Abstract
Background:
Sensory neuropathy (SN) is common in patients with HIV. Hepatitis C (HCV) coinfection is often cited as an HIV-SN risk factor, but data to support this are lacking. This collaboration aimed to examine the association between HCV serostatus and SN risk among ambulatory HIV-positive patients.
Methods:
Patients with HIV were assessed in cross-sectional studies in Baltimore, Jakarta, Johannesburg, Kuala Lumpur, Melbourne, and Sydney for SN (defined by both supportive symptoms and signs). HCV seropositivity was assessed as an SN risk using a χ2 test, followed by logistic regression modeling to correct for treatment exposures and demographics.
Results:
A total of 837 patients of African, Asian, and Caucasian descent were studied. HCV seroprevalence varied by site (Baltimore n = 104, 61% HCV+; Jakarta 96, 51%; Johannesburg 300, 1%; Kuala Lumpur 97, 10%; Melbourne 206, 16%; Sydney 34, 18%). HCV seropositivity was not associated with increased SN risk at any site, but was associated with reduced SN risk in Melbourne (p = 0.003). On multivariate analyses, the independent associations with SN were increasing age, height, and stavudine exposure. HCV seropositivity was not independently associated with an increased SN risk at any site, but associated independently with reduced SN risk in Baltimore (p = 0.04) and Melbourne (p = 0.06).
Conclusions:
Hepatitis C (HCV) seropositivity was not associated with increased sensory neuropathy risk among HIV-positive patients at any site. While we were unable to assess HCV RNA or liver damage, the data suggest that HCV coinfection is not a major contributor to HIV-SN.
GLOSSARY
HCV = hepatitis C; SN = sensory neuropathy.
Sensory neuropathy is a common complication of HIV infection and some HIV treatments.1–4 Hepatitis C (HCV) can also be complicated by peripheral neuropathy, with and possibly without cryoglobulinemia.5–7 Since HCV and HIV infection share risk factors and frequently coexist,8 concerns of possible synergistic effects of these viruses on the peripheral nervous system have been raised.3,9 However, this has not been evaluated systematically.10
We surveyed outpatients with HIV infection for the presence of neuropathy at 6 centers with varying HIV management practices and rates of HCV coinfection. Here we use data from all sites to address whether HCV seropositivity is associated with an increased risk for symptomatic neuropathy among ambulatory patients with HIV. We have recently developed algorithms for the evaluation of neuropathy risk.11,12 Here we systematically evaluate whether HCV seropositivity should be considered in this context.
METHODS
Cross-sectional data were analyzed from 7 studies of HIV-associated neuropathy performed at 6 sites in 5 countries. All studies were approved by the local Human Research and Ethics Committee and all participants gave written, informed consent to participate. All studies recruited ambulatory adults (aged at least 17 years) with HIV infection attending hospital clinics for HIV care.
Study sites involved in this work were as follows.
Moore Clinic, Johns Hopkins Hospital, Baltimore, MD. Patients were recruited in 2002–2003 onto an NIH-funded project examining the pathogenesis and risks for HIV-associated neuropathies.4
Pokdisus AIDS Clinic, Cipto Mangunkusumo Hospital/Faculty of Medicine University Indonesia, Jakarta, Indonesia. Patients were recruited in a neuropathy screening program in 2006.11
Virology Clinic, Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa. Patients were recruited in a neuropathy screening program in 2008–2009.13
Infectious Diseases Clinic, University of Malaya Medical Centre, Kuala Lumpur, Malaysia. Patients were recruited in a neuropathy screening program in 2005–2006.11
Infectious Diseases Clinic, The Alfred Hospital, Melbourne, Australia. Patients were recruited in 2001 and 2006 in 2 separate neuropathy screening programs.14 Only data collected in 2006 are included for patients assessed in both studies (n = 34).
Immunology B (HIV) Clinic, St Vincent's Hospital, Sydney, Australia. Patients were recruited in a neuropathy screening program in 2009.
All patients were assessed for neuropathy using the AIDS Clinical Trials Group Brief Peripheral Neuropathy Screen.15 Neuropathy was defined as present if the individual had one or more of the lower limb neuropathic symptoms elicited using this tool (pain, aching or burning, pins and needles, or numbness) and one or more signs (absent ankle reflexes or reduced vibration sense at the great toe—vibration of a 128-Hz tuning fork felt for 10 seconds or less). Patient height was measured and data on other possible risk factors for neuropathy were collected from the medical file. HCV antibody status was assessed using commercially available assays performed in the hospital laboratory at each study site, as follows.
Baltimore: Abbott AxSYM HCV version 3.0 microparticle enzyme immunoassay (Abbott Laboratories, Abbott Park, IL).
Jakarta: ORTHO HCV version 3.0 ELISA (Ortho-Clinical Diagnostics, Raritan, NJ).
Johannesburg: Abbott AxSYM HCV version 3.0 microparticle enzyme immunoassay (Abbott Laboratories).
Kuala Lumpur: Abbott ARCHITECT chemiluminescent microparticle enzyme immunoassay (Abbott Laboratories).
Melbourne: Abbott AxSYM HCV version 3.0 microparticle enzyme immunoassay until 2004. From 2004, Abbott ARCHITECT chemiluminescent microparticle enzyme immunoassay (Abbott Laboratories).
Sydney: Abbott ARCHITECT chemiluminescent microparticle enzyme immunoassay (Abbott Laboratories).
Statistical analyses were performed using Stata 10.1 (StataCorp). Rates of HCV seropositivity were compared between patients with and without neuropathy using χ2 tests. Univariate analyses used χ2 tests (dichotomous variables), Wilcoxon rank sum test (non-normally distributed continuous variables), or unpaired t tests (normally distributed continuous variables) to determine other factors associated with neuropathy. Multivariate analyses of neuropathy risk factors within each cohort and overall were then undertaken using logistic regression modeling. This included HCV antibody status, factors previously associated with neuropathy risk (increasing age and stavudine exposure), and any factor with p < 0.1 on univariate analysis. Variables other than HCV antibody status were then removed in a reverse selection procedure to obtain the models shown.
RESULTS
A total of 837 patients were surveyed at 6 sites between 2001 and 2009. The cohorts had different demographic profiles, rates of HCV seropositivity, and exposure to stavudine, reflecting patient populations at each site (table 1). On univariate analysis, HCV seropositivity was not associated with an increased risk of neuropathy among patients at any site (table 2). In Melbourne, HCV seropositivity was associated with a reduced risk of neuropathy.
Table 1 Description of the cohorts surveyed
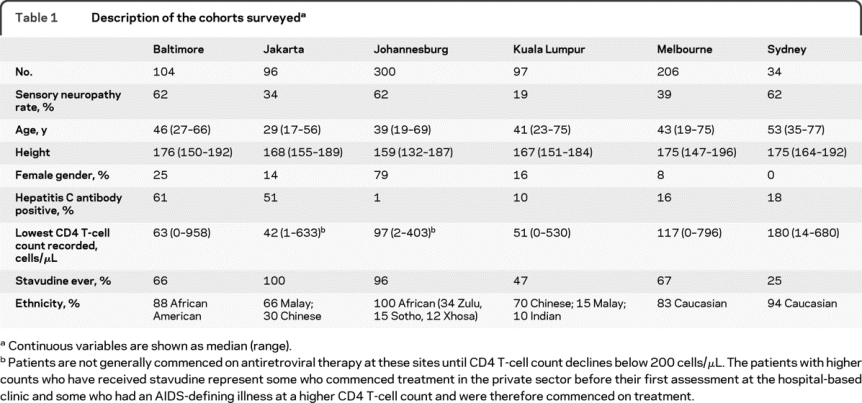
Table 2 Proportion of patients who were hepatitis C antibody positive at each site by sensory neuropathy status
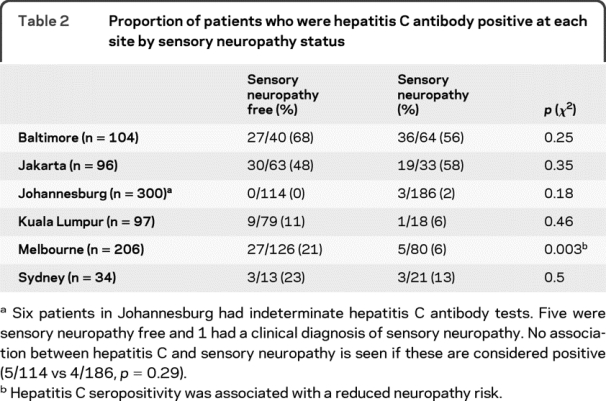
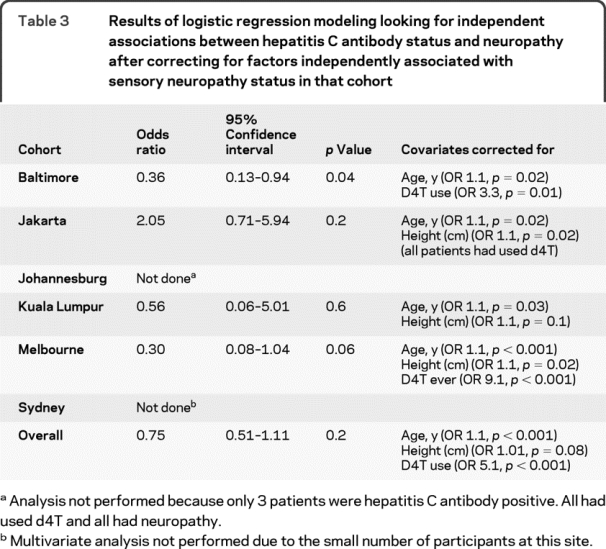
Logistic regression modeling was then used to correct for other risk factors that may have masked an association between HCV serostatus and neuropathy. HCV seropositivity was not independently associated with increased neuropathy risk in any cohort surveyed, nor in the combined data set. After correcting for patient age and stavudine use, an association between HCV antibody status and a reduced risk of neuropathy emerged in the Baltimore cohort, but became less apparent in Melbourne patients (table 3). Increasing age, a history of stavudine use, and increasing height were independently associated with neuropathy risk overall.
Table 3 Results of logistic regression modeling looking for independent associations between hepatitis C antibody status and neuropathy after correcting for factors independently associated with sensory neuropathy status in that cohort
DISCUSSION
We found no association between HCV seropositivity and increased neuropathy risk in any cohort of patients with HIV studied. These data represent more than 800 patients of African, Asian, and Caucasian descent in cohorts with varying HCV infection rates and antiretroviral treatment exposures. Differences in HCV seropositivity probably reflect the predominant mode of HIV infection, but definitive data are not available from the clinic populations sampled. The lack of an association with increased neuropathy risk in any cohort suggests that HCV coinfection is not a major risk factor for neuropathy among ambulatory patients with HIV.
The apparent association between HCV seropositivity and a reduced risk of neuropathy among patients with HIV in Melbourne raises the question of whether coinfected patients (often IV drug users) may have lower rates of antiretroviral drug use and hence exposure to potentially neurotoxic agents such as stavudine. However, 60% of HCV seropositive patients and 68% of HCV antibody negative patients in Melbourne had used stavudine (p = 0.3, χ2 test). HCV coinfected patients were younger than HIV monoinfected patients in the Melbourne cohort (mean age 40 vs 45 years, p = 0.008, unpaired t test). However, multivariate analysis correcting for age and stavudine use (table 3) revealed a continuing trend associating HCV coinfection with a reduced risk of neuropathy. A similar association emerged in the Baltimore cohort.
A protective effect from HCV coinfection against neuropathy in patients with HIV is difficult to explain biologically. HCV can infect peripheral nerves16,17 and the risk of neuropathy among monoinfected patients is well documented.5–7 The association observed between HCV and a reduced rate of neuropathy likely reflects factors not measured in this study that may influence neuropathy risk. For example, patients surveyed in Jakarta and Johannesburg had similarly high rates of stavudine exposure. However, neuropathy was more common in Johannesburg, where few patients are HCV coinfected. The difference in neuropathy prevalence is only partly explained by the younger age of the Jakarta cohort, so other factors such as genetics18–20 or other coinfections21 may also play a role.
The limitations of this work include our inability to examine HCV RNA levels or evidence of liver damage as these are not routinely monitored at all sites. We therefore cannot exclude an association between neuropathy and HCV viremia or chronic liver disease. Similarly, we cannot exclude the possibility that some patients with HCV viremia may have tested antibody negative, as previously described in a small proportion of patients with HIV,22 nor that some antibody-positive patients may have been aviremic. However, a recent study comparing neurocognitive function in patients with HIV with detectable vs undetectable HCV viral loads also showed no difference in neuropathy rates between these groups.23
PCR-based screening for HCV was not available in Johannesburg, but the observed low rate of HCV infection is consistent with other studies conducted in Southern Africa,24–29 including a study of HIV-infected individuals28 and a survey of HIV-infected patients presenting with liver disease.29 Our data were obtained using the AxSYM 3.0 (Abbott) assay that detects antibodies to proteins that are conserved across HCV genotypes.30 The manufacturers claim this includes genotype 5 (the most common type of HCV seen in South Africa31,32) but no genotype 5 sensitivity data are available for evaluation. The low prevalence of HCV in this cohort is attributed to HIV transmission via sexual contact (an uncommon route of HCV transmission) rather than through injecting drug use. It may also be significant that patients in this cohort were screened for HCV at the time of neuropathy assessments rather than at HIV diagnosis, so their HIV disease may have been more advanced. The 6 indeterminate results (table 2) may reflect patients with HCV infection but poor antibody responses due to immune suppression.33 However, as 5 of these 6 patients were neuropathy free, their indeterminate status would not alter our finding that HCV seropositivity is not associated with an increased neuropathy risk in patients with HIV.
A more extensive examination of risk factors for neuropathy in HIV (including more detailed demographics, treatment exposures, clinical factors, viral studies, and host genetics) will be required to exclude a minor contribution from HCV status to the individual patient's risk of symptomatic neuropathy. We used a simple clinical definition of neuropathy. Although we have validated this definition against epidermal nerve fiber density and quantitative sensory threshold testing,15 we cannot exclude associations between HCV coinfection and subclinical neuropathology or impaired sensory function in patients with HIV.
Despite these limitations, this work suggests that HCV coinfection does not increase neuropathy risks among ambulatory HIV-positive patients. This finding is strengthened by the inclusion of cohorts from settings with a range of HIV treatment practices, HCV seroprevalence, and ethnicity. While an individual coinfected patient may be at risk for peripheral nerve complications from HCV, these data suggest that HCV coinfection has minimal impact on the much greater neuropathy risk imposed by HIV and some HIV treatments.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. C.L. Cherry.
ACKNOWLEDGMENT
The authors thank all the patients who volunteered to take part in this work and Yong Yean Kong for facilitating work in Kuala Lumpur.
DISCLOSURE
Dr. Cherry has served on scientific advisory boards for Roche and Gilead Sciences, Inc.; serves as Basic Science Editor for the ASHM Journal Club and on the editorial boards of The Open AIDS Journal and the Journal of the International Association of Physicians in AIDS Care; serves as a consultant to CNSBio; and has received research support from Roche and CNSBio. Dr. Affandi reports no disclosures. Dr. Brew serves on scientific advisory boards for Biogen Idec, GlaxoSmithKline, and Merck Serono; receives royalties from the publication of HIV Neurology (Oxford University Press, 2001) and Palliative Neurology (Cambridge University Press, 2005); has received speaker honoraria from GlaxoSmithKline, Boehringer Ingelheim, Tibotec Therapeutics, and Abbott; and receives research support from NHMRC (Australia). Dr. Creighton receives research support from the NIH (NIMH N01MH22005 [Research Coordinator] and NIMH 907647 [Research Coordinator]). Dr. Djauzi, Dr. Hooker, and Dr. Imran report no disclosures. Dr. Kamarulzaman serves on a scientific advisory board for Johnson & Johnson Asia Pacific; serves on the editorial board of International Journal of Drug Policy; and receives publishing royalties for contributions to UpToDate. Dr. Kamerman has received travel expenses for lectures and educational activities not funded by commercial entities; has received speaker honoraria from Merck & Co., Inc. and Novartis; has served as a consultant for Aspen Pharmacare Holdings Limited and Partners in Research; and receives research support from Adcock Ingram Pharmaceuticals, Aspen Pharmacare Holdings Limited (donations of antiretroviral drugs for investigator-initiated preclinical research), the National Research Foundation (South Africa), and the Iris Ellen Hodges Trust of the University of the Witwatersrand. Dr. McArthur receives royalties from the publication of Current Therapy in Neurologic Disease, 7th Edition (Mosby, 2006); and receives research support from Biogen Idec, the NIH (R01 MH075673 [PI], RO1 NS44807 [PI], RO1 NS49465 [PI], and R01 MH067831 [Co-I]), the National Multiple Sclerosis Society, and from the Foundation for Peripheral Neuropathy. Dr. Moore serves on the editorial advisory boards of HIV/AIDS–Research and Palliative Care; has received speaker honoraria from Bristol-Myers Squibb and Merck Serono; serves as a consultant to Bristol-Myers Squibb; and receives research support from Pfizer Inc., the NIH (R01DA011602 [PI], U01AI069918 [PI], R01AA16893 [PI], and K24DA00432 [PI]), and the Agency for Healthcare Research and Quality (200600025 [PI]). Dr. Price has received research support from Australia's National Health and Medical Research Council. Dr. Smyth reports no disclosures. Dr. Tan has received funding for travel from Merck Serono and Biogen Idec. Dr. Vanar, Dr. Wadley, Dr. Wesselingh, and Dr. Yunihastuti report no disclosures.
Address correspondence and reprint requests to Dr. C.L. Cherry, Infectious Diseases Unit, The Alfred Hospital, Melbourne, Victoria 3004, Australia kcherry@burnet.edu.au
Study funding: The data reported here were collected in studies related to HIV-associated neuropathy that were funded by the Australian Centre for HIV and Hepatitis Research, Australia's National Health and Medical Research Council, the National Institutes of Health (NS 26643), and the South African National Research Foundation.
Disclosure: Author disclosures are provided at the end of the article.
Received October 20, 2009. Accepted in final form February 11, 2010.
REFERENCES
- 1.Keswani S, Pardo C, Cherry C, Hoke A, McArthur J. HIV-associated sensory neuropathies. AIDS 2002;16:2105–2117. [DOI] [PubMed] [Google Scholar]
- 2.Cherry C, McArthur J, Hoy J, Wesselingh S. Nucleoside analogues and neuropathy in the era of HAART. J Clin Virol 2003;26:195–207. [DOI] [PubMed] [Google Scholar]
- 3.Brew BJ. The peripheral nerve complications of human immunodeficiency virus (HIV) infection. Muscle Nerve 2003;28:542–552. [DOI] [PubMed] [Google Scholar]
- 4.Cherry C, Skolasky R, Lal L, et al. Antiretroviral use and other risks for HIV-associated neuropathies in an international cohort. Neurology 2006;66:867–873. [DOI] [PubMed] [Google Scholar]
- 5.Nemni R, Sanvito L, Quattrini A, Santuccio G, Camerlingo M, Canal N. Peripheral neuropathy in hepatitis C virus infection with and without cryoglobulinemia. J Neurol Neurosurg Psychiatry 2003;74:1267–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santoro L, Manganelli F, Briani C, et al. Prevalence and characteristics of peripheral neuropathy in hepatitis C virus population. J Neurol Neurosurg Psychiatry 2006;77:626–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ammendola A, Sampaolo S, Ambrosone L, et al. Peripheral neuropathy in hepatitis-related mixed cryoglobulinemia: Electrophysiologic follow-up study. Muscle Nerve 2005;31:382–385. [DOI] [PubMed] [Google Scholar]
- 8.Clifford D, Yang Y, Evans S. Neurologic consequences of hepatitis C and human immunodeficiency virus coinfection. J Neurovirol 2005;11(suppl 3):67–71. [DOI] [PubMed] [Google Scholar]
- 9.Estanislao L, Morgello S, Simpson D. Peripheral neuropathies associated with HIV and hepatitis C co-infection: a review. AIDS 2005;19(suppl 3):S135–S139. [DOI] [PubMed] [Google Scholar]
- 10.Cornblath D, Hoke A. Recent advances in HIV neuropathy. Curr Opin Neurol 2006;19:446–450. [DOI] [PubMed] [Google Scholar]
- 11.Cherry C, Affandi J, Imran D, et al. Age and height predict neuropathy risk in HIV patients prescribed stavudine. Neurology 2009;73:315–320. [DOI] [PubMed] [Google Scholar]
- 12.Affandi J, Price P, Imran D, Yuhaningsih E, Djauzi S, Cherry C. Can we predict neuropathy risk before stavudine prescription in a resource limited setting? AIDS Res Hum Retroviruses 2008;24:1281–1284. [DOI] [PubMed] [Google Scholar]
- 13.Wadley A, Cherry C, Price P, et al. HIV-associated sensory neuropathy: a comparison of rates and risk factors in Australia and South Africa. Cape Town, South Africa: International AIDS Society Conference; 2009.
- 14.Smyth K, Affandi J, McArthur J, et al. Prevalence and risk factors for HIV-associated neuropathy in Melbourne, Australia 1993–2006. HIV Med 2007;8:367–373. [DOI] [PubMed] [Google Scholar]
- 15.Cherry C, Wesselingh S, Lal L, McArthur J. Evaluation of a clinical screening tool for HIV-associated sensory neuropathies. Neurology 2005;65:1778–1781. [DOI] [PubMed] [Google Scholar]
- 16.Authier F, Bassez G, Payan C, et al. Detection of genomic viral RNA in nerve and muscle of patients with HCV neuropathy. Neurology 2003;60:808–812. [DOI] [PubMed] [Google Scholar]
- 17.De Martino L, Sampaolo S, Tucci C, et al. Viral RNA in nerve tissues of patients with hepatitis C infection and peripheral neuropathy. Muscle Nerve 2003;27:102–104. [DOI] [PubMed] [Google Scholar]
- 18.Cherry C, Affandi J, Rosenow A, McArthur J, Wesselingh S, Price P. Cytokine genotype suggests a role for inflammation in nucleoside analog-associated sensory neuropathy (NRTI-SN) and predicts an individual's NRTI-SN risk. AIDS Res Hum Retroviruses 2008;24:117–123. [DOI] [PubMed] [Google Scholar]
- 19.Hulgan T, Haas D, Haines J, et al. Mitochondrial haplogroups and peripheral neuropathy during antiretroviral therapy: an adult AIDS clinical trials group study. AIDS 2005;19:1341–1349. [DOI] [PubMed] [Google Scholar]
- 20.Kallianpur A, Hulgan T, Canter J, et al. Hemochromatosis (HFE) gene mutations and peripheral neuropathy during antiretroviral therapy. AIDS 2006;20:1503–1513. [DOI] [PubMed] [Google Scholar]
- 21.Woolley I, Faragher M, Ugoni A, Spelman D. An analysis of factors associated with HIV-related peripheral neuropathy. Neurol Infect Epidemiol 1997;1997:33–37. [Google Scholar]
- 22.George S, Gebhardt J, Klinzman D, et al. Hepatitis C virus viremia in HIV-infected individuals with negative HCV antibody tests. J Acquir Immune Defic Syndr 2002;31:154–162. [DOI] [PubMed] [Google Scholar]
- 23.Clifford D, Smurzynski M, Park L, et al. Effects of active HCV replication on neurologic status in HIV RNA virally suppressed patients. Neurology 2009;73:309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdool Karim S, Tait D. Hepatitis C virus infection in urban and rural Natal/KwaZulu. S Afr Med J 1993;83:191–193. [PubMed] [Google Scholar]
- 25.Ellis L, Brown D, Conradie J, et al. Prevalence of hepatitis C in South Africa: detection of anti-HCV in recent and stored serum. J Med Virol 1990;32:249–251. [DOI] [PubMed] [Google Scholar]
- 26.Tucker T, Voigt M, Bird A, et al. Hepatitis C virus infection rate in volunteer blood donors from the Western Cape: comparison of screening tests and PCR. S Afr Med J 1997;87:603–605. [PubMed] [Google Scholar]
- 27.Vardas E, Sitas F, Seidel K, Casteling A, Sim J. Prevalence of hepatitis C virus antibodies and genotypes in asymptomatic, first-time blood donors in Namibia. Bull World Health Organ 1999;77:965–972. [PMC free article] [PubMed] [Google Scholar]
- 28.Amin J, Kaye M, Skidmore S, Pillay D, Cooper D, Dore G. HIV and hepatitis C coinfection within the CAESAR study. HIV Med 2004;5:174–179. [DOI] [PubMed] [Google Scholar]
- 29.Ocama P, Katwere M, Piloya T, et al. The spectrum of liver diseases in HIV infected individuals at an HIV treatment clinic in Kampala, Uganda. Afr Health Sci 2008;8:8–12. [PMC free article] [PubMed] [Google Scholar]
- 30.Hennig H, Schlenke P, Kirchner H, Bauer I, Schulte-Kellinghaus B, Bludau H. Evaluation of newly developed microparticle enzyme immunoassays for the detection of HCV antibodies. J Virol Methods 2000;84:181–199. [DOI] [PubMed] [Google Scholar]
- 31.Smuts H, Kannemeyer J. Genotyping of hepatitis C virus in South Africa. J Clin Microbiol 1995;33:1679–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verbeeck J, Peigue-Lafeuille H, Ross R, et al. HCV genotype 5: epidemiology and spread of an uncommon genotype. J Clin Virol 2008;41:170–171. [DOI] [PubMed] [Google Scholar]
- 33.Cribier B, Rey D, Schmitt C, Lang J, Kirn A, Stoll-Keller F. High hepatitis C viraemia and impaired antibody response in patients coinfected with HIV. AIDS 1995;9:1131–1136. [DOI] [PubMed] [Google Scholar]


