Abstract
Previous studies have identified interleukin 6 (IL-6) as an important cytokine with prognostic significance in ovarian cancer. Activation of the IL-6-Stat3 pathway contributes to tumor cell growth, survival and drug resistance in several cancers, including ovarian cancer. To explore potential therapeutic strategies for interrupting signaling through this pathway, we assessed the ability of CDDO-Me, a synthetic triterpenoid, to inhibit IL-6 secretion, Stat3 phosphorylation, Stat3 nuclear translocation and paclitaxel sensitivity in several cell line model systems. These studies demonstrated that CDDO-Me significantly inhibits IL-6 secretion in paclitaxel-resistant ovarian cancer cells and specifically suppresses IL-6- or oncostatin M- induced Stat3 nuclear translocation. Treatment with CDDO-Me significantly decreases the levels of Stat3, Jak2, and Src phosphorylation in ovarian and breast cancer cell lines with constitutively activated Stat3. This inhibition of the IL-6-Stat3 pathway correlated with suppression of the anti-apoptotic Stat3 target genes Bcl-XL, survivin, and Mcl-1, and with apoptosis induction as measured by monitoring PARP and its cleavage product, as well as by quantitative measurement of the apoptosis-associated CK18Asp396. Furthermore, CDDO-Me increases the cytotoxic effects of paclitaxel in the paclitaxel-resistant ovarian cancer cell line OVCAR8TR (2 to 5 fold) and of cisplatin in the cisplatin-resistant ovarian cancer cell line A2780cp70 (2 to 4 fold). Our data confirm that CDDO-Me interrupts the signaling of multiple kinases involved in the IL-6-Stat3 and Src signaling pathways. Inhibition is likely achieved through multiple points within these pathways. In a model system of established acquired drug resistance, CCDO-Me is effective at partially reversing the drug-resistance phenotype.
Keywords: CDDO-Me, IL-6, Stat3, Ovarian cancer, Multidrug resistance
Introduction
Ovarian cancer is the most lethal gynecological malignancy in the United States. Typically, women with this malignancy present with advanced stage disease; however, with aggressive surgical management and subsequent paclitaxel and platinum-based chemotherapy most these women can be returned to a state of microscopic disease or minimal macroscopic residual tumor and, more importantly, to a better quality of life. Unfortunately, the progression of this residual disease, with the eventual acquisition of drug resistance, ultimately leads to significant morbidity and eventual mortality [29]. There is no therapy to overcome drug resistance in ovarian cancer. The development of chemoresistance is associated with many events, such as defective apoptotic signaling in response to drugs, overexpression of antiapoptotic proteins such interleukin 6 (IL-6), Bcl-2, BcL-XL, or survivin, and overexpression of multidrug resistance (MDR) gene protein pgp1[6-9, 12]. Successful management of ovarian cancer would be greatly aided by novel agents that interfere with both intrinsic and acquired mechanisms of drug resistance mechanisms.
Regulation of the apoptotic process is controlled through a complex interplay of pro- and anti-apoptotic signals that together set an apoptotic threshold. IL-6 is a progrowth, proangiogenic and anti-apoptotic cytokine that has been elevated in serum and ascites of patient with ovarian cancer as well as in culture supernatant of multi-drug resistant cell lines [8, 19-21]. In many tumors, elevated IL-6 levels in the serum is predictive of poor clinical outcome. Recently, a study of a mouse model of ovarian cancer has demonstrated a pro-angiogenic role of this cytokine in ovarian cancer [19]. Oncostatin M, another member of IL-6 cytokine family, is constitutively expressed in ovarian cancer [23]. IL-6 and oncostatin induce intracellular signaling through Stat3. Binding of IL-6 with IL-6R on cell surface activates the Janus kinase (Jak) family of protein tyrosine kinases, which then phosphorylate and activate cytoplasmic Stat3 protein [5]. Activated and dimerized Stat3 translocates to the nucleus where it binds to specific DNA response elements and induces expression of Stat3-regulated gene expression, including a collection of anti-apoptotic genes. In certain model systems, Stat3 has can serve as classic oncogene inducing transformation in part through inhibition of apoptosis [27]. We have previously shown that progressive paclitaxel resistance in vitro is associated with increasing IL-6 expression in ovarian cancer cell lines and showed that transfection of IL-6 (a Stat3 activating ligand) into some cell lines induced paclitaxel resistance [7, 10]. Recently, several studies have demonstrated that Stat3 is highly activated in high-grade as well as recurrent drug resistant ovarian cancer tumor cells [8, 18, 22, 25]. Constitutive activation of the Stat3 pathway has been shown to confer resistance to chemotherapy-induced apoptosis in epithelial malignancies [2].
CDDO-Me, also known as RTA-402, NSC713200, is a novel C-28 methyl ester synthetic oleanane triterpenoid. When CDDO-Me applied to macrophages at low concentrations, it demonstrates a variety of anti-inflammatory effects; at higher concentrations the compound inhibits cancer cell growth and proliferation in a wide variety of cell lines [17]. CDDO-Me is consistently more active than the parental compound, CDDO [15]. CDDO-Me also induces apoptosis in various human cancer and leukemic cells. CDDO-Me-induced apoptosis is associated with activation of caspase 3 and 8, cytochrome c, SOCS-1, and SHP-1, and inhibition of NF-kB, Cox2, and VEGF. SOCS-1 and SHP-1 have been shown to inhibit Stat3 phosphorylation [17, 24, 28]. To date, however, the effect of triterpenoids on multidrug resistant ovarian cancer cells is undefined.
In this study, we investigated the effect of CDDO-Me on constitutive expression of IL-6 and activation of Stat3 in multidrug-resistant ovarian cancer cells, and whether a combination of minimally or non-toxic doses of CDDO-Me induces apoptosis, overcomes drug resistance, or enhances drug sensitivity of paclitaxel-resistant ovarian cancer cells.
Materials and Methods
Cell Lines, antibodies and drugs
The human ovarian cancer cell line SKOV-3, human breast cancer cell line MDA-MB-468, and the hamster kidney cell line BHK-21 were obtained from the American Type Tissue Collection (Rockville, MD). Dr. Patricia Donahoe (Massachusetts General Hospital, Boston, MA) provided the human OVCAR8 ovarian cancer cell line. The pEGFP-Stat3 vector was obtained from Amersham Biosciences (Buckinghamshire, UK). The paclitaxel-resistant SKOV-3TR and OVCAR8TR lines were established as previously reported [7, 9]. Briefly, the paclitaxel-resistant cell lines were selected over a period of eight months by continuous culture in media containing step-wise increases in paclitaxel. The cisplatin-resistant ovarian cancer cell lines A2780CP70 was kindly supplied by Dr. T. C. Hamilton (Fox Chase Cancer Center, Philadelphia, PA). Paclitaxel and Cisplatin were obtained through unused residual clinical material at the Massachusetts General Hospital. CDDO-Me was kindly provided by Dr. Jeff Supko (Massachusetts General Hospital). G418 was purchased from Invitrogen (Carlsbad, CA).
The rabbit polyclonal antibodies to Stat3, Src, pSrc, pJak2, Mcl-1, Bcl-2 and BcL-XL, and the mouse monoclonal antibodies to phosphorylated-Stat3, survivin were purchased from Cell Signaling Technologies (Cambridge, MA). The mouse monoclonal antibody to actin and MTT were purchased from Sigma-Aldrich (St. Louis, MO). The Pgp1 monoclonal antibody C219 was purchased from Signet (Dedham, MA). Goat anti-rabbit-HRP and goat anti-mouse-HRP were purchased from Bio-Rad (Hercules, CA). SuperSignal® West Pico Chemiluminescent Substrate was purchased from PIERCE (Rockford, IL).
Cell culture
All the cell lines were cultured in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 100-units/ml penicillin and 100 μg/ml streptomycin (Invitrogen). Cells were incubated at 37°C in 5% CO2-95% air atmosphere and passaged twice every 6 days. Paclitaxel-resistant cell lines were periodically cultured in paclitaxel to confirm their drug resistance. Cells were free on mycoplasma contamination as tested by MycoAlert(R) Mycoplasma Detection Kit from Cambrex (Rockland, ME).
IL-6 measurements
Previously, we have found that IL-6 is secreted in high levels by several paclitaxel-resistant ovarian cancer cell lines, including SKOV-3TR and OVCAR8TR [7, 8]. SKOV-3TR and OVCAR8TR cells were plated at 1×105 cells per well in 24-well plates and treated with CDDO-Me in both a time- and dose-dependent manner. The supernatants were collected at different time points and kept at −20° C. IL-6 levels in culture supernatants were measured using a quantitative ELISA kit (R&D Systems, Minneapolis, MN) as described previously [7, 8]. The absorbance of each well was read using a BT 2000 Microkinetics Reader (Bio-Tek Instrument Inc. Winooski, VT) at 450 nm. A standard curve was constructed to quantitate the cytokine concentrations in the controls and samples.
Western blotting
Total cell lysates were prepared, and Western blot analysis was performed as previously described. Briefly, the cells were lysed in 1× RIPA lysis buffer (Upstate Biotechnology, Charlottesville, VA) and protein concentration was determined by the DC Protein Assay (Bio-Rad). Twenty-five micrograms of total protein were resolved on NuPage™ 4-12% Bis-Tris Gels (Invitrogen) and immunoblotted with specific antibodies. Primary antibodies were incubated in TBS (pH 7.4) with 0.1% Tween-20 with gentle agitation overnight at 4°C. Horseradish peroxidase (HRP)-conjugated secondary antibodies (Bio-Rad) were incubated in TBS (pH 7.4) with 5% nonfat milk (Bio-Rad) and 0.1% Tween-20, at a 1:2000 dilution for one hour at room temperature with gentle agitation. Positive immunoreactions were detected by using SuperSingal® West Pico Chemiluminescent Substrate.
Real-time analysis of the effect of CDDO-Me on Stat3 nucleocytoplasmic translocation
To study the effects of CDDO-Me on Stat3 nuclear translocation in live cells, a real-time cell-based assay was used as described below. Stable transfectants of expressing the EGFP-Stat3 were generated with the BHK-21 hamster kidney cell line and the resistant ovarian cancer cell line OVCAR8TR using standard lipofection techniques with G418 selection. EGFP-Stat3 expressing cells were seeded at a density of 4000 cells per well in 96-well flat bottom plates and incubated overnight at 37°C. The cells were then treated with CDDO-Me compound at 0.5 μM or 1 μM for four hours. After incubation, human recombinant IL-6 (R&D Systems, Minneapolis, MN) was added to the wells to a final concentration of 30 ng/ml for an additional hour of incubation. Human recombinant oncostatin M (R&D Systems) was added to OVCAR8 TR to a final concentration of 60 ng/ml for an additional hour of incubation. IL-6 or oncostatin-dependent nuclear translocation of EGFP-Stat3 was analyzed using an Olympus 1×71 fluorescence microscope and the pictures were captured as digital images using IPLab Software from Scanalytics (Rockville, MD).
Cytotoxicity assay
The cytotoxic effects of CDDO-Me on OVCAR8TR, A2780cp70 were assessed using the MTT assay as previously described [3, 7]. Briefly, 2×103 cells per well were plated in triplicate in 96-well plates. Cells were plated in RPMI 1640 medium in the presence or absence of indicated concentration of paclitaxel or cisplatin and CDDO-Me. After seven days of culture at 37°C, 10 μL of MTT (5 mg/ml in PBS) was added to each well and the plates were incubated for four hours. The resulting formazan product was dissolved with acid-isopropanol and the absorbance at a wavelength of 490nm (A490) was read on a BT 2000 Microkinetics Reader with the acid-isopropanol solution serving as blank. The IC50 was defined as the paclitaxel concentration required to decrease the A490 to 50% of the control (no paclitaxel) value.
Apoptosis assay
Whole-cell lysates were immunoblotted with specific antibodies to PARP (Cell Signaling Technologies) and its cleavage products. Positive immunoreactions were detected by using Super Signal® West Pico Chemiluminescent Substrate. Quantification of apoptosis was also evaluated using the M30-Apoptosense ELISA assay kit, as per manufacturer's instructions (Peviva AB, Bromma, Sweden; ref. [11]). OVCAR8 and OVCAR8TR cells were seeded at 8000 cells/per well in a 96-well plate for 24 hours before the addition of paclitaxel, cisplatin, and CDDO-Me. The cells were then treated with 0.01 μM paclitaxel, 1 μM cisplatin, 0.3 μM CDDO-Me or a combination of either of the cytotoxics and CDDO-Me for an additional 24 hours. The cells were then lysed by adding 10 μl 10% NP-40 per well, and the manufacturer's instructions for the apoptosis assay were then followed.
Results
CDDO-Me inhibits IL-6 secretion in multidrug resistant ovarian cancer cells
Initial experiments investigated the effect of CDDO-Me on IL-6 secretion in paclitaxel-resistant ovarian cancer cells OVCAR8 TR and SKOV-3TR. As seen in Figure 1, CDDO-Me significantly inhibits IL-6 secretion in these cell lines in both a time- and dose-dependent manner (Fig.1). The reduction of IL-6 secretion in these CDDO-Me treated cells was not due to the inhibit cell proliferation, as the concentration of 0.01 μM (in OVCAR8 TR) and 0.05 μM (in SKOV-3TR) of CDDO-Me already showed inhibit IL-6 expression (Fig.1A and Fig.1C). Such non-toxic low doses of CDDO-Me showed have no effect on these cells growth and proliferation (data not shown). CDDO-Me-mediated inhibition of IL-6 secretion was observed by 4 h, and with a concentration as low as 0.3 μM (Fig. 1B). The levels of IL-6 secretion and Stat3 phosphorylation in the parental cell lines have been reported in our previous studies [7, 8].
Fig.1.
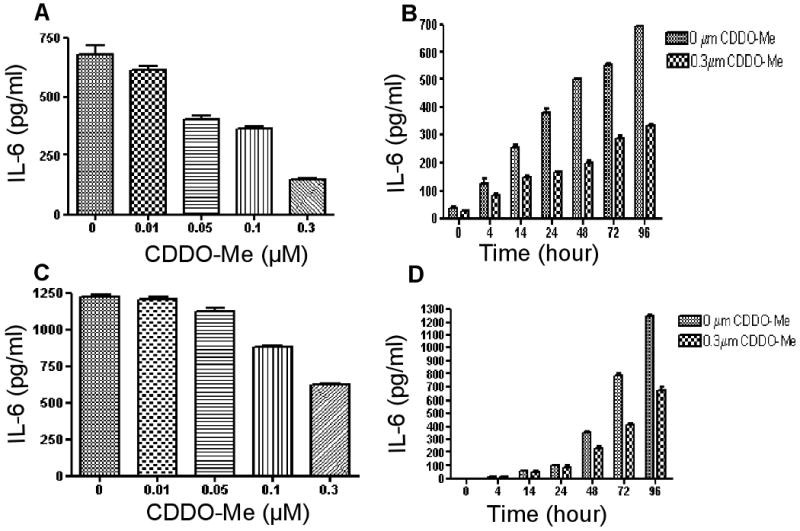
Effect of CDDO-Me on IL-6 secretion in OVCAR8TR and SKOV-3TR. IL-6 levels were measured using IL-6 ELISA on supernatants obtained from cultures of OVCAR8TR and SKOV-3TR cells treated with CDDO-Me in both a time- and dose-dependent manner. Results are mean ± SD of 3 independent experiments. A and C: CDDO-Me inhibits IL-6 protein synthesis in a dose-dependent manner. ELISA analysis of IL-6 levels isolated from OVCAR8TR (A) SKOV-3TR(C) that were cultured for 96 h in the presence of various concentrations of CDDO-Me as indicated. B and D: CDDO-Me inhibits IL-6 protein synthesis in a time-dependent manner. ELISA analysis of IL-6 levels isolated from OVCAR8TR (B) SKOV-3TR (D) that were cultured for the time indicated (in hours) in the presence of 0.3 μM of CDDO-Me.
CDDO-Me inhibits IL-6- or oncostain-induced nuclear translocation of Stat3
In resting cells, most Stat3 is cytoplasmic until the addition of human IL-6 or oncostatin M, which induces a series of events, including phosphorylation and translocation of Stat3 molecules to the nucleus. Exposure of cells to CDDO-Me for 4 h, followed by an hour-long incubation in either IL-6 or oncostatin significantly blocked IL-6- or oncostatin M-dependent translocation of EGFP-Stat3 (Fig.2).
Fig. 2.
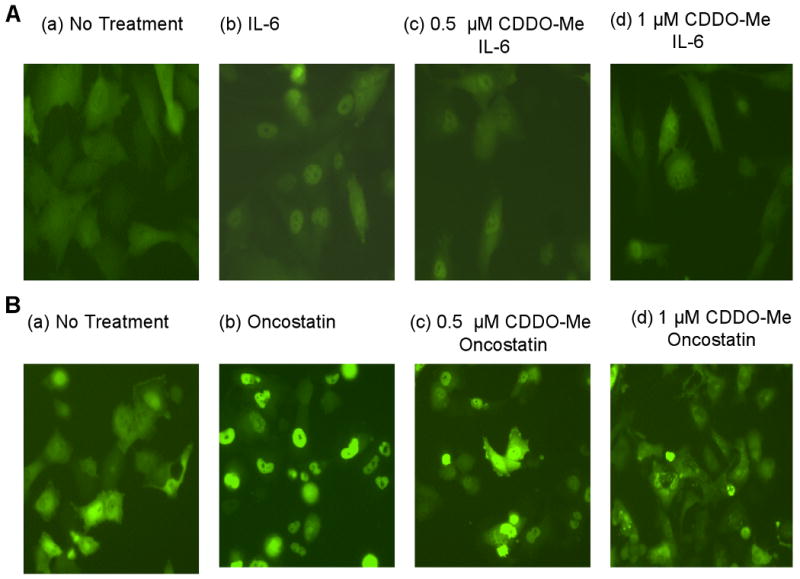
CDDO-Me inhibits EGFP-Stat3 nuclear translocation in BHK-21 and OVCAR8 TR cells. BHK-21 or OVCAR8 TR which stably express the EGFP-Stat3 fusion protein were incubated for 4 hours with CDDO-Me (0.5 μM, 1 μM) followed immediately thereafter with the addition of IL-6 or oncostatin M to a final concentration of 30 ng/ml rIL-6. Cells were photographed one hour later. Subcellular localization of the fusion protein was assessed by fluorescence microscopy. A. BHK-21 derived pEGFP-Stat3 expression cells with CDDO-Me and IL-6 treatment as indicated. B. OVCAR8TR-derived pEGFP-Stat3 expression cells with CDDO-Me and oncostatin treatment as indicated.
CDDO-Me inhibits Stat3 phosphorylation levels
Because IL-6 and oncostatin signals are mediated through Stat3 phosphorylation, we then investigated whether CDDO-Me inhibits Stat3 phosphorylation in paclitaxel-resistant cell lines OVCAR8TR and SKOV-3 TR, and breast cancer cell line MDA-MB-468. All these cells have been shown to express high constitutive levels of phospho-Stat3 (pStat3). The cells were incubated either with a range of concentrations of CDDO-Me for 24 h or with 1 μM or 10 μM for 0, 2h, 4h, 8h and 24h. Western blot analysis demonstrated that CDDO-Me reduced phospho-Stat3 (pStat3) expression in a dose- and time- dependent manner (Fig. 3). CDDO-Me-induced inhibition could be observed as early 2 h (10 μM) or 8 h (1 μM), and with a concentration as low as 0.1μM. In OVCAR8TR cells, with a four hours exposure to CDDO-Me at 10 μM was associated with a near complete absence of Stat3 phosphorylation without a significant change in the expression of total Stat3 protein. Similar results were found in SKOV-3TR and breast cancer cell line MBA-MD-468 cell lines (data not shown).
Fig. 3.

CDDO-Me inhibits Stat3 phosphorylation in paclitaxel-resistant ovarian cancer OVCAR8 TR cells. The OVCAR8 TR cells were treated with CDDO-Me as indicated and then total protein was harvested and 25 μg of cellular proteins were subjected to immunoblotting with specific antibodies as indicated. A. CDDO-Me reduces pStat3 levels OVCAR8TR cells as time dependent manner. OVCAR8TR cells were cultured for the time indicated (in hours) in the presence of 1 μM or 10 μM of CDDO-Me. B. CDDO-Me reduces pStat3 levels OVCAR8TR cells as dose dependent manner. OVCAR8TR cells were cultured for 24 h in the presence of various concentrations of CDDO-Me as indicated.
CDDO-Me has no effect on Pgp1 expression
Moreover, while CDDO-Me significantly inhibits Stat3 phosphorylation in OVCAR8TR cells, no significant change in Pgp1 expression was observed in these Pgp1-overexpressing cell lines (Fig. 3).
CDDO-Me suppresses phosphotyrosine levels of Jak2 and Src
We have shown that CDDO-Me suppresses pStat3 levels, suggesting that this compound might interfere with the function of one or more of the upstream tyrosine kinases such as Jak or Src. Evaluation of the effect of CDDO-Me on the phosphotyrosine levels of Jak1, Jak2, Akt and Src in the OVCAR8TR cell lines in vitro demonstrated significant suppression of tyrosine-phosphorylated Jak2 and Src levels (Fig. 3). Tyrosine phosphorylation of Jak1 and Akt was unaffected in comparison to the inhibition of Jak2 or Src tyrosine phosphorylation at these concentrations and under these conditions (data not shown).
CDDO-Me inhibits Stat3-mediated anti-apoptotic protein expression and induces apoptosis in human cancer cells
Inhibition of Stat3 phosphorylation and nuclear transport would be predicted to effect the apoptotic threshold. Therefore, we examined whether exposure of paclitaxel-resistant cells lines to CDDO-Me would result in apoptosis and decreased expression of the anti-apoptotic proteins Bcl-XL, MCL-1, Bcl-2, and survivin. PARP cleavage, a apoptotic associated biochemical event, was detected at 8 hours after incubation with 1 μM CDDO-Me that correlated with concentrations and exposure time required for blocking Stat3 phosphorylation (Fig. 3 and Fig. 4). Incubation with CDDO-Me for 24 hours significantly down-regulated BcL-XL and survivin expression in OVCAR 8TR (Fig. 4). Furthermore, OVCAR8TR cells treated with low doses (0.2 μM) of CDDO-Me for 5 days also showed a decreased in pStat3, pJak2, and pSrc levels as well as anti-apoptotic gene BcL-XL (Fig. 5). In the control experiments, CDDO-Me did not significantly alter the level of total Stat3, total Jak2 and total Src protein expression (Fig.5).
Fig. 4.

CDDO-Me down-regulated Stat3 targeted anti-apoptotic proteins and induces apoptosis in cancer cells. OVCAR8TR was treated with CDDO-Me in time (A) or dose (B) dependent manner. Total cellular proteins were subjected to immunoblotting with specific antibodies to PARP, MCL-1, Bcl-2, Bcl-XL, surviving and β-actin as described in “Materials and Methods”. A: CDDO-Me reduces anti-apoptotic proteins in OVCAR8TR cells as time dependent manner. OVCAR8TR cells were cultured for the time indicated (in hours) in the presence of 1 μM or 10 μM of CDDO-Me. B: CDDO-Me reduces antiapoptotic proteins in OVCAR8TR cells as dose dependent manner. OVCAR8TR cells were cultured for 24 h in the presence of various concentrations of CDDO-Me as indicated.
Fig. 5.
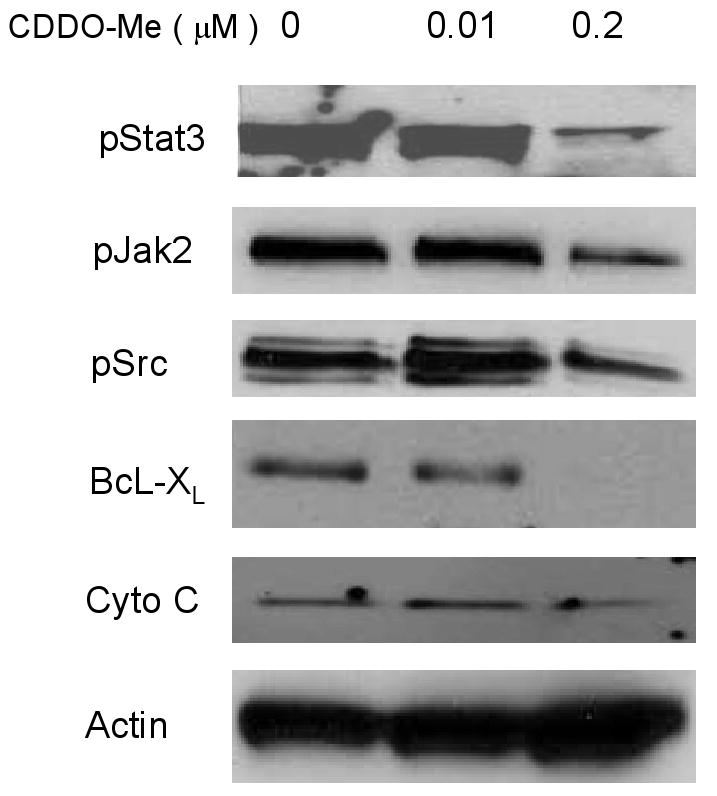
Low dose of DDO-Me inhibits Stat3 phosphorylation and down-regulated Stat3 targeted anti-apoptotic proteins. The OVCAR8 TR cells were treated with CDDO-Me at low dose (0.2 μM) for 96 h and then total protein was harvested and 25 μg of cellular proteins was subjected to immunoblotting with specific antibodies as indicated.
CDDO-Me enhances apoptosis and reduces resistance in paclitaxel resistant human cancer cells
Inhibition of Stat3 signaling in, OVCAR8 and OVCAR8TR would be predicted to increase drug sensitivity to paclitaxel alone and cisplatin. The addition of CDDO-Me to cells exposed to either paclitaxel or cisplatin resulted in at least additive apoptosis in both sensitive and resistant ovarian cancer cells (Fig. 6A). Additionally, MTT assay that measures a combination of cellular proliferation and cytotoxicity also demonstrated that CDDO-Me has an at least additive effect on paclitaxel-induced cell death (Fig. 6B).
Fig. 6.

CDDO-Me induces apoptosis and overcome drug resistance in drug resistance ovarian cancer cells. A: CDDO-Me inhibits cell growth and induces apoptosis in OVCAR8 and OVCAR8TR ovarian cancer cells. OVCAR8TR cells were seeded at a density of 8000 cells per well in a 96-well plate for 24 h. The cells were then treated with 0.01 μM paclitaxel, 1 μM cisplatin, 0.3 μM CDDO-Me or combination of the two drugs for an additional 24 hours. The cells were lysed with 10% NP-40 and the M30-Apoptosense ELISA assay was performed as described in “Materials and Methods”. B: MTT assay of CDDO-Me together with paclitaxel or cisplatin overcome drug resistance in paclitaxel resistant OVCAR8TR or A2780cp70 cells.
These effects were also seen in OVCAR8TR cells exposed to sublethal doses of paclitaxel and CDDO-Me (0.3 μM) (figure 6A). Additionally, the MTT cytotoxicity assay demonstrated that CDDO-Me increased paclitaxel-induced cell death and partially overcomes paclitaxel resistance (Figure 6B). CDDO-Me (0.3 μM) increases the cytotoxic effects of paclitaxel in the paclitaxel-resistant ovarian cancer cell line OVCAR8TR (2 to 5 fold) and cisplatin in the cisplatin-resistant ovarian cancer cell line A2780cp70 (2 to 4 fold) (Fig. 6B).
Discussion
Previous studies with CDDO or CDDO-Me compound have demonstrated that these compounds have the capabilities of modifying a wide variety of proteins through nucleophilic attack and Michaels addition, particularly at vulnerable –SH groups. Cells with altered redox potential are predicted to be sensitive to the myriad of biologic effects seen with CDDO compound (16). Because large collections of regulatory proteins have reactive –SH groups, the targets of CDDO are multiple and the interaction between CDDO and targets may result in broad collection of direct and indirect effects. The altered redox potential of malignant cells as compared to their normal counterparts, is thought to provide a rationale why this agent may has a useful therapeutic window in the treatment of cancer, inflammation, and perhaps cancer prevention [16, 17].
The IL-6-Stat3 signaling pathway plays a significant role in growth and proliferation of tumor cells. Several lines of evidence have demonstrated that IL-6 production and Stat3 activation may prevent cell death and lead to immunosuppression and drug resistance through upregulation of survival proteins in ovarian cancer [8, 19, 22, 25]. Therefore, IL-6 and Stat3 proteins emerged as important targets for ovarian cancer therapy. In this study, we demonstrated that CDDO-Me inhibits IL-6 secretion and Stat3 activation in paclitaxel-resistant ovarian cancer.
Our findings further demonstrated that CDDO-Me inhibits IL-6- and oncostatin M-induced Stat3 nuclear translocation. Moreover, we show that CDDO-Me also inhibits the activation of Jak and Src. As both Jak and Src activation could induce Stat3 activation, inhibition of Jak2 or Src is the likely mechanism for CDDO-Me-mediated inhibition of Stat3 phosphorylation and nuclear translocation. Several previous studies have found CDDO-Me to be a potent inhibitor of both constitutive and inducible NF-κB activation [1, 17, 26]. The suppression of NF-κB occurred through inhibition of IκBα kinase activation [1]. Activation of NF-κB has been shown to promote IL-6 expression in tumor cells [4, 13]. Our results show CDDO-Me inhibits IL-6 secretion, Jak2 and Src phosphorylation; therefore, CDDO-Me likely inhibits the IL-6-Stat3 pathway at multiple points. These results are consistent with a series of studies showing that CDDO-related compounds are not monofunctional drugs that uniquely target single steps in signal transduction pathways [17, 26, 28]
Paclitaxel and platinum compounds such as cisplatin have been the most effective and widely used means of treating ovarian cancer. The development of multi-drug resistance, however, has posed major obstacle to the efficacy of chemotherapy and cancer treatment. Several studies have shown that IL-6, Stat3 pathway is often overexpressed and activated in many paclitaxel-resistant ovarian cancer cells as compared with paired parental cell lines that are paclitaxel relatively sensitive. The inhibition of IL-6 secretion and Stat3 activation in paclitaxel-resistant cells was accompanied by a rapid induction of apoptosis, which is consistent with the role of the IL-6-Stat3 pathway in suppressing apoptosis [2, 8, 14]. The response of cells to apoptotic stimuli depends on the balance between pro- and anti-apoptotic members of apoptosis family. The overexpression of both Bcl-XL and Bcl-2, survivin have been shown to prevent mitochondrial apoptosis in tumor cells [6, 12]. Western blot analysis demonstrated that CDDO-Me treatment results in the down regulation of these antiapoptotic proteins in paclitaxel-resistant cells.
The recent focus on the development of IL-6 and Jak inhibitors emphasizes the relevance of the Stat pathway as a therapeutic target [27, 28]. The ability of CDDO-Me to affect IL-6-Stat3 signaling predicts the potential for its application in many disease settings other than cancer in which aberrant activation of IL-6-Stat3 pathways is observed [17]. Our observation that the MDR1 (Pgp1) positive OVCAR8TR cells are sensitive to CDDO-Me-induced cell death and the additive toxicity of CDDO-Me with paclitaxel suggests that CDDO-Me could induce apoptosis in paclitaxel-resistant cells through mechanisms independent on the inhibition of Pgp1 protein.
In summary, we show that CDDO-Me inhibits IL-6 secretion, Stat3 phosphorylation, and consequent Stat3 nuclear translocation in ovarian cancer cells, including drug resistant lines. In addition, this compound induces apoptosis in these lines including paclitaxel-resistant lines. We hypothesize this apoptotic response may be due, at least in part, to the inhibition of the Stat-3 pathway although interruption of other pro-survival pathways may also play an important role in this process. These preclinical studies provide the framework for clinical evaluation of CDDO-Me, either as a monotherapy or in combination with paclitaxel or cisplatin, to treat ovarian cancer and overcome drug resistance.
Acknowledgments
This project was supported by the Ovarian Cancer SPORE at the DF/HCC 1P50CA105009, and a Grant from the Ovarian Cancer Education and Awareness Network (OCEAN). Dr. Duan is supported, in part, through a grant from the Marsha Rivkin Ovarian Cancer Research Foundation and Wechsler fund. Dr. Seiden is supported by a Mid Career Development Award, 1K24 CA109416-01A1. In addition, we would like to acknowledge Dr. Donald Kufe at Dana-Farber Cancer Institute for providing useful advices during the drafting of this manuscript.
References
- 1.Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem. 2006;281:35764–9. doi: 10.1074/jbc.M607160200. [DOI] [PubMed] [Google Scholar]
- 2.Barre B, Vigneron A, Perkins N, Roninson IB, Gamelin E, Coqueret O. The STAT3 oncogene as a predictive marker of drug resistance. Trends Mol Med. 2006 doi: 10.1016/j.molmed.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of radiosensitivity. Cancer Res. 1987;47:943–6. [PubMed] [Google Scholar]
- 4.Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, Anderson KC. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104–12. [PubMed] [Google Scholar]
- 5.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–5. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 6.Diaz N, Minton S, Cox C, Bowman T, Gritsko T, Garcia R, Eweis I, Wloch M, Livingston S, Seijo E, Cantor A, Lee JH, Beam CA, Sullivan D, Jove R, Muro-Cacho CA. Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clin Cancer Res. 2006;12:20–8. doi: 10.1158/1078-0432.CCR-04-1749. [DOI] [PubMed] [Google Scholar]
- 7.Duan Z, Feller AJ, Penson RT, Chabner BA, Seiden MV. Discovery of differentially expressed genes associated with paclitaxel resistance using cDNA array technology: analysis of interleukin (IL) 6, IL-8, and monocyte chemotactic protein 1 in the paclitaxel-resistant phenotype. Clin Cancer Res. 1999;5:3445–53. [PubMed] [Google Scholar]
- 8.Duan Z, Foster R, Bell D, Mahoney J, Wolak K, Vaidya A, Hampel C, Lee E, Seiden MV. Signal Transducers and Activators of Transcription 3 Pathway Activation in Drug-Resistant Ovarian Cancer. Clin Cancer Res. 2006;12 doi: 10.1158/1078-0432.CCR-06-0861. [DOI] [PubMed] [Google Scholar]
- 9.Duan Z, Lamendola DE, Duan Y, Yusuf RZ, Seiden MV. Description of paclitaxel resistance-associated genes in ovarian and breast cancer cell lines. Cancer Chemother Pharmacol. 2005;55:277–85. doi: 10.1007/s00280-004-0878-y. [DOI] [PubMed] [Google Scholar]
- 10.Duan Z, Lamendola DE, Penson RT, Kronish KM, Seiden MV. Overexpression of IL-6 but not IL-8 increases paclitaxel resistance of U-2OS human osteosarcoma cells. Cytokine. 2002;17:234–42. doi: 10.1006/cyto.2001.1008. [DOI] [PubMed] [Google Scholar]
- 11.Erdal H, Berndtsson M, Castro J, Brunk U, Shoshan MC, Linder S. Induction of lysosomal membrane permeabilization by compounds that activate p53-independent apoptosis. Proc Natl Acad Sci U S A. 2005;102:192–7. doi: 10.1073/pnas.0408592102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, Yoder S, Enkemann S, Eschrich S, Lee JH, Beam CA, Cheng J, Minton S, Muro-Cacho CA, Jove R. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12:11–9. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- 13.Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, Munshi N, Dang L, Castro A, Palombella V, Adams J, Anderson KC. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277:16639–47. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 14.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–12. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Konopleva M, Tsao T, Ruvolo P, Stiouf I, Estrov Z, Leysath CE, Zhao S, Harris D, Chang S, Jackson CE, Munsell M, Suh N, Gribble G, Honda T, May WS, Sporn MB, Andreeff M. Novel triterpenoid CDDO-Me is a potent inducer of apoptosis and differentiation in acute myelogenous leukemia. Blood. 2002;99:326–35. doi: 10.1182/blood.v99.1.326. [DOI] [PubMed] [Google Scholar]
- 16.Liby K, Royce DB, Williams CR, Risingsong R, Yore MM, Honda T, Gribble GW, Dmitrovsky E, Sporn TA, Sporn MB. The synthetic triterpenoids CDDO-methyl ester and CDDO-ethyl amide prevent lung cancer induced by vinyl carbamate in A/J mice. Cancer Res. 2007;67:2414–9. doi: 10.1158/0008-5472.CAN-06-4534. [DOI] [PubMed] [Google Scholar]
- 17.Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–69. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 18.Meinhold-Heerlein I, Bauerschlag D, Hilpert F, Dimitrov P, Sapinoso LM, Orlowska-Volk M, Bauknecht T, Park TW, Jonat W, Jacobsen A, Sehouli J, Luttges J, Krajewski M, Krajewski S, Reed JC, Arnold N, Hampton GM. Molecular and prognostic distinction between serous ovarian carcinomas of varying grade and malignant potential. Oncogene. 2005;24:1053–65. doi: 10.1038/sj.onc.1208298. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65:10794–800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penson RT, Kronish K, Duan Z, Feller AJ, Stark P, Cook SE, Duska LR, Fuller AF, Goodman AK, Nikrui N, MacNeill KM, Matulonis UA, Preffer FI, Seiden MV. Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int J Gynecol Cancer. 2000;10:33–41. doi: 10.1046/j.1525-1438.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 21.Plante M, Rubin SC, Wong GY, Federici MG, Finstad CL, Gastl GA. Interleukin-6 level in serum and ascites as a prognostic factor in patients with epithelial ovarian cancer. Cancer. 1994;73:1882–8. doi: 10.1002/1097-0142(19940401)73:7<1882::aid-cncr2820730718>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 22.Rosen DG, Mercado-Uribe I, Yang G, Bast RC, Jr, Amin HM, Lai R, Liu J. The role of constitutively active signal transducer and activator of transcription 3 in ovarian tumorigenesis and prognosis. Cancer. 2006;107:2730–40. doi: 10.1002/cncr.22293. [DOI] [PubMed] [Google Scholar]
- 23.Savarese TM, Campbell CL, McQuain C, Mitchell K, Guardiani R, Quesenberry PJ, Nelson BE. Coexpression of oncostatin M and its receptors and evidence for STAT3 activation in human ovarian carcinomas. Cytokine. 2002;17:324–34. doi: 10.1006/cyto.2002.1022. [DOI] [PubMed] [Google Scholar]
- 24.Shishodia S, Sethi G, Konopleva M, Andreeff M, Aggarwal BB. A synthetic triterpenoid, CDDO-Me, inhibits IkappaBalpha kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of nuclear factor kappaB-regulated gene products in human leukemic cells. Clin Cancer Res. 2006;12:1828–38. doi: 10.1158/1078-0432.CCR-05-2044. [DOI] [PubMed] [Google Scholar]
- 25.Silver DL, Naora H, Liu J, Cheng W, Montell DJ. Activated signal transducer and activator of transcription (STAT) 3: localization in focal adhesions and function in ovarian cancer cell motility. Cancer Res. 2004;64:3550–8. doi: 10.1158/0008-5472.CAN-03-3959. [DOI] [PubMed] [Google Scholar]
- 26.Yore MM, Liby KT, Honda T, Gribble GW, Sporn MB. The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole blocks nuclear factor-kappaB activation through direct inhibition of IkappaB kinase beta. Mol Cancer Ther. 2006;5:3232–9. doi: 10.1158/1535-7163.MCT-06-0444. [DOI] [PubMed] [Google Scholar]
- 27.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 28.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 29.Yusuf RZ, Duan Z, Lamendola DE, Penson RT, Seiden MV. Paclitaxel resistance: molecular mechanisms and pharmacologic manipulation. Curr Cancer Drug Targets. 2003;3:1–19. doi: 10.2174/1568009033333754. [DOI] [PubMed] [Google Scholar]


