Abstract
Background:
Patients with acute neurologic symptoms may have other causes simulating ischemic stroke, called stroke mimics (SM), but they may also have averted strokes that do not appear as infarcts on neuroimaging, which we call neuroimaging-negative cerebral ischemia (NNCI). We determined the safety and outcome of IV thrombolysis within 3 hours of symptom onset in patients with SM and NNCI.
Methods:
Patients treated with IV tissue plasminogen activator (tPA) within 3 hours of symptom onset were identified from our stroke registry from June 2004 to October 2008. We collected admission NIH Stroke Scale (NIHSS) score, modified Rankin score (mRS), length of stay (LOS), symptomatic intracerebral hemorrhage (sICH), and discharge diagnosis.
Results:
Among 512 treated patients, 21% were found not to have an infarct on follow-up imaging. In the SM group (14%), average age was 55 years, median admission NIHSS was 7, median discharge NIHSS was 0, median LOS was 3 days, and there were no instances of sICH. The most common etiologies were seizure, complicated migraine, and conversion disorder. In the NNCI group (7%), average age was 61 years, median admission NIHSS was 7, median discharge NIHSS was 0, median LOS was 3 days, and there were no instances of sICH. Nearly all SM (87%) and NNCI (91%) patients were functionally independent on discharge (mRS 0–1).
Conclusions:
Our data support the safety of administering IV tissue plasminogen activator to patients with suspected acute cerebral ischemia within 3 hours of symptom onset, even when the diagnosis ultimately is found not to be stroke or imaging does not show an infarct.
GLOSSARY
- AIS
= acute ischemic stroke;
- CI
= confidence interval;
- DWI
= diffusion-weighted imaging;
- ED
= emergency department;
- LOS
= length of stay;
- mRS
= modified Rankin score;
- NIHSS
= NIH Stroke Scale;
- NNCI
= neuroimaging-negative cerebral ischemia;
- OR
= odds ratio;
- sICH
= symptomatic intracerebral hemorrhage;
- SM
= stroke mimics;
- tPA
= tissue plasminogen activator.
Although there is worldwide consensus among disease experts and independent regulators regarding the utility of IV tissue plasminogen activator (tPA) for acute ischemic stroke (AIS), there is concern about administering IV tPA to patients who present with clinical features suggestive of AIS but have an alternative diagnosis.1 AIS causes neurologic deficits that commonly occur in other disorders2 for which tPA has no benefit and may carry an increased risk for hemorrhage. When patients present to the emergency department (ED) with acute neurologic deficits, time is important to make a rapid evaluation if IV tPA is being considered.3 The short time window of 3 hours from symptom onset to administer tPA (per Food and Drug Administration label) may not allow physicians to make a correct diagnosis. A quick history and neurologic examination, often with the NIH Stroke Scale (NIHSS), along with a CT scan to rule out hemorrhage, comprise the main components of the evaluation. Such an approach may miss several other disorders that mimic stroke or cannot reliably differentiate conditions that masquerade as stroke, such as seizure, complicated migraine, or functional deficits. Does specificity matter in patients presenting with suspected stroke once a brain hemorrhage has been ruled out? Many would argue that it is wrong to give a treatment that is potentially dangerous to patients who do not have the target disease.4 Vascular imaging with CT angiography, magnetic resonance angiography, or transcranial Doppler ultrasound can visualize large artery occlusions and diffusion-weighted imaging (DWI) is highly sensitive to detect ischemic stroke, but these modalities are not universally available at primary and community hospitals.
In the absence of additional imaging, it is estimated from studies that 3%–7% of patients treated with IV tPA for assumed acute cerebral ischemia have a stroke mimic (SM).5,6 However, these studies collectively reported 13 patients with SMs who were treated with tPA. No large study has addressed the question of safety of thrombolysis in patients with SMs or in patients who did not have an infarct on MRI and were not found to have another diagnosis aside from stroke (i.e., averted stroke). Herein, we report a retrospective study of over 100 patients who presented to our ED with symptoms concerning for acute cerebral ischemia but who ultimately were found to have an SM or who had no supporting evidence for stroke on subsequent imaging and were discharged with an averted stroke, which we call neuroimaging-negative cerebral ischemia.
METHODS
IV tPA protocol.
The UT Stroke Program follows established guidelines to administer IV tPA to suspected acute stroke patients.7 All patients who present to our ED with suspected acute cerebral ischemia are evaluated by a member of our stroke team. All patients undergo a rapid neurologic evaluation with the NIHSS and a cranial CT scan to rule out hemorrhage. Patients who, in the judgment of the treating physician, may have acute cerebral ischemia within 3 hours of symptom onset, even when other diagnoses are being considered, are treated with IV tPA as soon as possible after presentation to the ED. Our protocol follows from the National Institute of Neurological Disorders and Stroke trial,7 in which eligibility is based on whether the patient has measurable deficits on the NIHSS scale. Any patient with a disabling deficit including mild hemiparesis, hemianopsia, or aphasia is considered for IV tPA. All treated patients are monitored either in our stroke unit with continuous cardiac monitoring or the neurointensive care unit for at least 24 hours after initiation of thrombolysis. An MRI or CT scan is routinely obtained within 24 hours after treatment.
Study design.
We conducted a retrospective study of patients derived from our prospective stroke registry. We determined the safety and outcome of IV thrombolysis within 3 hours of symptom onset in patients who presented to our ED and were found, during hospitalization, not to have an ischemic stroke. Patients treated with full-dose, 0.9 mg/kg, IV tPA within 3 hours of symptom onset were identified from June 2004 to October 2008. We collected NIHSS on admission and discharge, discharge modified Rankin Scale (mRS) score, length of stay (LOS), past medical history, and symptomatic intracerebral hemorrhage (sICH), as defined in the National Institute of Neurological Disorders and Stroke trial,7 and discharge diagnosis. Initial and follow-up neuroimaging with CT and/or MRI were performed as a part of our routine stroke evaluation. All patients who met inclusion for this study had undergone neuroimaging initially with CT upon presentation, multimodal MRI (DWI, magnetic resonance angiography, fluid-attenuated inversion recovery, and gradient echo imaging) within 24 hours of presentation, and then CT or multimodal imaging before hospital discharge. Diagnosis of SM was based on the absence of acute ischemia/infarct on pre and post IV tPA treatment neuroimaging (<24 hours from the time of onset) and follow-up neuroimaging (>24 hours from the time of onset) in addition to an alternate discharge diagnosis. Diagnosis of neuroimaging-negative cerebral ischemia (NNCI) was based on presentation of acute cerebral ischemia in the ED, the absence of acute ischemia/infarct on pre and post IV tPA treatment neuroimaging (<24 hours from the time of onset), and follow-up posttreatment neuroimaging (>24 hours from the time of onset).
Standard protocol approvals, registrations, and patient consents.
This project was approved by the University of Texas– Houston Health Science Center Institutional Review Board.
Statistics.
Statistical analyses were performed using SPSS 15.0 (SPSS Inc, Chicago, IL). Continuous variables were reported as mean ± SD when the distribution was normal and median with range for non-normal distributions. Categorical variables were analyzed using χ2, Fisher exact test, or analysis of variance where appropriate.
RESULTS
Over the study period, we treated 512 suspected stroke patients with full dose IV tPA (0.9 mg/kg). A total of 106 (21%) were not found to have an infarct on posttreatment DWI obtained within 1 day of hospitalization and subsequent posttreatment CT or MRI performed 24 hours after admission. Among the 106 patients, 69 had an SM (14% of the 512 patients) and 37 (7% of the 512 patients) had NNCI. Twelve patients were excluded from analysis because they did not undergo follow-up posttreatment neuroimaging. Follow-up MRI after 24 hours was done in 64% of patients, follow-up CT was done in 79%, and both a follow-up MRI and CT was done in 44% of patients.
Demographics of SM and NNCI patients.
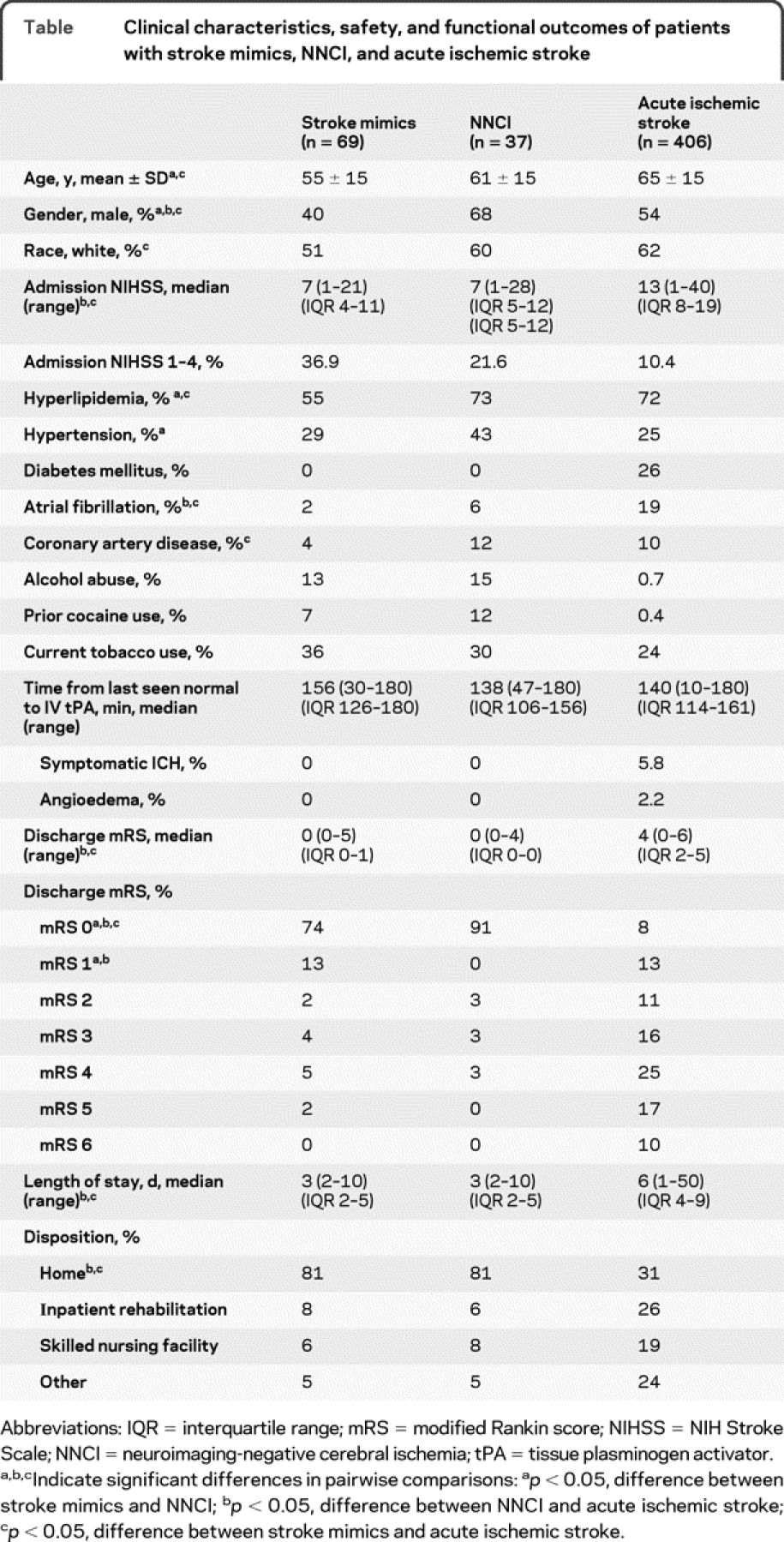
Demographics of SM and NNCI patients are detailed in the table. In the SM group (n = 69), the average age was 55 ± 15 years, 60% were female, and 51% were white. The median NIHSS on admission was 7. In the NNCI group (n = 37), the average age was 61 ± 15 years, 32% were female, and 60% were white. The median NIHSS on admission was 7. There were no significant differences between SM and NNCI groups except there were higher rates of hypertension and hyperlipidemia in the NNCI group and higher rates of CAD in the SM group. In comparison with the SM group, patients with AIS were older (65 ± 15 years) and had higher admission NIHSS on admission (median 13) and higher rates of hyperlipidemia, atrial fibrillation, and coronary artery disease. In comparison with the NNCI group, AIS patients had higher admission NIHSS scores and higher rates of hyperlipidemia and atrial fibrillation. There was a higher percentage of patients with NIHSS 1–4 in the SM group compared to the other groups. In fact, when compared to AIS patients, SMs were 5 times more likely to have an NIHSS of 1–4 (odds ratio [OR] 5.1, 95% confidence interval [CI] 2.8–9.2, p < 0.0001). None of the SM or NNCI patients presented with pure sensory symptoms.
Table Clinical characteristics, safety, and functional outcomes of patients with stroke mimics, NNCI, and acute ischemic stroke
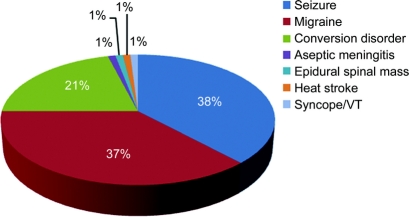
Etiologies of SM.
Etiologies of SM are shown in the figure. The most common etiologies in the SM group were seizure (38%), complicated migraine (37%), and conversion disorder (21%). Case vignettes for each of these conditions are provided in appendix e-1 on the Neurology® Web site at www.neurology.org. In those patients diagnosed with a seizure, 2 patients presented with a history of motor activity before IV tPA administration and the rest of the patients had, during hospitalization, witnessed motor activity consistent with a seizure, an EEG showing epileptiform features explaining the presenting symptoms, or symptoms that resolved with IV Ativan but not tPA. There were single cases of aseptic meningitis, heatstroke, cardiac syncope due to arrhythmia, and spinal epidural mass.
Figure Etiologies of stroke mimics
Etiologies of NNCI.
The presumptive diagnosis was either a TIA or partial/complete recanalization and reperfusion after tPA. Based on the TOAST classification, etiologies were cardioembolic (3.3%), large artery (6.7%), small artery (8.3%), cryptogenic (30%), and other (51.7%). The other category included NNCI patients who had cocaine detected on a positive urinary drug screening, leading to a presumptive clinical diagnosis of cocaine-associated vasospasm.
Safety outcomes.
None of the patients with SM or NNCI had a systemic hemorrhage, intracerebral hemorrhage, or angioedema (table). One patient was found to have an epidural cervical spinal mass after tPA infusion was completed. The patient was taken for surgical decompression of the mass, which was found to be a tumor that had showed signs of hemorrhage. In comparison, patients with AIS had a 6% incidence of sICH and 2% incidence of angioedema.
Functional outcomes.
SM and NNCI patients had a median NIHSS on discharge of 0 and the median LOS was 3 days (table). The majority were functionally independent on discharge (mRS 0–1) and the median discharge mRS was 0 in both groups. A total of 81% were discharged home and the remaining patients had baseline neurologic deficits or functional weakness for which a neuroanatomic etiology could not be found. They were transferred to other services: inpatient rehabilitation, skilled nursing facility, or an inpatient medical floor. In comparison, patients with AIS had a median 6-day LOS and 21% were functionally independent on discharge (mRS 0–1).
Excluded patients.
We also analyzed the 12 patients excluded from this study and found an average age of 48 ± 13 years, median admission NIHSS 9, no instances of sICH, and median discharge mRS 0. There were no significant differences in stroke severity or other baseline characteristics compared with the SM or NNCI group.
DISCUSSION
It is of paramount importance to study the safety of IV tPA in patients with suspected acute cerebral ischemia who are ultimately found on hospital workup not to have a stroke. Within 3 hours after the onset of symptoms, it may be difficult to determine whether acute neurologic signs are due to stroke or to another cause. ED physicians are uncomfortable differentiating a hemiplegic migraine, for example, from stroke.4 The concern is that exposing patients who do not have ischemic stroke to an added risk of hemorrhage with tPA is inappropriate.4
Over a 4-year period, we discovered that 21% of our patients who had presented to our ED with symptoms suggestive of acute cerebral ischemia and had received Food and Drug Administration– approved IV tPA did not have a confirmed infarct on subsequent DWI. Among all treated patients, the incidence of SM was 14% and NNCI was 7%. Our stroke center has a wide referral base and a high volume of hyperacute patients with neurologic problems requiring emergency stroke workup to consider administration of standard of care IV tPA. The incidence of SM in our patient population is higher than other reported studies, which might reflect a greater number of patients referred and evaluated for thrombolysis, and possibly a lower threshold to treat with IV tPA at our center. Our treatment approach is to consider any patient for thrombolysis with a measurable deficit that is disabling such as a mild hemiparesis. In addition, we used serial monitoring of patients with imaging as part of the definition of SM and NNCI groups. All SM and NNCI patients had follow-up posttreatment neuroimaging. Previous studies did not consistently use posttreatment imaging as a selection criterion for SM.5,6,8 Our method to define SM may be a limiting factor to determine the actual SM rate among hospitals without primary stroke centers.
To our knowledge, this is the largest reported series of patients with SM treated with tPA. In contrast to patients with AIS, they were younger, were more often women, and had significantly lower admission NIHSS scores. SM patients had a more favorable functional status and disposition at discharge compared with AIS patients. Other studies with a smaller sample number have not found demographic differences between AIS and SM patients.5 SM patients were diagnosed with a variety of territorial syndromes: right and left MCA, posterior circulation, and subcortical presentations. A prior study of 7 treated SM patients found mainly left MCA symptoms. Consistent with prior studies,5,6,8 the main SM etiologies were seizures, migraine, and conversion disorder. However, we also treated patients with tPA who were subsequently diagnosed with aseptic meningitis, a cervical epidural mass, and cardiac syncope.
Neuroimaging before and after treatment in the absence of an alternative diagnosis other than acute cerebral ischemia allowed us to distinguish an NNCI group from the SM group. Rapid, sometimes dramatic recovery from stroke-like syndromes within 24 hours after IV thrombolysis has previously been reported.6,9 As per the WHO criteria, acute focal deficits resolving after treatment might be described as TIAs. Such patients may have recanalized rapidly after tPA before imaging could show damage. These patients have also been described as having an averted stroke.10 Our findings are in agreement with a recent study showing that tPA was safe to administer in 23 patients with suspected cerebral ischemia who were found not to have an infarct and were diagnosed with TIA.10 Alternatively, small strokes may not be detected on neuroimaging, including DWI. False negative DWI studies have been described in acute ischemic stroke within the first 24 hours of symptom onset in which follow-up studies confirmed the “missing” infarct.11 However, in our dataset, all patients had imaging not only before treatment but at least 24 hours after treatment. Given the absence of an infarct and that no diagnosis other than averted stroke could be found, we believed this condition merited its own category separate from SM. Pretreatment vascular studies and perfusion imaging would have been helpful to better define if NNCI patients had evidence for ischemia or evidence for recanalization after thrombolysis. It is also possible that some patients in this group had a nonischemic etiology that was not found during hospitalization.
Despite finding that we gave IV tPA to 14% of patients with suspected acute cerebral ischemia who were ultimately diagnosed with an SM, we found no instances of symptomatic or systemic hemorrhage despite a range of nonstroke diagnoses. It may not be surprising to have found no instances of sICH in these patients since they did not have a stroke. Patients treated with IV tPA for acute myocardial infarction have less than 1% incidence of intracerebral hemorrhage, and the tPA dose for stroke is lower.12 The patient who presented with a cervical epidural mass and acute focal deficits required surgery, during which it was found that the mass had bled after tPA. However, we believe the patient's symptoms did not worsen because of the hemorrhage. Most patients with SM and NNCI had shorter hospital stays, significant improvement on the NIHSS score, and excellent functional outcomes.
This study has important limitations including its retrospective design and single center experience from a comprehensive stroke center. Therefore, extrapolating the results to community hospitals with lower volumes of stroke patients must be done with caution. The small sample size may possibly introduce sample and selection bias. In the National Institute of Neurological Disorders and Stroke trial,7 the rate of ICH in the treated group was 6.41% (95% CI 3.69%–9.13%). Thus, the margin of error with a 95% CI was 2.72%. The sample size needed to obtain this rate is 312. Thus, while our sample of 405 AIS patients is adequate to observe the same rate of ICH as in the National Institute of Neurological Disorders and Stroke trial with at least 95% confidence, the sample size in our study of SM and NNCI patients was not powered to detect such a difference. Thus, this retrospective study is vulnerable to type II error. Certain patients were excluded from our analysis if they were unable to undergo MRI secondary to large body size or presence of implants. While our study suggests that the safety risk is low in patients who later turn out to have an SM, our data should not be used to excuse a less than thorough evaluation. All patients enrolled in this study and treated with tPA met published Food and Drug Administration and American Heart Association guidelines for treatment, and we do not advocate that these criteria should be shortcut. However, even if these criteria are followed, the diagnosis of stroke will remain based on clinical judgment. Our data would support that if the clinician thinks that the patient may be experiencing a stroke, and the patient meets accepted guidelines, the patient should be treated.
Overall, our data together with others5,6,10 support administering IV tPA to patients presenting in the 3-hour window with suspected AIS, even when alternate etiologies are being considered. Taken collectively, the preponderance of data from this study and the literature suggests that delaying IV tPA administration in the ED in order to obtain further investigations beyond cranial CT in the form of imaging or EEG may not be warranted, even if the diagnosis ultimately is not AIS. However, some centers are able to rapidly obtain additional testing such as CT angiography with CT or even an MRI in the ED setting. Because the risk of emergently treating SMs such as migraine are low, and there is loss of potential benefit in withholding treatment if the patient is indeed having a stroke, we favor IV tPA treatment if the patient meets Food and Drug Administration and American Heart Association criteria for thrombolysis. Subsequent studies after tPA initiation can be performed to better clarify the etiology in cases where the diagnosis is uncertain. Further prospective investigations are needed to establish the safety of thrombolysis in SM and NNCI.
DISCLOSURE
Dr. Chernyshev, Dr. Martin-Schild, Dr. Albright, Dr. Barreto, Dr. Misra, and Dr. Acosta report no disclosures. Dr. Grotta serves on a scientific advisory board for Lundbeck, Inc.; serves on the editorial board of the International Journal of Stroke; holds US Patents 6,500,834, 6,503,915, and 6,503,916 (issued: 1/7/03): A Composition and Method for Treatment of Cerebral Ischemia and received a license fee payment from InnerCool technology; receives royalties from the publication of Acute Stroke Care: A Manual from the University of Texas–Houston Stroke Team (Cambridge, 2007) and Stroke: Pathophysiology, Diagnosis, and Management (Churchill Livingstone, 2004); has received speaker honoraria for lectures not sponsored by industry; and receives research support from the NIH/NINDS (P50 NS 044227 [PI], R01 NS052971 [Co-I], P01 NS046588-02 [Co-I], and T32-NS0074212-11 [PI]) and from the Burnett Family Stroke Fund, and from the Harold Farb Research Fund. Dr. Savitz serves on a scientific advisory board for Grupo Ferrer Internacional S.A.; has received travel expenses and/or honoraria for lectures or educational activities not funded by industry; serves as an Associate Editor of Experimental and Translational Stroke Medicine; has received honoraria from Johnson & Johnson; and receives research support from Athersys, the NIH R21NS064316 (PI) and R21 HD060978 (PI), the American Heart Association, and the Howard Hughes Medical Institute.
Supplementary Material
Address correspondence and reprint requests to Dr. Sean I. Savitz, Department of Neurology, University of Texas Medical School at Houston, Houston, TX 77030
Editorial, page 1336
Supplemental data at www.neurology.org
e-Pub ahead of print on March 24, 2010, at www.neurology.org.
Study funding: Supported by National Institutes of Health (NIH) training grant T32NS04712 and P50 NS044227 and American Heart Association 0475008N.
Disclosure: Author disclosures are provided at the end of the article.
Received August 11, 2009. Accepted in final form December 10, 2009.
REFERENCES
- 1.D'Addesio JP. Should thrombolytic therapy be the first-line treatment for acute ischemic stroke? N Engl J Med 1998;338:762; author reply 762–763. [PubMed]
- 2.Hemmen TM, Meyer BC, McClean TL, Lyden PD. Identification of nonischemic stroke mimics among 411 code strokes at the University of California, San Diego, Stroke Center. J Stroke Cerebrovasc Dis 2008;17:23–25. [DOI] [PubMed] [Google Scholar]
- 3.Saver JL. Time is brain: quantified. Stroke 2006;37:263–266. [DOI] [PubMed] [Google Scholar]
- 4.Caplan LR, Mohr JP, Kistler JP, Koroshetz W. Should thrombolytic therapy be the first-line treatment for acute ischemic stroke? Thrombolysis: not a panacea for ischemic stroke. N Engl J Med 1997;337:1309–1310; discussion 1313. [DOI] [PubMed]
- 5.Winkler DT, Fluri F, Fuhr P, et al. Thrombolysis in stroke mimics: frequency, clinical characteristics, and outcome. Stroke 2009;40:1522–1525. [DOI] [PubMed] [Google Scholar]
- 6.Scott PA, Silbergleit R. Misdiagnosis of stroke in tissue plasminogen activator-treated patients: characteristics and outcomes. Ann Emerg Med 2003;42:611–618. [DOI] [PubMed] [Google Scholar]
- 7.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 8.Vroomen PC, Buddingh MK, Luijckx GJ, De Keyser J. The incidence of stroke mimics among stroke department admissions in relation to age group. J Stroke Cerebrovasc Dis 2008;17:418–422. [DOI] [PubMed] [Google Scholar]
- 9.Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med 2004;351:2170–2178. [DOI] [PubMed] [Google Scholar]
- 10.Uchino K, Massaro L, Hammer MD. Transient ischemic attack after tissue plasminogen activator: aborted stroke or unnecessary stroke therapy? Cerebrovasc Dis 2009;29: 57–61. [DOI] [PubMed] [Google Scholar]
- 11.Oppenheim C, Stanescu R, Dormont D, et al. False-negative diffusion-weighted MR findings in acute ischemic stroke. AJNR Am J Neuroradiol 2000;21:1434–1440. [PMC free article] [PubMed] [Google Scholar]
- 12.The GUSTO investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993;329:673–682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.