SUMMARY
Emerging in vitro and epidemiological studies indicate an association between virally-induced impaired type I interferon (IFN-I) production and enhanced susceptibility to opportunistic infections, which represent a major health problem. Here, we provide in vivo evidence that lymphocytic choriomeningitis virus (LCMV) infection of its natural host, the mouse, dramatically diminishes the unique capacity of plasmacytoid dendritic cells (pDCs) to secrete high levels of systemic IFN-I. While both acute and persistent LCMV infections suppress pDC IFN-I response, only the persistent virus induces a long-lasting diversion of this innate immune pathway. LCMV infection selectively affects the hallmark function of pDCs to produce prodigious amounts of IFN-I but does not alter their secretion of other cytokines, chemokines and maturation. This results in reduced IFN-I production and relates to impaired NK cell responses in LCMV-infected mice challenged with murine cytomegalovirus (MCMV) as an opportunistic pathogen. This innate immune-defect is associated with a compromised host ability to counteract early MCMV spread. Thus, an explanation for understanding the occurrence of opportunistic infections following in vivo viral insults is uncovered and has important implications for the alleviation and treatment of such medical complications.
Keywords: plasmacytoid dendritic cells, type I interferon, virus infection, immunosuppression, opportunistic infections
INTRODUCTION
Virus infections are often associated with a transient or long-lasting generalized suppression of the host immune response. Dramatic examples of the global impact of virus-induced immunosuppression are the 40 million individuals infected worldwide with human immunodeficiency virus type-1 (HIV-1) with more than 3 million deaths per year, mainly as a result of opportunistic secondary infections (Mathers and Loncar, 2006). Similarly, measles virus (MV) that currently infects over 30 million individuals worldwide leads to approximately 500,000 deaths annually also primarily as a consequence of secondary infections (Griffin, 1995; Guilbert, 2003). Both of these infections illustrate the clinical relevance of virus-induced immunosuppression and the importance of a healthy immune system to ward off infections with opportunistic pathogens. In addition to the well-established contribution of altered adaptive immunity to viral-induced immunosuppression (Chisari and Ferrari, 1995; Klenerman and Hill, 2005; Oldstone, 2006; Shoukry et al., 2004; Wherry and Ahmed, 2004), in vitro and epidemiological evidences indicate an association between defective production of type I interferons (IFN-I) and more frequent opportunistic infections (Hosmalin and Lebon, 2006; Schlender et al., 2005; Siegal, 2003; Siegal et al., 2001).
IFN-I orchestrate numerous biological and cellular processes and are a critical link between innate and adaptive immunity (Garcia-Sastre and Biron, 2006; Le Bon and Tough, 2002). Although any cell can potentially produce IFN-I upon virus infection, plasmacytoid DCs (pDCs), also known as IFN producing cells, are a unique cell type specialized to rapidly produce prodigious amount of these innate mediators following infection by multiple viruses (Asselin-Paturel and Trinchieri, 2005; Colonna et al., 2004; Kadowaki and Liu, 2002; Le Bon and Tough, 2002; Liu, 2005; McKenna et al., 2005). Indeed, pDCs dedicate 50% of their transcription to make IFN-I mRNA and following viral stimulation synthesize a significantly broad range of these closely related cytokines including IFN-α, IFN-β, IFN-ω, IFN-λ and IFN-τ in some cases without requiring viral replication (Lee et al., 2007; Liu, 2005). Through the secretion of such important innate factors, pDCs orchestrate a systemic antiviral state programmed to directly control viral growth and communicate danger signals to other innate cells such as natural killer (NK) cells (McKenna et al., 2005). To accomplish this key role, pDCs use the Toll-like receptor (TLR) system (Kato et al., 2006), including TLR-7 (Diebold et al., 2004; Heil et al., 2004; Lund et al., 2004) and TLR-9 (Bauer et al., 2001; Jarrossay et al., 2001; Kadowaki et al., 2001; Krug et al., 2001; Lund et al., 2003), which rapidly trigger IFN-I transcription through a MyD88 dependent signaling pathway. TLR-9 mediates recognition of CpG-rich regions in the genome of DNA viruses and bacteria including herpes simplex virus 1 and 2, murine cytomegalovirus (MCMV) (Delale et al., 2005) and mycobaterium tuberculosis (Bafica et al., 2005). TLR-9 also senses malaria pigment hemozoin, extending its role to host defense against parasite infections (Coban et al., 2005). On the other hand, TLR-7 recognizes single stranded (ss)RNA viruses such as HIV, influenza and vesicular stomatitis virus (VSV) through the binding of uridine and guanosine rich sequences (Diebold et al., 2004; Heil et al., 2004; Lund et al., 2004). pDCs produce most of the IFN-α protein within the first 24 h following viral stimulation and then differentiate into mature DCs with enhanced antigen presenting capacity (Liu, 2005). In vitro studies indicated that after the first wave of IFN-I production, pDCs are refractory upon secondary stimulations to produce these cytokines (Jarrossay et al., 2001). However, how these observations translate into an in vivo acute and persistent viral infection in terms of systemic IFN-I production, innate response and susceptibility to secondary opportunistic infections is unclear.
In the present report we use lymphocytic choriomeningitis virus (LCMV) infection in its natural host, the mouse, to investigate the ability of pDCs from acutely or persistently infected hosts to produce systemic IFN-I during in vivo challenge with TLR ligands. We examine how this response is associated with pDC numbers and functional status. Finally, we evaluate the biological significance of virus-altered pDC-IFN-I-production in the context of opportunistic infection with murine cytomegalovirus (MCMV) and VSV in terms of systemic IFN-I levels, activation and function of NK cells, and early innate control of the secondary pathogen.
RESULTS
Rapid silencing of systemic IFN-I production during both acute and persistent viral infections
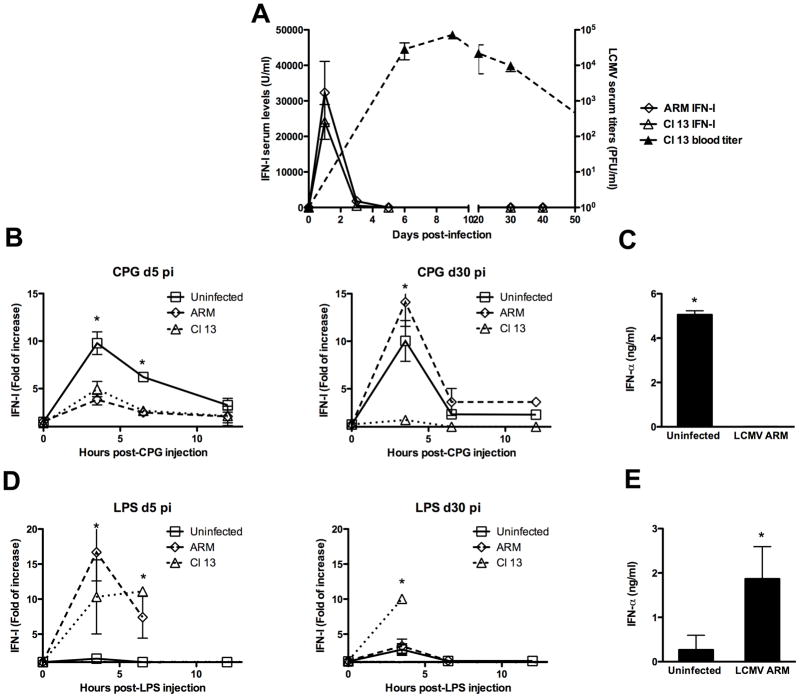
To gain insight on systemic IFN-I regulation during acute and persistent viral infections, we analyzed sera IFN-I levels throughout the course of LCMV infection. LCMV Armstrong 53b (ARM) and Clone 13 (Cl 13) isolates were used to model in vivo acute and persistent infection, respectively (Ahmed et al., 1984). Mice were infected intravenously (iv) with 2 × 106 PFU of LCMV and the levels of IFN-I were determined in sera at 0, 1, 3, 5, 30 and 40 days post-infection (pi) (Figure 1A, left axis). ARM and Cl 13 infected mice exhibited similar levels and kinetics of systemic IFN-I in sera, which peaked at day 1 after inoculation. By 5 days post-infection, systemic levels of IFN-I returned to baseline and remained undetectable throughout the course of the infection regardless of whether viral clearance (ARM) or persistence (Cl 13) occurred. These data indicate that even in the presence of persistent viral replication of 1 × 104 to 1 × 106 PFU/ml in blood and several tissues with LCMV Cl 13 (Ahmed et al., 1984; Moskophidis and Zinkernagel, 1995), the initial wave of systemic IFN-I is silenced. This observation suggested that LCMV infection was deregulating innate immunity and raised the dual issue of the host ability to re-induce IFN-I systemically at latter times, and what the biological impact of IFN-I silencing would be upon subsequent stimulations or infections.
Figure 1. Systemic IFN-I response during LCMV infection.
Mice were infected iv with 2 × 106 PFU of ARM or Cl 13 LCMV. Eye-bleed samples were collected before infection (day 0) and at 1, 3, 5, 30 and 40 days pi. IFN-I activity was measured by luciferase bioassay (left y axis) and LCMV titer by plaque assay (right y axis) (A). Uninfected ARM- and Cl 13-infected mice at day 5 or 30 pi were injected with 5 μg of CpG (B–C) or LPS (D–E). Blood samples were collected before injection (0 h) and at 3.5, 6.5 and 12 h post-stimulation. IFN-I activity was measured by luciferase bioassay and the fold of increase respect to levels at 0 h is shown (B and D). ELISA was used to quantify IFN-α in samples collected at 3.5 h after stimulation from day-5 ARM infected mice (C and E). The mean ± s. d. obtained with 3 to 4 mice per group is shown (* LCMV-infected compared to uninfected group p<0.01). Data are representative of two to eight independent experiments.
Differential deregulation of TLR-induced IFN-I production during LCMV infection
TLRs are well-characterized pattern recognition receptors that induce IFN-I in response to microbial stimulation (Akira, 2006; Beutler et al., 2006; Janeway and Medzhitov, 2002). We investigated whether acute and/or persistent LCMV infection impacted the ability of the host to increase systemic IFN-I levels in response to TLR stimulation. We focused on TLR-9 and TLR-4 stimulation as representative MyD88 dependent and independent pathways of IFN-I induction, respectively (Akira, 2006; O’Neill, 2006). Uninfected, ARM (acute) and Cl 13 (persistent) infected mice at day 5 and 30 pi were injected iv with 5 μg of CpG-ODN (TLR-9 ligand) or lypopolysaccharide (LPS, TLR-4 ligand). The levels of IFN-I were determined by luciferase bioassay (Jiang et al., 2005) in sera at 0, 3.5, 6.5, and 12 h post-stimulation (Figure 1B and D). As previously reported (Asselin-Paturel et al., 2005) uninfected mice rapidly responded to CpG challenge by elevating systemic IFN-I levels in sera displaying a peak response at about 3.5 h post-stimulation (Figure 1B). In contrast, following inoculation of CpG at day 5 pi, both ARM and Cl 13 infected mice presented a dramatic reduction in IFN-I levels when compared to uninfected controls. The inhibited IFN-I response was transient for ARM-infected mice but long term for Cl 13-infected mice since such mice still failed to respond to CpG at 30 and 40 days pi when LCMV-ARM-infected mice had returned to normal (Figure 1B and data not shown). Similar results were obtained when IFN-α was quantified by ELISA at day 5 after ARM infection (Figure 1C). Importantly, the defective IFN-I production during LCMV infection was not due to an enhanced degradation of the administered CpG or a general failure to respond to TLR-9, since upon CpG injection LCMV-infected mice produced higher levels of IFN-γ compared to uninfected controls and succumbed to death within 24 and 48 h after TLR-9 stimulation (supplementary Figure 1 and data not shown). As reported before, minimal amounts of IFN-I were detected in uninfected mouse sera after in vivo administration of 5 μg of LPS (Asselin-Paturel et al., 2005) and Figure 1D). In sharp contrast to the TLR-9 response, IFN-I was over-produced in response to LPS in mice infected with ARM at day 5 pi and Cl 13 at day 5 and 30 pi, inducing lethality between 6.5 and 24 h after stimulation (Figure 1D and data not shown). These results were confirmed by quantification of IFN-α by Elisa at day 5 after ARM infection (Figure 1E) and agree with the previously reported enhanced sensitivity to IFN-I-mediated LPS endotoxin shock during acute LCMV infection (Doughty et al., 2001; Durbin et al., 2003; Nguyen and Biron, 1999). Finally, we tested the ability of LCMV infected mice to enhanced systemic IFN-I levels upon TLR-3 stimulation. Uninfected and ARM infected mice at day 5 pi were injected with 5 μg of the TLR-3 ligand polyribocytidylic acid, poly(I:C), and serum IFN-I level were quantified (Supplementary Figure 2). We found that LCMV infection also inhibited TLR-3 IFN-I response as indicated by the profound reduction in IFN-I levels in LCMV-infected mice compared to uninfected controls that received the same dose of Poly (I:C).
Altogether, these data indicate for the first time that during in vivo virus infection, the ability of the innate immune system to produce IFN-I upon discrete TLR stimulations can be differentially modulated. Specifically during LCMV infection, TLR-9 and TLR-3 IFN-I responses are abrogated while TLR-4 signaling is enhanced. Moreover, while acute and persistent LCMV infection deregulate TLR-IFN-I response, only the persistent infection induces a long-lasting diversion of these innate pathways.
Quantitative and qualitative defects of pDCs during acute and persistent LCMV infection
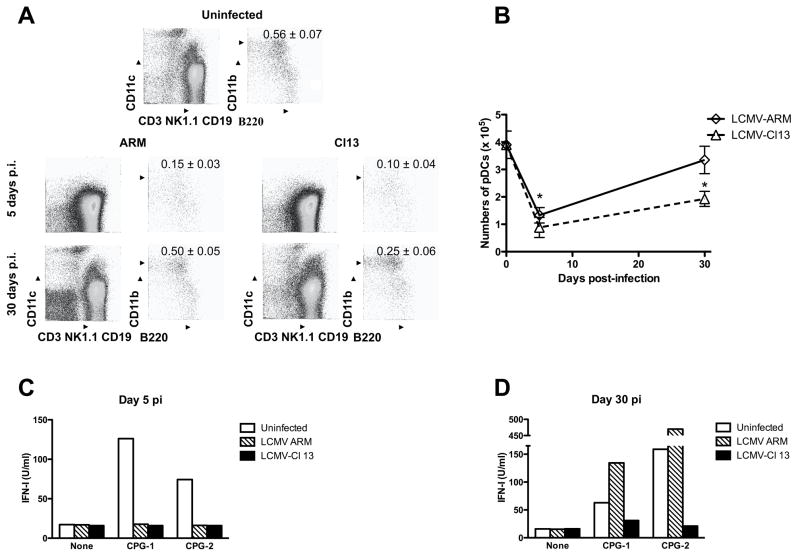
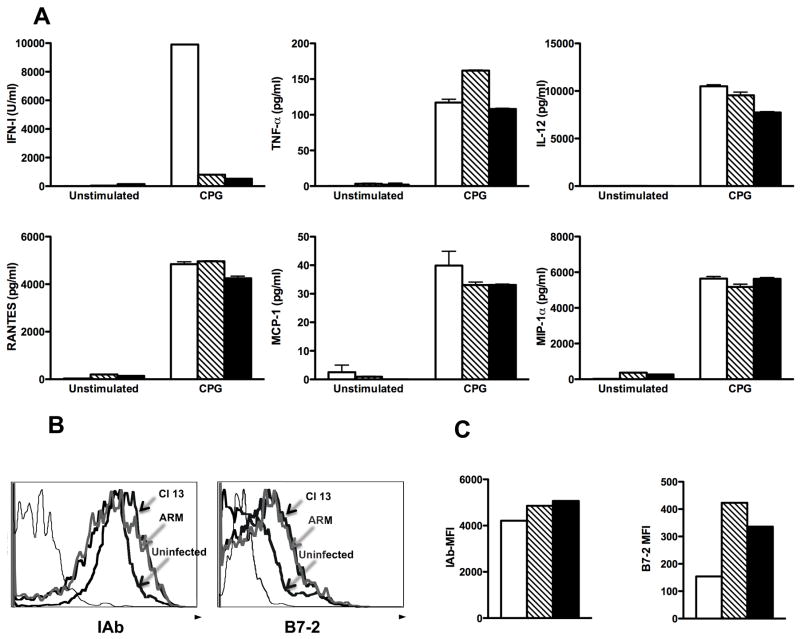
pDCs are responsible for the production of IFN-I upon CpG-ODN administration (Asselin-Paturel et al., 2005). We next investigated whether the affected TLR-9 IFN-I secretion during in vivo LCMV infection was due to a quantitative or qualitative defect on pDCs, or both. To address this question, we first assessed the percentage and numbers of pDCs (defined as CD11c+ B220+CD11b−CD3−CD19−NK1.1−) by FACS in spleens from uninfected, ARM and Cl 13 infected mice at day 5 and 30 pi (Figure 2A and B, respectively). Both ARM and Cl 13 infection induced a significant reduction in pDC percentages and numbers at day 5 pi. However, by day 30 pi pDCs showed complete recovery in ARM infected mice, but Cl 13 infected mice still exhibited a decreased number of these cells. Earlier we reported a reduced number of pDCs in the bone marrow of LCMV infected mice (Zuniga et al., 2004). These data indicate that LCMV infection affects the availability of pDCs in primary and secondary lymphoid organs, and suggest that this likely contributes to the suppressed production of IFN-I observed in vivo. However, the altered pDC numbers did not completely account for the profound suppression of IFN-I production in LCMV infected mice upon in vivo CpG challenge. At day 30 pi pDCs were only ~50% reduced while CpG-induced IFN-I levels in Cl 13 infected mice were almost undetectable. This led to the next series of studies to examine the functional capacity of the remaining pDCs to produce IFN-I upon CpG stimulation. CD11c+B220+120G8highCD11b−CD3−CD19−NK1.1− pDCs from spleens of uninfected, ARM or Cl 13 infected mice were purified by FACS-sort (purity ≥ 98%) at day 5 and 30 pi and cultured in the absence or in the presence of CpG during 12 to 15 h. Levels of IFN-I in the supernatant were determined by luciferase bioassay (Figure 2C and D). Two different phosphorothioate-backbone CpG sequences that induce significant IFN-I production by mouse pDCs (Asselin-Paturel et al., 2005; Boonstra et al., 2003; Gilliet et al., 2002) were used. In accordance with the in vivo data, production of IFN-I in response to CpG-ODNs was significantly impaired in pDCs from day-5 ARM and Cl 13 infected mice as compared to uninfected controls (Fig 2C). Remarkably, at day 30 pi only pDCs from Cl 13 infected mice displayed lower IFN-I production in response to CpG challenge (Fig 2D), indicating a long-lasting pDC intrinsic defect during persistent LCMV infection. We next evaluated pDC IFN-I production in response to TLR-7 stimulation with Loxoribine. As shown in Supplementary Figure 3, pDCs from ARM and Cl 13 infected mice also failed to produce IFN-I upon TLR-7 stimulation at day 5 pi and continued to be suppressed for 30 days after Cl 13, but not ARM, infection (Supplementary Figure 3). To further, investigate the pDC functional status during LCMV infection, we fully analyzed the ability of pDCs from uninfected, ARM and Cl 13 infected mice to respond to TLR signaling in terms of other cytokines, chemokines and maturation markers. For that, the production of TNF-α, IL-12, RANTES, Monocyte chemoattractant protein-1 (MCP-1), Macrophage Inflammatory Protein-1α (MIP-1α) and the expression of MHC class II and B7-2 molecules were evaluated in pDCs 15 h after in vitro CPG stimulation (Figure 3). Strikingly, the production of the additional cytokines and chemokines studied were not altered by LCMV ARM or Cl 13 infection, showing similar levels in culture supernatants from uninfected and infected mice. The expression of MHC class II molecule was also comparable among pDCs from uninfected and LCMV infected mice upon CPG stimulation while B7-2 expression was up-regulated in the cells from infected mice. Importantly, the levels of IFN-I in the same cell culture supernatant were dramatically reduced, consistently with the results described in Figure 2C. These data indicate that LCMV infection selectively alters the most unique function of pDCs without affecting their more redundant functional properties.
Figure 2. Quantitative and qualitative pDC alterations during LCMV infection.
Spleen cells were obtained from uninfected (day 0), ARM and Cl 13 infected mice (day 5 and 30 pi). (A and B) Cells were stained with the indicated markers, and analyzed by FACS A) The numbers indicate the mean ± s.d. percentage of pDCs (CD11c+B220+CD11b−CD3−NK1.1−CD19−) within total spleen cells obtained with 4 mice per group. B) The absolute number of pDCs was calculated by the total number of splenocytes and the percentage of pDCs (* LCMV-infected compared to uninfected (day 0) group p<0.01). (C and D) Cells were processed for FACS–purification of pDCs defined as CD11c+B220+120G8highCD11b−CD3−NK1.1−CD19− from pools of 3 to 4 mice per group at day 5 (C) or 30 (D) pi and cultured with medium alone (none), CpG-1668 (CPG-1; 1mM) or CpG-2 (1 mM). Levels of IFN-I were measured in the supernatants at 12–15 h post-culture by luciferase bioassay. Data are representative of two additional independent experiments.
Figure 3. Characterization of pDC-TLR response during LCMV infection.
Spleen pDCs (5 × 105 cells/ml) were FACS–purified as CD11c+B220+120G8highCD11b−CD3−NK1.1−CD19− from uninfected (n=3, white bars), ARM (n=10, striped bars) or Cl 13 (n=10, black bars) infected mice at day 5 pi. Cells were cultured with medium alone (none) or CpG-1668 during 15 h. A) IFN-I activity was measured in the supernatant by luciferase bioassay, other cytokines and chemokines indicated were determined by multi-plex ELISA. (B and C) CPG-stimulated pDCs were stained with anti-IAb and anti B7-2 mAbs and analyzed by FACS. B) Histrograms depict expression of the indicated molecules. Thin line, unstained control. C) Mean fluorescence intensity (MFI) for each molecule is shown.
In Toto, these results indicate that both acute and persistent in vivo LCMV infection disable a fundamental pillar of immunity not only by dampening the amount of pDCs available for innate response but also interfering with their unique capacity to massively produce IFN-I upon TLR stimulation.
TLR9-IFN-I impairment during LCMV infection is independent of IFN-IR, IL-10, IFN-γ, TNF-α and T cells
Since the inhibition of TLR IFN-I production by pDCs occurs to similar extends during ARM (minimal DC infection) and Cl 13 (robust replication in DCs) infections, it is likely that this alteration is at least in part consequence of pDC exposure to the infectious environment. In a search for a factor responsible for TLR-9-IFN-I inhibition during LCMV infection, we analyzed the participation of elements with demonstrated ability to suppress IFN-I production, which are present during LCMV infection.
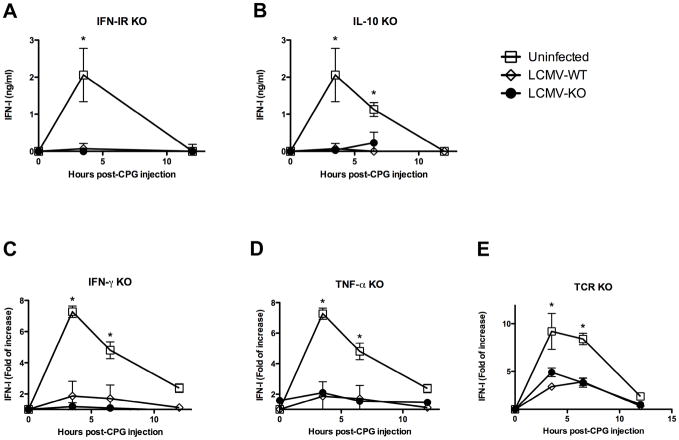
The role of IFN-I was of particular interest since it is secreted to high levels early during LCMV infection (Figure 1A) and it could exert a negative feedback loop that silences its own production. To test this possibility, we injected CPG into WT and IFN-I receptor (R) knockout (ko) mice infected with LCMV ARM at day 5 pi or infected with LCMV Cl 13 at day 30 pi (Figure 4A and data not shown, respectively). The levels of IFN-I in serum were measured at 3.5, and 12 h post CPG injection. We found similar degree of IFN-I suppression in WT and IFN-IR ko mice infected with ARM and Cl 13, indicating that IFN-I negative feedback is not responsible for the inhibition of TLR9-IFN-I production during LCMV infection.
Figure 4. TLR-9 IFN-I inhibition is independent of IFN-IR, IL-10, IFN-γ, TNF-α and T cells.
A) IFN-IR ko (A), IL-10 ko (B), IFN-γ ko (C), TNF-α ko (D) and TCR ko (E) mice at day 5 after ARM infection were injected with 5 μg of CPG. Uninfected and ARM-infected WT mice were processed in parallel as controls. Blood samples were collected at the indicated time points. Elisa was used to quantify IFN-α levels (A and B). IFN-I activity was measured by luciferase bioassay and fold of increase respect to levels at 0 h is shown (C, D and E). The mean ± s. d. obtained with 3 to 4 mice per group is shown. (* LCMV-infected compared to uninfected group p<0.01).
IL-10 has been demonstrated to inhibit pDC IFN-I production (Duramad et al., 2003; Gary-Gouy et al., 2002). Moreover, endogenous IL-10 is responsible for the suppression of CD8 T cell responses during persistent LCMV Cl 13 infection (Brooks et al., 2006; Ejrnaes et al., 2006). Thus, we investigated the participation of IL-10 in the pDC-IFN-I inhibition during in vivo acute and persistent LCMV infection. To evaluate the role of IL-10 during acute LCMV infection, we determined the levels of systemic IFN-I upon CPG injection into WT and IL-10 ko mice at day 5 pi with ARM. We found that both WT and IL10-ko mice exhibited similarly low levels of IFN-I as compared to uninfected controls. Similarly, administration of anti-IL10R mAb at day 20 post-Cl 13 infection, 8 hours before CPG injection, fail to restore IFN-I production (data not shown). These data indicate that IL-10 is not involved in IFN-I suppression during LCMV infection.
We next tested the participation of IFN-γ and TNF-α in IFN-I inhibition during viral infection, since these two cytokines are produced during LCMV infection (Zinkernagel, 2002) and can suppress pDC responses (Palucka et al., 2005 (Fallarino et al., 2005). For that IFN-γ ko and TNF-α ko mice were infected with LCMV ARM and stimulated with CPG at day 5 pi (Figure 4C and D, respectively). Again, we could not detect recovery of IFN-I response in the absence of these cytokines in LCMV-infected mice, indicating that neither IFN-γ or TNF-α are mediating IFN-I suppression during LCMV infection.
Finally, we explored the possibility that T cells or T cell-derived factors would be involved in TLR-IFN-I hampering during viral infection, as it has been recently shown that adaptive immune response can weaken innate immunity (Kim et al., 2007). To accomplish this, we investigated CPG-induced IFN-I production in TCR ko mice infected with LCMV ARM and Cl 13 (Figure 4E and data not shown, respectively). We found that T cells are not mediating TLR9-IFN-I inhibition during LCMV ARM or Cl 13 infection as judged by the similar levels of systemic IFN-I responses in WT and TCR deficient mice.
Based on the published results that the evaluated factors, which are present during LCMV infection, can inhibit IFN-I production; these results were quite unexpected. However, they illuminate the complexity and uniqueness of the molecular mechanism/s responsible for the TLR-IFN-I inhibition during LCMV infection and pose a challenge for future investigations.
Defective IFN-I response during secondary infections with unrelated pathogens
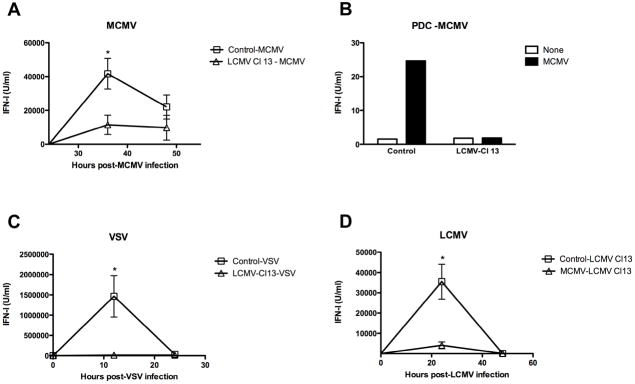
TLR-9 plays a critical role for host defense against multiple microbes including DNA viruses and mycobacteria (Bafica et al., 2005; Coban et al., 2005; Delale et al., 2005; Krug et al., 2004; Tabeta et al., 2004) Thus, the effect of the long-term inhibition of TLR-9 IFN-I production during Cl 13 infection to compromise the ability of the host to mount an effective innate immune response and enhance the susceptibility to an opportunistic infection was evaluated. We used murine cytomegalovirus (MCMV) as a model of opportunistic infection since initial IFN-I production 36 h after MCMV infection is known to be pDC-TLR-9 dependent (Asselin-Paturel et al., 2001; Delale et al., 2005; Krug et al., 2004; Tabeta et al., 2004). Uninfected or day 30-LCMV-Cl 13 infected mice were given 1 × 104 PFU of MCMV and systemic levels of IFN-I determined in sera 36 and 48 h later (Figure 5A). As anticipated (Orange and Biron, 1996), control mice that received a primary infection with MCMV triggered a robust IFN-I secretion in sera as compared to uninfected mice. In contrast, a profound reduction of IFN-I in sera from Cl 13 infected mice receiving MCMV as a secondary infection was observed when compared to mice receiving MCMV as a primary infection. Next, FACS-purified splenic CD11c+B220+120G8highCD11b−CD3−CD19−NK1.1− pDCs from controls or Cl 13 infected mice at 36 h after infection with MCMV were cultured for 15h and the levels of IFN-I in the supernatant measured (Figure 5B). pDCs from control mice infected with MCMV produced high levels of IFN-I in culture supernatant while, in contrast, pDCs obtained from Cl 13-MCMV infected mice failed to enhance IFN-I levels. These findings indicate that LCMV Cl 13 infection disrupts IFN-I secretion by pDCs in response to in vivo MCMV infection. To further examine the extend of IFN-I disruption during chronic LCMV infection, we tested IFN-I production in LCMV Cl 13 infected mice upon a different secondary infection with VSV (Figure 5C). We found that mice with a progressing LCMV Cl 13 infection failed to elicit systemic IFN-I levels upon VSV secondary infection. This is consistent with the previously reported TLR-7-dependent IFN-I response during VSV infection (Lund et al., 2004) and the impaired TLR-7 IFN-I production by pDCs that we described in Supplementary Figure 3. Finally, we investigated whether primary infection with MCMV compromises systemic IFN-I production upon LCMV secondary infection. We observed that an ongoing MCMV infection indeed compromises systemic IFN-I response upon a subsequent infection with LCMV Cl 13 (Figure 5D). These data support the idea that pDC-IFN-I inhibition is a general event during primary viral infections that prevents the systemic IFN-I elevation upon exposure to distinct opportunistic pathogens.
Figure 5. IFN-I production upon secondary viral infections.
Uninfected and LCMV Cl 13 infected mice (day 20–30 pi) were injected with MCMV (A and B) or with VSV (C). In D uninfected or MCMV-infected mice (day 2.5 pi) were injected with LCMV Cl 13. (A, C and D) Blood samples were collected at the indicated times after secondary infection (indicated on top of each graph) and IFN-I activity was measured by luciferase bioassay. The mean obtained from 6 mice per group is shown. (* dual viral infection compared to single viral infection p<0.01). Results are representative from 1 or 2 independent experiments. B) FACS–purified spleen pDCs were pooled from 4 mice per group and cultured with medium alone. Levels of IFN-I were measured in the supernatants at 15 h post-culture. Results are representative of 3 independent experiments.
Impaired NK cell response in LCMV infected mice upon secondary MCMV exposure
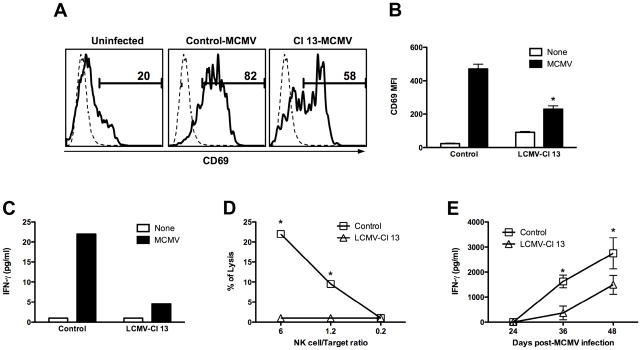
TLR-induction of DC/NK cell cross-talk is a key hallmark of innate response with many viral infections (Degli-Esposti and Smyth, 2005; Della Chiesa et al., 2005). It has been demonstrated that during MCMV infection, production of IFN-I by pDCs is vital for NK cell activation and function (Dalod et al., 2003; Delale et al., 2005; Krug et al., 2004; Tabeta et al., 2004). To evaluate the biological impact of the LCMV impaired pDC-IFN-I secretion on the orchestration of a subsequent innate response we examined NK cell activation and functional status upon MCMV infection. We determined the expression of the activation marker CD69 on NK cells from control or Cl 13 infected mice 36 h after MCMV infection (Figure 6A and B). Control mice showed both a higher percentage of CD69+NK1.1+CD3− NK cells (Figure 6A) as well as an enhanced expression of CD69 on these cells (Figure 6B). Although NK cells from Cl 13 infected mice showed an increased activation phenotype compared to uninfected controls (Fig 6B, white bars), they presented a significantly lower percentage of activated NK cells (Figure 6A) and a limited up-regulation of CD69 upon MCMV infection compared to control-MCMV infected mice (Fig 6B, black bars). To investigate NK cell functions during MCMV infection, NK1.1+CD3− NK cells were purified by FACS-Sort (purity ≥ 98%) from control or Cl 13 infected mice at 36 h post-MCMV infection. Cells were cultured for 15 h and IFN-γ production quantified in the supernatant (Figure 6C) while harvested cells were incubated with 51Cr labeled YAC-1 cells to evaluate cytotoxicity in a 51Cr release assay (Figure 6D) (Orange et al., 1995). Results indicated a significant reduction in both IFN-γ production and cytotoxic capacity of NK cells obtained from Cl 13-MCMV co-infected mice compared to control MCMV infected mice. To test whether the NK cell dysfunction that we observed ex-vivo compromised the in vivo production of IFN-γ, levels of this cytokine were measured by ELISA in sera from control and Cl 13 infected mice at 24, 36 and 44 hs after MCMV infection (Figure 6E). Interestingly, Cl 13 infected mice displayed a significant reduction in systemic IFN-γ levels after MCMV infection in comparison to control mice infected with MCMV.
Figure 6. NK cell function upon MCMV infection.
Uninfected and Cl 13 infected mice (day 30 pi) were injected MCMV. A) Spleen cells were stained with anti NK1.1, anti-CD3, and anti-CD69 mAb and the levels of CD69 on NK1.1+CD3− cells determined by FACS. Histograms depicting CD69 expression are shown in the left panel and mean fluorescence intensity (MFI) is shown in the bar graph in the right panel. (* Cl 13-MCMV co-infected compared to Control-MCMV group p<0.01). Results are representative of 2 independent experiments. B and C) FACS–purified spleen Nk1.1+CD3− cells were pooled from 3 mice per group and cultured with medium alone. After 15 h, levels of IFN-γ were measured in the supernatants (B) and cells were cultured with 51Cr-labeled YAC-1 targets in a cytotoxicity assay (C). Results are representative of 2 to 4 independent experiments. D) Blood samples were collected at 24, 36 and 44 h post MCMV infection. IFN-γ activity was measured by ELISA and the mean ± s. d. obtained from 3 mice per group is shown. (* Cl 13-MCMV co-infected compared to Control-MCMV group p<0.05). Results are representative of 2 independent experiments.
In concert, these results indicate that the long-term inhibition of TLR-9-IFN-I production during Cl 13 infection is biologically meaningful, resulting in impaired pDCs and NK cell innate responses upon subsequent encounter with an unrelated opportunistic pathogen.
Control of early MCMV replication is compromised in LCMV-infected mice
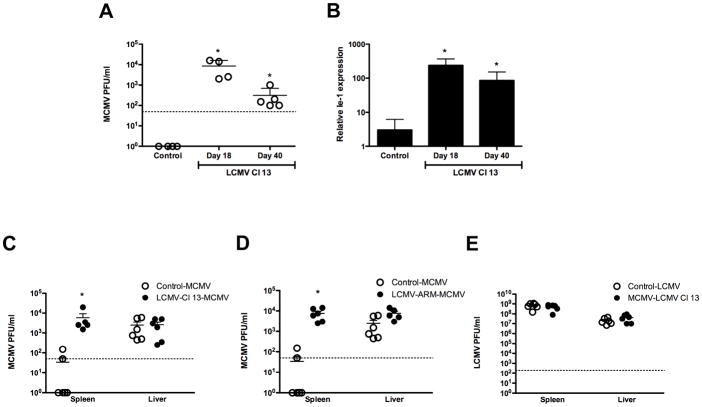
Early activation of pDCs and NK cells is decisive for counteracting MCMV spread (Dalod et al., 2003; Degli-Esposti and Smyth, 2005). The last set of experiments investigates the biological impact of LCMV diversion of innate response on the containment of MCMV infection. Infectious titers of MCMV in spleens from control, day-18 and day-40 Cl 13 infected mice 4 days after MCMV infection were determined (Figure 7A). Control mice that received a primary infection with MCMV had less than 50 PFU/ml of MCMV by 4 days pi. In contrast, 3 to 4 log of infectious MCMV was detected in Cl 13-MCMV co-infected mice. These results were further supported by real time PCR amplification of MCMV early inducible gene (Ie-1) (Figure 7B), showing a significantly higher expression of Ie-1 in MCMV-Cl 13 infected mice compared to control-MCMV infected mice.
Figure 7. Secondary virus replication in spleen and liver.
Mice uninfected, infected with LCMV Cl 13 at day 18 to 40 pi (A, B and C) or infected with LCMV ARM at day 5 pi (D) were injected with MCMV. In E, uninfected or MCMV-infected mice at day 2.5 pi were challenged with LCMV. Spleens (A–E) and livers (C–E) were collected at day 4 pi. (A, C, D and E) Titers of secondary virus were determined by plaque assay as described in material and methods section. Each symbol represents an individual mouse and dotted line indicate the detection limit of the plaque assay B) MCMV Ie-1 gene was determined by Q-PCR. Results are representative from 2 independent experiments. The mean ± s. d. obtained from 4–6 mice per group is shown. (* dual viral infection compared to single viral infection p<0.01).
Different mechanisms are responsible in C57BL/6 mice for resistance against MCMV in the spleen (mostly NK cells) and in the liver (mainly IFN-γ). Therefore, we analyzed in parallel MCMV PFU in spleens and livers of control-MCMV infected mice versus LCMV-MCMV co-infected mice (Figure 7C). The results indicate that while LCMV-Cl13 infected mice that received MCMV as a secondary infection exhibit ~2 log higher MCMV titers in the spleen, no significant differences could be appreciated in the liver. The impaired NK cell activation, cytotoxicity and IFN-γ production that we described in Figure 6A, B, C and D, respectively, is consistent with the higher viral titers in spleen. However, the similar growth of MCMV in the liver was an unexpected finding considering the reduced systemic IFN-γ levels detected in the serum of LCMV-MCMV co-infected mice (Figure 6E). It is likely that the existent LCMV-specific CD8 T cells in the liver would enhance the local levels of IFN-γ in this organ during LCMV-MCMV co-infection thus compensating the decreased systemic levels of this cytokine.
We next investigated whether the failure to control MCMV secondary infection also occurs during a primary acute infection with LCMV-ARM, within the time window of altered pDC response. We performed experiments evaluating secondary MCMV infection at day 5 after acute LCMV-ARM infection (Figure 7D). As expected by the altered pDC-IFN-I response observed at this time point after ARM infection (Figure 1B, 1C, 2C and 3A), the spleen MCMV titers were significantly elevated in LCMV-ARM-MCMV dual infected mice compared to single MCMV-infected mice. Similarly to Cl 13 chronic infection, no increase could be appreciated in liver MCMV titers in MCMV-LCMV ARM co-infected mice.
Next, we evaluated whether the early containment of VSV secondary infection was also compromised. Strikingly, both control and Cl-13 infected mice that received VSV as primary and secondary infection, respectively, were able to completely eradicate infectious virus by day 4 pi (data not shown). Finally, we investigated early LCMV Cl 13 containment in mice with a progressing MCMV infection. We did not detect any difference in spleen or liver LCMV titers between single LCMV infection and dual MCMV (primary)-LCMV (secondary) co-infection (Figure 7E).
These data indicate that the deleterious effect of LCMV infection on innate immune response(s) contributes to disarming the host capacity to contain early replication of an extraneous pathogen such as MCMV. However, the results obtained with VSV and LCMV secondary infection emphasize that the biological impact that IFN-I inhibition has on the early control of a secondary pathogen depends on the unique nature (i.e. viral properties, dose, route) of the secondary infection.
DISCUSSION
In vitro and epidemiological data support an association between increased incidence of opportunistic infections during HIV or MV infections and the inability of infected cells to produce IFN-I (Hosmalin and Lebon, 2006; Schlender et al., 2005; Siegal, 2003; Siegal et al., 2001). This report provides an explanation on how this impaired production of IFN-I contributes to pathogenesis in vivo. Compelling evidence show that in vivo infection with LCMV interferes with the unique ability of pDCs to secrete prodigious amount of IFN-I upon TLR stimulation. This innate immune-deficiency is sustained over a long period during a persistent infection and is a direct consequence of quantitative and qualitative alterations on pDCs. Furthermore, this virally induced immune-defect is biologically meaningful in preventing an effective innate response (i.e. systemic IFN-I production and NK cell function) by the host upon encountering opportunistic pathogen, such as MCMV, and relates to an early spread and delayed clearance of the secondary infection.
Like other viruses, LCMV induces enhanced systemic IFN-I early after infection (Biron, 1998). Both ARM and Cl 13 LCMV, the former that generates a successful T cell response to terminate an acute infection while the later results in T cell exhaustion and persistent infection, induce comparable levels of systemic IFN-I in sera of infected mice, which peaked at day 1 pi. At present the cellular source of this early wave of systemic IFN-I during LCMV infection is unclear. Although pDCs secrete IFN-I during LCMV infection (Jung et al., 2008; Montoya et al., 2005), the early peak of systemic IFN-I was still detected in the absence of pDCs (Louten et al., 2006). Importantly, systemic IFN-I is rapidly silenced during both acute and chronic LCMV infection, indicating that the production and presence of high viral load in blood and several tissues (Ahmed et al., 1984; Moskophidis and Zinkernagel, 1995) is not sufficient to maintain enhanced levels of IFN-I in serum. The inhibition of systemic IFN-I early after LCMV infection is in sharp contrast to the local IFN-I production by spleen and bone marrow DCs that support chronic infection by Cl 13 and manifest a sustained production of IFN-I for at least 50 days p.i. (Diebold et al., 2004; Hahm et al., 2005). Thus, it is possible that the virus has evolved selective strategies to block systemic IFN-I response and/or that the host is armed with immune-regulatory mechanisms to prevent sustained-systemic IFN-I production that could lead to immunopathology. Moreover, the capacity of LCMV infected mice to re-induce IFN-I levels in the sera varies depending on the stimuli used. TLR-9 and TLR-3-IFN-I responses are dramatically abrogated during LCMV infection while, in contrast, TLR-4 signaling is enhanced. These novel observations demonstrate that an ongoing natural viral infection can differentially modulate the ability of the innate immune system to produce IFN-I upon in vivo TLR stimulation; specifically suppressing selective pathways while re-enforcing others. The differential de-regulation of TLR-IFN-I production may be applicable to other viral infections that, as LCMV (Doughty et al., 2001; Durbin et al., 2003; Nguyen and Biron, 1999), induce an enhanced response to LPS and/or susceptibility to endotoxic shock (Baqui et al., 2000; Bender et al., 1993; Fejer et al., 2005; Nansen and Randrup Thomsen, 2001). In addition, response to in vivo TLR-9 stimulation is of particular interest since treatment with TLR-9 agonists has been proposed as therapy for chronic viral diseases and subsequent clinical trials have been initiated (Vollmer, 2006; Wilson et al., 2006).
LCMV infection affects pDCs at multiple levels. LCMV causes a numeric defect in this cell population in both periphery and bone marrow. This is likely related to the virus ability to block DC development from early undifferentiated progenitors (Hahm et al., 2005; Sevilla et al., 2004) and to redirect the differentiation of immature bone marrow pDCs into CD11b+ cDCs (Zuniga et al., 2004). In addition, LCMV infection alters the quality of the remaining pDCs by disabling their distinctive function (i.e. IFN-I production) without affecting secretion of other cytokines and maturation. The ability of LCMV or other viruses to selectively affect a unique function in neurons, endocrine cells or immune cells while sparing other differentiated functions thereby disrupting homeostasis and causing disease has been previously reported (de la Torre et al., 1996; de la Torre and Oldstone, 1992; Oldstone, 2002; Oldstone et al., 1982). The ability of viruses to interfere with IFN-I biosynthesis has been usually associated with virus replication within the affected cells, which interferes with IFN-I induction, transcription, RNA processing and/or translation (Garcia-Sastre and Biron, 2006). Specifically, LCMV infection of A549 cells inhibits IRF-3 phosphorylation and IFN-β synthesis and this could be contributing to the diminished IRF-3-dependent, Poly (I:C)-induced-IFN-I response during LCMV infection (Martinez-Sobrido et al., 2006). In the LCMV murine model used in the present study the parental virus ARM infects a minimal proportion of conventional DCs and pDCs while the Cl 13 variant, which differs from ARM by only 2 amino acids in the glycoprotein and the polymerase, infects a significantly higher number of DCs (Borrow et al., 1995 and unpublished observations; Sevilla et al., 2000). ARM and Cl 13 infection both result in pDC IFN-I inhibition suggesting that virus replication within pDCs is not a prerequisite for the suppression of TLR IFN-I production but that the exposure of pDCs to the infectious environment is likely responsible for this defect. This could result from an advanced maturation status of pDC triggered by the virus infection and is consistent with a previous report demonstrating that DC exposure to herpes simplex virus in vivo, prevent them to produce IFN-α after re-challenge with the same virus in vitro (Bjorck, 2004).
We investigated the biological implications of the impaired TLR-IFN-I response during LCMV infection by using MCMV as a model of opportunistic infections. MCMV is a well-characterized mouse natural pathogen (Krmpotic et al., 2003) which is recognized by pDCs through TLR-9. As a result, a massive IFN-I response is initiated and is crucial for direct anti-viral effect and the orchestration of other innate cells, i.e. NK cells (Biron, 1999; Dalod et al., 2003; Degli-Esposti and Smyth, 2005; Delale et al., 2005; Krug et al., 2004; Tabeta et al., 2004). We noted that LCMV infection compromises MCMV and VSV innate responses as indicated by the reduced levels of systemic IFN-I. Moreover, also impaired IFN-γ and defective activation and function of pDCs and NK cells were observed during super-infection of LCMV-infected mice with MCMV. NK cell dysfunction has been described previously during several viral infections (Ahmad and Alvarez, 2004; Fauci et al., 2005) and our data suggest that their in vivo alteration could be in part a consequence of impaired TLR-IFN-I response. In keeping to the altered innate response, LCMV infected mice fail to control MCMV early replication supporting the concept that interference of TLR-IFN-I response during an in vivo viral infection facilitates co-infections with opportunistic pathogens. However, the ablation of IFN-I did not make any difference in the early control of VSV or LCMV secondary infections, indicating that the biological significance of IFN-I suppression is related to the specific characteristics of the secondary challenge. It is important to mention that other innate immune defects during Cl 13 infection, such as poor IL-12 production upon secondary MCMV infection (Supplementary Figure 4), may couple to the impaired IFN-I response to determine the early MCMV spread. Indeed, our failure to restore MCMV containment by the sole injection of recombinant IFN-β during LCMV Cl13 infection (Supplementary Figure 5) suggests multi-factorial events acting in conjunction to discredit innate defense during persistent viral infection. This integrally altered innate response would facilitate early microbial spread and could potentially compromise the CD8 T cell response against secondary pathogens, which is suppressed during persistent LCMV Cl 13 infection (Tishon et al., 1993). Notably, many other serious human opportunistic infections are sensed through TLR-9 (Bafica et al., 2005; Coban et al., 2005) and inhibition of TLR-9 IFN-I response is likely facilitating their growth in the virally infected host. Our findings are in agreement with a previous report indicating that transient IFN-I exhaustion during acute infection with Semliki Forest virus (SFV) associates with enhanced susceptibility to secondary infections (Alsharifi et al., 2006).
LCMV infection in its natural host, the mouse, has been a rosetta stone model to illuminate the occurrence and cause of multiple host-virus interactions in vivo (Zinkernagel, 2002). Particularly, the use of LCMV-Cl 13 has served as a model that uncovered T cell exhaustion during persistent infection (Gallimore et al., 1998; Zajac et al., 1998), mechanisms of viral immune-evasion (Oldstone, 2006) and potential translational treatments for human infections (Barber et al., 2006; Blattman et al., 2003; Brooks et al., 2006). The present study set the basis to use LCMV model to understand the de-regulation of innate immunity during acute and chronic viral diseases.
In summary, the results reported here indicate that inhibition of systemic TLR-IFN-I production by pDCs during in vivo viral infection interferes with efficient and effective innate immune response to a subsequent infection and sets a stage for enhanced susceptibility of the host to opportunistic pathogens. Further, our data support the concept that an in vivo virus infection in addition to disordering adaptive immune response can also discredit the innate immune system, thus favoring microbial spread and evolution.
EXPERIMENTAL PROCEDURES
Viruses
The ARM53b and Cl 13 LCMV clones were grown, identified and quantified as described (Ahmed et al., 1984; Borrow et al., 1995; Salvato et al., 1991). Smith strain of MCMV was obtained from Bruce Beutler, The Scripps Research Institute (TSRI). MCMV stocks were prepared from salivary glands of BALB/c mice given 1 × 104 PFU intraperitoneally (ip) (Tabeta et al., 2004). Homogenates of pooled salivary glands were made in sterile PBS and viral titers determined by plaque assay as described (Orange et al., 1995). New Jersey VSV strain was obtained from Dr. Robert Lamb (Northwestern University, IL) and viral titers determined on baby hamster kidney cells after 1 h absorption at 37°C and 48 h of 1% agarose overlay.
Mice
C57BL/6 mice were obtained from the closed breeding colony of The Scripps Research Institute. IL-10 ko, TNF-α ko and IFN-γ ko mice were obtained from The Jackson Laboratory. IFNR-I ko mice were obtained from Dr. Jonathan Sprent (TSRI) and TCR ko mice were a gift from Dr. Stephen Hedrick (UCSD). All ko mice used were in C57BL/6 background. Mice at 6–8 weeks of age were infected by iv inoculation of LCMV (2 × 106 PFU either ARM or Cl 13), iv injection with VSV (5 × 107 PFU) or ip injection of MCMV (1 × 104 PFU). Where indicated LCMV Cl 13 infected mice received 1 × 104 units of recombinant IFN-β ip 1 h before MCMV infection (Research Diagnostics) For in vivo treatment with TLR ligands, mice were injected in the tail vein with LPS (5 μg in 200 μl of PBS, Alexis), Poly(I:C) (5 μg in 200 μl of PBS, Invivogene) or CpG-ODN (IDT, Inc) preparations. CPG preparations consisted of 5 μg of CpG-1668 (TCCATGACGTTCCGATGCT) mixed with 30 μl of a cationic liposome preparation (DOTAP; F. Hoffmann-La Roche Ltd.) and 170 μl PBS. For IL-10 signalling blockade during persistent infection, day-20-Cl-13 infected mice received 500 μg of anti-IL-10R mAb (clone 1B1.3a, BD Biosciences) 8 h before CPG injection. Mouse handling conformed to the requirements of the National Institutes of Health guidelines and The Scripps Research Institute Animal Research Committee.
Cell purification
Spleens were removed and incubated with collagenase D (1 mg/ml, Roche, Indianapolis, IN) for 20 min at 37°C. Splenocytes were harvested by homogenization through a 100 μm tissue strainer and red blood cells removed by lysis following incubation with tris–ammonium chloride buffer for 4 min. Single cell suspension of splenocytes were incubated for 30 minutes with rat monoclonal antibodies (mAbs) specific for murine CD3 and CD19 (E–bioscience, San Diego, CA) followed by magnetic cell sorting using anti–rat immunoglobulin–coated magnetic beads (Invitrogene, Carlsbad, CA) according to manufacturer’s instructions. Cell were immunostained and pDCs (defined as CD11c+B220+120G8highCD11b−CD3−CD19−NK1.1−) and NK cells (defined as CD3−NK1.1+) were separated using a FACS-Aria Sorter (Becton Dickinson, San Jose, CA). Purity was >98%.
Flow Cytometry
Antibodies used were either purchased from BD Pharmingen or E-bioscience (San Diego, CA) to stain splenocytes: CD11b-PE-CY7, anti-CD11c-APC, anti-CD19-Percp-Cy5.5, anti-NK1.1-Percp-Cy5.5, anti-CD3- Percp-Cy5.5, anti-B220-APC-CY7, anti-B220-FITC, anti-NK1.1-PE, anti-CD69-FITC, anti-IAb-Alexa-700 and anti B7-2-PE. Anti-120G8 monoclonal antibody was kindly provided by Giorgio Trinchieri and conjugated to Alexa 488 following manufacture instructions (Molecular Probes, Invitrogen, Carlsbad, CA). Prior to staining all cell preparations were blocked with 3.3 μg/ml anti-mouse CD16/CD32 (Fc block, BD Pharmingen) in PBS containing 1% FBS for 10 min. The Fc block was also included in all 20 min surface stains. Cells were acquired using the Digital LSR II flow cytometer (Becton Dickinson, San Jose, CA). Flow cytometric data were analyzed with the FlowJo software.
pDC cultures and in vitro stimulation
Unless otherwise indicated, pDCs were plated at 1 × 105 or cells/ml in 100 μl RPMI complete medium (10% FBS, 2 mM L–glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 15 μM β–mercaptoethanol). For pDC stimulation, cells were cultured overnight in the presence of HPLC purified CpG-1668 (TCCATGACGTTCCGATGCT phosphorothioate-modified; IDT, Inc), CpG-2 (TCA TTG GAA AAC GTT CTT CGG GGC G phosphorothioate-modified; IDT, Inc) at 1 μM or Loxoribine (Invivogen, San Diego, CA) at 100 μM and supernatants then collected.
Cytokine meassurements
IFN-I activity was measured with reference to a recombinant mouse IFN-β standard (Research Diagnostics) using a L-929 cell line transfected with an interferon-sensitive luciferase obtained from Bruce Beutler, The Scripps Research Institute (Jiang et al., 2005). Where indicated the amount of IFN–α production was determined by ELISA (PBL–Biomedical, Picataway, NJ). IFN-γ was quantified by ELISA (e-bioscience, San Diego, CA). TNF-α, IL-12, RANTES, MCP-1 and MIP-1 α were quantified by multiplex ELISA (Quansys Biosciences, Utah).
NK cell IFN-γ production and cytotoxicity
FACS-purified NK cells were cultured at 106 cells/ml for 15 h in complete medium (10% FBS, 2 mM L–glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM β–mercaptoethanol) at 37°C in 5% CO2. Supernatants were harvested and stored at −70°C for IFN-γ quantification by ELISA. NK cell cytotoxic activity was assessed by 51Cr release, in a 4-h assay at 37°C, from YAC-1 target cells, as described (Orange et al., 1995).
Real-time RT-PCR for quantification of MCMV mRNA
Total RNA was extracted from spleen homogenates using RNeasy kit (Qiagen) digested with DNase I (RNase-free DNase set; Qiagen) and reverse transcribed into cDNA. Quantification of cDNA was performed using SYBR Green PCR kit (Applied Byosistem) and Real-Time PCR Detection System (ABI). The primers to amplify a single specific fragment of 137 bp for Ie-1 forward (GAGTCTGGAACCGAAACCGT) and reverse GTCGCTGTTATCATTCCCCAC) were described (Delale et al., 2005). The relative RNA levels of Ie-1 were normalized against cellular glyceraldehydes 3-phosphate dehydrogenase (GAPDH) RNA.
Statistical analysis
Statistical differences were determined by Student t test or one way analysis of variance (ANOVA) with InStat 3.0 software (GraphPad, CA.). p<0.05 were considered significant.
Supplementary Material
Acknowledgments
We acknowledge Marilyn Mendoza for technical help during the initial phase of this study. We thank Dr Juan Carlos de la Torre and Dr. Dorian McGavern for critically reading the manuscript. This is publication No. 18651 from Molecular and Neuroscience Integrative Department, The Viral Immunobiology Laboratory, The Scripps Research Institute (TSRI). This work was supported by grants from AI55540 and AI49527. EIZ was supported by Pew Foundation Latin American Fellowship and LL by NIH training grant NS041219.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad A, Alvarez F. Role of NK and NKT cells in the immunopathogenesis of HCV-induced hepatitis. J Leukoc Biol. 2004;76:743–759. doi: 10.1189/jlb.0304197. [DOI] [PubMed] [Google Scholar]
- Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- Alsharifi M, Regner M, Blanden R, Lobigs M, Lee E, Koskinen A, Mullbacher A. Exhaustion of type I interferon response following an acute viral infection. J Immunol. 2006;177:3235–3241. doi: 10.4049/jimmunol.177.5.3235. [DOI] [PubMed] [Google Scholar]
- Asselin-Paturel C, Brizard G, Chemin K, Boonstra A, O’Garra A, Vicari A, Trinchieri G. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med. 2005;201:1157–1167. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005;202:461–465. doi: 10.1084/jem.20051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med. 2005;202:1715–1724. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baqui AA, Jabra-Rizk MA, Kelley JI, Zhang M, Falkler WA, Jr, Meiller TF. Enhanced interleukin-1beta, interleukin-6 and tumor necrosis factor-alpha production by LPS stimulated human monocytes isolated from HIV+ patients. Immunopharmacol Immunotoxicol. 2000;22:401–421. doi: 10.3109/08923970009026002. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Sprenger H, Gong JH, Henke A, Bolte G, Spengler HP, Nain M, Gemsa D. The potentiating effect of LPS on tumor necrosis factor-alpha production by influenza A virus-infected macrophages. Immunobiology. 1993;187:357–371. doi: 10.1016/s0171-2985(11)80350-5. [DOI] [PubMed] [Google Scholar]
- Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- Biron CA. Role of early cytokines, including alpha and beta interferons (IFNalpha/beta), in innate and adaptive immune responses to viral infections. Semin Immunol. 1998;10:383–390. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- Biron CA. Initial and innate responses to viral infections--pattern setting in immunity or disease. Curr Opin Microbiol. 1999;2:374–381. doi: 10.1016/s1369-5274(99)80066-6. [DOI] [PubMed] [Google Scholar]
- Bjorck P. Dendritic cells exposed to herpes simplex virus in vivo do not produce IFN-alpha after rechallenge with virus in vitro and exhibit decreased T cell alloreactivity. J Immunol. 2004;172:5396–5404. doi: 10.4049/jimmunol.172.9.5396. [DOI] [PubMed] [Google Scholar]
- Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, O’Garra A. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J Exp Med. 2003;197:101–109. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P, Evans CF, Oldstone MB. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- Coban C, Ishii KJ, Kawai T, Hemmi H, Sato S, Uematsu S, Yamamoto M, Takeuchi O, Itagaki S, Kumar N, et al. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med. 2005;201:19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- Dalod M, Hamilton T, Salomon R, Salazar-Mather TP, Henry SC, Hamilton JD, Biron CA. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J Exp Med. 2003;197:885–898. doi: 10.1084/jem.20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5:112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- Delale T, Paquin A, Asselin-Paturel C, Dalod M, Brizard G, Bates EE, Kastner P, Chan S, Akira S, Vicari A, et al. MyD88-dependent and -independent murine cytomegalovirus sensing for IFN-alpha release and initiation of immune responses in vivo. J Immunol. 2005;175:6723–6732. doi: 10.4049/jimmunol.175.10.6723. [DOI] [PubMed] [Google Scholar]
- Della Chiesa M, Sivori S, Castriconi R, Marcenaro E, Moretta A. Pathogen-induced private conversations between natural killer and dendritic cells. Trends Microbiol. 2005;13:128–136. doi: 10.1016/j.tim.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Doughty L, Nguyen K, Durbin J, Biron C. A role for IFN-alpha beta in virus infection-induced sensitization to endotoxin. J Immunol. 2001;166:2658–2664. doi: 10.4049/jimmunol.166.4.2658. [DOI] [PubMed] [Google Scholar]
- Duramad O, Fearon KL, Chan JH, Kanzler H, Marshall JD, Coffman RL, Barrat FJ. IL-10 regulates plasmacytoid dendritic cell response to CpG-containing immunostimulatory sequences. Blood. 2003;102:4487–4492. doi: 10.1182/blood-2003-07-2465. [DOI] [PubMed] [Google Scholar]
- Durbin J, Doughty L, Nguyen K, Caligiuri M, Van Deusen J, Biron C. The role of STAT1 in viral sensitization to LPS. J Endotoxin Res. 2003;9:313–316. doi: 10.1179/096805103225002575. [DOI] [PubMed] [Google Scholar]
- Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5:835–843. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- Fejer G, Szalay K, Gyory I, Fejes M, Kusz E, Nedieanu S, Pali T, Schmidt T, Siklodi B, Lazar G, Jr, et al. Adenovirus infection dramatically augments lipopolysaccharide-induced TNF production and sensitizes to lethal shock. J Immunol. 2005;175:1498–1506. doi: 10.4049/jimmunol.175.3.1498. [DOI] [PubMed] [Google Scholar]
- Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- Gary-Gouy H, Lebon P, Dalloul AH. Type I interferon production by plasmacytoid dendritic cells and monocytes is triggered by viruses, but the level of production is controlled by distinct cytokines. J Interferon Cytokine Res. 2002;22:653–659. doi: 10.1089/10799900260100132. [DOI] [PubMed] [Google Scholar]
- Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, O’Garra A, Liu YJ. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DE. Immune responses during measles virus infection. Curr Top Microbiol Immunol. 1995;191:117–134. doi: 10.1007/978-3-642-78621-1_8. [DOI] [PubMed] [Google Scholar]
- Guilbert JJ. The world health report 2002 - reducing risks, promoting healthy life. Educ Health (Abingdon) 2003;16:230. doi: 10.1080/1357628031000116808. [DOI] [PubMed] [Google Scholar]
- Hahm B, Trifilo MJ, Zuniga EI, Oldstone MB. Viruses evade the immune system through type I interferon-mediated STAT2-dependent, but STAT1-independent, signaling. Immunity. 2005;22:247–257. doi: 10.1016/j.immuni.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Hosmalin A, Lebon P. Type I interferon production in HIV-infected patients. J Leukoc Biol. 2006;80:984–993. doi: 10.1189/jlb.0306154. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, et al. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- Jung A, Kato H, Kumagai Y, Kumar H, Kawai T, Takeuchi O, Akira S. Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T-cell response via MyD88. J Virol. 2008;82:196–206. doi: 10.1128/JVI.01640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki N, Liu YJ. Natural type I interferon-producing cells as a link between innate and adaptive immunity. Hum Immunol. 2002;63:1126–1132. doi: 10.1016/s0198-8859(02)00751-6. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Akira S. Cell type specific involvement of RIG-I in antiviral responses. Nippon Rinsho. 2006;64:1244–1247. [PubMed] [Google Scholar]
- Kim KD, Zhao J, Auh S, Yang X, Du P, Tang H, Fu YX. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13:1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- Krmpotic A, Bubic I, Polic B, Lucin P, Jonjic S. Pathogenesis of murine cytomegalovirus infection. Microbes Infect. 2003;5:1263–1277. doi: 10.1016/j.micinf.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, Giese T, Engelmann H, Endres S, Krieg AM, Hartmann G. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol. 2002;14:432–436. doi: 10.1016/s0952-7915(02)00354-0. [DOI] [PubMed] [Google Scholar]
- Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- Louten J, van Rooijen N, Biron CA. Type 1 IFN deficiency in the absence of normal splenic architecture during lymphocytic choriomeningitis virus infection. J Immunol. 2006;177:3266–3272. doi: 10.4049/jimmunol.177.5.3266. [DOI] [PubMed] [Google Scholar]
- Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Sobrido L, Zuniga EI, Rosario D, Garcia-Sastre A, de la Torre JC. Inhibition of the type I interferon response by the nucleoprotein of the prototypic arenavirus lymphocytic choriomeningitis virus. J Virol. 2006;80:9192–9199. doi: 10.1128/JVI.00555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna K, Beignon AS, Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J Virol. 2005;79:17–27. doi: 10.1128/JVI.79.1.17-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya M, Edwards MJ, Reid DM, Borrow P. Rapid activation of spleen dendritic cell subsets following lymphocytic choriomeningitis virus infection of mice: analysis of the involvement of type 1 IFN. J Immunol. 2005;174:1851–1861. doi: 10.4049/jimmunol.174.4.1851. [DOI] [PubMed] [Google Scholar]
- Moskophidis D, Zinkernagel RM. Immunobiology of cytotoxic T-cell escape mutants of lymphocytic choriomeningitis virus. J Virol. 1995;69:2187–2193. doi: 10.1128/jvi.69.4.2187-2193.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nansen A, Randrup Thomsen A. Viral infection causes rapid sensitization to lipopolysaccharide: central role of IFN-alpha beta. J Immunol. 2001;166:982–988. doi: 10.4049/jimmunol.166.2.982. [DOI] [PubMed] [Google Scholar]
- Nguyen KB, Biron CA. Synergism for cytokine-mediated disease during concurrent endotoxin and viral challenges: roles for NK and T cell IFN-gamma production. J Immunol. 1999;162:5238–5246. [PubMed] [Google Scholar]
- Oldstone MB. Viral persistence: parameters, mechanisms and future predictions. Virology. 2006;344:111–118. doi: 10.1016/j.virol.2005.09.028. [DOI] [PubMed] [Google Scholar]
- O’Neill LA. How Toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Orange JS, Biron CA. Characterization of early IL-12, IFN-alphabeta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J Immunol. 1996;156:4746–4756. [PubMed] [Google Scholar]
- Orange JS, Wang B, Terhorst C, Biron CA. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J Exp Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc Natl Acad Sci U S A. 2005;102:3372–3377. doi: 10.1073/pnas.0408506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvato M, Borrow P, Shimomaye E, Oldstone MB. Molecular basis of viral persistence: a single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with suppression of the antiviral cytotoxic Tlymphocyte response and establishment of persistence. J Virol. 1991;65:1863–1869. doi: 10.1128/jvi.65.4.1863-1869.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlender J, Hornung V, Finke S, Gunthner-Biller M, Marozin S, Brzozka K, Moghim S, Endres S, Hartmann G, Conzelmann KK. Inhibition of tolllike receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J Virol. 2005;79:5507–5515. doi: 10.1128/JVI.79.9.5507-5515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla N, Kunz S, Holz A, Lewicki H, Homann D, Yamada H, Campbell KP, de La Torre JC, Oldstone MB. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J Exp Med. 2000;192:1249–1260. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla N, McGavern DB, Teng C, Kunz S, Oldstone MB. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J Clin Invest. 2004;113:737–745. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoukry NH, Cawthon AG, Walker CM. Cell-mediated immunity and the outcome of hepatitis C virus infection. Annu Rev Microbiol. 2004;58:391–424. doi: 10.1146/annurev.micro.58.030603.123836. [DOI] [PubMed] [Google Scholar]
- Siegal F. Interferon-producing plasmacytoid dendritic cells and the pathogenesis of AIDS. Res Initiat Treat Action. 2003;8:10–13. [PubMed] [Google Scholar]
- Siegal FP, Fitzgerald-Bocarsly P, Holland BK, Shodell M. Interferonalpha generation and immune reconstitution during antiretroviral therapy for human immunodeficiency virus infection. Aids. 2001;15:1603–1612. doi: 10.1097/00002030-200109070-00002. [DOI] [PubMed] [Google Scholar]
- Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer J. TLR9 in health and disease. Int Rev Immunol. 2006;25:155–181. doi: 10.1080/08830180600743107. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HL, Dar A, Napper SK, Marianela Lopez A, Babiuk LA, Mutwiri GK. Immune mechanisms and therapeutic potential of CpG oligodeoxynucleotides. Int Rev Immunol. 2006;25:183–213. doi: 10.1080/08830180600785868. [DOI] [PubMed] [Google Scholar]
- Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel RM. Lymphocytic choriomeningitis virus and immunology. Curr Top Microbiol Immunol. 2002;263:1–5. doi: 10.1007/978-3-642-56055-2_1. [DOI] [PubMed] [Google Scholar]
- Zuniga EI, McGavern DB, Pruneda-Paz JL, Teng C, Oldstone MB. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat Immunol. 2004;5:1227–1234. doi: 10.1038/ni1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.