Abstract
n-3 polyunsaturated fatty acids (PUFA) are widely used for chemotheraphy/chemoprevention of chronic diseases. However, the molecular mechanism(s) by which the bioactive n-3 PUFA (eicosapentaenoic acid and docosahexaenoic acid) modulate effector pathways are not fully elucidated. Multiple experimental approaches, including use of animal models, cell lines, and human clinical trials, have been utilized to dissect the complex effectors. It is imperative to link these different experimental approaches together in order to interpret outcomes in the context of human physiology and pathophysiology. Unfortunately, the adoption of a broad array of model systems and a wide range of fatty acid exposures (i.e. doses) has made it difficult to interpret biological outcomes. Therefore, in this mini-review we discuss the impact of (a) molecular structure of bioactive fatty acids, (b) dose relevance relative to human consumption, (c) enrichment of fatty acids in sera and tissues following dietary intake, and (d) limitations of cell/tissue culture studies.
Introduction
Long-chain polyunsaturated fatty acids (PUFA) are subcategorized into n-3 [alpha-linolenic acid (ALA, 18:3), eicosapentaenoic acid (EPA, 20:5), docosapentaenoic acid (DPA, 22:5), and docosahexaenoic acid (DHA, 22:6)] and n-6 [linoleic acid (LA, 18:2), arachidonic acid (ARA, 20:4)] families according to the position of the first double bond from the methyl end of the acyl chain. A plethora of data from epidemiological studies and clinical trials investigating the effect of increased consumption of n-3 PUFA either in the form of fish or fish oil supplements suggest that, compared to n-6 PUFA, n-3 PUFA favorably modulate multiple biological processes involved in coronary heart diseases [1, 2], cancers [3–7], immune diseases [8–10], and brain health [11]. In general, studies involving cell culture and animal models utilizing fish oil, purified n-3 PUFA in triglyceride, free fatty acid or ethyl ester form, support the epidemiological and clinical observations [12–14]. However, the interpretation of experimental data with regard to physiological relevance is complicated by atleast two issues: (a) bioavailability/bioactivity of different forms of n-3 PUFA and (b) the dose and local concentration of effective n-3 PUFA in tissues. In this review, we will probe these issues specifically from the perspective of immune effector cell model systems.
Molecular forms of n-3 PUFA and their bioavailability
In processed fish oils available as supplements DHA is predominantly localized to the sn-2 position compared to EPA which is more randomly esterified to all three positions of the triglyceride backbone [15]. In this form, DHA is primarily absorbed as the monoglyceride [16]. This contrasts, for example, with seal oil (also rich in n-3 PUFA), where EPA, DPA and DHA are preferentially located both in the sn-1 and sn-3 positions in the triglyceride molecule [17]. Fatty acids can be easily released from the sn-1 and sn-3 positions by pancreatic lipase and are directly absorbed [15].
There are reports that fish consumption is more effective at increasing serum EPA and DHA levels in humans compared to supplementation with fish oil [18]. With respect to intramolecular fatty acid distribution, the randomization of n-3 PUFA within fish oil triglycerides does not appear to have an effect on the apparent digestibility of the individual fatty acids [17]. In addition, De Schrijver et al [19] concluded that once n-3 PUFA are absorbed, their effect on lipid metabolism in the rat is not determined by the dietary source. Manipulation of fatty acid content of an oil may increase the susceptibility of the oil to oxidation relative to its unmodified counterpart [20]. Interestingly, liposomes based on natural phospholipids enriched in n-3 PUFA may have enhanced bioavailability compared to standard fish oil [21]. Lastly, with respect to in vivo models to evaluate n-3 PUFA bioavailability, it is important to note that most of the studies conducted to date have been in rats.
What is a relevant dose of n-3 PUFA in experimental models?
In general, the typical American consumes 0.7–1.6 g of n-3 PUFA per day, equivalent to approximately 0.2–0.7% of total calories [22, 23]. Much of this is as ALA, the plant n-3 PUFA, and intake of fish-derived long chain n-3 PUFA (i.e., EPA and DHA) was reported to be less than 0.1–0.2 g per day. In contrast, in human clinical trials, 1–9 g/day (0.45–4% of calories) n-3 PUFA, mainly in the form of EPA and/or DHA has been used [24–27]. With respect to physiological relevance, this range is similar to levels consumed by Greenland Inuit (i.e., 6–14 g/day, which corresponds to 2.7–6.3% of daily energy) [28, 29]. Similarly, traditional Japanese diets contain 1–2% of daily energy as long chain n-3 PUFA [30, 31]. Therefore, it seems reasonable for animal feeding studies designed to probe the biological properties of n-3 PUFA relevant to humans, that 4% (wt/wt) fish oil or 1% purified n-3 PUFA ethyl esters be used. This level of intake delivers ~2.4% of total energy as n-3 PUFA, which is within the range consumed by humans and used in human clinical trials.
Enrichment of DHA in serum and tissues
Conquer et al. reported the amount of n-3 PUFA in serum total phospholipids and non-esterified fatty acids (NEFA) in subjects supplemented with 1.5 g DHA/day (~0.7% of calories) [22]. The circulating phospholipid form of DHA (402 μM) was predominant in serum compared to NEFA (12.7 μM) (Table 1). Levels approximating 130 μM DHA in total phospholipids and 1.5 μM in NEFA were detected in the control group. Overall, DHA supplementation (at 1.5 g/day) increased phospholipid DHA 3-fold, compared to a 0.5-fold increase in EPA. In contrast to DHA enrichment in human sera, Switzer et al [13] demonstrated only a modest enrichment of DHA and DPA in mouse serum total phospholipids following consumption of a diet containing 4% (wt/wt) fish oil, which supplied 0.87% of total calories as DHA. Notably, EPA (9.9 μM) was significantly enriched in mouse serum compared to the n-6 PUFA rich corn oil fed control group (Table 1).
Table 1.
Enrichment of n-3 PUFA in sera by dietary intake.
| Model | Dose | Treatment | Control | References | |
|---|---|---|---|---|---|
| Human serum | 1.5 g DHA/d for 6 wk vs 0 g DHA | Serum total phospholipids | 402 μM DHA | 133 μM DHA | [22] |
| 7.9 mol% DHA | 2.8 mol% DHA | ||||
| 0.5 mol% DPA | 0.9 mol% DPA | ||||
| 1.5 mol% EPA | 1.0 mol% EPA | ||||
| Non-esterified fatty acid | 12.7 μM DHA | 1.5 μM DHA | |||
| 2.2 mol% DHA | 0. μmol% DHA | ||||
| 0.0 mol% DPA | 0.0 mol% DPA | ||||
| 0.1 mol% EPA | 0.1 mol% EPA | ||||
| Mouse serum | 4% fish oil+ 1% corn oil for 2 wk vs 5% corn oil | Serum total phospholipids | 6.7 μM DHA | 6.7 μM DHA | [13] |
| 0.2 μM DPA | 0.0 μM DPA | ||||
| 9.9 μM EPA | 0.0 μM EPA | ||||
With regard to tissue enrichment following n-3 PUFA consumption, Damsgaard et al. demonstrated that DHA, DPA and EPA are highly enriched in human peripheral blood mononuclear cells (PBMC) following the intake of 5 mL fish oil/d for 8 wk (Table 2) [28]. Significant amounts of n-3 PUFA (DHA, DPA and EPA) were also observed in subjects consuming 5 mL olive oil (negative control), likely indicating the contribution of the previous dietary consumption of n-3 PUFA. In comparison, in the mouse model, we have shown that, following fish oil feeding (4%, wt/wt), CD4+ T-cell membrane lipid raft and non-raft membrane fractions become enriched in DHA and EPA [13]. Fish oil supplementation increased mainly DHA levels in T-cell membrane rafts (4-fold) and non-rafts (1.9-fold) of CD4+ T cells compared to T-cells from 5% corn oil fed mice. Leslie et al. [32] demonstrated that macrophages from mice fed a diet containing 5% fish oil were highly enriched in DHA (9.8 mol%), as well as in total n-3 PUFA (22.3 mol%), compared to 5% corn oil fed control mice (Table 2).
Table 2.
Enrichment of n-3 PUFA in immunocytes by dietary intake.
| Model | Dose | Treatment | Control | References | |
|---|---|---|---|---|---|
| Human PBMC | 5 mL fish oil/d vs 5 mL olive oil | Total phospholipids | 3.4 mol% DHA | 2.7 mol% DHA | [28] |
| 4.0 mol% DPA | 2.6 mol% DPA | ||||
| 2.6 mol% EPA | 0.3 mol% EPA | ||||
| 10.0 mol% n-3 PUFA | 5.6 mol % n-3 PUFA | ||||
| Mouse CD4+ T-cells | 4% fish oil + 1% corn oil for 2 wk vs 5% corn oil | Phospholipid in raft fraction | 2.5 mol% DHA | 0.6 mol% DHA | [13] |
| 0.8 mol% EPA | 0.0 mol% EPA | ||||
| Phospholipid in non-raft fraction | 4.5 mol% DHA | 1.3 mol% DHA | |||
| Mouse macrophages | 5% fish oil for 9 wk vs 5% corn oil | Total phospholipids in macrophages | 9.8 mol% DHA | 0.1 mol% DHA | [32] |
| 22.3 mol% n-3 PUFA | 0.1 mol% n-3 PUFA | ||||
EPA/DHA enrichment in cell culture
Cell culture studies are convenient and advantageous in some circumstances. However, interpretation of cell culture data in terms of biological outcomes is not always straightforward, since the experimental conditions may be somewhat contrived and perhaps far removed with respect to physiological relevance. To assess the effect of dietary fatty acids on specific tissues, animals are typically fed n-3 PUFA enriched diets followed by the isolation of tissues/cells to be activated in media, ex vivo. However culture itself can modify the fatty acid composition of cells. Switzer et al. [13] demonstrated n-3 PUFA enrichment in the non-raft fraction of murine CD4+ T-cells induced by 4% fish oil feeding for 2 wk dropped from 7.7 mol% to 4.1 mol% by culturing the cells in 5% fetal bovine serum supplemented culture medium for 5 d, whereas n-3 PUFA in the raft fraction remained at 2.2 mol% after culture. The loss of fatty acids in the culture might result in the loss of a diet-induced phenotype, and therefore, possible misinterpretation of the treatment effect. To overcome these limitations, cell culture in the presence of homologous serum in the medium has been used to maintain a significant amount of fatty acids in cell membranes [13]. Indeed, the n-3 PUFA level in the non-raft fraction of CD4+ T-cells from 4% fish oil fed mice increased from 4.1 to 12.2 mol% following 5 d in culture with homologous serum. This also complicates interpretation, since it is difficult to rule out a direct effect of n-3 PUFA containing homologous serum. Of interest, recently, Fan et al. [33] demonstrated that CD4+ T-cells from Fat-1 transgenic mice, which generate endogenous n-3 PUFA by n-3 desaturase, maintained the initial amount of n-3 PUFA in T-cell membranes after 72 h in culture without homologous serum supplementation. These data indicate that Fat-1-containing cells express a physiologically relevant, n-3 PUFA enriched, membrane fatty acid composition which is resistant to conventional cell culture-induced depletion.
Li et al. [34] reported enrichment of n-3 PUFA into both lipid raft and non-raft membrane phospholipid fractions upon incubation of human Jurkat CD4+ T-cells with 50 μM DHA (Table 3). However, DHA content (15.3 mol% in rafts and 15.0 mol% in non-rafts) was higher compared to dietary enrichment of n-3 PUFA in human PBMC (10.0 mol% in total phospholipids) or murine CD4+ T-cells (2.2 mol% in rafts and 7.7 mol% in non-rafts) as described above. Therefore, the effect of the concentration of fatty acids used in cell culture studies should be carefully considered with respect to physiological relevance. As a precautionary note involving n-3 PUFA enrichment in cells, we recently noted that “lipid bodies” form when human Jurkat CD4+ T-cells are incubated in the presence of 50 μM DHA. This impairs the ability of these cells to form an immunological synapse with co-incubated human Raji B-cells (Figure 1). Therefore, investigators performing cell culture studies involving the supplementation of media with fatty acids should remain vigilant of the off-target and perhaps toxic effects of long chain PUFA in culture.
Table 3.
n-3 PUFA enrichment in human Jurkat CD4+ T-cells by DHA treatment in cell culture.
| Dose | Treatment | Control | References | |
|---|---|---|---|---|
| 50 μM DHA for 48 h vs 50 μM AA | Phospholipid in raft fraction | 15.3 mol% DHA | 0.3 mol% DHA | [34] |
| 1.0 mol% DPA | 0.3 mol% DPA | |||
| 1.2 mol% EPA | 0.6 mol% EPA | |||
| Phospholipid in non-raft fraction | 15.0 mol% DHA | 3.0 mol% DHA | ||
| 0.0 mol% DPA | 0.7 mol% DPA | |||
| 0.0 mol% EPA | 0.0 mol% EPA | |||
Figure 1.
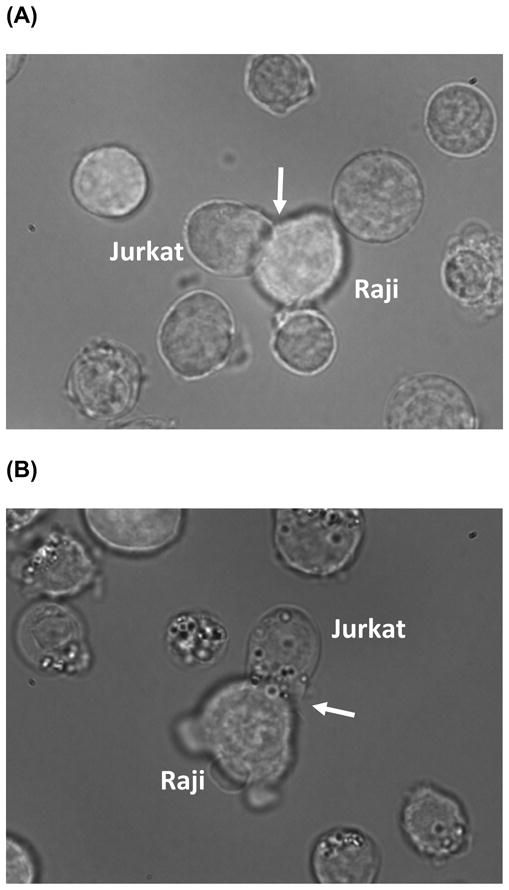
The formation of “lipid bodies” following DHA incubation imaged by bright field microscopy. (A) Fetal bovine serum (FBS, control) or (B) 50 μM DHA was added to FBS (72 h) treated human Jurkat CD4+ T-cells co-cultured with human Raji B-cells primed with superantigen Styphyllococal Enterotoxin E to form an immunological synapse (arrow).
Conclusion
In this report, we reviewed the potential complicating effects of the molecular form and dose of n-3 PUFA on biological endpoints. Clearly, the interpretation of experimental outcomes can be confounded by the failure to consider the effects of the molecular form and the dose of the fatty acid used and also the incorporation into discrete intracellular domains.
Acknowledgments
Supported by USDA 2008-34402-19195 Vegetable & Fruit Improvement Center, NIH grants DK071707, CA59034 and P30ES09106.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stone NJ. Fish consumption, fish oil, lipids, and coronary heart disease. Circulation. 1996;94:2337–2340. doi: 10.1161/01.cir.94.9.2337. [DOI] [PubMed] [Google Scholar]
- 2.Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2002;112:298–304. doi: 10.1016/s0002-9343(01)01114-7. [DOI] [PubMed] [Google Scholar]
- 3.Lupton JR, Chapkin RS. Chemopreventive effects of Omega-3 fatty acids. In: Kelloff GJ, Hawk ET, Sigman CC, editors. Cancer Chemo prevention; Vol I: Promising Cancer Chemopreventive Agents. Humana Press; Totowa, NJ: 2004. pp. 591–608. [Google Scholar]
- 4.Augustsson K, Michaud DS, Rimm EB, Leitzmann MF, Stampfer MJ, Willett WC, Giovannucci E. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:64–67. [PubMed] [Google Scholar]
- 5.Caygill CP, Hill MJ. Fish, n-3 fatty acids and human colorectal and breast cancer mortality. Eur J Cancer Prev. 1995;4:329–332. doi: 10.1097/00008469-199508000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Chapkin RS, McMurray DN, Davidson LA, Patil BS, Fan YY, Lupton JR. Bioactive dietary long-chain fatty acids: emerging mechanisms of action. Br J Nutr. 2008;100:1152–1157. doi: 10.1017/S0007114508992576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia Q, Lupton JR, Smith R, Weeks BR, Callaway E, Davidson LA, Kim W, Fan YY, Yang P, Newman RA, Kang JX, McMurray DN, Chapkin RS. Reduced colitis-associated colon cancer in Fat-1 (n-3 fatty acid desaturase) transgenic mice. Cancer Res. 2008;68:3985–3991. doi: 10.1158/0008-5472.CAN-07-6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins Leukot Essent Fatty Acids. 2009;81:187–191. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley DS. Modulation of human immune and inflammatory responses by dietary fatty acids. Nutrition. 2001;17:669–673. doi: 10.1016/s0899-9007(01)00576-7. [DOI] [PubMed] [Google Scholar]
- 10.Mehta LR, Dworkin RH, Schwid SR. Polyunsaturated fatty acids and their potential therapeutic role in multiple sclerosis. Nat Clin Pract Neurol. 2009;5:82–92. doi: 10.1038/ncpneuro1009. [DOI] [PubMed] [Google Scholar]
- 11.Green KN, Martinez-Coria H, Khashwji H, Hall EB, Yurko-Mauro KA, Ellis L, LaFerla FM. Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J Neurosci. 2007;27:4385–4395. doi: 10.1523/JNEUROSCI.0055-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolar SS, Barhoumi R, Lupton JR, Chapkin RS. Docosahexaenoic acid and butyrate synergistically induce colonocyte apoptosis by enhancing mitochondrial Ca2+ accumulation. Cancer Res. 2007;67:5561–5568. doi: 10.1158/0008-5472.CAN-06-4716. [DOI] [PubMed] [Google Scholar]
- 13.Switzer KC, Fan YY, Wang N, McMurray DN, Chapkin RS. Dietary n-3 polyunsaturated fatty acids promote activation-induced cell death in Th1-polarized murine CD4+ T-cells. J Lipid Res. 2004;45:1482–1492. doi: 10.1194/jlr.M400028-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang P, Kim W, Zhou L, Wang N, Ly LH, McMurray DN, Chapkin RS. Dietary fish oil inhibits antigen-specific murine Th1 cell development by suppression of clonal expansion. J Nutr. 2006;136:2391–2398. doi: 10.1093/jn/136.9.2391. [DOI] [PubMed] [Google Scholar]
- 15.Ackman RG. The absorption of fish oils and concentrates. Lipids. 1992;27:858–862. doi: 10.1007/BF02535864. [DOI] [PubMed] [Google Scholar]
- 16.Sadou H, Leger CL, Descomps B, Barjon JN, Monnier L, Crastes de Paulet A. Differential incorporation of fish-oil eicosapentaenoate and docosahexaenoate into lipids of lipoprotein fractions as related to their glyceryl esterification: a short-term (postprandial) and long-term study in healthy humans. Am J Clin Nutr. 1995;62:1193–1200. doi: 10.1093/ajcn/62.6.1193. [DOI] [PubMed] [Google Scholar]
- 17.Christensen MS, Hoy CE, Redgrave TG. Lymphatic absorption of n - 3 polyunsaturated fatty acids from marine oils with different intramolecular fatty acid distributions. Biochim Biophys Acta. 1994;1215:198–204. doi: 10.1016/0005-2760(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 18.Elvevoll EO, Barstad H, Breimo ES, Brox J, Eilertsen KE, Lund T, Olsen JO, Osterud B. Enhanced incorporation of n-3 fatty acids from fish compared with fish oils. Lipids. 2006;41:1109–1114. doi: 10.1007/s11745-006-5060-3. [DOI] [PubMed] [Google Scholar]
- 19.De Schrijver R, Vermeulen D, Backx S. Digestion and absorption of free and esterified fish oil fatty acids in rats. Lipids. 1991;26:400–404. doi: 10.1007/BF02537207. [DOI] [PubMed] [Google Scholar]
- 20.Hamam F, Shahidi F. Synthesis of structured lipids containing medium-chain and omega-3 fatty acids. J Agric Food Chem. 2006;54:4390–4396. doi: 10.1021/jf052540r. [DOI] [PubMed] [Google Scholar]
- 21.Cansell M, Nacka F, Combe N. Marine lipid-based liposomes increase in vivo FA bioavailability. Lipids. 2003;38:551–559. doi: 10.1007/s11745-003-1341-0. [DOI] [PubMed] [Google Scholar]
- 22.Conquer JA, Holub BJ. Effect of supplementation with different doses of DHA on the levels of circulating DHA as non-esterified fatty acid in subjects of Asian Indian background. J Lipid Res. 1998;39:286–292. [PubMed] [Google Scholar]
- 23.Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, Hargrove RL, Zhao G, Etherton TD. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:179S–188S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 24.Rees D, Miles EA, Banerjee T, Wells SJ, Roynette CE, Wahle KW, Calder PC. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. Am J Clin Nutr. 2006;83:331–342. doi: 10.1093/ajcn/83.2.331. [DOI] [PubMed] [Google Scholar]
- 25.Kelley DS, Taylor PC, Nelson GJ, Mackey BE. Dietary docosahexaenoic acid and immunocompetence in young healthy men. Lipids. 1998;33:559–566. doi: 10.1007/s11745-998-0240-8. [DOI] [PubMed] [Google Scholar]
- 26.Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with gamma-linolenic acid or fish oil decreases T lymphocyte proliferation in healthy older humans. J Nutr. 2001;131:1918–1927. doi: 10.1093/jn/131.7.1918. [DOI] [PubMed] [Google Scholar]
- 27.Kelley DS, Taylor PC, Nelson GJ, Schmidt PC, Ferretti A, Erickson KL, Yu R, Chandra RK, Mackey BE. Docosahexaenoic acid ingestion inhibits natural killer cell activity and production of inflammatory mediators in young healthy men. Lipids. 1999;34:317–324. doi: 10.1007/s11745-999-0369-5. [DOI] [PubMed] [Google Scholar]
- 28.Damsgaard CT, Frokiaer H, Lauritzen L. The effects of fish oil and high or low linoleic acid intake on fatty acid composition of human peripheral blood mononuclear cells. Br J Nutr. 2008;99:147–154. doi: 10.1017/S0007114507791900. [DOI] [PubMed] [Google Scholar]
- 29.Feskens EJ, Kromhout D. Epidemiologic studies on Eskimos and fish intake. Ann N Y Acad Sci. 1993;683:9–15. doi: 10.1111/j.1749-6632.1993.tb35688.x. [DOI] [PubMed] [Google Scholar]
- 30.Nagata C, Takatsuka N, Shimizu H. Soy and fish oil intake and mortality in a Japanese community. Am J Epidemiol. 2002;156:824–831. doi: 10.1093/aje/kwf118. [DOI] [PubMed] [Google Scholar]
- 31.Okuyama H, Kobayashi T, Watanabe S. Dietary fatty acids--the N-6/N-3 balance and chronic elderly diseases. Excess linoleic acid and relative N-3 deficiency syndrome seen in Japan. Prog Lipid Res. 1996;35:409–457. doi: 10.1016/s0163-7827(96)00012-4. [DOI] [PubMed] [Google Scholar]
- 32.Leslie CA, Gonnerman WA, Ullman MD, Hayes KC, Franzblau C, Cathcart ES. Dietary fish oil modulates macrophage fatty acids and decreases arthritis susceptibility in mice. J Exp Med. 1985;162:1336–1349. doi: 10.1084/jem.162.4.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan YY, Kim W, Callaway E, Smith R, Jia Q, Zhou L, McMurray DN, Chapkin RS. fat-1 transgene expression prevents cell culture-induced loss of membrane n-3 fatty acids in activated CD4+ T-cells. Prostaglandins Leukot Essent Fatty Acids. 2008;79:209–214. doi: 10.1016/j.plefa.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Wang M, Tan L, Wang C, Ma J, Li N, Li Y, Xu G, Li J. Docosahexaenoic acid changes lipid composition and interleukin-2 receptor signaling in membrane rafts. J Lipid Res. 2005;46:1904–1913. doi: 10.1194/jlr.M500033-JLR200. [DOI] [PubMed] [Google Scholar]


