Abstract
Background:
Commonly used organophosphate and organochlorine pesticides inhibit acetylcholinesterase at synapses in the somatic, autonomic, and central nervous systems and may therefore have lasting effects on the nervous system. Few studies have examined the relationship of pesticide exposure and risk of dementia or Alzheimer disease (AD). We sought to examine the association of occupational pesticide exposure and the risk of incident dementia and AD in later life.
Methods:
Residents of the agricultural community of Cache County, UT, who were aged 65 years and older as of January 1995, were invited to participate in the study. At baseline, participants completed detailed occupational history questionnaires that included information about exposures to various types of pesticides. Cognitive status was assessed at baseline and after 3, 7, and 10 years. Standardized methods were used for detection and diagnosis of dementia and AD. Cox proportional hazards survival analyses were used to evaluate the risk of incident dementia and AD associated with pesticide exposure.
Results:
Among 3,084 enrollees without dementia, more men than women reported pesticide exposure (p < 0.0001). Exposed individuals (n = 572) had more years of education (p < 0.01) but did not differ from others in age. Some 500 individuals developed incident dementia, 344 with AD. After adjustment for baseline age, sex, education, APOE ε4 status, and baseline Modified Mini-Mental State Examination scores, Cox proportional hazards models showed increased risks among pesticide-exposed individuals for all-cause dementia, with hazard ratio (HR) 1.38 and 95% confidence interval (CI) 1.09–1.76, and for AD (HR 1.42, 95% CI 1.06–1.91). The risk of AD associated with organophosphate exposure (HR 1.53, 95% CI 1.05–2.23) was slightly higher than the risk associated with organochlorines (HR 1.49, 95% CI 0.99–2.24), which was nearly significant.
Conclusions:
Pesticide exposure may increase the risk of dementia and Alzheimer disease in late life.
GLOSSARY
- 3MS
= Modified Mini-Mental State Examination;
- AD
= Alzheimer disease;
- CI
= confidence interval;
- DQ
= Dementia Questionnaire;
- DSM-III-R
= Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised;
- HR
= hazard ratio.
Few researchers have evaluated the potential association between pesticide exposure and later risk of Alzheimer disease (AD). Studies of this potential association are of particular interest for several important reasons. First, some pesticides are acetylcholinesterase inhibitors or have similar effects.1 There is evidence that long-term pesticide exposure may have lasting toxic effects on the CNS.2,3 Second, the use of pesticides has increased dramatically in the last 50 years.4 As noted previously,5 the Environmental Protection Agency reports that “In the U.S., more than 18,000 products are licensed for use . . . each year >2 billion pounds of pesticides are applied to crops, homes, schools, parks, and forests . . . .”6 These numbers highlight a potentially important public health issue. Finally, older adults are more likely to have been exposed to persistent pesticides such as DDT, which was in use from the 1940s until its ban in 1972. The peak year for DDT use was 1959, when 80 million pounds were applied across the United States.7
There have only been a few case-control studies and 2 relatively small cohort studies on the association between pesticide exposure and AD.8 Larger sample sizes and better methods of exposure classification are needed in order to get a clear picture of the potential risk associated with occupational exposure to pesticides. Our objective was to evaluate the effects of occupational pesticide exposure on AD risk in a well-characterized cohort of elderly individuals who reside in the agricultural community of Cache County, UT.
METHODS
Cache County is known as one of Utah's primary agricultural regions. Cache leads the state in barley production; other products include winter wheat, spring wheat, dry beans, corn for silage, apples, and alfalfa hay.9 Roughly 12% of the population under study reported farming as a primary occupation.
Standard protocol approvals, registrations, and patient consents.
Procedures and protocols of the Cache County Memory Study, a prospective cohort study, were approved by the Institutional Review Boards of Utah State University, Duke University, and The Johns Hopkins University. Informed consent was obtained from all study participants at each stage of the study. Spouses or next of kin gave consent when participants were unable to provide it.
Ascertainment of dementia status.
The Cache County Memory Study methods have been described in detail previously.10,11 At the start of the study, all residents of the county aged 65 and older in 1995 were invited to participate.10 Baseline cognitive screening and risk factor questionnaires were completed by a total of 5,092 participants (90% of the population aged 65 or older). A version of the Modified Mini-Mental State Examination (3MS)12 adapted for epidemiologic studies13 in combination with the Dementia Questionnaire (DQ)14 were used as a 2-stage screening tool at the baseline evaluation and 3 years later at the first follow-up. At baseline, individuals falling below a predefined cutpoint in screening and all participants aged 90+ (at baseline) or aged 85+ (at subsequent evaluations) were assessed with a full clinical evaluation for dementia and for milder cognitive syndromes. Final diagnoses were assigned at consensus conferences using standard criteria. DSM-III-R15 and the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria16 were used for AD diagnoses and the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l'Enseignement en Neurosciences criteria,17 as operationalized by Tatemichi et al.,18 was used to diagnose vascular dementia. Dementia onset age was defined as the year in which a participant unambiguously met DSM-III-R criteria for dementia.
Exposure assessment.
At baseline, participants completed detailed occupational questionnaires, providing information about their work histories and associated exposures to various substances. This structured, detailed questionnaire was administered in person and was used to classify occupations and assess occupational exposures including cues for exposures to specific agents. Initial questions determined if participants held a job to support their family and the kind of work they performed for the majority of their lives. If a person had multiple jobs, they were asked if they worked at each type of job for 5 years or more. If the 5-year threshold was met, the participant was asked about industry, employer, and primary duties. A second series of detailed questions were asked about exposures on the job. If pesticide use was endorsed, further probe questions were used to determine the type of pesticide, the frequency of use, and the duration of use in years. There were no questions about the use of safety equipment or the intensity of exposure. Questions addressed pesticides in general and 4 specific types of pesticides including organophosphates, carbamates, organochlorines (DDT), and methyl bromides. Responses to the occupational questionnaire were coded by an occupational health nurse according to the Dictionary of Occupational Titles.19 Participants were classified as exposed if they endorsed any pesticide exposure.
Analytic approach.
Demographic characteristics of the exposed and unexposed members of the sample were compared using χ2 tests for categorical variables and t tests were used for continuous variables. Cox proportional hazard analysis was used to assess the relationship between prior pesticide exposure and the risk of incident dementia and AD. Unexposed participants were used as the reference group; age on study was used as the time axis. Participants were censored at dementia onset or last observation if dementia was not diagnosed. Additional models were constructed to address the risk associated with organophosphates and organochlorines specifically. Models were adjusted for factors known to independently influence the risk of dementia and AD including age at baseline (centered at age 65), sex, education in years, and APOE ε4 status. We also included a term for baseline 3MS scores to control for baseline cognitive status. In addition, we tested whether the estimated hazard of the association between pesticide exposure and dementia was proportional over the analytic time scale by including an interaction term between the variable for pesticide exposure and age on study. Analyses were performed using SAS statistical software.20
RESULTS
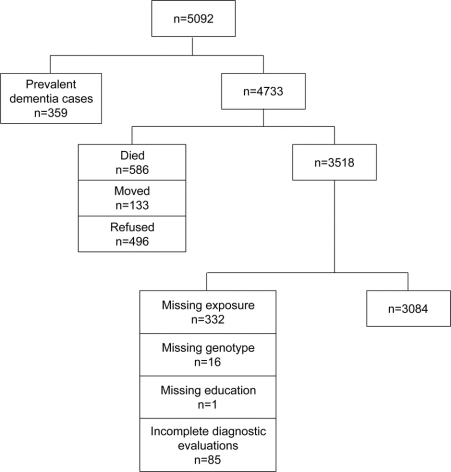
At baseline, a total of 5,092 participants were screened for dementia and completed detailed risk factor questionnaires. Each participant was classified by pesticide exposure. We excluded from the current analysis 359 individuals with prevalent dementia. A total of 586 individuals died prior to the first follow-up evaluation, 133 moved out of the area, and 496 refused to participate, leaving a potential sample of 3,518 individuals (figure). Another 434 individuals were excluded from the analysis due to missing data (exposure = 332, genotype = 16, education = 1, and diagnosis = 85). The final analytic sample comprised a total of 3,084 individuals without dementia at baseline who provided self-report information about pesticide exposures; 572 reported some pesticide exposure. Over 40% of those exposed reported farming as the primary occupation for most of their working lives. Follow-up evaluations were conducted 3, 7, and 10 years after the initial evaluation; cognitive status was reevaluated at each time point. The average duration of follow-up was 7.2 (3.5) years. Compared to those included in the study sample, those who were excluded tended to be older (p < 0.0001), were more likely to be female (p < 0.0001), and had fewer years of education (p < 0.0001). There were no differences between groups in APOE status (1 or more APOE ε4 alleles vs no ε4 alleles) (p = 0.49) or pesticide exposure status (p = 0.86) among those providing such information (data not shown).
Figure Sample selection flowchart
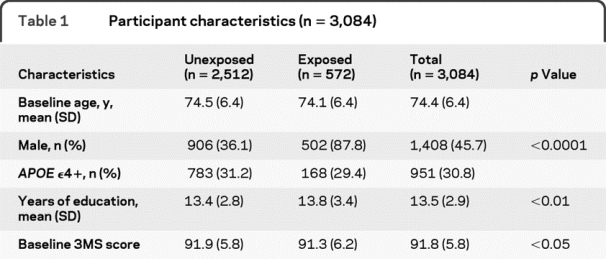
Among those retained in the analytic sample, more men reported exposures than women (p < 0.0001); there were no differences in baseline age between the exposed and unexposed groups (overall mean age 74.4, SD 6.4) (table 1). Those who reported exposure had more education (p < 0.01). This difference is due to the fact that men had higher mean education levels than women (14.1 vs 13.0 years, p < 0.0001) and there were few women in the exposed group. There was a small but significant difference between groups' baseline 3MS scores. Of 572 individuals reporting pesticide exposure, 316 were exposed to organophosphates, 256 were exposed to organochlorines, 25 were exposed to carbamates, and 28 reported exposure to methyl bromides. A number of individuals reported more than one type of exposure (n = 186) and 164 individuals' exposures were not classified because participants' responses either indicated an agent that was not among the 4 major classes or their responses were nonspecific. A total of 500 individuals were identified with incident dementia; 344 had a primary or secondary diagnosis of AD. A total of 108 individuals who reported exposure to pesticides were later diagnosed with dementia.
Table 1 Participant characteristics (n = 3,084)
Initial univariable Cox proportional hazard models for incident dementia are presented in table 2. Additional models were created adjusting for baseline age (centered at age 65), education, and APOE ε4 status because these factors are all known to significantly influence the risk of dementia and AD. We included a gender term because prior work in our study has shown a difference in risk for AD between men and women11; in the current sample, most of the exposed individuals are men. A term for baseline 3MS score was used to control for global cognitive status at baseline; age on study was used as the time axis. Results showed there was an increased risk of dementia in those exposed to any pesticide (hazard ratio [HR] 1.38, 95% confidence interval [CI] 1.09–1.76). When the outcome was restricted to AD only (including cases with AD as the primary or secondary diagnoses) the risk was similar (HR 1.42, 95% CI 1.06–1.91) (table 3). A test of the proportionality assumption indicated that the hazards associated with each group were proportional (p = 0.17).
Table 2 Cox proportional hazards ratios for dementia incidence by pesticide exposure
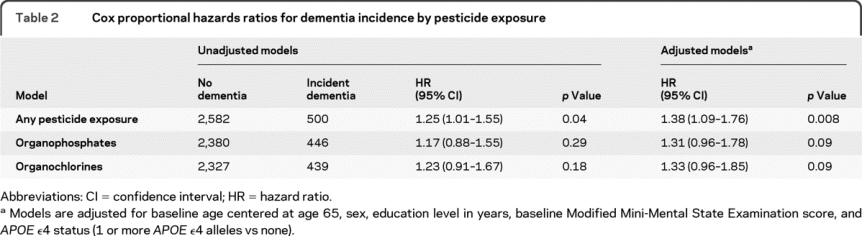
Table 3 Cox proportional hazards ratios for Alzheimer dementia incidence by pesticide exposure
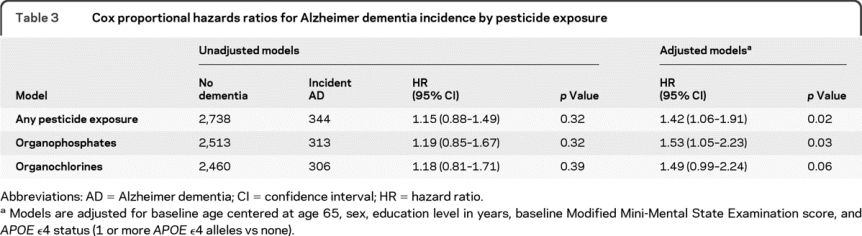
Separate models were created to test the association between risk of incident dementia or AD and specific pesticides. In these models, we set aside individuals who reported exposure to pesticides other than the group under study in order to clearly distinguish between the exposed and unexposed groups. A total of 256 individuals reporting exposures other than organophosphates were set aside in the analysis of organophosphates and 316 individuals were set aside in the analysis of organochlorines. The risk of dementia associated with exposure to organophosphates after adjustment for baseline age, sex, education, baseline 3MS score, and APOE ε4 status was increased among the exposed (HR 1.31, 95% CI 0.96–1.78) although marginally nonsignificant (p = 0.09). The risk of AD was 53% higher (HR 1.53, 95% CI 1.05–2.23). In the evaluation of organochlorines, we found similarly marginal increased risks for dementia after adjustment for baseline age, sex, education, baseline 3MS score, and APOE ε4 status (HR 1.33, 95% CI 0.96–1.85) and for AD (HR 1.49, 95% CI 0.99–2.24). Because 177 participants reported exposures to both organophosphates and organochlorines, we performed an additional analysis to determine if the risk for dementia and AD was increased in this group, but none of our findings were significant (data not shown).
DISCUSSION
Thus far, there have been few reports on the association between pesticides and AD.2,21–25 Findings have been equivocal and the samples under study have been relatively small. Methods of exposure determination in these studies vary, ranging from proxy reports22–24 to self-administered mail-in questionnaires25 to structured in-person interviews.2,24 Determination of the level of occupational pesticide exposure is particularly difficult because it depends on the duration of use, the method of application, and whether safety equipment was used, or used properly.5 An individual who lives proximal to treated fields may also be exposed. Exposure classification can therefore be a problem in studies of the effects of pesticides on the risk of dementia. Case-control studies in dementia research, which necessarily rely on informant reports, are likely to produce somewhat conservative estimates as proxy informants have been shown to underreport pesticide exposures.26,27 Evaluations of data from cohort studies have also shown that informant reports tend to underestimate exposures while reports from farmers themselves tend to be reliable.27,28
Using self-reported exposure data, we showed that occupational pesticide exposure (including all forms endorsed) was associated with an increased risk of dementia. When the outcome was restricted to AD, the association remained. Two classes of pesticides, organophosphates and organochlorines, were associated with an increased risk of dementia and more specifically AD, although the associations were only marginal. We found a stronger risk for dementia with “any pesticide” exposure than for specific types likely because the “any pesticide” group included a number of exposures that could not be accurately classified. As a result, there were more individuals in the exposed group as well as a greater number of outcome events under study in the all-cause dementia group. In the analysis of specific classes of pesticides, we set aside individuals who claimed pesticide exposures other than the exposure of interest in order to reduce noise in the analysis. As a result, we found stronger risks for AD among those exposed to organophosphates and organochlorines than for all-cause dementia. We should note that 177 individuals reported exposure to both and were included in both analyses. A post hoc evaluation showed that there was no additional increased risk in individuals exposed to both pesticides. The fact that the associations we found were slightly stronger when the outcome was restricted to AD suggests that effects of pesticide exposure may be specific to AD and not other forms of dementia.
Our findings are generally consistent with another study that found an elevated risk of AD associated with occupational pesticide exposure.2 Others have found elevated risks but probably lacked the power to find significant associations.21,24,25 In a carefully conducted study from the PAQUID cohort,2 pesticide exposures were evaluated in great detail. In-person questionnaires were used in combination with job coding classifications; a panel of experts assessed the likelihood of exposure associated with each job classification and a job exposure matrix was produced. Cumulative exposures were estimated by taking the product of the duration of exposure and the levels of exposure per the exposure matrix. Place of residence in proximity to vineyards was also taken into consideration. A significant association between pesticide exposure and later onset of AD in men was found (adjusted relative risk 2.36, 95% CI 1.02–5.63). These findings highlight the importance of exposure ascertainment as exposure to pesticides can be particularly difficult to determine.5
In the current study, our exposure ascertainment is derived from in-person structured interviews with cues for types of pesticides. Results of investigations into the reliability of data collection methods used in the Agricultural Health Study27 suggest that our methods may be more accurate than those applied in studies that use mail back questionnaires25 and reports from proxy informants which were necessarily used in case-control studies.22–24 The Agricultural Health Study analysis showed that structured questionnaires with cues yield better information than open-ended questioning.27 Farmers' self-reported pesticide use has shown good reliability27,29 regardless of the age or education level of participants.29 It is possible that farmers' recollection of pesticide use is strengthened because of the certification processes required for pesticide applicators. At the same time, the reliability of these reports decreases when more specific details are requested.29
There are several strengths and limitations of note in this study. First, this is one of the largest samples used to investigate this question to date, and the follow-up period is relatively long (mean 7.2 years). We have used well-established methods for identification and classification of dementia status. The sample is relatively stable, with low in and out migration, and high response rates (90% participation at baseline) dramatically reducing nonresponder bias.30 They are also relatively homogenous with regard to education levels and access to health care. Over 90% of the sample are members of the Church of Jesus Christ of Latter-Day Saints, a factor which influences the amount of alcohol and tobacco use in the population. For these reasons, the sample may tend to be healthier, with lower rates of chronic disease. While it is unlikely that these factors influence the effects of pesticide exposures, it is possible that our findings may not be generalizable to other populations. Some nonspecific pesticide exposures were recorded and some individuals reported multiple exposures (177 were exposed to both organophosphates and organochlorines). While we recorded exposures to other pesticides (carbamates and methyl bromides), not enough were reported to analyze them separately. In general, although many forms of pesticides are available, most farmers choose from about 5.27 The “any pesticide” category additionally captured exposures that either did not fit into one of the 4 major classifications that we targeted or the subject's report of the particular agent was nonspecific such that it could not be classified. In order to reduce measurement error in our analyses of organophosphates and organochlorines, we set aside individuals who reported exposure to other pesticides or exposures that were not classified. It is possible that some reports among the unclassified exposures were not related to occupational exposure and could be considered measurement error.
Although participants were queried about the duration and extent of exposures, these questions were completed with less frequency. We choose to classify participants as ever/never users in order to retain the largest possible sample for analysis. This decision is bolstered by prior reports which indicate that ever/never use of pesticides is reported more reliably than responses to more detailed questions.29,31 Regardless of our efforts, there is always the possibility of misclassification. In the current study, it is likely that the misclassification is nondifferential with regard to disease status. Given that exposures are classified dichotomously and the outcome is determined exhaustively, it is likely that the resulting effect sizes are conservative.29,32,33
These findings add to a small but growing literature suggesting that exposure to pesticides may have adverse long-term effects on the nervous system, thereby increasing the risk of AD in late life. Future epidemiologic work should focus on specific types of pesticides and the intensity of exposure to fully characterize the potential association with AD. Toxicologic studies may help elucidate the biologic mechanism for the association.
ACKNOWLEDGMENT
The authors thank the study participants; the neurogenetics laboratory of the Bryan AD Research Center at Duke University for APOE genotyping; and study coordinators Nancy Sassano (Utah State University) and Michelle McCart (Duke University) and Cara Brewer, Tony Calvert, Tiffany Newman, Roxane Pfister, Sarah Schwartz, and Joslin Werstak of Utah State University for technical assistance in data collection and entry.
DISCLOSURE
Dr. Hayden serves as Senior Editor of Alzheimer's & Dementia and receives research support from the NIH (R01-AG1380 [investigator], K01-AG029336 [PI], and P30-AG028377 [administrative support]). Dr. Norton receives research support from the NIH (AG-11380 [Project manager], AG-11380 [Subcontract PI], AG-18712 [Co-I], AG-211386 [Co-I], and AG-31272 [PI]). Dr. Darcey receives research support from Cobalt Development Institute and the CDC/NIOSH (educational training grant). Dr. Østbye has received travel expenses and honoraria for educational activities not funded by commercial entities; has received/receives research support from the NIH (NIDDK R01-DK-07549 [PI], NCI R01-CA-114392-01A1 [Co-I], and NIDDK R01-DK-64986 [PI]); and has served as a legal consultant for AstraZeneca. Dr. Zandi receives research support from the NIH; work on this project was supported by AG-11380 [Subcontract PI]. Dr. Breitner serves on scientific advisory boards for the N. Bud Grossman Center for Research in Memory and Care, the Florida ADRC (NIA and State of FL), and for the Chicago Health and Aging Project; serves on the editorial boards of Alzheimer's Disease and Associated Disorders, PLOS-One, Academics, Alzheimer's and Dementia, and the American Journal of Alzheimer's Disease; and receives research support from the NIH (NIA U01-AG-15477 [PI]) and the Minnesota Medical Foundation. Dr. Welsh-Bohmer has served on a scientific advisory board for Medivation, Inc.; has received funding for travel and speaker honoraria from Medivation, Inc. and Elan Corporation/Wyeth; has served/serves as an Associate Editor of Neuropsychology Review, on the editorial boards of Alzheimer's & Dementia, the Journal International Neuropsychological Society, and the Journal of Experimental & Clinical Neuropsychology, and as a consulting editor for Aging, Neuropsychology, and Cognition and Neuropsychology; holds US Patent 6867236 (issued 2005): use patent for nonsteroidal anti-inflammatory compounds for the treatment of Alzheimer's disease; receives royalties from the publication of Geriatric Neuropsychology: Assessment and Intervention (Guildford Publications, 2006); and receives research support from the NIH (NIA AG11380 [PI] and NIA AG028377 [PI]).
Address correspondence and reprint requests to Dr. Kathleen M. Hayden, DUMC, Joseph and Kathleen Bryan ADRC, 2200 West Main Street, Suite A-200, Durham, NC 27705 khayden@duke.edu
Study funding: Supported by NIH/NIA R01-AG1380 and P30-AG 028377 [APOE genotyping].
Disclosure: Author disclosures are provided at the end of the article.
Received October 23, 2009. Accepted in final form February 5, 2010.
REFERENCES
- 1.Carlock LL, Chen WL, Gordon EB, et al. Regulating and assessing risks of cholinesterase-inhibiting pesticides: divergent approaches and interpretations. J Toxicol Environ Health B Crit Rev 1999;2:105–160. [DOI] [PubMed] [Google Scholar]
- 2.Baldi I, Lebailly P, Mohammed-Brahim B, Letenneur L, Dartigues JF, Brochard P. Neurodegenerative diseases and exposure to pesticides in the elderly. Am J Epidemiol 2003;157:409–414. [DOI] [PubMed] [Google Scholar]
- 3.van Wendel de Joode B, Wesseling C, Kromhout H, Monge P, Garcia M, Mergler D. Chronic nervous-system effects of long-term occupational exposure to DDT. Lancet 2001;357:1014–1016. [DOI] [PubMed] [Google Scholar]
- 4.Osteen C. Pesticide use trends and issues in the United States. In: Pimentel D, Lehman H, eds. The Pesticide Question: Environment, Economics, and Ethics. New York: Chapman & Hall; 1993: 307–336. [Google Scholar]
- 5.Kamel F, Hoppin JA. Association of pesticide exposure with neurologic dysfunction and disease. Environ Health Perspect 2004;112:950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Environmental Protection Agency. Promoting Safety for America's Future: Office of Pesticide Programs FY 2002 Annual Report: 2003 EPA-735-R-03-001. Washington, DC: Environmental Protection Agency; 2003. [Google Scholar]
- 7.Environmental Protection Agency. DDT Ban Takes Effect; press release December 21, 1972. Available at: http://www.epa.gov/history/topics/ddt/01.htm. Accessed July 21, 2009.
- 8.Santibanez M, Bolumar F, Garcia AM. Occupational risk factors in Alzheimer's disease: a review assessing the quality of published epidemiological studies. Occup Environ Med 2007;64:723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooperation Extension Services ED. Cache County Agriculture Profile. Logan, UT: Utah State University; 2005–2006.
- 10.Breitner JC, Wyse BW, Anthony JC, et al. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology 1999;53:321–331. [DOI] [PubMed] [Google Scholar]
- 11.Miech RA, Breitner JC, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90s for men, later for women: The Cache County Study. Neurology 2002;58:209–218. [DOI] [PubMed] [Google Scholar]
- 12.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48:314–318. [PubMed] [Google Scholar]
- 13.Tschanz JT, Welsh-Bohmer KA, Plassman BL, Norton MC, Wyse BW, Breitner JC. An adaptation of the modified mini-mental state examination: analysis of demographic influences and normative data: The Cache County Study. Neuropsychiatry Neuropsychol Behav Neurol 2002;15:28–38. [PubMed] [Google Scholar]
- 14.Silverman JM, Breitner JC, Mohs RC, Davis KL. Reliability of the family history method in genetic studies of Alzheimer's disease and related dementias. Am J Psychiatry 1986;143:1279–1282. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, third edition revised: DSM-III-R. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 17.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 18.Tatemichi TK, Sacktor N, Mayeux R. Dementia associated with cerebrovascular disease, other degenerative diseases, and metabolic disorders. In: Terry RD, Katzman R, Bick KL, eds. Alzheimer Disease. New York: Raven Press; 1994: 123–166. [Google Scholar]
- 19.US Department of Labor. Dictionary of Occupational Titles, 4th ed. Washington, DC: US Government Printing Office; 1991. [Google Scholar]
- 20.SAS 9.1, version 9.1. Cary, NC: SAS Institute; 2002–2003.
- 21.The Canadian Study of Health and Aging: risk factors for Alzheimer's disease in Canada. Neurology 1994;44:2073–2080. [DOI] [PubMed] [Google Scholar]
- 22.French LR, Schuman LM, Mortimer JA, Hutton JT, Boatman RA, Christians B. A case-control study of dementia of the Alzheimer type. Am J Epidemiol 1985;121:414–421. [DOI] [PubMed] [Google Scholar]
- 23.Gauthier E, Fortier I, Courchesne F, Pepin P, Mortimer J, Gauvreau D. Environmental pesticide exposure as a risk factor for Alzheimer's disease: a case-control study. Environ Res 2001;86:37–45. [DOI] [PubMed] [Google Scholar]
- 24.Gun RT, Korten AE, Jorm AF, et al. Occupational risk factors for Alzheimer disease: a case-control study. Alzheimer Dis Assoc Disord 1997;11:21–27. [DOI] [PubMed] [Google Scholar]
- 25.Tyas SL, Manfreda J, Strain LA, Montgomery PR. Risk factors for Alzheimer's disease: a population-based, longitudinal study in Manitoba, Canada. Int J Epidemiol 2001;30:590–597. [DOI] [PubMed] [Google Scholar]
- 26.Teschke K, Olshan AF, Daniels JL, et al. Occupational exposure assessment in case-control studies: opportunities for improvement. Occup Environ Med 2002;59:575–593; discussion 594. [DOI] [PMC free article] [PubMed]
- 27.Blair A, Zahm SH. Patterns of pesticide use among farmers: implications for epidemiologic research. Epidemiology 1993;4:55–62. [DOI] [PubMed] [Google Scholar]
- 28.Brown LM, Dosemeci M, Blair A, Burmeister L. Comparability of data obtained from farmers and surrogate respondents on use of agricultural pesticides. Am J Epidemiol 1991;134:348–355. [DOI] [PubMed] [Google Scholar]
- 29.Blair A, Tarone R, Sandler D, et al. Reliability of reporting on life-style and agricultural factors by a sample of participants in the Agricultural Health Study from Iowa. Epidemiology 2002;13:94–99. [DOI] [PubMed] [Google Scholar]
- 30.Norton MC, Breitner JCS, Welsh KA, Wyse BW. Characteristics of nonresponders in a community survey of the elderly. J Am Geriatr Soc 1994;42:1252–1256. [DOI] [PubMed] [Google Scholar]
- 31.Engel LS, Seixas NS, Keifer MC, Longstreth WT, Jr., Checkoway H. Validity study of self-reported pesticide exposure among orchardists. J Expo Anal Environ Epidemiol 2001;11:359–368. [DOI] [PubMed] [Google Scholar]
- 32.Rothman KJ, Greenland S. Modern Epidemiology, 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 33.Wacholder S, Hartge P, Lubin JH, Dosemeci M. Non-differential misclassification and bias towards the null: a clarification. Occup Environ Med 1995;52:557–558. [DOI] [PMC free article] [PubMed] [Google Scholar]