Abstract
Background:
Treatment of aneurysmal subarachnoid hemorrhage (SAH) has changed substantially over the last 25 years but there is a lack of reliable population-based data on whether case-fatality or functional outcomes have improved.
Methods:
We determined changes in the standardized incidence and outcome of SAH in the same population between 1981 and 1986 (Oxford Community Stroke Project) and 2002 and 2008 (Oxford Vascular Study). In a meta-analysis with other population-based studies, we used linear regression to determine time trends in outcome.
Results:
There were no reductions in incidence of SAH (RR = 0.79, 95% confidence interval [CI] 0.48–1.29, p = 0.34) and in 30-day case-fatality (RR = 0.67, 95% CI 0.39–1.13, p = 0.14) in the Oxford Vascular Study vs Oxford Community Stroke Project, but there was a decrease in overall mortality (RR = 0.47, 0.23–0.97, p = 0.04). Following adjustment for age and baseline SAH severity, patients surviving to hospital had reduced risk of death or dependency (modified Rankin score > 3) at 12 months in the Oxford Vascular Study (RR = 0.51, 0.29–0.88, p = 0.01). Among 32 studies covering 39 study periods from 1980 to 2005, 7 studied time trends within single populations. Unadjusted case-fatality fell by 0.9% per annum (0.3–1.5, p = 0.007) in a meta-analysis of data from all studies, and by 0.9% per annum (0.2–1.6%, p = 0.01) within the 7 population studies.
Conclusion:
Mortality due to subarachnoid hemorrhage fell by about 50% in our study population over the last 2 decades, due mainly to improved outcomes in cases surviving to reach hospital. This improvement is consistent with a significant decrease in case-fatality over the last 25 years in our pooled analysis of other similar population-based studies.
GLOSSARY
- CI
= confidence interval;
- mRS
= modified Rankin score;
- OCSP
= Oxford Community Stroke Project;
- OXVASC
= Oxford Vascular Study;
- SAH
= subarachnoid hemorrhage;
- WFNS
= World Federation of Neurosurgical Societies.

e–Pub ahead of print
Several systematic reviews based on separate observational studies have tended to show declining trends in case-fatality following subarachnoid hemorrhage (SAH) over the last 3 decades.1–4 If real, these trends have important implications for planning clinical services. SAH occurs at a relatively young age compared to other stroke types; if more patients survive SAH but are severely disabled, the burden of post-SAH care will increase considerably. There may also be consequences for the cost-effectiveness of screening programs for unruptured aneurysms. If SAH outcomes are improving, conservative treatment may become preferable for some patient groups in which the risks and benefits of treating unruptured aneurysms are very closely matched.5
However, indirect estimation of apparent time trends from multiple separate studies performed at different times is subject to several sources of bias, including differences between studies in case-finding and diagnostic criteria, differences in the age and sex distributions of each study population, and advances in diagnostic techniques over time. The most reliable method for determining time trends in outcomes is repeated studies within the same population over time, using consistent case finding methods and diagnostic criteria,6 but few such studies have been reported and to our knowledge there have been no recent population-based studies that have correlated trends in case-fatality with more detailed analyses of changes in treatment and functional outcomes. We therefore studied time trends in SAH outcomes by determining changes in outcome over time in our own population-based study, and by pooling our results with other studies of time trends within single populations.
METHODS
Oxford Vascular Study and Oxford Community Stroke Project.
All cases of SAH which were first-ever strokes were ascertained from the first 6 years (April 2002–March 2008) of the Oxford Vascular Study (OXVASC), the methods of which have been described previously.7 In brief, the OXVASC study is a prospective population-based study of all vascular events including stroke, occurring in a population of 91,106 individuals, registered with 63 general practitioners in 9 primary care practices in Oxfordshire, all of which had participated in the Oxford Community Stroke Project (OCSP).8 Only 2 practices involved in OCSP could not be included in OXVASC, and data from all 4 years of the OCSP study (1981–1984, 1986) have been reanalyzed for this comparison, comprising a mid-study population estimate of 87,861 individuals that excludes cases from these 2 practices.
We reviewed case records, postmortem reports, and brain imaging for all patients diagnosed with SAH in both OCSP and OXVASC. SAH was diagnosed in the context of a consistent clinical presentation and 1) brain imaging showing subarachnoid blood or 2) an adequate postmortem examination or 3) the presence of xanthochromia in a CSF sample.8 SAH associated with primary intracerebral hemorrhage, or secondary to trauma, drug use, arterial dissection, or vasculitis, was excluded. The overall rate of imaging and/or autopsy was 98% in OXVASC and 89% in OCSP.
In each case, we derived the World Federation of Neurosurgical Societies (WFNS) score9 using details of the initial clinical presentation, and collected data on vascular imaging and treatment. Functional outcomes at 12 months post SAH were assessed in both studies using the modified Rankin score (mRS). Premorbid risk factor and medication data were obtained by questioning patients and relatives, and by reviewing medical records.
We calculated the incidence rates of SAH in both studies, and standardized these to the 2001 census population of England and Wales. Confidence intervals for incidence were derived assuming a Poisson distribution for the number of events. Poisson regression models adjusted for overdispersion were used to produce the relative incidence of all SAH, and the relative 30-day case-fatality following SAH in OXVASC vs OCSP after adjustment for age and sex. The relative risk of death or dependency (mRS ≥ 3) at 12 months, adjusted for age and clinical severity of SAH at presentation (dichotomized as WFNS score at presentation ≤3 or >3), was calculated in a similar way. Clinical and risk factor data from OXVASC and OCSP were compared using Fisher exact test and Student t test.
Standard protocol approvals, registrations, and patient consents.
OXVASC has local research ethics committee approval and written informed consent or assent is obtained for all patients participating in the study.
Systematic review.
We performed a MEDLINE search using combinations of the following search terms: “subarachnoid hemorrhage/haemorrhage” or “stroke,” and “outcome” or “mortality” or “case-fatality,” and “population” or “community” or “epidemiology,” limited to studies publishing outcome data from 1980 onwards, to include the same period of observation as our population-based study. The reference lists of retrieved studies were also searched. Studies were included if they met the following criteria: 1) use of a population-based study design in which the study population was representative of the population in general, and all death certificates had been reviewed or all nonhospitalized deaths had been reviewed with the coroner; 2) the upper age limit of the study was not <75 years and the lower limit was not >35 years; 3) results for SAH were reported separately if the study was about stroke in general; 4) brain imaging or autopsy was performed in at least 80% of the population; 5) study period was not longer than 10 years unless separate results were given per decade; 6) crude case-fatality rates were given or could be calculated from the data presented; 7) there were at least 5 cases of SAH in any study period. We also included our own data from OXVASC and OCSP.
For each study we recorded the mid-calendar year of the study, total numbers with SAH, crude case-fatality rates, time of follow-up, age and sex distribution of the population with SAH, and the percentage with brain imaging or autopsy. We used linear regression analysis to describe the relationship between crude case-fatality rates and the mid-calendar year of each included study, weighted by the inverse of the standard error of the case-fatality of each study. Age and sex differences between studies were taken into account by entering the mean age and percentage of women in the study into the weighted linear regression model, when these data were available.
Time trends within single populations were also analyzed using outcome data taken at the 2 furthest timepoints within each study, which were required to be at least 5 years apart. The results are expressed as the absolute percentage change in case-fatality per calendar year.
RESULTS
OXVASC and OCSP.
In the first 6 years of OXVASC there were 883 incident strokes, of which 38 (4.3%) were due to SAH. Of the incident cases, 2 died prior to reaching hospital and were diagnosed at postmortem, 35 were diagnosed on brain imaging, and 1 was diagnosed on the basis of CSF xanthochromia with a consistent clinical presentation but negative brain imaging.
Over a period of 4 years in OCSP, there were 557 incident strokes in the population corresponding to OXVASC, and 27 (5.0%) were due to SAH. One patient died before reaching hospital and was diagnosed at postmortem. Of the remaining cases surviving to reach hospital, 3 cases were diagnosed at postmortem, 18 were diagnosed on brain and vascular imaging, and 5 were diagnosed on the basis of having a typical clinical presentation plus the presence of CSF xanthochromia.
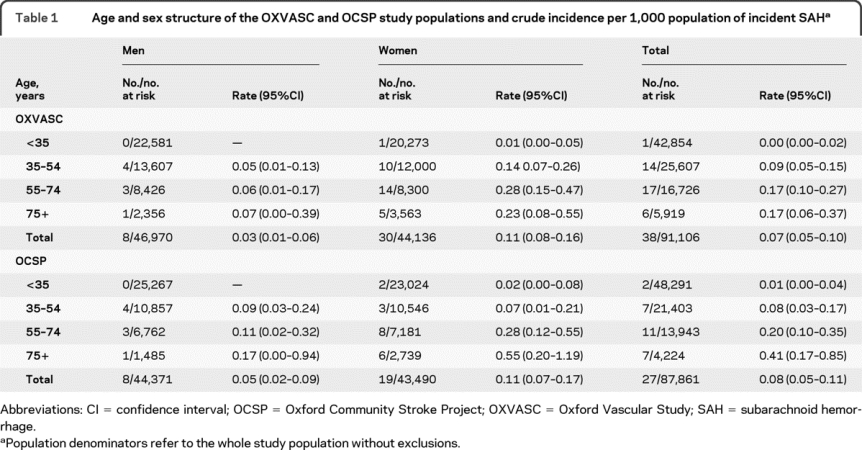
The age and sex structure of the study populations and crude incidence per 1,000 of SAH are given in table 1. After adjustment to the 2001 census population of England and Wales, the overall incidence of SAH in OXVASC was 0.07 (0.05–0.10) and in OCSP it was 0.10 (0.06–0.13). The decrease in SAH incidence was not significant (rate ratio = 0.79, 95% confidence interval [CI] 0.48–1.29, p = 0.34).
Table 1 Age and sex structure of the OXVASC and OCSP study populations and crude incidence per 1,000 population of incident SAH
Demographic and premorbid clinical risk factor data for cases with SAH in OXVASC and OCSP are shown in table e-1 on the Neurology® Web site at www.neurology.org. The mean age and sex distribution of patients in both studies were similar. There was a nonsignificant fall in the proportion of current smokers in OXVASC compared to OCSP, and in the proportion of patients with a past history of hypertension or on treatment for hypertension.
Although SAH severity (distribution of WFNS grades at presentation) was comparable in OXVASC and OCSP (figure e-1), a greater proportion of patients surviving to reach hospital underwent vascular imaging in OXVASC compared to OCSP (24 [67%] vs 6 [23%], p = 0.001), and treatment to secure aneurysms (18 [50%] vs 5 [19%], p = 0.02). The median delay (IQR) in days to any interventional treatment was also shorter in OXVASC (2 [1–5] vs 14 [11–16], p = 0.001). In OCSP, all 5 treated aneurysms were secured by surgical clipping, whereas in OXVASC, 15/18 (83%) treated aneurysms were secured by endovascular embolization.
Age- and sex-adjusted 30-day case fatality tended to be lower in OXVASC vs OCSP (43% vs 67%, RR = 0.67, 95% CI 0.39–1.13, p = 0.14), contributing to a 53% reduction in age and sex-standardized mortality rates between the 2 study periods (RR = 0.47, 0.23–0.97, p = 0.04). Among survivors to reach hospital, the relative risk of death or dependency (mRS ≥ 3) at 12 months, adjusted for age and SAH severity, was halved in OXVASC (0.51, 0.29–0.88, p = 0.01). The range of modified Rankin scores at 12 months in each study is compared in figure 1.
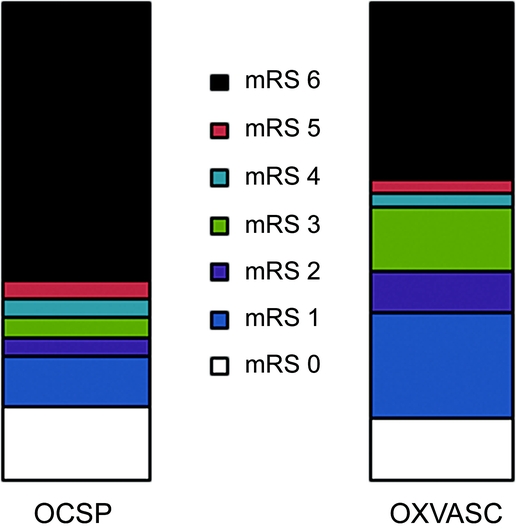
Figure 1 Twelve-month modified Rankin Scale scores (mRS) in patients surviving to reach hospital in Oxford Community Stroke Project (OCSP) and Oxford Vascular Study (OXVASC)
Systematic review.
The MEDLINE search produced 16,657 articles, from which we identified 186 potentially relevant studies. Including our population-based study, there were 31 studies that met our inclusion criteria10–39 (covering 41 study periods from 1980 to 2005). Details of the included studies are shown in table 2.
Table 2 Characteristics of population-based studies reporting case-fatality after SAH included in the systematic review
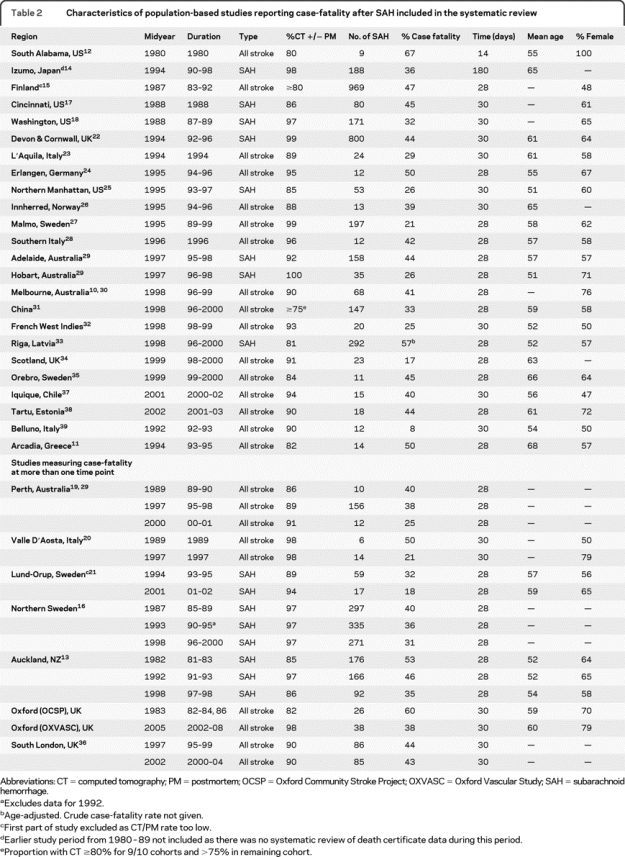
Weighted linear regression analysis of all studies showed that case-fatality decreased by 0.9% per annum (95% CI 0.3–1.5%, p = 0.007). Exclusion of 2 studies12,14 which did not report 1-month outcomes, and 1 study with an exceptionally low case-fatality rate of 8%,39 did not substantially alter the results. Eighteen studies including our own11,13,21–25,27-29,31-33,35,37,38 reported the mean age of cases with SAH and proportion of women. After adjustment for age and sex, there was a similar but nonsignificant reduction in case-fatality of 0.9% per annum (−0.2 to 2.0%, p = 0.10).
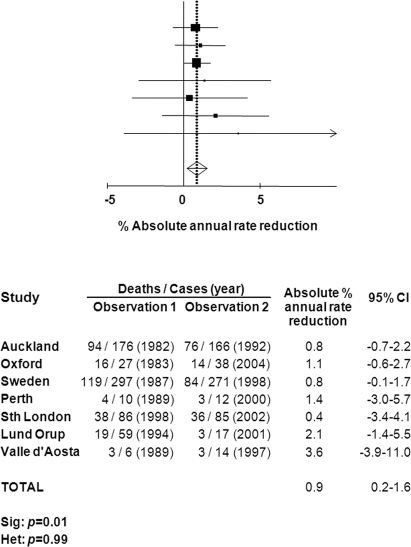
Seven studies including our own (table 2) measured 1-month case-fatality at more than 1 timepoint, separated by an interval of at least 5 years.13,14,16,19–21,36 Data from the Perth Community Stroke Study from June 1995 to June 199619 were published separately from data from December 1996 to February 1998,29 but these were combined for this analysis because the periods of study were so close. A pooled analysis of time trends within study populations, based on the difference in 1-month case-fatality taken from the 2 furthest available timepoints (figure 2), demonstrated a 0.9% reduction in case-fatality per annum (0.2–1.6, p = 0.01). Unadjusted estimates of case-fatality at different timepoints within each study are plotted alongside the weighted regression line using all data from the 7 population-based studies in figure 3. All studies demonstrated a trend of declining case-fatality rates over time, and the rate of that decline was similar to the results of the weighted linear regression analysis from all studies, with the exception of the Valle d'Aosta study and the Perth Community Stroke Study, which had very small numbers of cases of SAH in at least 2 of the periods examined.19,20,29
Figure 2 Absolute % annual rate reduction in case fatality among studies reporting outcomes at 2 different timepoints
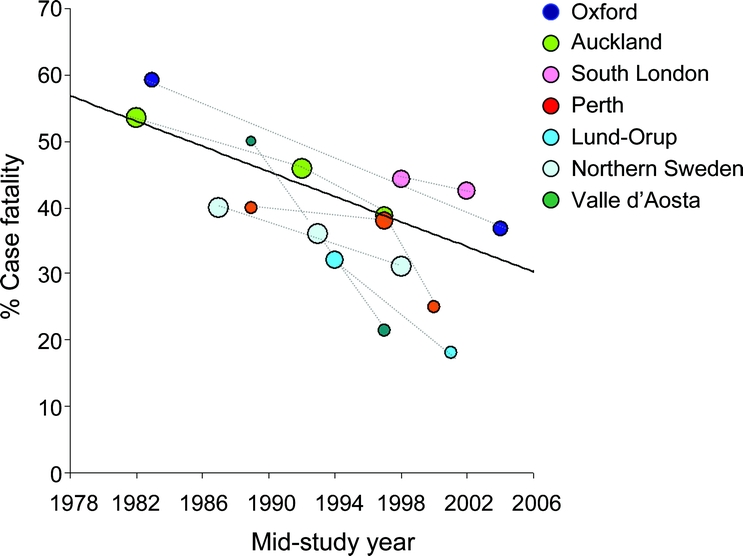
Figure 3 Crude 1-month case fatality vs year for each population-based study reporting time-trend data plotted alongside the weighted regression line combining all 7 studies
Size of each circle proportional to the log of the number of cases in each study.
DISCUSSION
Although a significant reduction in the incidence of SAH has not occurred in the last 25 years in our Oxfordshire population, mortality has halved largely due to improved survival among hospitalized patients, without a corresponding increase in the proportion of survivors with severe disability. Further evidence of improved outcome after SAH comes from our meta-analysis of time trends in case-fatality within other population-based studies showing a 0.9% absolute annual reduction in crude 30-day case-fatality rates, and a similar sized reduction in case-fatality in a meta-analysis of all population-based studies that measured case-fatality at more than one timepoint within the same population.
Strengths of our population-based study include consistent case-finding methods and diagnostic criteria over time, and detailed data collection during both study periods. Consequently, we could show that the improvement in outcomes is unlikely to be due to better detection of milder cases through improved diagnostic techniques, as the distribution of stroke severity was similar in both study periods. Instead it is more likely to reflect improvements in the management of SAH. In OXVASC, these include the earlier and more frequent use of neurovascular investigations and interventions compared to OCSP, and greater use of endovascular therapies.
There have been few data published from other population-based studies examining trends in case-fatality following SAH despite ongoing improvements in treatment. Nevertheless, our pooled analysis of these studies demonstrates a significant fall in case-fatality per annum over the last 30 years, consistent with time trends observed in our Oxfordshire population. The majority of data in the pooled analysis come from the Auckland13,29 and Northern Swedish populations,16 with the latter study showing a significant decline in case-fatality. Smaller studies including the Perth Community Stroke Study (1989–2001),19,29 the Lund Registry,21 and the Valle d'Aosta study (1989–1997)20 also reported reductions in case-fatality of around 40%, but were not adequately powered to show a significant fall, and the South London Stroke Registry36 may not have covered a long enough period of time to examine the cumulative effect of changes in SAH management.
Each of the methods we used to study trends in outcome has limitations. Our population-based study is limited by small numbers of fatal cases of SAH in each study period, meaning that chance effects cannot be excluded. There were also few time trend data from other population-based studies. The more indirect method of studying outcomes from separate population-based studies at different timepoints included larger numbers of patients from a wider range of regions, but was subject to bias from differences in case-finding and diagnostic criteria between studies. Nevertheless, the consistency of the results from each of these analyses suggests that the trend for improving outcomes following SAH is real. Furthermore, the 55% reduction in mortality that has occurred in our own population-based study is comparable to the reduction in mortality reported by the Oxford Linkages study in an analysis of routinely collected data within the wider Oxfordshire population over a similar time period.40
A further source of bias in analyzing time trends in SAH outcome is the improvement in availability and quality of brain imaging, which has reduced misdiagnosis of SAH and probably improved the detection of milder cases in later study periods. We have tried to minimize this source of bias by limiting our analysis to studies in which the proportion of stroke classified by brain imaging or postmortem was >80% throughout the study. Furthermore, as has been discussed, this bias does not entirely explain the improvements in outcome observed in our own population-based study, as the distributions of stroke severity at presentation in OCSP and OXVASC were similar, and functional outcomes among hospitalized cases still improved after adjustment for differences in age and stroke severity between both study periods. Instead it is more likely that the lower rate of brain imaging/autopsy in OCSP meant that more cases of fatal SAH were missed, resulting in an underestimation of the reduction in case-fatality.
We found no significant decline in the incidence of SAH. If a real decline has occurred, then it has been modest compared to the 40% reduction in stroke incidence overall in OXVASC compared to OCSP.8 This observation is consistent with the findings of recent systematic reviews of trends in SAH incidence worldwide.3,4
Our finding that the SAH outcomes are improving has important implications for clinical practice and research. SAH occurs at a relatively young age compared to other stroke types, and a concern is that if more patients now survive SAH but are severely disabled, the burden of rehabilitation or residential care will increase considerably. However, our results show that there has not been a substantial increase in the proportion of survivors with more severe disability. There will also be consequences for the cost-effectiveness of programs to screen for and treat unruptured aneurysms. The risks and benefits of treating unruptured aneurysms may be closely matched depending on patient age,9 as well as size and location of the aneurysm, and if outcomes following SAH are improving, then conservative treatment may become more cost-effective for some patient groups. Finally, our findings have implications for planning randomized trials of treatment in SAH. Given the trend for improving outcomes and greater early survival, greater numbers of patients will need to be recruited to achieve adequate statistical power.
ACKNOWLEDGMENT
The authors thank Professor B. Stegmayr of the Northern Sweden WHO MONICA project; Professor C. Wolfe and Dr. N. Smeeton of the South London Stroke Registry; and Professor A. Lindgren, Professor B. Norrving, and Dr. B. Hansen of the Lund Stroke Register for providing additional unpublished data for the systematic review.
DISCLOSURE
Dr. Lovelock has received funding for travel from Sanofi-Aventis. Prof. Rinkel receives royalties from the publication of Stroke: Practical Management 3rd edition (Blackwell Publishing, 2007) and receives research support from the Netherlands Heart Foundation, Hersenstichting Nederland (The Netherlands Brain Foundation), and Netherlands Organisation of Health Research. Prof. Rothwell serves on scientific advisory boards for Bayer-Schering Pharma, Servier Laboratories, Pfizer Inc., and Biotronic; has received travel for funding and/or honoraria from Sanofi-Aventis, Servier Laboratories, AstraZeneca, Boehringer-Ingelheim, and Bayer-Schering Pharma; and serves on the editorial boards of Lancet Neurology, Stroke, and Cerebrovascular Diseases, and as Assistant Editor for the International Journal of Stroke.
Supplementary Material
Address correspondence and reprint requests to Professor P.M. Rothwell, Stroke Prevention Research Unit, University Department of Clinical Neurology, John Radcliffe Hospital, Oxford, UK, OX3 9DU peter.rothwell@clneuro.ox.ac.uk
Editorial, page 1486
Supplemental data at www.neurology.org
e-Pub ahead of print on April 7, 2010, at www.neurology.org.
Study funding: OXVASC is funded by the UK Medical Research Council, the Dunhill Medical Trust, the Stroke Association, the BUPA Foundation, the National Institute for Health Research (NIHR), the Thames Valley Primary Care Research Partnership, and the NIHR Oxford Biomedical Research Centre.
Disclosure: Author disclosures are provided at the end of the article.
Received September 17, 2009. Accepted in final form February 8, 2010.
REFERENCES
- 1.Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 2009;8:635–642. [DOI] [PubMed] [Google Scholar]
- 2.Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 1997;28:660–664. [DOI] [PubMed] [Google Scholar]
- 3.de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry 2007;78:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009;8:355–369. [DOI] [PubMed] [Google Scholar]
- 5.Wiebers DO, Whisnant JP, Huston J 3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003;362:103–110. [DOI] [PubMed] [Google Scholar]
- 6.Al-Shahi Salman R, Sudlow CL. Case fatality after subarachnoid haemorrhage: declining, but why? Lancet Neurol 2009;8:598–599. [DOI] [PubMed] [Google Scholar]
- 7.Rothwell PM, Coull AJ, Giles MF, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet 2004;363:1925–1933. [DOI] [PubMed] [Google Scholar]
- 8.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. A prospective study of acute cerebrovascular disease in the community: The Oxfordshire Community Stroke Project: 1981–86: 2: incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 1990;53:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teasdale GM, Drake CG, Hunt W, et al. A universal subarachnoid hemorrhage scale: report of a committee of the world federation of neurosurgical societies. J Neurol Neurosurg Psychiatry 1988;51:1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thrift AG, Dewey HM, Sturm JW, et al. Incidence of stroke subtypes in the North East Melbourne Stroke Incidence Study (nemesis): differences between men and women. Neuroepidemiology 2009;32:11–18. [DOI] [PubMed] [Google Scholar]
- 11.Vemmos KN, Bots ML, Tsibouris PK, et al. Prognosis of stroke in the south of Greece: 1 year mortality, functional outcome and its determinants: The Arcadia Stroke Registry. J Neurol Neurosurg Psychiatry 2000;69:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross CR, Kase CS, Mohr JP, Cunningham SC, Baker WE. Stroke in South Alabama: incidence and diagnostic features: a population-based study. Stroke 1984;15:249–255. [DOI] [PubMed] [Google Scholar]
- 13.Truelsen T, Bonita R, Duncan J, Anderson NE, Mee E. Changes in subarachnoid hemorrhage mortality, incidence, and case fatality in New Zealand between 1981–1983 and 1991–1993. Stroke 1998;29:2298–2303. [DOI] [PubMed] [Google Scholar]
- 14.Inagawa T. Trends in incidence and case fatality rates of aneurysmal subarachnoid hemorrhage in Izumo City, Japan, between 1980–1989 and 1990–1998. Stroke 2001;32:1499–1507. [DOI] [PubMed] [Google Scholar]
- 15.Immonen-Raiha P, Mahonen M, Tuomilehto J, et al. Trends in case-fatality of stroke in Finland during 1983 to 1992. Stroke 1997;28:2493–2499. [DOI] [PubMed] [Google Scholar]
- 16.Stegmayr B, Eriksson M, Asplund K. Declining mortality from subarachnoid hemorrhage: changes in incidence and case fatality from 1985 through 2000. Stroke 2004;35:2059–2063. [DOI] [PubMed] [Google Scholar]
- 17.Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 1994;25:1342–1347. [DOI] [PubMed] [Google Scholar]
- 18.Longstreth WT, Jr., Nelson LM, Koepsell TD, van Belle G. Clinical course of spontaneous subarachnoid hemorrhage: a population-based study in King County, Washington. Neurology 1993;43:712–718. [DOI] [PubMed] [Google Scholar]
- 19.Islam MS, Anderson CS, Hankey GJ, et al. Trends in incidence and outcome of stroke in Perth, Western Australia during 1989 to 2001: The Perth Community Stroke Study. Stroke 2008;39:776–782. [DOI] [PubMed] [Google Scholar]
- 20.D'Alessandro G, Bottacchi E, Di Giovanni M, et al. Temporal trends of stroke in Valle D'Aosta, Italy: incidence and 30-day fatality rates. Neurol Sci 2000;21:13–18. [DOI] [PubMed] [Google Scholar]
- 21.Hallstrom B, Jonsson AC, Nerbrand C, Norrving B, Lindgren A. Stroke incidence and survival in the beginning of the 21st century in southern Sweden: comparisons with the late 20th century and projections into the future. Stroke 2008;39:10–15. [DOI] [PubMed] [Google Scholar]
- 22.Pobereskin LH. Incidence and outcome of subarachnoid haemorrhage: a retrospective population based study. J Neurol Neurosurg Psychiatry 2001;70:340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carolei A, Marini C, Di Napoli M, et al. High stroke incidence in the prospective community-based l'Aquila registry (1994–1998): first year's results. Stroke 1997;28:2500–2506. [DOI] [PubMed] [Google Scholar]
- 24.Kolominsky-Rabas PL, Sarti C, Heuschmann PU, et al. A prospective community-based study of stroke in Germany: The Erlangen Stroke Project (ESPRO): incidence and case fatality at 1, 3, and 12 months. Stroke 1998;29:2501–2506. [DOI] [PubMed] [Google Scholar]
- 25.Labovitz DL, Halim AX, Brent B, Boden-Albala B, Hauser WA, Sacco RL. Subarachnoid hemorrhage incidence among whites, blacks and Caribbean Hispanics: The Northern Manhattan Study. Neuroepidemiology 2006;26:147–150. [DOI] [PubMed] [Google Scholar]
- 26.Ellekjaer H, Holmen J, Indredavik B, Terent A. Epidemiology of stroke in Innherred, Norway, 1994 to 1996: incidence and 30-day case-fatality rate. Stroke 1997;28:2180–2184. [DOI] [PubMed] [Google Scholar]
- 27.Khan FA, Engstrom G, Jerntorp I, Pessah-Rasmussen H, Janzon L. Seasonal patterns of incidence and case fatality of stroke in Malmo, Sweden: The Stroma Study. Neuroepidemiology 2005;24:26–31. [DOI] [PubMed] [Google Scholar]
- 28.Di Carlo A, Inzitari D, Galati F, et al. A prospective community-based study of stroke in Southern Italy: The Vibo Valentia Incidence of Stroke Study (VISS): methodology, incidence and case fatality at 28 days, 3 and 12 months. Cerebrovasc Dis 2003;16:410–417. [DOI] [PubMed] [Google Scholar]
- 29.Epidemiology of aneurysmal subarachnoid hemorrhage in Australia and New Zealand: incidence and case fatality from the Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS). Stroke 2000;31:1843–1850. [DOI] [PubMed] [Google Scholar]
- 30.Thrift AG, Dewey HM, Macdonell RA, McNeil JJ, Donnan GA. Incidence of the major stroke subtypes: initial findings from the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 2001;32:1732–1738. [DOI] [PubMed] [Google Scholar]
- 31.Zhang LF, Yang J, Hong Z, et al. Proportion of different subtypes of stroke in China. Stroke 2003;34:2091–2096. [DOI] [PubMed] [Google Scholar]
- 32.Smadja D, Cabre P, May F, et al. Epidemiology of stroke in Martinique, French West Indies: part I: methodology, incidence, and 30-day case fatality rate. Stroke 2001;32:2741–2747. [DOI] [PubMed] [Google Scholar]
- 33.Keris V, Buks M, Macane I, et al. Aneurysmal subarachnoid hemorrhage in Baltic population: experience from Latvia (1996–2000). Eur J Neurol 2002;9:601–607. [DOI] [PubMed] [Google Scholar]
- 34.Syme PD, Byrne AW, Chen R, Devenny R, Forbes JF. Community-based stroke incidence in a Scottish population: The Scottish Borders Stroke Study. Stroke 2005;36:1837–1843. [DOI] [PubMed] [Google Scholar]
- 35.Appelros P, Nydevik I, Seiger A, Terent A. High incidence rates of stroke in Orebro, Sweden: further support for regional incidence differences within Scandinavia. Cerebrovasc Dis 2002;14:161–168. [DOI] [PubMed] [Google Scholar]
- 36.Smeeton NC, Heuschmann PU, Rudd AG, et al. Incidence of hemorrhagic stroke in black Caribbean, black African, and white populations: the South London Stroke Register, 1995–2004. Stroke 2007;38:3133–3138. [DOI] [PubMed] [Google Scholar]
- 37.Lavados PM, Sacks C, Prina L, et al. Incidence, 30-day case-fatality rate, and prognosis of stroke in Iquique, Chile: a 2-year community-based prospective study (PISCIS project). Lancet 2005;365:2206–2215. [DOI] [PubMed] [Google Scholar]
- 38.Vibo R, Korv J, Roose M. The third stroke registry in Tartu, Estonia, from 2001 to 2003. Acta Neurol Scand 2007;116:31–36. [DOI] [PubMed] [Google Scholar]
- 39.Lauria G, Gentile M, Fassetta G, et al. Incidence and prognosis of stroke in the Belluno Province, Italy: first-year results of a community-based study. Stroke 1995;26:1787–1793. [DOI] [PubMed] [Google Scholar]
- 40.Goldacre MJ, Duncan M, Griffith M, Rothwell PM. Mortality rates for stroke in England from 1979 to 2004: trends, diagnostic precision, and artifacts. Stroke 2008;39:2197–2203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.