Abstract
Background
In the mid-1990s, Cochlear Corporation introduced a cochlear implant (CI) to the market that was equipped with hardware that made it possible to record electrically evoked compound action potentials (ECAPs) from CI users of all ages. Over the course of the next decade, many studies were published that compared ECAP thresholds with levels used to program the speech processor of the Nucleus CI. In 2001 Advanced Bionics Corporation introduced the Clarion CII cochlear implant (the Clarion CII internal device is also known as the CII Bionic Ear). This cochlear implant was also equipped with a system that allowed measurement of the ECAP. While a great deal is known about how ECAP thresholds compare with the levels used to program the speech processor of the Nucleus CI, relatively few studies have reported comparisons between ECAP thresholds and the levels used to program the speech processor of the Advanced Bionics CI.
Purpose
To explore the relationship between ECAP thresholds and behavioral measures of perceptual dynamic range for the range of stimuli commonly used to program the speech processor of the Advanced Bionics CI.
Research Design
This prospective and experimental study uses correlational and descriptive statistics to define the relationship between ECAP thresholds and perceptual dynamic range measures.
Study Sample
Twelve postlingually deafened adults participated in this study. All were experienced users of the Advanced Bionics CI system.
Data Collection and Analysis
ECAP thresholds were recorded using the commercially available SoundWave software. Perceptual measures of threshold (T-level), most comfortable level (M-level), and maximum comfortable level (C-level) were obtained using both “tone bursts” and “speech bursts.” The relationship between these perceptual and electrophysiological variables was defined using paired t-tests as well as correlation and linear regression.
Results
ECAP thresholds were significantly correlated with the perceptual dynamic range measures studied; however, correlations were not strong. Analysis of the individual data revealed considerable discrepancy between the contour of ECAP threshold versus electrode function and the behavioral loudness estimates used for programming.
Conclusion
ECAP thresholds recorded from Advanced Bionics cochlear implant users always indicated levels where the programming stimulus was audible for the listener. However, the correlation between ECAP thresholds and M-levels (the primary metric used to program the speech processor of the Advanced Bionics CI), while statistically significant, was quite modest. If programming levels are to be determined on the basis of ECAP thresholds, care should be taken to ensure that stimulation is not uncomfortably loud, particularly on the basal electrodes in the array.
Keywords: Auditory evoked potential, cochlear implant, compound action potential, electrical stimulation, neural response imaging
INTRODUCTION
Multichannel cochlear implants first became available in the United States more than two decades ago. Since that time, cochlear implantation has become the treatment of choice for children with severe-to-profound, bilateral sensorineural hearing loss. This technology has been shown to be safe and, for most individuals, leads to significantly improved speech perception abilities. As typical performance levels with cochlear implants (CI) improved, candidacy criteria were relaxed. In 2000 the Food and Drug Administration approved CIs for children as young as one year of age. Since that time many children with hearing loss have received a CI on, or in some cases shortly before, their first birthday. Increasing numbers of children with multiple developmental delays are now also being considered candidates for a CI. As a result, there is a pressing need for methods of programming the speech processor of the CI that do not rely solely on behavioral loudness judgments. While a range of different electrophysiological measures can be used for this purpose, one of the most commonly used is the electrically evoked compound action potential (ECAP).
Electrically Evoked Compound Action Potentials
ECAPs are far-field recordings of the synchronized response of multiple auditory neurons to electrical stimulation. This peripherally generated evoked potential is characterized by a single negative peak (N1) with a latency of approximately 0.2–0.4 msec. ECAP amplitudes can be as large as 1500 μV. Threshold and slope of ECAP growth function are thought to depend, at least to some extent, on the size of the stimulable neural population and the proximity of the stimulating and recording electrodes to those auditory nerve fibers (Miller et al, 1994).
ECAPs were originally measured in adults who used a CI with six intracochlear electrodes that could be accessed directly via a percutaneous connector mounted on the temporal bone (Brown et al, 1990). The primary contribution of this early study was to develop a method of minimizing stimulus artifact in the responses recorded from an intracochlear electrode.
In 1995 the Nucleus CI24M internal device was introduced. This device was equipped with Neural Response Telemetry (NRT) that allowed the intracochlear electrodes to be used both for stimulation and recording purposes. The introduction of this technology made it possible, for the first time, to record ECAPs from individuals who used a CI without a percutaneous connector and without the use of additional recording equipment. Those facts, coupled with the fact that the response was a direct measure of the response of the auditory nerve to electrical stimulation and that it was not adversely affected by muscle artifact, made the ECAP recording a very attractive tool for both clinicians and researchers (Abbas et al, 1999).
The Relationship between ECAP Thresholds and MAP Levels
Following the introduction of CIs with NRT capabilities, many investigative teams began exploring the relationship between ECAP thresholds and the stimulation levels used to program the speech processor. Brown et al (2000) and Hughes et al (2000) initially showed that correlations between ECAP thresholds recorded at low stimulation rates required for measuring the peripheral neural response and MAP (a program for fitting a speech processor) T-level (sound detection threshold) and C-level (maximum comfortable level) recorded at the higher rates used to program the speech processor, while significant, were not strong. However, they proposed a technique to improve the correlation by combining the electrophysiological data with a limited amount of behavioral information. Specifically, they proposed shifting the ECAP threshold versus electrode contour up or down based on a single behavioral measure of threshold or maximum comfort level for one electrode in the intracochlear electrode array.
Other techniques for predicting behavioral levels from ECAP measures were also proposed. For example, Franck and Norton (2001) suggested combining ECAP thresholds with the slope of the ECAP growth function on each electrode to predict C-levels. Smoorenburg et al (2002) assessed the “tilt” of the threshold versus electrode functions. They found a considerably stronger correlation (r = 0.82) between the tilt of the ECAP thresholds and that of T-levels than the correlation between the absolute ECAP thresholds and T-levels (r = 0.64). They proposed using ECAP thresholds to set initial stimulation levels on each electrode before activating the processor in a live voice mode and globally shifting the stimulation profile to approximate T- and C-levels. In addition to simple shifts upward or downward in the ECAP threshold versus electrode function, Smoorenburg also suggested that the tilt and/or curvature of the stimulation profiles could be varied to improve the accuracy with which MAP levels could be predicted.
None of these methods were perfect; moreover, most of the studies reported correlations between NRT measures and the levels used to program the relatively low-rate SPEAK processor that operated at 250 pps (pulses per second)/channel. Over the past decade, the speed of CI speech processors has increased significantly. Today, most CI recipients use speech processing strategies that employ stimulation rates in excess of 900 pps/channel, and some use stimulation rates as high as 3200 pps/channel. As the rate of stimulation used by the speech processors has increased, the strength of the relationship between ECAP thresholds and the behavioral measures of dynamic range has tended to decrease. For example, Franck and Norton (2001) reported finding a correlation of ECAP thresholds and behavioral detection thresholds of 0.76 when both measures were obtained using an 80 Hz pulse train. However, when ECAP thresholds (measured using a stimulation rate of 80 pps) were compared with behavioral thresholds for a 250 Hz pulse train, the correlation coefficient decreased (r = 0.59). In addition, although the ECAP measures generally fall within the individual’s dynamic range for both children and adults, a number of individuals have ECAP thresholds that approximate or exceed their C-levels. And while the ECAP thresholds follow the contour of the behavioral threshold versus electrode function for some users, that is not always the case (Brown et al, 1998, 2000; Hughes et al, 2000; Franck and Norton, 2001; Smoorenburg et al, 2002). Despite these limitations, NRT technology has become an accepted clinical practice and, while not replacing behavioral measures, has proven to provide a reasonable approximation of MAP levels for a child who is too young or otherwise unable to participate actively in the programming process (Seyle and Brown, 2002).
Neural Response Imaging (NRI)
In 2001 Advanced Bionics Corporation introduced the Clarion CII cochlear implant. This was followed several years later with the HiRes 90K cochlear implant. Both devices have 16 independent current sources and are capable of high-rate sequential or simultaneous analog stimulation. The Neural Response Imaging (NRI) software used with the Advanced Bionics CI system operates as a module within the SoundWave programming software and, as a result, rapidly moved from the research bench into clinical practice (Frijns et al, 2002).
While both the NRI and NRT recording systems measure the same underlying neural response (the ECAP), the way they handle contamination of the neural response by stimulus artifact is very different. Cochlear Corporation’s NRT software uses a forward masking technique first described by Brown et al (1990) while the NRI system uses alternating stimulus polarities to help minimize contamination of the neural response by stimulus artifact.
In addition to differences between the neural telemetry systems, the speech processors of the two cochlear implants are also programmed very differently. The speech processor of the Cochlear Corporation device is programmed by setting detection levels (T-levels) and maximum comfort levels (C-levels) for a train of biphasic current pulses presented to individual electrodes at rates that range from 900 to 2400 pps/channel. In contrast, the speech processor of the Advanced Bionics CI is typically programmed by setting the most comfortable levels (M-levels) using a series of pulses that are delivered randomly to a group of three to four electrodes at rates that can be as high as 5200 pps/channel. This type of stimulation has been called a “speech burst” by Advanced Bionics. When the speech burst programming mode is used, both pulse duration and stimulation rate change as the stimulation level is changed. At low stimulation levels, pulse durations as narrow as 10.8 μsec/phase and stimulation rates as high as 2800 pps/channel are produced when HiRes-S (HiResolution—Sequential) processing is selected and 5200 pps/channel when the HiRes-P (HiResolution—Paired) processing strategy is used. Unlike the Cochlear Corporation CI, programming the speech processor of the Advanced Bionics CI does not require the use of T-levels or C-levels. The single metric used to program the Advanced Bionics speech processor is the M-level. While the SoundWave software provides clinicians with the capability of setting T-levels manually, this is not necessary. T-levels are often set to zero or to 10% of measured M-levels for all electrodes. In addition, current levels increase linearly as the programming levels increase while a logarithmic function is used to control increases in current level for Cochlear Corporation CIs.
The differences outlined above are significant and could reasonably be expected to affect the relationship between ECAP thresholds and the behavioral levels required to program the speech processor. Unfortunately, while numerous studies have reported comparisons between ECAP thresholds and MAP levels for Nucleus CI users, relatively few studies have directly explored the relationship between ECAP thresholds recorded using the NRI system and programming levels for individuals who use the Advanced Bionics system.
Han et al (2005) used the NRI software to investigate the correlation between ECAP thresholds and M-levels in nine Clarion CII cochlear implant users. They recorded ECAP thresholds in two ways: first, using visual detection thresholds and, second, by recording an ECAP growth function and then using linear regression analysis to estimate the stimulation level that would result in an ECAP response with a 0 μV amplitude. Advanced Bionics used the abbreviation tNRI to refer to ECAP thresholds established in this way. ECAPs were successfully recorded in 89% of the subjects. Significant correlations were also found between M-levels and ECAP visual detection thresholds (r = 0.74) and tNRI levels (r = 0.68). M-levels were recorded on average 20 Advanced Bionics clinical programming units (CUs) lower than ECAP visual detection thresholds and 12 CUs higher than tNRI levels. Han et al (2005) suggested programming the speech processor of the Advanced Bionics CI by setting M-levels initially at 50% of the ECAP thresholds determined using the visual detection method.
Eisen and Franck (2004) compared tNRI levels with T- and M-levels measured in 16 pediatric CI users who ranged in age from 2.2 to 12.8 yr. These investigators found significant but moderate correlations between tNRI levels and M-levels measured using a “tone burst” stimulus. They reported finding the strongest correlations between tNRI levels and M-levels for the most apical electrodes. The strength of this relationship decreased for more basal stimulating electrodes.
More recently, Wolfe and Kasulis (2008) studied 19 HiRes 90K CI users who ranged in age from 12 to 81 yr. Unlike previous investigations, they reported correlations not between ECAP thresholds and behavioral levels for a tone burst stimulus but between ECAP thresholds and M-levels recorded using a speech burst. Speech bursts are 500 ms biphasic pulse trains, but the individual pulses in the train are randomly strobed across a set of up to four intracochlear electrodes. The advantage of this stimulus over the more traditional tone burst stimulus where the pulses are presented to a single intracochlear electrode is that perceptual loudness estimates will reflect integration across electrodes. Wolfe and Kasulis (2008) reported finding mean ECAP thresholds at approximately 65% of M-levels when stimulating the most apical electrode group (electrodes 1-4) and at and 90% of M-level for the most basal electrode group (electrodes 13–16). The correlation between M-levels and the visual detection thresholds also varied as a function of the stimulating electrode group from approximately 0.9 for the most apical electrode group (electrodes 1–4) to 0.39 for the most basal electrode group (electrodes 13–16). Wolfe and Kasulis (2008) noted the substantial intersubject variability that existed between ECAP thresholds and the M-levels and that these electrophysiological measures should be used only loosely to guide the programming process.
All three studies have limitations. For example, none provided details relative to how the levels used to program the speech processor were obtained. Han et al (2005) simply states that the M-levels were obtained from the program the subject used most frequently in normal listening situations. No information was provided regarding the methods used to determine or define the M-levels. Additionally, the Advanced Bionics CI can be programmed using either a HiRes-S or HiRes-P programming strategy. Using the HiRes-S strategy, all enabled electrodes are stimulated in sequence; a single electrode is stimulated at any given time. Using the HiRes-P strategy, pairs of spatially separated electrodes are stimulated in sequence; two of the enabled electrodes are stimulated at any given time. In the paired strategy, the same number of electrodes can be stimulated in half the time, and a faster stimulation rate can be achieved. Therefore, the HiRes-P strategy uses stimulation rates that are approximately twice as fast as stimulation rates used with the HiRes-S strategy. Previous studies, however, did not specify which coding strategy was used for the behavioral loudness estimates. In addition, comparison of these studies with previously published data obtained from the Nucleus CI users is difficult due to differences between NRI and NRT and in how the speech processors are programmed. Most of the studies using the Advanced Bionics device only report correlations between ECAP thresholds recorded using an alternating stimulus polarity scheme with M-levels, while studies focusing on the Nucleus CI system report ECAP thresholds recorded using a forward masking technique to minimize stimulus artifact with behavioral measures of T- and C-level.
The Goal of This Study
The primary goal of this study was to explore the relationship between ECAP thresholds obtained using the NRI module within the SoundWave programming software and measures of perceptual dynamic range (T-, M-, and C-levels) for the stimuli commonly used to program the speech processor of the Advanced Bionics CI. Based on previous research—primarily conducted using Nucleus CI users—we hypothesize that there will be significant, but modest, positive correlations between ECAP thresholds and behavioral programming levels. A slightly weaker correlation between ECAP thresholds and behavioral levels is anticipated when ECAP thresholds are compared with behavioral dynamic range measures recorded using speech bursts as opposed to tone bursts. It is hoped that results of this study will allow clinicians who work with the Advanced Bionics CI users to more confidently incorporate NRI measures into clinical practice.
METHOD
Subjects
Twelve postlingually deafened adult cochlear implant recipients who ranged in age from 32 to 86 yr (mean = 58.9 yr; SD = 16.5 yr) participated in this study. All twelve subjects were implanted with an Advanced Bionics CII or HiRes 90K cochlear implant at the University of Iowa Hospitals and Clinics between 2001 and 2008. Six subjects used bilateral cochlear implants. For the purposes of this study and due to time constraints, data from only one ear of these bilateral CI users was included. The ear with better ECAP responses or wider dynamic ranges was typically selected. If the two ears were about the same, the test ear was randomly selected.
Table 1 lists pertinent demographic information about the subjects. All 12 subjects had at least 1 yr of experience with their CI prior to participating in this research. All had normal cochlear anatomy and full electrode insertions. Two subjects had two open-circuit electrodes (S3: electrode 2 and 6, S7: electrode 15 and 16). These electrodes were excluded from testing. Four subjects used the HiRes-P speech coding strategy on a regular basis. Five used the HiRes-S speech coding strategy, and the remaining three used the HiRes-S strategy with the Fidelity 120 feature for everyday listening.
Table 1. Demographic Information about the Study Participants.
| Subject Number | Gender | Device | Ear | Age (yr) | Duration of CI use (yr) | Etiology | Strategy |
|---|---|---|---|---|---|---|---|
| 1 | F | Clarion CII | Right | 65.8 | 7.0 | Hereditary | HiRes-S |
| 2 | F | HiRes90K | Left | 70.0 | 4.1 | Hereditary | HiRes-P |
| 3 | F | Clarion CII | Left | 44.0 | 7.0 | Unknown | HiRes-P |
| 4 | M | Clarion CII | Right | 32.5 | 6.7 | Unknown | HiRes-S |
| 5 | F | HiRes90K | Left | 74.2 | 1.1 | Sudden SNHL | HiRes-S F120 |
| 6 | F | HiRes90K | Right | 69.5 | 5.0 | Hereditary/noise | HiRes-S F120 |
| 7 | F | HiRes90K | Left | 56.0 | 3.0 | Toxic shock syndrome | HiRes-P |
| 8 | F | Clarion CII | Left | 45.5 | 7.0 | Unknown | HiRes-P |
| 9 | F | Clarion CII | Left | 86.6 | 6.9 | Hereditary | HiRes-S |
| 10 | F | HiRes90K | Left | 63.0 | 3.2 | Unknown | HiRes-S F120 |
| 11 | M | HiRes90K | Right | 65.0 | 4.1 | Unknown | HiRes-S |
| 12 | F | Clarion CII | Left | 33.5 | 7.8 | Hereditary | HiRes-S |
General Procedure
Both electrophysiological and psychophysical measures were collected in a single session that typically lasted 4–5 hr. All testing was done using a clinic-owned Platinum Speech Processor (PSP) and the SoundWave clinical programming software. Prior to testing, electrode impedance was measured. Then, each of the four stimulating electrodes used in this study (3, 7, 11, and 15) were activated and the stimulation level systematically increased across the subject’s dynamic range. The goal of this initial screening procedure was to note levels where the stimulus approached compliance voltage limits and to insure that stimulation did not result in nonauditory sensations. During the electrophysiological recording session, the subject was asked to sit in a comfortable chair and read, nap, or watch closedcaptioned videos while listening to a series of pulsatile stimuli. During the psychophysical procedures, subjects were asked to listen to a series of stimuli and report the loudness for each stimulus burst using a ten-point loudness ranking scale commonly used for programming the Advanced Bionics cochlear implant.
Electrophysiological Procedure
ECAPs were measured using the SoundWave NRI software. Advanced Bionics CIs have 16 intracochlear electrodes numbered sequentially. Electrode 16 is the electrode located closest to the base of the cochlea. In this study, ECAP thresholds were measured on electrodes 3, 7, 11, and 15. These four stimulation electrodes were chosen because they span the electrode array and include one electrode from each of the four electrode groups typically used when programming the speech processor with speech bursts. Recording electrodes were located two electrodes away from the stimulating electrode in an apical direction. Subject 7 had an open circuit at electrode 15. For this subject, electrode 14 instead of electrode 15 was used for stimulation, and electrode 12 was used to record the response.
The NRI system uses a biphasic pulse stimulus with duration of 32 msec per phase and no interphase gap to record the ECAP. The stimulation rate is 30 pps, and stimuli are presented in a monopolar stimulation mode. In order to minimize contamination of the response by stimulus artifact, the polarity of the stimulus pulse is alternated in sequential presentations. Most of these stimulation and recording parameters cannot be modified by the user within the NRI module of the SoundWave software. In this study, all ECAP recordings were obtained using 128 averages and a gain of 300. Full ECAP growth functions were recorded by systematically increasing the stimulation level in steps of 13 CUs (approximately 32 μA) from an initial level of 50 CUs (approximately 120 μA) to a maximum level of 400 CUs (approximately 963 μA) or until either compliance voltage was reached or the subject expressed some discomfort.
Estimating ECAP Threshold
The NRI software automatically measures peak-to-peak amplitude of the ECAP, plots those values relative to stimulation level, and uses linear regression to estimate ECAP threshold by determining the current level that would result in a response with an amplitude of 0 μV. This stimulation level is tNRI and is the metric typically proposed by Advanced Bionics to be used as a guideline for fitting the speech processor of the CI. This technique works well if the ECAP growth function on which the estimate of ECAP threshold is based is well fit by a straight line and is based on a reasonable number of suprathreshold responses. That is not always possible because voltage compliance becomes an issue for some subjects at higher stimulation levels and other subjects find stimuli that are presented above ECAP threshold to be uncomfortably loud.1 In this study, care was taken to insure that extrapolated ECAP thresholds were based on a growth function that consisted of no fewer than six points, that the ECAP amplitude measures obtained using the automatic peak picking software were appropriate, and that the points used to estimate slope of the ECAP growth function were well fit using a linear model.
An alternative method of establishing ECAP threshold is to record the lowest stimulation level where the ECAP can be visually identified by an experienced observer. Visual detection paradigms are widely used in clinical practice. Visual detection levels would be expected to be higher than tNRI levels because the response is required to exceed the noise floor of the measurement system. The noise floor of the NRI system is approximately 20 μV peak to peak. Additionally, fewer suprathreshold ECAP responses are needed to determine visual detection thresholds than to establish a tNRI level. For comparison purposes, in this study both tNRI and visual detection ECAP thresholds were recorded.
Psychophysical Procedure
The speech processor of the Advanced Bionics CI can be programmed in several different ways. One approach is to stimulate a single channel at a time. This is accomplished by selecting a tone burst stimulus within the programming software. When the tone burst stimulation is selected, 500 msec bursts of biphasic current pulses are applied to a selected individual intracochlear electrode in a monopolar stimulation mode. In this study, when tone bursts were used to measure the perceptual dynamic range, the stimulus pulse duration was fixed manually at 32.3 μsec/phase. This results in a stimulation rate of 967 pps/channel when the HiRes-S programming strategy is selected. The 32.3 μsec/phase pulse duration was selected because that is the pulse duration used for measuring ECAP thresholds with the NRI module of the programming software. The stimulation rate (967 pps/channel) is fast enough to be representative of the stimulation rates used clinically but significantly slower than the pulse rate used when programming levels were obtained using speech bursts.
In this study, perceptual dynamic range measures were obtained using the HiRes-S strategy programmed using the speech burst stimulation mode. A speech burst is a 500 msec biphasic pulse train consisting of individual pulses randomly strobed across a set of up to four intracochlear electrodes. As the stimulation level of the speech burst is increased, the pulse duration and stimulation rate vary automatically. To achieve the fastest possible stimulation rates, the pulse duration is initially very short, 10.8 msec/phase. This results in a rate of 2900 pps/channel. As stimulation level in CUs is increased, the amplitude of the pulse train increases to the system’s limit. When increases in pulse amplitude are no longer possible, the pulse duration is then automatically lengthened. This results in a lower stimulation rate. Further increases in phase duration with the accompanying decrease in rate automatically occur as needed to achieve higher stimulation levels. The point at which pulse durations automatically change will depend on an individual’s electrode impedance values. For an individual with low impedances, a high stimulation level of 500 CUs would be needed to achieve a speech burst with a pulse duration of 32.3 μsec/phase and a stimulation rate of 967 pps/channel.
The differences between tone burst and speech burst stimuli are substantial. However, both are used widely in clinical practice. In the current study, for comparative purposes, behavioral dynamic range measures were obtained using both types of stimuli. When the tone burst stimulus was used, the electrodes tested included 3, 7, 11, and 15. When the speech burst stimulus was used, the stimulation was applied to the following electrode groups: 1–4, 5–8, 9–12, and 13–16.
Psychophysical Dynamic Range Measures
Perceived loudness was estimated using a ten-point rating scale distributed by Advanced Bionics Corporation. This scale is commonly used for programming and defines rankings of 0 as “no sound,” 6 as “most comfortable,” 9 as “upper loudness limit,” and 10 as “too loud.” Subjects were asked to listen to a specific stimulus and assign a rank to that stimulus in terms of perceived loudness. T-levels were measured first using steps of 5 CUs and a modified Hughson-Westlake approach. T-level was defined as the stimulation level ranked by the subject as a 1 on the ten-point scale for two out of the three ascending runs.
Once T-level was established, the stimulus level was systematically increased until the subject indicated that it had become uncomfortably loud. The subject was required to rank each stimulus presented in terms of perceived loudness using the ten-point scale and the whole process was repeated three times. It was common for subjects to assign the same loudness ranking to several different stimulation levels and for the ranking assigned to a specific stimulation level to vary somewhat on the three ascending runs. A step size of 10 CUs was used for the first ascending run, and a step size of 5 CUs was used for the second and third ascending runs. The results of all three runs were combined. M- and C-levels were defined as the median of the stimulation levels that were assigned a rank of 6 or 9 respectively.
RESULTS
Two different approaches for defining ECAP threshold have been described in the literature. One approach is to determine the visual detection threshold for the response. The second approach is to use linear regression techniques to determine the “tNRI” level.
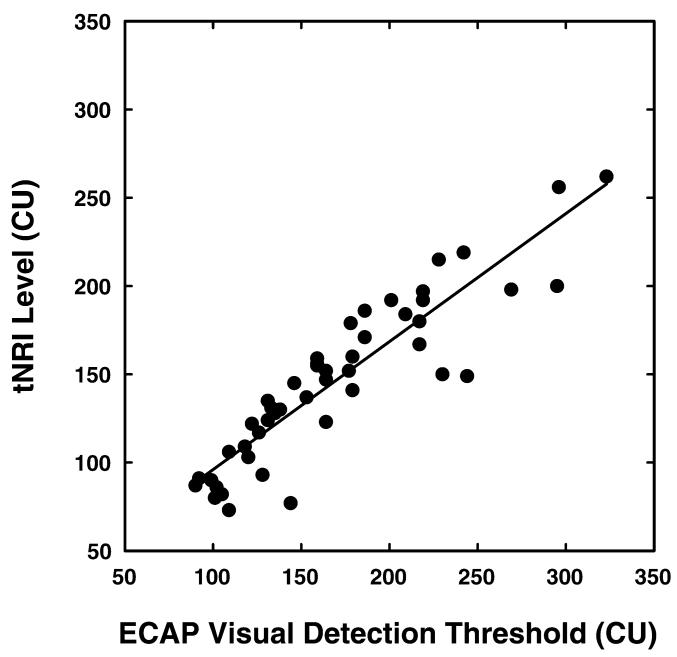
Visual detection thresholds and tNRI levels may not always be equivalent. For comparison purposes, in this study, both measures of threshold were obtained. These results are shown in Figure 1. Visual detection thresholds were estimated independently by two experienced researchers, and interjudge agreement was high (46 of 48 thresholds matched). Visual detection thresholds ranged from 90 to 323 CUs (mean 170 CUs, SD 57.1); tNRI levels ranged from 73 CUs to 262 CUs (mean 147 CUs, SD 45.8). Paired t-test analysis revealed that these two measures were significantly different (t-value: 6.48, p< 0.0001) with visual detection thresholds on average 23 CUs higher than the tNRI levels. However, the two threshold estimation procedures were strongly correlated with each other (r = 0.905, p < 0.0001). Because these two measures of determining ECAP were strongly correlated, all subsequent analyses were based on ECAP thresholds measured using the visual detection method.
Figure 1.
The correlation between ECAP thresholds determined using visual detection techniques and ECAP thresholds obtained using the tNRI procedure. Stimulation level is specified in clinical programming units (CUs).
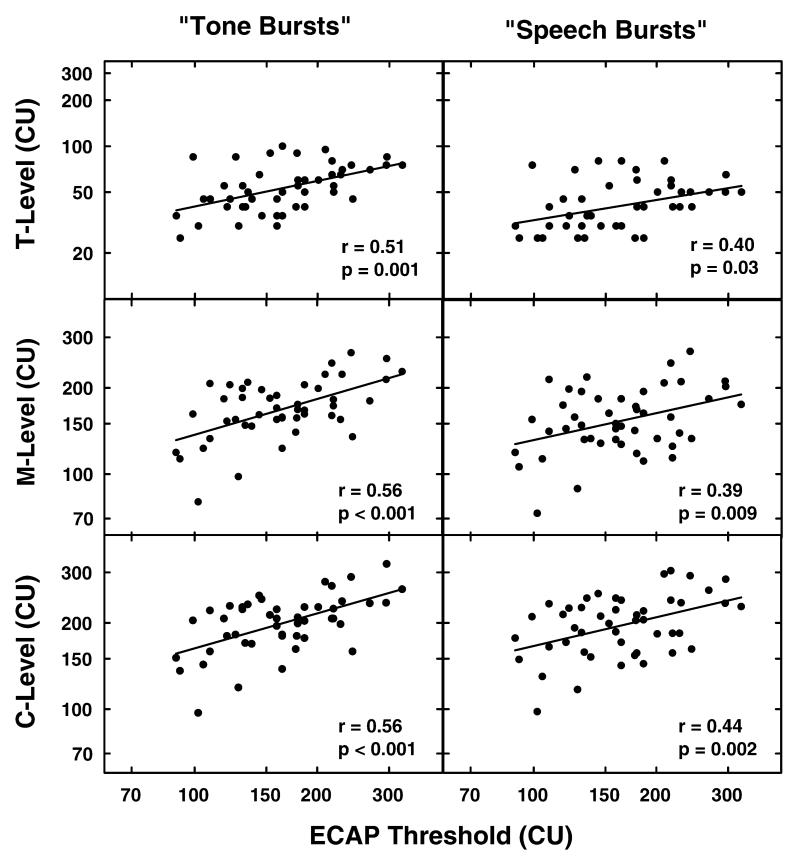
Figure 2 shows the relationship between ECAP thresholds and programming levels obtained using both tone burst and speech burst stimuli. The three graphs in the left column of Figure 2 show the correlation between ECAP thresholds and T-, M-, and C-levels measured using tone bursts. The three graphs in the right column show the relationship between ECAP thresholds and T-, M-, and C-levels as measured using the speech burst. Our primary goal in including this figure is to allow comparison of these results with results published previously using the Nucleus CI. The programming software used with the Nucleus CI uses a scale where current is increased approximately logarithmically. Advanced Bionics software uses a linear scale. In order to facilitate comparisons across devices, the stimulation levels in Figure 2 have been plotted on a log axis. The correlation coefficients, computed using log-log coordinates and shown on the figure, were all significant but relatively modest. Slightly better correlations were found between ECAP thresholds and programming levels obtained using the tone burst as opposed to the speech burst stimulus.
Figure 2.
ECAP thresholds determined using the visual detection paradigm are compared with the behavioral measures of T-, M-, and C-level. In the three graphs on the left, ECAP threshold is compared with T-, M-, and C-levels obtained using a tone burst stimulus. In the three graphs on the right, ECAP threshold is compared with T-, M-, and C-levels obtained using a speech burst stimulus. Stimulation level is specified in clinical programming units (CUs).
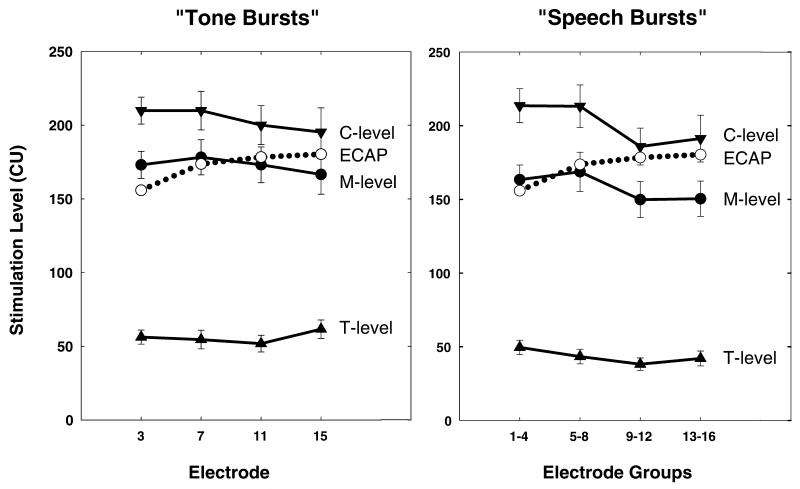
Figure 3 shows average ECAP thresholds plotted relative to loudness estimates obtained using both the tone burst and speech burst stimuli. This figure shows the effect of stimulating electrode on the results. Paired t-tests revealed that T- and M-levels were significantly higher for tone bursts than speech bursts (p < 0.0001). C-levels, however, were not found to be significantly different (p = 0.412). Regardless of the programming mode (tone burst versus speech burst), mean ECAP recordings lie within the subjects’ dynamic range for loudness (i.e., above T- and below C-levels). When tone burst stimuli are used, ECAP thresholds approximate M-levels for the two more apical stimulating electrodes; however, in this average data, the contour of the ECAP threshold versus electrode curve does not follow the contour of the behavioral loudness versus electrode estimates. The discrepancy between the contour of the mean ECAP threshold versus electrode curve and the contour of the mean behavioral loudness versus electrode measure is more apparent when speech burst as opposed to tone burst stimuli are used.
Figure 3.
The graph on the left shows the relationship between mean ECAP thresholds and dynamic range as a function of stimulating electrode when tone burst stimulation was used. The graph on the right shows the same ECAP thresholds plotted relative to the measures of behavioral dynamic range obtained using a speech burst stimulus. Error bars indicate plus and minus one standard error around the mean. ECAP thresholds are shown with open circles and dotted lines. The solid lines and solid symbols are used for the behavioral measures of dynamic range. Stimulation level is specified in clinical programming units (CUs).
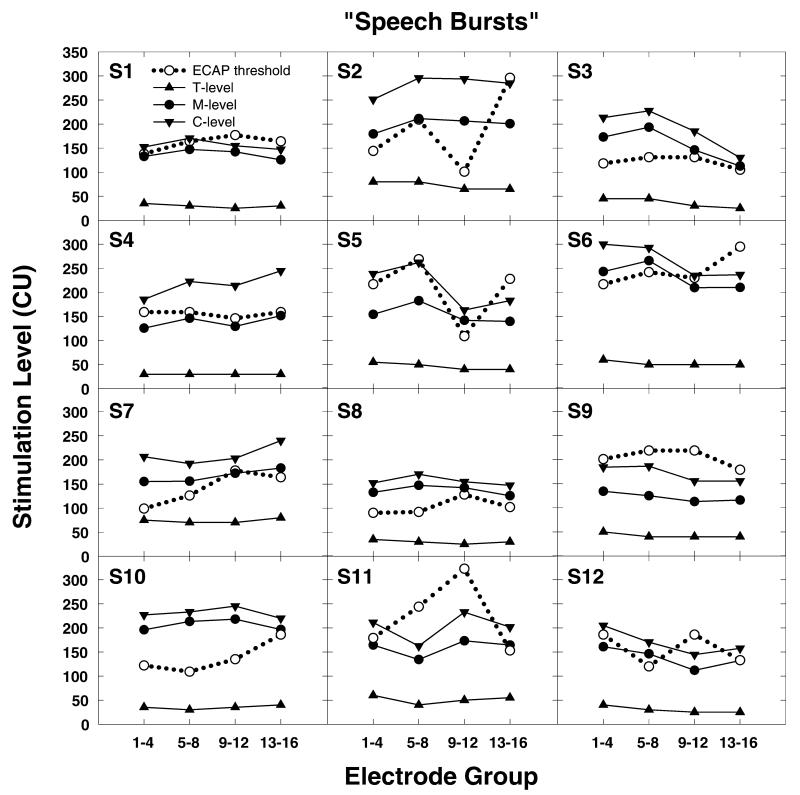
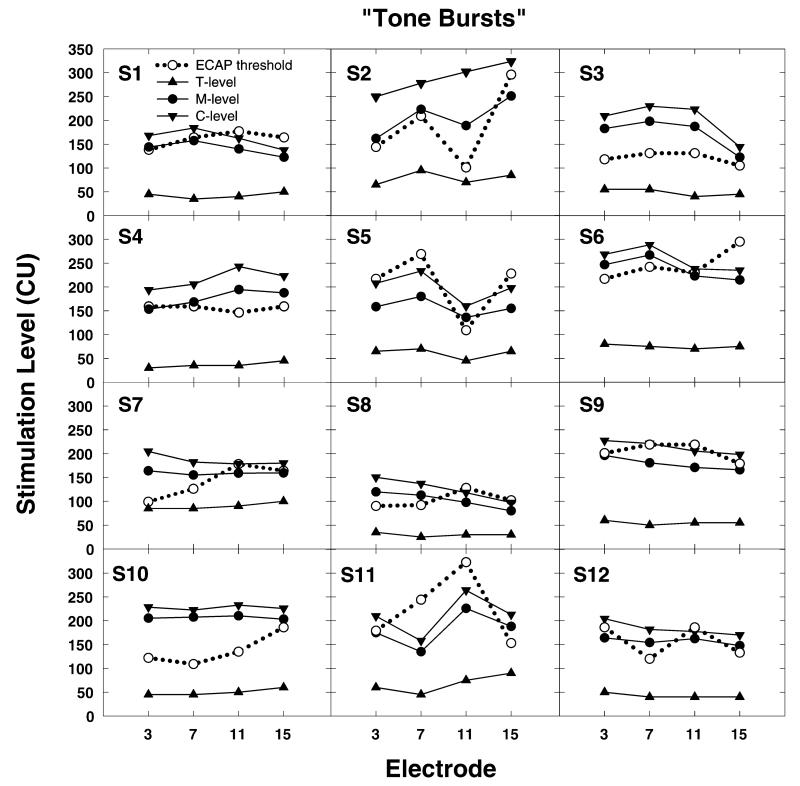
While group data are useful to understand trends, it is also important to examine the variance that can be expected in the individual data. Figure 4 shows results obtained from all 12 subjects using the speech burst stimuli. Figure 5 shows the same results obtained using the tone burst stimuli. As is evident from the group data, ECAP threshold is always obtained at a level where the stimulus used to program the processor of the CI should be clearly audible. However, for several subjects (e.g., S9 and S11), ECAP thresholds not only exceed M-levels but also exceed C-levels. Additionally, the correspondence between the contour of the ECAP threshold versus electrode curve and the equal loudness versus electrode curve is poor for many subjects. Moreover, the relative discrepancy between the ECAP thresholds and behavioral loudness estimates seems to be most apparent for the electrodes in the more basal electrode groups (electrodes 9–12 and 13–16).
Figure 4.
Individual data from all 12 study participants showing the relationship between loudness judgments obtained using a speech burst stimulus and ECAP thresholds. Loudness measures are plotted using filled symbols and solid lines. ECAP thresholds are plotted using open circles and dotted lines. Stimulation level is specified in clinical programming units (CUs).
Figure 5.
Individual data from all 12 study participants showing the relationship between loudness judgments obtained using a tone burst stimulus and ECAP thresholds. Loudness measures are plotted using filled symbols and solid lines. ECAP thresholds are plotted using open circles and dotted lines. Stimulation level is specified in clinical programming units (CUs).
DISCUSSION
The purpose of this study was to explore the relationship between ECAP thresholds obtained using the Neural Response Imaging (NRI) software and measures of perceptual dynamic range for the kinds of stimuli used to program the speech processor of the Advanced Bionics CI. Toward that end, ECAPs were recorded on four electrodes for all 12 study participants. Both visual detection and extrapolation techniques (tNRI) were used to determine ECAP threshold. For each study participant, careful measures of their perceptual dynamic range were then obtained using the HiRes-S programming strategy with both tone burst and speech burst stimuli.
As expected, visual detection thresholds for the ECAP were recorded at stimulation levels that were significantly higher than the tNRI levels (see Figure 1). In this study the mean difference was 23 CUs. Han et al (2005) also measured ECAP thresholds using both visual detection and tNRI methods, and reported visual detection thresholds were, on average, 32 CUs higher than tNRI levels. Our results show that the two threshold measures are highly correlated with each other. Han et al (2005) do not report correlations between visual detection thresholds and tNRI levels, but based on the individual data they show that the two measures appear comparable.
We hypothesized—based on results published primarily from studies with Nucleus CI users—that we would find modest but statistically significant positive correlations between ECAP thresholds and behavioral programming levels. We predicted that these correlations would be stronger when comparing ECAP thresholds and behavioral levels obtained using tone burst as opposed to speech burst stimuli. Figure 2 shows ECAP thresholds plotted relative to behavioral measures of T-, M-, and C-level for both tone burst and speech burst stimuli. As predicted, modest positive correlations between ECAP thresholds and behavioral levels were found. When tone burst stimuli were used, the correlation between ECAP thresholds and behavioral loudness judgments was slightly better than when speech burst stimuli were used. This finding might have been anticipated given the fact that the discrepancy in the stimulation rates and pulse durations used for electrophysiological versus behavioral testing is larger when speech bursts as opposed to tone bursts are used.
The results shown in Figure 2 illustrate the range of variance in the data. Figure 3 shows mean ECAP, T-, M-, and C-levels (±1 SE) plotted as a function of the stimulating electrode. On average, ECAP thresholds are recorded near M-levels but tend to increase as the place of stimulation within the cochlea is moved in an apical to basal direction. This trend—toward higher physiological thresholds as the place of stimulation is moved toward the base of the cochlea—has been observed previously for small groups of Advanced Bionics CI users (Han et al, 2005; Akin et al, 2006; Wolfe and Kasulis, 2008) and for Nucleus CI users (Brown et al, 2000; Hughes et al, 2000). This trend may reflect better neural survival near the apex of the cochlea; alternatively, it may reflect systematic differences in the size of the electrical field that results from stimulation at the two ends of the electrode array. In contrast to the physiological data, average M-levels for this group of postlingually deafened adults tend to decrease as the place of stimulation is moved from the apex toward the base of the cochlea. As a result, ECAP thresholds more closely approximate M-levels for electrodes in the apical region of the intracochlear array and C-levels for electrodes near the base of the cochlea. This trend is particularly apparent when speech burst stimuli are used for programming (see Figure 3).
In clinical research, cross-subject correlations such as those shown in Figures 2 and 3 can reveal general trends but do not adequately reflect how much variance across electrodes might be expected for an individual CI user. Figures 4 and 5 show comparisons between ECAP thresholds and behaviorally based measures of dynamic range obtained using either speech burst (Fig. 4) or tone burst stimuli (Fig. 5). Considerable variance between the ECAP versus stimulating electrode functions and the behavioral measures of dynamic range is evident. Additionally, for several subjects, ECAP thresholds are obtained at stimulation levels that exceed both M- and C-levels on some or all of the electrodes tested (e.g., S1, S5, S9, S11).
To date, only two studies have reported comparisons between ECAP thresholds with the levels used to program the speech processor of individual Advanced Bionics CI users (Eisen and Franck, 2004; Han et al, 2005). Both of these earlier studies show variance in ECAP thresholds across electrodes, and for many individuals, the extent of variance in the ECAP threshold versus electrode functions is greater than the cross electrode variance in M-level. The same trends are apparent in the current study. Neither of the previously published studies report C-levels, but both show examples of subjects for whom ECAP thresholds are recorded at levels that exceed M-level.
The rather substantial amount of cross-electrode variance that is apparent in our data coupled with the observation that that the ECAP thresholds can exceed C-levels, particularly for the more basal electrodes in the array, presents a significant challenge for clinicians who would like to use NRI measures to program the speech processor of the Advanced Bionics CI. While presentation of the programming stimulus at levels sufficient to evoke an ECAP will typically insure audibility, the possibility of overstimulation needs to be considered.
The results shown in Figures 2-5, while somewhat disappointing, are generally comparable to results that have been reported previously for Nucleus CI (Brown et al, 2000; Cullington, 2000; Hughes et al, 2000; Polak et al, 2005; Smoorenburg et al, 2002). ECAP thresholds measured using the NRT system of the Nucleus cochlear implant typically fall within the range of levels used to program the speech processor, but considerable discrepancy has also been reported for Nucleus CI users between the contour of the ECAP versus electrode function and the T- and/or C-level versus electrode functions (Brown et al, 1998, 2000; Cullington, 2000; Franck and Norton, 2001; Hughes et al, 2001; Smoorenburg et al, 2002).
Based on the results of the current study, it appears that if it is not possible to obtain behavioral estimates of M-level, the best option may be to record ECAP thresholds for one or two electrodes in the apical half of the electrode array and then use these values to set M-levels for a speech processor programmed using tone bursts with a duration of 32 μsec/phase and sequential stimulation.
CONCLUSION
ECAP thresholds were measured for all 12 study participants using the NRI system of the Advanced Bionics CI. Like previous studies with Nucleus CI users, modest correlations between ECAP thresholds and behavioral estimates of T-, M-, and C-level were found. However, considerable cross-electrode and cross-subject variability was also apparent in these data. Moreover, the cross- electrode variance in the ECAP threshold data did not correspond well with the cross-electrode variance in the loudness estimates. The best correlation found was between ECAP thresholds and M-levels recorded for electrodes in the apical half of the electrode array. These correlations were strongest when the HiRes-S programming strategy was used with a 32 μsec/phase pulse duration and tone burst rather than speech burst stimulation. This should supplement previously published literature and should provide clinicians with a more complete picture of how the electrophysiological measures compare with behavioral dynamic range measures for individuals who use the Advanced Bionics CI.
Acknowledgments
The authors would like to thank all participants and the University of Iowa Hospitals and Clinics for their aid in acquiring participants for this project.
This work was supported in part by grants from the NIH/NIDCD (P50DC00242), NIH/NCRR (RR00059), and the Iowa Lions Sight and Hearing Foundation.
Abbreviations
- CI
cochlear implant
- C-level
maximum comfortable level
- CU
Advanced Bionics clinical programming unit
- ECAP
electrically evoked compound action potential
- HiRes-P
HiResolution–Paired
- HiRes-S
HiResolution–Sequential
- MAP
program for fitting a speech processor
- M-level
most comfortable level
- NRI
Neural Response Imaging
- NRT
Neural Response Telemetry
- pps
pulses per second
- T-level
sound detection threshold
Footnotes
The maximum voltage output in a current source. Increases in the amplitude of biphasic pulses are not possible once the voltage compliance level has been reached. In such cases, pulse duration must be lengthened in order to increase stimulus charge.
REFERENCES
- Abbas PJ, Brown CJ, Shallop JK, et al. Summary of results using the nucleus CI24M implant to record the electrically evoked compound action potential. Ear Hear. 1999;20(1):45–59. doi: 10.1097/00003446-199902000-00005. [DOI] [PubMed] [Google Scholar]
- Akin I, Kuran G, Saka C, Vural M. Preliminary results on correlation between neural response imaging and ‘most comfortable levels’ in cochlear implantation. J Laryngol Otol. 2006;120(4):261–265. doi: 10.1017/S0022215106000442. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, Gantz B. Electrically evoked whole-nerve action potentials: data from human cochlear implant users. J Acoust Soc Am. 1990;88(3):1385–1391. doi: 10.1121/1.399716. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, Gantz BJ. Preliminary experience with neural response telemetry in the nucleus CI24M cochlear implant. Am J Otol. 1998;19(3):320–327. [PubMed] [Google Scholar]
- Brown CJ, Hughes ML, Luk B, Abbas PJ, Wolaver A, Gervais J. The relationship between EAP and EABR thresholds and levels used to program the nucleus 24 speech processor: data from adults. Ear Hear. 2000;21(2):151–163. doi: 10.1097/00003446-200004000-00009. [DOI] [PubMed] [Google Scholar]
- Cullington H. Preliminary neural response telemetry results. Br J Audiol. 2000;34(3):131–140. doi: 10.3109/03005364000000123. [DOI] [PubMed] [Google Scholar]
- Eisen MD, Franck KH. Electrically evoked compound action potential amplitude growth functions and HiResolution programming levels in pediatric CII implant subjects. Ear Hear. 2004;25(6):528–538. doi: 10.1097/00003446-200412000-00002. [DOI] [PubMed] [Google Scholar]
- Franck KH, Norton SJ. Estimation of psychophysical levels using the electrically evoked compound action potential measured with the neural response telemetry capabilities of Cochlear Corporation’s CI24M device. Ear Hear. 2001;22(4):289–299. doi: 10.1097/00003446-200108000-00004. [DOI] [PubMed] [Google Scholar]
- Frijns JH, Briaire JJ, de Laat JA, Grote JJ. Initial evaluation of the Clarion CII cochlear implant: speech perception and neural response imaging. Ear Hear. 2002;23(3):184–197. doi: 10.1097/00003446-200206000-00003. [DOI] [PubMed] [Google Scholar]
- Han DM, Chen XQ, Zhao XT, et al. Comparisons between neural response imaging thresholds, electrically evoked auditory reflex thresholds and most comfortable loudness levels in CII bionic ear users with HiResolution sound processing strategies. Acta Otolaryngol. 2005;125(7):732–735. doi: 10.1080/00016480510026890. [DOI] [PubMed] [Google Scholar]
- Hughes ML, Brown CJ, Abbas PJ, Wolaver AA, Gervais JP. Comparison of EAP thresholds with MAP levels in the nucleus 24 cochlear implant: data from children. Ear Hear. 2000;21(2):164–174. doi: 10.1097/00003446-200004000-00010. [DOI] [PubMed] [Google Scholar]
- Hughes ML, Vander Werff KR, Brown CJ, et al. A longitudinal study of electrode impedance, the electrically evoked compound action potential, and behavioral measures in nucleus 24 cochlear implant users. Ear Hear. 2001;22(6):471–486. doi: 10.1097/00003446-200112000-00004. [DOI] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Robinson BK. The use of long-duration current pulses to assess nerve survival. Hear Res. 1994;78(1):11–26. doi: 10.1016/0378-5955(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Polak M, Hodges A, Balkany T. ECAP, ESR and subjective levels for two different nucleus 24 electrode arrays. Otol Neurotol. 2005;26(4):639–645. doi: 10.1097/01.mao.0000178145.14010.25. [DOI] [PubMed] [Google Scholar]
- Smoorenburg GF, Willeboer C, van Dijk JE. Speech perception in nucleus CI24M cochlear implant users with processor settings based on electrically evoked compound action potential thresholds. Audiol Neurootol. 2002;7(6):335–347. doi: 10.1159/000066154. [DOI] [PubMed] [Google Scholar]
- Seyle K, Brown CJ. Speech perception using maps based on neural response telemetry measures. Ear Hear. 2002;23(Suppl.):72S–79S. doi: 10.1097/00003446-200202001-00009. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Kasulis H. Relationships among objective measures and speech perception in adult users of the HiResolution Bionic Ear. Cochlear Implants Int. 2008;9(2):70–81. doi: 10.1179/cim.2008.9.2.70. [DOI] [PubMed] [Google Scholar]