which were visual naming exclusive. McNemar symmetry χ2 (p = 0.007) suggested a higher probability of visual naming decline, contrasted with a lower probability of exclusive auditory naming decline.
In light of previous reports of more severe visual naming decline in patients without HS relative to patients with HS, we attempted to examine these 2 subgroups separately. Unfortunately, the current sample of left MTLE patients contained only 8 patients without HS, which is insufficient for valid statistical analysis. Similar to previous reports, qualitatively, visual naming decline appeared greater in patients without HS. To illustrate using TOT, which is the most sensitive naming performance measure,2 mean pre–postoperative VNT TOT difference scores from the 2 left MTLE subgroups were as follows: left non-HS: 4.4 (6.8), left HS: 2.1 (5.1). This pattern was not observed among right MTLE patients: right non-HS: −0.43 (2.3); right HS: 0.54 (1.5). Other subgroup test scores are available in table e-1 on the Neurology® Web site at www.neurology.org.
Regarding CVLT scores, results of 1-way, multivariate ANOVA comparing preoperative CVLT scores between groups revealed poorer CVLT short delay and long delay scores in the left MTLE group (both p < 0.01). Results of repeated measures, multivariate 2-way (group by time) ANOVA revealed main effects of both group and time (all p < 0.01). Most importantly, these were modified by group by time interactions (table 3), reflecting postoperative memory decline in the left, but not the right MTLE group.
Table 3 Preoperative and postoperative CVLT scores and results of repeated measures multivariate analysis of variance
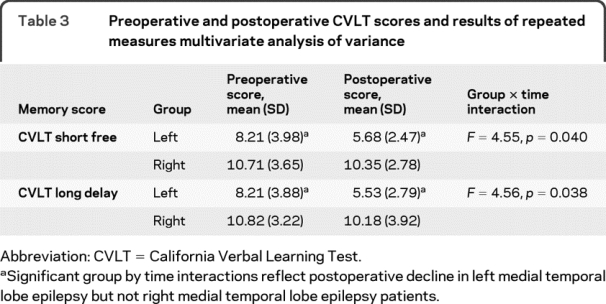
Postoperative decline in both visual naming and verbal memory might suggest a common neural substrate for these 2 functions, and potentially, common cognitive mechanisms. However, Pearson correlations revealed no relation between naming and verbal memory scores (all p > 0.09). Unexpectedly, with the exception of RT, visual naming and verbal memory correlated in right MTLE patients (p < 0.03).
Consistent with previous findings, Pearson correlations indicated that subjective word-finding difficulty correlated with Auditory Naming TOT (r = 0.31, p = 0.04) and RT (r = 0.32, p = 0.03), yet not with any visual naming measures (all p > 0.26).
Seizure outcome.
Given the clinical significance of seizure freedom, seizure outcome was analyzed by dichotomizing patients as seizure free (i.e., Engel classification,22 class I) vs not seizure free (i.e., classes II, III, and IV). Results of Fisher exact analysis indicated no group difference in the proportion of seizure-free patients (p = 0.71). Seizure outcome rates in each group were as follows: left MTLE: class I: 76%, class II: 20%, class III: 0, class IV: 4%; right MTLE: class I: 85%, class II: 5%, class III: 5%, class IV: 5%.
DISCUSSION
Declines in verbal memory and visual naming are a common consequence of left AMTLR in patients with left MTLE. To investigate whether auditory naming might also be at risk, we tested auditory naming, visual naming, and verbal memory before and after AMTLR in patients with left or right MTLE. Consistent with well-established findings, left MTLE patients exhibited significant visual naming and verbal memory decline. In contrast, and consistent with our hypothesis, auditory naming remained relatively stable postoperatively. As expected, visual naming, auditory naming, or verbal memory did not decline following right AMTLR.
Consistent with previous findings, preoperatively, auditory naming was poorer than visual naming in left MTLE patients, and auditory naming was poorer in left than right MTLE patients.2 Accordingly, the stability of auditory naming following left AMTLR could potentially be explained as an absence of change due to impaired baseline performance. However, visual naming was also impaired at baseline, yet declined postoperatively, and auditory naming, while relatively impaired, was nowhere near floor levels in the left MTLE group. Thus, decline in visual but not auditory naming appeared to reflect differential effects of left temporal resection.
For patients considering left AMTLR, potential changes in memory and language are often a significant concern and must be weighed against the benefits of improved seizure outcome. Although numerous studies report visual naming decline, to our knowledge, effects of AMTLR on auditory naming have not yet been described. Prior to dominant temporal resection, patients are typically counseled regarding the risk of naming decline, with naming portrayed as a unitary entity. Given the current data, rather than discussing the risk of global naming decline, perhaps patients can be informed of the well-documented risk of visual naming decline, with potentially lower risk to auditory naming. Moreover, as auditory naming but not visual naming correlates significantly with subjective ratings of word-finding difficulty, arguably, auditory naming might better reflect everyday word-finding difficulty.2
Another clinical implication involves the use of stimulation language mapping to preserve naming. Investigators reporting a relation between visual naming and hippocampus contend that mapping may have minimal value if naming decline is inevitable with hippocampal removal.10,12 Although group analyses indicated significant visual naming decline in the left MTLE sample, individual patient analysis revealed variability in naming outcome, indicating that naming decline is not necessarily inevitable. As it is quite unlikely that visual naming is mediated solely by the hippocampus, it might be important to determine whether naming sites are located in neocortical regions within resection boundaries. Additionally, given the discrepancies between visual and auditory naming observed here and in other studies, perhaps caution is warranted in drawing generalizations regarding naming based only on visual naming results. Most relevant, we have found that removal of stimulation-identified auditory naming sites is associated with postoperative decline on both visual and auditory naming measures.7
With respect to neuroanatomic correlates, verbal memory decline following left AMTLR is understood to be a direct consequence of hippocampal removal and naming decline has typically been attributed to resection of lateral temporal cortex. This reasoning is in line with traditional neurocognitive models of memory in which episodic verbal memory is mediated by the left hippocampal region and naming (an aspect of semantic memory) is supported by left lateral temporal neocortex.23,24 Potentially at odds with this view, however, are findings that suggest a relation between visual naming and the integrity of the hippocampus. These findings include poorer visual naming in patients with left HS compared to those with structurally normal hippocampi,10 greater visual naming decline following left AMTLR in patients with structurally normal left hippocampi than in patients with compromised hippocampal integrity due to HS,11 and significant correlations between hippocampal metabolism, measured by 1H- magnetic resonance spectroscopy, and visual naming performance.12,13 The current visual naming results are consistent with these reports, and suggest that, despite its critical role in visual naming, the hippocampus may not play an essential role in auditory naming, at least in individuals with left MTLE.
With regard to study limitations, the current results might not generalize to normal individuals or other clinical populations. Our population included only MTLE patients who may have undergone developmental changes related to early brain insult and neural plasticity. Additionally, clinically based research frequently does not allow control of potentially important factors. Although our focus was the effect of hippocampal removal on auditory naming, AMTLR removes additional medial structures and portions of lateral temporal cortex. Assessment of auditory and visual naming in patients who undergo targeted hippocampectomy, perhaps via gamma knife surgery,25 might better answer questions regarding unique hippocampal contribution to modality-specific naming processes. Additionally, generalizations from the current study might be limited by the relatively small sample. Nevertheless, results suggest relatively preserved auditory naming despite visual naming decline in left MTLE patients who undergo left AMTLR, which is useful information for physicians counseling these patients regarding surgical risks.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Marla J. Hamberger.
ACKNOWLEDGMENT
The authors thank Alicia Williams, MA, for assistance with data management.
DISCLOSURE
Dr. Hamberger serves on the editorial board of Epilepsy and Behavior and receives research support from the NIH (NINDS R01 NS35140 [PI]). Dr. Seidel reports no disclosures. Dr. McKhann serves on the editorial board of Neurosurgery; receives royalties for the publication of Fundamentals of Operative Techniques in Neurosurgery (Thieme, 2002); receives research support from the NOH (K08 NS 48064 [PI]), the Tuberous Sclerosis Alliance; and is involved (without financial support) in clinical trials supported by Neuropace, Inc. and St. Jude Medical/Advanced Neuromodulation Systems. Dr. Goodman serves on a scientific advisory board for Neuropace, Inc. and is involved (without financial support) in clinical trials supported by Neuropace, Inc. and St. Jude Medical/Advanced Neuromodulation Systems.
Supplementary Material
Address correspondence and reprint requests to Dr. Marla J. Hamberger, The Neurological Institute, 710 West 168th Street, Box 100, New York, NY 10032 mh61@columbia.edu
Editorial, page 1484
Supplemental data at www.neurology.org
e-Pub ahead of print on March 24, 2010, at www.neurology.org.
Disclosure: Author disclosures are provided at the end of the article.
Received June 1, 2009. Accepted in final form February 4, 2010.
REFERENCES
- 1.Bell BD, Davies KG. Anterior temporal lobectomy, hippocampal sclerosis, and memory: recent neuropsychological findings. Neuropsychol Rev 1998;8:25–41. [DOI] [PubMed] [Google Scholar]
- 2.Hamberger MJ, Seidel WT. Auditory and visual naming tests: normative and patient data for accuracy, response time and tip-of-the-tongue. J Int Neuropsychol Soc 2003;9:479–489. [DOI] [PubMed] [Google Scholar]
- 3.Bell B, Seidenberg M, Hermann B, Douville K. Visual and auditory naming in patients with left or bilateral temporal lobe epilepsy. Epilepsy Res 2003;55:29–37. [DOI] [PubMed] [Google Scholar]
- 4.Hamberger MJ, Goodman RR, Perrine K, Tammy T. Anatomical dissociation of auditory and visual naming in the lateral temporal cortex. Neurology 2001;56:56–61. [DOI] [PubMed] [Google Scholar]
- 5.Ojemann GA, Dodrill CB. Predicting postoperative language and memory deficits after dominant hemisphere anterior temporal lobectomy by intraoperative stimulation mapping. Boston: American Association of Neurological Surgeons; 1981:76–77. [Google Scholar]
- 6.Ojemann GA. Brain organization for language from the perspective of electrical stimulation mapping. Behav Brain Res 1983;6:189–230. [Google Scholar]
- 7.Hamberger MJ, Seidel WT, McKhann GM, et al. Brain stimulation reveals critical auditory naming cortex. Brain 2005;128:2742–2749. [DOI] [PubMed] [Google Scholar]
- 8.Haglund M, Berger M, Shamseldin M, et al. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery 1994;34:567–576. [DOI] [PubMed] [Google Scholar]
- 9.Ojemann GA, Ojemann J, Lettich E, Berger M. Cortical language localization in left-dominant hemisphere: an electrical stimulation mapping investigation in 117 patients. J Neurosurg 1989;71:316–326. [DOI] [PubMed] [Google Scholar]
- 10.Davies K, Bell B, Bush A, et al. Naming decline after left anterior temporal lobectomy correlates with pathological status of resected hippocampus. Epilepsia 1998;39:407–419. [DOI] [PubMed] [Google Scholar]
- 11.Seidenberg M, Hermann B, Wyler AR, et al. Neuropsychological outcome following anterior temporal lobectomy in patients with and without syndrome of mesial temporal lobe epilepsy. Neuropsychology 1998;12:303–316. [DOI] [PubMed] [Google Scholar]
- 12.Sawrie SM, Martin RC, Gilliam FG, et al. Visual confrontation naming and hippocampal function: A neural network study using quantitative (1)H magnetic resonance spectroscopy. Brain 2000;123:770–780. [DOI] [PubMed] [Google Scholar]
- 13.Martin RC, Sawrie S, Hugg J, et al. Cognitive correlates of H MRSI-detected hippocampal abnormalities in temporal lobe epilepsy. Neurology 1999;53:2052–2058. [DOI] [PubMed] [Google Scholar]
- 14.Mesalum MM. Large-scale neurocognitive networks and distributed processing for attention, language and memory. Ann Neurol 1990;28:597–613. [DOI] [PubMed] [Google Scholar]
- 15.Hamberger MJ, Seidel WT, Goodman RR, et al. Evidence for cortical reorganization of language in patients with hippocampal sclerosis. Brain 2007;130:2942–1950. [DOI] [PubMed] [Google Scholar]
- 16.Wada J, Rasmussen T. Intracarotid injection of sodium Amytal for the lateralization of cerebral speech dominance: experimental and clinical observations. J Neurosurg 1960;17:266–282. [DOI] [PubMed] [Google Scholar]
- 17.Privitera MD, Morris GL, Gilliam F. Postictal language assessment and lateralization of complex partial seizures. Ann Neurol 1991;30:391–396. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test, 2nd ed. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 19.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Manual (2nd ed). San Antonio: Psychological Corporation; 2000. [Google Scholar]
- 20.Spencer DD, Spencer SS, Mattson RH, et al. Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery 1984;15:667–671. [DOI] [PubMed] [Google Scholar]
- 21.Hamberger MJ, Seidel WT. Localization of cortical dysfunction based on auditory and visual naming performance. J Int Neuropsychol Soc 2009;15:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engel J, Van Ness PC, Rasmussen TB, Ojemann GA. Outcome with respect to epileptic seizures. In: Jr. EJ, ed. Surgical Treatment of the Epilepsies, 2nd ed. New York: Raven Press; 1993:609–621. [Google Scholar]
- 23.Tulving E. How many memory systems are there? Am Psychol 1985;40:285–398. [Google Scholar]
- 24.Zola-Morgan S, Squire LR. Neuroanatomy of memory. Annu Rev Neurosci 1993;16:547–563. [DOI] [PubMed] [Google Scholar]
- 25.Barbaro NM, Quigg M, Broshek DK, et al. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol 2009;65:167–175. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler Adult Intelligence Test–Revised manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- 27.Wechsler D. Wechsler Adult Intelligence Scale–III manual. New York: The Psychological Corporation; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.