Abstract
Background:
Cerebral amyloid angiopathy (CAA) typically presents with lobar intracerebral macrohemorrhages (ICH) or microbleeds (MBs). Several case reports also found superficial siderosis (SS) in patients with CAA. We aimed to assess the value of SS for the in vivo diagnosis of CAA, and tested whether the inclusion of SS as a criterion alters the sensitivity and specificity of the Boston criteria for CAA-related hemorrhage.
Methods:
We retrospectively analyzed the T2*-weighted MRIs of 38 patients with histopathologically proven CAA and of 22 control patients with histopathologically proven non-CAA ICHs regarding the presence of ICHs, MBs, and SS. We compared the sensitivity and specificity of the classic Boston criteria to that of modified criteria, which included SS as a criterion.
Results:
ICHs were present in 71% of the patients with CAA, and in all control patients. MBs were found in 47.4% of patients with CAA and in 22.7% of controls. SS was detected in 60.5% of patients with CAA, but in none of the controls. The classic criteria had a sensitivity of 89.5% for CAA-related hemorrhage, while inclusion of SS increased their sensitivity to 94.7% (not significant). On the contrary, the specificity of the Boston criteria was 81.2% both for the classic and for the modified criteria.
Conclusions:
Superficial siderosis (SS) occurs with high prevalence in cerebral amyloid angiopathy (CAA) and is rare in non-CAA forms of intracerebral hemorrhages. Thus, we propose that inclusion of SS in the Boston criteria might enhance their sensitivity for CAA-related hemorrhage without loss of specificity.
GLOSSARY
- CAA
= cerebral amyloid angiopathy;
- ICH
= intracerebral macrohemorrhages;
- MB
= microbleed;
- PDw
= proton density–weighted;
- SAH
= subarachnoid hemorrhage;
- SS
= superficial siderosis;
- T2*w
= T2*-weighted.
Cerebral amyloid angiopathy (CAA) is defined as the deposition of β-amyloid in the walls of the cortical and leptomeningeal vessels. Intracerebral macrohemorrhages (ICH) are the most important clinical presentation, and cerebral microbleeds (MBs) are a common magnetic resonance sign of CAA.1
The Boston criteria for CAA-related hemorrhage can be used to attribute an ICH with increasing certainty to CAA by using clinical data, imaging signs, and—if available—histopathologic findings. The definite diagnosis requires a full postmortem examination.2
Recently, superficial siderosis (SS) has been described as a potential magnetic resonance marker of CAA,3–5 but systematic studies on the value of this sign are lacking. Thus, the aims of our study were to assess the prevalence of SS in histologically proven CAA and non-CAA forms of ICH, and to test whether the addition of SS as a criterion alters the sensitivity and specificity of the Boston criteria.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study was approved by our institutional ethical standard committees on human experimentation, and written informed consent for research was obtained from all patients participating in the study or from their guardians.
Study population and patient recruitment.
We included 2 patient groups: 1) patients with a histologically proven CAA and 2) patients with non-CAA forms of ICH, in which CAA was excluded by tissue examination (control group). Patients were retrospectively identified by electronic database searches for consecutive patients with histologically proven CAA and for patients with histologically proven non-CAA forms of ICH (figure 1). The inclusion criteria for all patients was the availability of an MRI with a T2*-weighted gradient-echo sequence (T2*w). In control cases, evidence of at least 1 acute ICH on MRI and no evidence of CAA in the tissue examination were further recommended. A total of 38 CAA patients (16 male, mean age 70 ± 6.4 years) and 22 control patients (12 male, mean age 54 ± 18 years) matched the criteria and were included. Three patients of the CAA group have been published previously as a case report.4
Figure 1 Flow diagram
The retrospective identification of patients with cerebral amyloid angiopathy (CAA) and control patients.
Reference standard.
Neuropathologic diagnosis served as the reference standard. It was established based on postmortem brain examination (11 CAA cases, 8 controls), hematoma evacuation (9 CAA cases, 14 controls), or brain biopsy (18 CAA cases). CAA was histologically confirmed by evidence of replacement of vessel walls with amyloid.e1
Available imaging and data analysis.
MRI had been performed at 1.5 Tesla MR scanners, including T2*w (slice thickness: 5 mm, repetition time: 500 to 1,000 msec, echo time: 13.8 to 25 msec), fluid-attenuated inversion recovery, or proton density–weighted (PDw) images.
Analysis was done in consensus by 2 neuroradiologists with 7 and 12 years experience with analysis of cranial MRI. They were blinded to all clinical and patient information data, including the histopathologic diagnosis. T2*w images were systematically assessed for evidence of ICHs (>5 mm in diameter), MBs (<5 mm),6 and SS.4 PDw and FLAIR images were used to identify acute subarachnoid hemorrhages (SAH). The distribution of SS and SAH was classified as either focal (restricted to ≤3 sulci) or disseminated (≥4 sulci). The prevalence of MBs, SS, and acute SAH in both patient groups was calculated in percentages.
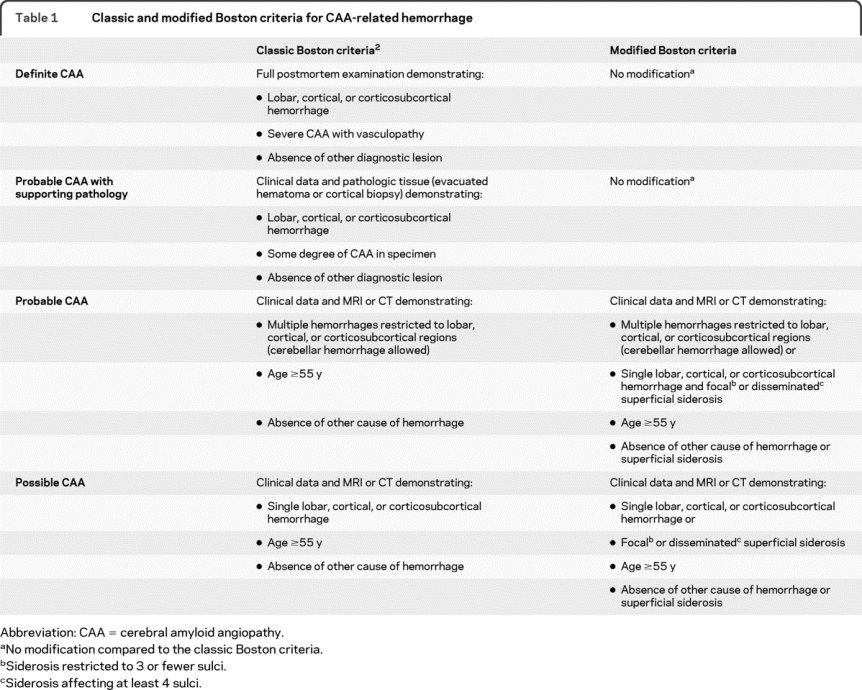
To test whether the addition of SS as a criterion alters the sensitivity and specificity of the Boston diagnostic criteria for CAA-related hemorrhage, we proposed the modified Boston criteria, in which SS is regarded as another hemorrhagic manifestation of CAA (table 1). Readers classified the patients according to both the conventional and the modified Boston criteria as possible, probable, or no CAA-related hemorrhage. MBs were also regarded as hemorrhages in terms of the criteria, as this has been shown to increase their sensitivity.6 The histologic information was not taken into account for the classification. It was assessed in how many cases the modification did influence the classification, and sensitivity and specificity of both sets of criteria were calculated including 95% CIs.
Table 1 Classic and modified Boston criteria for CAA-related hemorrhage
RESULTS
ICHs and MBs were present in 71% and 47.4% of the CAA patients and in 100% and 22.7% of the controls. Acute SAH was observed in 50% of the CAA patients (n = 19). SS was detected in 60.5% of CAA patients. It was focal in 34.2% of patients and disseminated in 26.3% of cases (figure 2). In 6 of the patients with SS no ICHs were present, and in 2 of them SS ± acute SAH were the only magnetic resonance–detectable hemorrhagic lesions, i.e., no MBs were found. In 13 patients with SS and ICH, SS was found not only adjacent to the ICH, but also at sites distant from the ICH. In one patient, SS affected both the supra- and the infratentorial compartment; in all other patients with SS, only the supratentorial sulci were involved. Neither SS nor acute SAH were observed in any of the control cases (table e-1 on the Neurology® Web site at www.neurology.org).
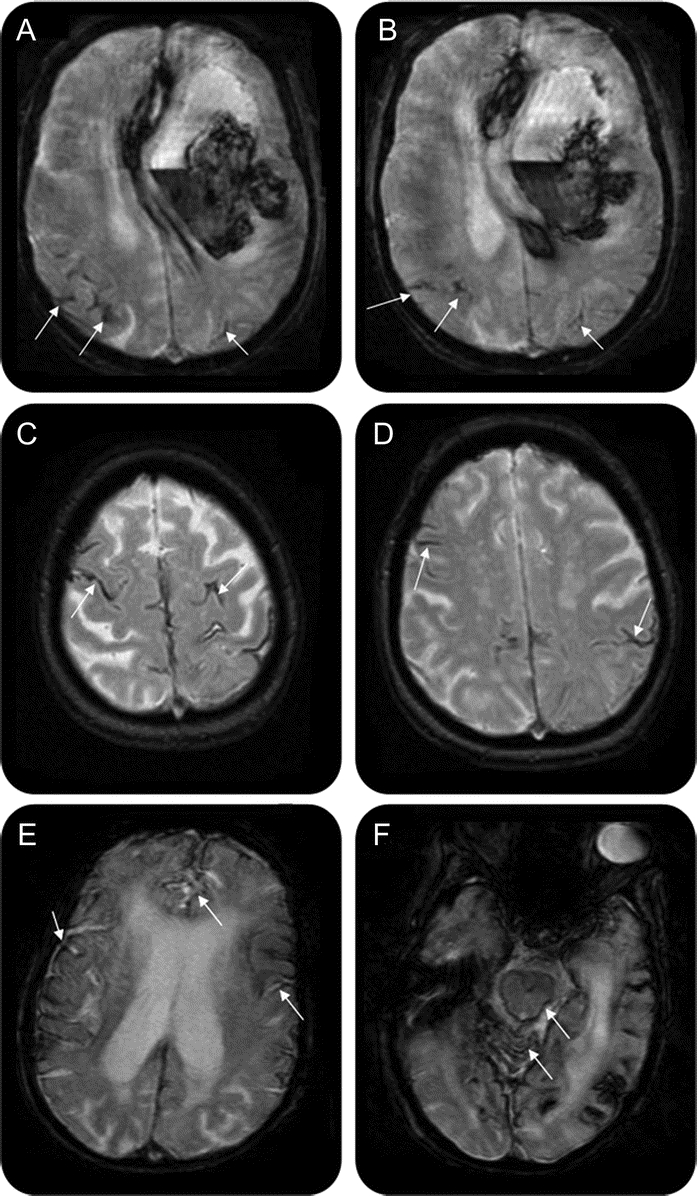
Figure 2 T2*-weighted MRI of 3 different patients with cerebral amyloid angiopathy (CAA)
(A, B) A 67-year-old woman with CAA with large left frontal intracerebral hemorrhage (ICH) and focal superficial siderosis in the right and left parietal lobes (arrows). In this patient, 2 microbleeds (MBs) were also present (not shown). (C, D) An 84-year-old woman with CAA with disseminated, bilateral frontal and parietal superficial siderosis (e.g., arrows), in whom no ICHs or MBs were present. (E, F) A 73-year-old woman with CAA with disseminated bilateral superficial siderosis (arrows in E) affecting also the infratentorial compartment (arrows in F). In this patient, 1 infratentorial MB was found (not shown), but no ICHs.
Inclusion of SS in the Boston criteria resulted in a diagnostic upgrading from no to possible CAA-related hemorrhage in 2 patients (who had SS as the only site of hemorrhage), and from possible to probable CAA in 5 patients (who otherwise had only a single ICH). The classic Boston criteria showed a sensitivity of 89.5% (95% CI 75.9–95.9), while inclusion of SS in the modified criteria increased their sensitivity to 94.7% (95% CI 82.7–98.5). This increase was not significant. The specificity of the Boston criteria was 81.2% (95% CI 61.5–92.7) for the classic as well as for the modified criteria.
DISCUSSION
The main findings in our study were 1) focal or disseminated, supratentorially located SS is commonly present in CAA but not in other types of ICH and 2) inclusion of SS as a criterion resulted in a diagnostic upgrading in 7 patients with CAA, without lowering the specificity of the Boston criteria.
It has been shown in animal models on experimental siderosis that repeated bleeding into the subarachnoid space leads to deposition of hemosiderin in the subpial layer of the brain.7 Thus, we propose that the same mechanism underlies the pathogenesis of SS observed in CAA, and favor a primary subarachnoid origin of the blood over a secondary spread of ICH into the subarachnoid space in most cases. This hypothesis is supported by the facts that we found SS distant from the localization of any ICH, and that SS was present in 6 patients who did not show any ICH. In accordance with these observations, a neuropathologic study revealed that—at least in some cases of CAA-related subcortical hematoma—the primary hemorrhage occurred in the subarachnoid space.8 It has even been suggested that a restricted SAH could be a warning sign, which can precede a lobar ICH in CAA.9
Compared to the well-described SS of the CNS, which mainly affects brainstem and posterior fossa,10 SS in CAA showed a preference for the cerebral convexity and only exceptionally occurred in the infratentorial compartment. This explains why patients with CAA lack the typical clinical presentation associated with the previously described form of SS, namely cerebellar and brainstem signs.
To facilitate noninvasive diagnosis of CAA-related hemorrhage, the Boston criteria have been developed in order to provide standardized diagnostic criteria applicable in vivo.2 Yet SS has been reported as the only bleeding manifestation in some patients with CAA, especially if they present with transient clinical manifestations, e.g., seizures.3 Regarding SS as another hemorrhagic manifestation of CAA, adding it as a criterion resulted in a diagnostic upgrading in 7 patients with CAA (2 of which had no other type of intracranial hemorrhage), whereas it did not lower the specificity of the Boston criteria for CAA-related hemorrhage.
There are some limitations to our study. 1) The difference in sensitivity between classic and modified Boston criteria was not significant, probably due to the limited number of cases. 2) A full postmortem examination was only available in 11 patients with CAA, but replacement of vessel walls with amyloid in hematoma or biopsy samples has been shown to be highly sensitive for CAA.e1 3) The inclusion criterion T2*w MRI may have contributed to a selection bias, as patients with large ICH often do not undergo MRI. 4) Due to the limited cases of histologically proven non-CAA forms of ICHs in which T2*w MRI was available, the control group was not age- and sex-matched to the CAA group, but younger on average. 5) In patients with isolated, localized supratentorial SS, there are several other causes of nontraumatic SAH which have to be taken into account as potential differential diagnosis, e.g., cortical vein thrombosis or vasculitis.e2
The sensitivity and specificity of SS for CAA have to be validated in larger prospective studies, and longitudinal follow-up studies of patients with SS should be performed.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. J. Linn.
DISCLOSURE
Dr. Linn has received research support from B. Braun Stiftung, Friedrich-Baur Stiftung, and the Förderprogramm für Forschung und Lehre, and a grant from the Bayerische Gleichstellungsförderung (BGF). Dr. Halpin, Dr. Demaerel, and J. Ruhland report no disclosures. Dr. Giese has received research support from Boehringer Ingelheim, the DFG, SFB596-B13, Lüneburg-Stiftung für Parkinsonforschung, and Studienstiftung des Deutschen Volkes. Dr. Dichgans served as a Section Editor for Stroke and receives research support from BMBF, Wellcome Trust, and the Foundation for Vascular Dementia Research. Dr. van Buchem and Dr. Bruckmann report no disclosures. Dr. Greenberg serves on a scientific advisory board for Hoffman-LaRoche; has received travel expenses and/or honoraria for lectures or educational activities not funded by industry; serves on the editorial boards of Stroke, Cerebrovascular Disease, Neurology®, and the Journal of Alzheimer's Disease and Other Dementias; and receives research support from the NIH (R01AG026484 [PI], K24NS056207 [PI], R01AG021084 [Co-I], R01NS042147 [PI], and U54NS057405 [Co-I]) and the Alzheimer's Association.
Supplementary Material
Address correspondence and reprint requests to Dr. Jennifer Linn, Department of Neuroradiology, University Hospital Munich, Marchioninistrasse 15, 81377 Munich, Germany linn@nrad.de
Supplemental data at www.neurology.org
Disclosure: Author disclosures are provided at the end of the article.
Received October 9, 2009. Accepted in final form February 3, 2010.
REFERENCES
- 1.Vinters HV. Cerebral amyloid angiopathy: a critical review. Stroke 1987;18:311–324. [DOI] [PubMed] [Google Scholar]
- 2.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001;56:537–539. [DOI] [PubMed] [Google Scholar]
- 3.Roch JA, Nighoghossian N, Hermier M, et al. Transient neurologic symptoms related to cerebral amyloid angiopathy: usefulness of T2*-weighted imaging. Cerebrovasc Dis 2005;20:412–414. [DOI] [PubMed] [Google Scholar]
- 4.Linn J, Herms J, Dichgans M, et al. Subarachnoid hemosiderosis and superficial cortical hemosiderosis in cerebral amyloid angiopathy. AJNR Am J Neuroradiol 2008;29:184–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vernooij MW, Ikram MA, Hofman A, Krestin GP, Breteler MM, van der Lugt A. Superficial siderosis in the general population. Neurology 2009;73:202–205. [DOI] [PubMed] [Google Scholar]
- 6.van Rooden S, van der Grond J, van den Boom R, et al. Descriptive analysis of the Boston criteria applied to a Dutch-type cerebral amyloid angiopathy population. Stroke 2009;40:3022–3027. [DOI] [PubMed] [Google Scholar]
- 7.Koeppen AH, Dickson AC, Chu RC, Thach RE. The pathogenesis of superficial siderosis of the central nervous system. Ann Neurol 1993;34:646–653. [DOI] [PubMed] [Google Scholar]
- 8.Takeda S, Yamazaki K, Miyakawa T, et al. Subcortical hematoma caused by cerebral amyloid angiopathy: does the first evidence of hemorrhage occur in the subarachnoid space? Neuropathology 2003;23:254–261. [DOI] [PubMed] [Google Scholar]
- 9.Katoh M, Yoshino M, Asaoka K, et al. A restricted subarachnoid hemorrhage in the cortical sulcus in cerebral amyloid angiopathy: could it be a warning sign? Surg Neurol 2007;68:457–460. [DOI] [PubMed] [Google Scholar]
- 10.Kumar N, Cohen-Gadol AA, Wright RA, Miller GM, Piepgras DG, Ahlskog JE. Superficial siderosis. Neurology 2006;66:1144–1152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




