Abstract
OBJECTIVES: To determine the frequency and types of respiratory viruses circulating in Boston long‐term care facilities (LTCFs) during a 3‐year period.
DESIGN: Observational.
SETTING: Thirty‐three Boston‐area LTCFs over a 3‐year period.
PARTICIPANTS: Residents of long‐term care who had previously participated in a trial of vitamin E supplementation and had paired serum samples available for viral analysis.
MEASUREMENTS: Viral antibody titers to eight respiratory viruses (influenza A and B, respiratory syncytial virus (RSV), parainfluenza virus serotype three (PIV‐3), PIV‐2, human metapneumovirus (hMPV), and coronaviruses 229E and OC43) were measured using enzyme immunoassay at baseline and 53 weeks. Infection was defined as a more than quadrupling of viral titers. Clinical data on respiratory illnesses were collected throughout the study period.
RESULTS: A total of 617 persons were enrolled in the trial. Of these, 382 (62%) had sera available for viral analysis. A total of 204 viral infections were documented in 157 subjects. Serological responses to all eight viruses were documented, with hMPV (12.8%) and coronavirus 229E (10.5%) being the most common and PIV‐2 (2.4%) the least common. The occurrence of bronchitis (P=.007), pneumonia (P=.02), and any lower respiratory tract infection (P=.002) was significantly associated with having a viral diagnosis.
CONCLUSION: A wide range of respiratory viruses cocirculates in LTCFs and contributes to respiratory illness morbidity in these populations.
Keywords: viral infections, long‐term care, human metapneumovirus
Respiratory tract infections are a major cause of morbidity and mortality in long‐term care facilities (LTCFs). 1 A wide range of bacterial and viral pathogens has been documented in nursing home settings, although the relative contribution of specific pathogens is not well defined. 2 , 3 Influenza and respiratory syncytial virus (RSV) are now accepted as significant pathogens in this population, but the roles of other viruses such as the newly described human metapneumovirus (hMPV) are largely unexplored. 4 Residents of LTCFs are at risk for nosocomial spread of respiratory viruses, and outbreaks of respiratory illness are not uncommon. 5 Although infections with these pathogens in young adults are generally mild, serious complications may occur in frail elderly persons. 6 Thus, residents of nursing homes are considered target groups for antivirals and vaccines. Although influenza remains the most important viral respiratory pathogen in this population, determining the types and frequencies of other viruses leading to lower respiratory infections (LRIs) in nursing homes may have importance for future studies of preventative measures and therapeutics. As a first step, serum samples that were available from a vitamin E supplementation trial conducted in a large number of LTCFs were analyzed for evidence of recent infection with eight common respiratory viruses, and viral diagnoses were correlated with the occurrence of respiratory illnesses.
METHODS
Subjects
Study participants were recruited from 33 LTCFs in the Boston, Massachusetts, area over a 3‐year period from 1998 to 2000. 7 Inclusion criteria were aged 65 and older; life expectancy greater than 6 months; not room bound; and absence of active cancer, tube feeding, urethral catheters, tracheostomy, chronic ventilator dependence, or long‐term steroid use. In addition, a serum albumin of at least 3.0 g/dL and willingness to receive influenza vaccine were required. The Tufts New England Medical Center institutional review board approved the study. All participants or their legal guardians provided informed consent.
Study Design
During the 3 years of study, 617 volunteers were enrolled in a vitamin E supplementation trial for a 52‐week intervention period. A serum sample was collected at the time of enrollment (April–August) and at Week 53, after 1 year of supplementation. Investigators trained study nurses to identify relevant respiratory symptoms and perform focused physical examinations, and prospective surveillance for respiratory illnesses was conducted throughout the year. Data from the nursing home medical records and findings by study nurses were used to classify respiratory illnesses. Respiratory tract infections were categorized according to standard definitions and included common cold, influenza‐like illness, pharyngitis, otitis media, sinusitis (defined as upper respiratory infection (URI)) and acute bronchitis and pneumonia (defined as LRI). 8 Patient samples were not collected at the time of illness for diagnostic microbiology.
Viral Serology
Enzyme immunoassay (EIA) was performed to detect viral‐specific immunoglobulin G for eight respiratory viruses (influenza A and B, respiratory syncytial virus (RSV), parainfluenza virus serotype three (PIV‐3), PIV‐2, human metapneumovirus (hMPV), and coronaviruses 229E and OC43) according to published methods. 9 , 10 , 11 , 12 Briefly, antigens were produced from virally infected whole‐cell lysates for all viruses except RSV. Purified viral surface glycoproteins were used as antigen for RSV EIA, according to published methods. 11 Paired serum samples were screened at a single dilution, and those showing a 1.5 times or more greater optical density reading from acute to convalescent specimens were further tested using full dilution to determine antibody titer. Serial two‐fold dilutions of each sample were tested in duplicate, and infection was defined as a more than quadrupling in antibody from baseline to Week 53. Because PIV‐1 and PIV‐3 antibodies cross‐react, infection with these viruses cannot be distinguished serologically. Therefore, a serological response to PIV‐3 antigen is designated PIV‐1/3.
Statistical Analysis
All analyses were performed using SAS for Windows, version 9.1.3 (SAS Institute, Inc., Cary, NC). Chi‐square and Fisher exact tests were used to compare proportions. Means were compared using the Student t‐test for independent samples. The association between clinical illness (yes or no) and number of viral infections diagnosed (0, 1, or 2) was assessed using the Mantel‐Haenszel chi‐square statistic for linear association as implemented in SAS PROC FREQ.
RESULTS
A total of 617 persons were enrolled in the trial. Of these, 452 (73%) completed the study, and 382 (62%) had paired sera available at baseline and 53 weeks for viral analysis. Differences between the baseline characteristics of those who did and did not undergo viral testing were not statistically significant (data not shown). Demographics and underlying medical conditions were also similar for the group with documented viral infection and those who tested negative for viral infection (Table 1).
Table 1.
Baseline Characteristics of Subjects with and without Viral Diagnoses
| Characteristic | Viral Testing Performed N=382 | Viral Positive n=157 | P‐Value |
|---|---|---|---|
| Age, mean (range) | 84.9 (65–102) | 85.3 (67–102) | .72 |
| Women, n (%) | 286 (74.9) | 123 (78.3) | .44 |
| Whites, n (%) | 363 (95.0) | 150 (95.5) | 1.00 |
| Medical history, n (%) | |||
| Chronic obstructive pulmonary disease | 89 (23.3) | 33 (21.0) | .65 |
| Coronary artery disease | 119 (31.2) | 48 (30.6) | .91 |
| Congestive heart failure | 68 (17.8) | 30 (19.1) | .71 |
| Hypertension | 202 (52.7) | 87 (55.4) | .63 |
| Diabetes mellitus | 77 (20.2) | 33 (21.0) | .81 |
| Malignancy | 39 (10.2) | 19 (12.1) | .54 |
| Dementia | 182 (47.6) | 78 (49.7) | .70 |
Overall, 204 viral infections were documented in 157 subjects. Thus, 41% of participants had at least one viral infection during the 1‐year study period. One hundred seventeen subjects had one infection, 34 had two infections, five had three infections, and one had evidence of four viral infections. Serological responses to all eight viruses were documented in the study patients, with hMPV and coronavirus 229E being the most common and PIV‐2 the least frequent (Table 2). Although the activity of individual viruses varied according to year studied, all viral infections were identified each year. Displayed graphically as cases per facility in each year (Figure 1), these data indicate the complex nature of viral respiratory disease in nursing homes. With the exception of two facilities in which 42% (1999) and 28% (2000) of residents tested were positive for hMPV and one facility with a 36% positive rate for influenza A during 1999, there did not appear to be predominance of a single pathogen within a nursing home. Rather, a multitude of pathogens circulating throughout the facilities during all 3 years was observed.
Table 2.
Serological Evidence of Viral Infection in Nursing Homes
| Year (Number of Subjects Tested) | Number of Infections | |||||||
|---|---|---|---|---|---|---|---|---|
| Respiratory Syncytial Virus | Influenza A | Influenza B | Human Metapneumovirus | Coronavirus OC43 | Coronavirus 229E | PIV‐3 | PIV‐2 | |
| 1 (99) | 10 | 12 | 4 | 11 | 7 | 9 | 5 | 1 |
| 2 (149) | 9 | 11 | 4 | 22 | 3 | 4 | 4 | 4 |
| 3 (134) | 6 | 1 | 11 | 16 | 13 | 27 | 6 | 4 |
| Total (382) | 25 | 24 | 19 | 49 | 23 | 40 | 15 | 9 |
| Percentage of tested | 6.5 | 6.3 | 5.0 | 12.8 | 6.0 | 10.5 | 3.9 | 2.4 |
PIV=parainfluenza virus.
Figure 1.
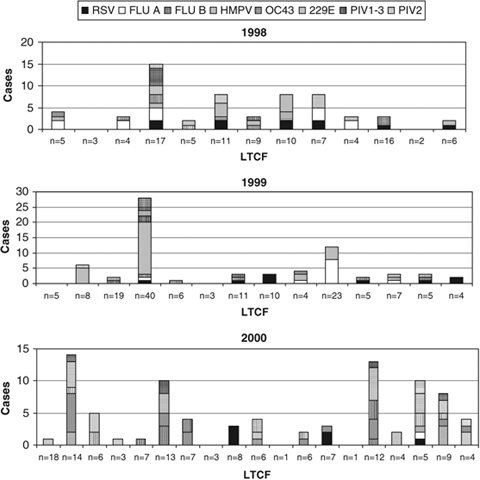
Number of viral infections diagnosed serologically during the 3‐year period. The individual nursing homes which participated in each year are shown on the horizontal axis with the number of individuals tested indicated. Each type of viral infection is expressed by a different shade in the bar graph. Some nursing homes participated in more than 1 year.RSV = respiratory syncytial virus; Flu A = influenza A virus; Flu B = influenza B virus; HMPV = human metapneumovirus; OC43 = coronavirus OC43; 229E = coronavirus 229E; PIV‐3 = parainfluenza virus serotype 3; PIV‐1 = parainfluenza virus serotype 1.
Because sera were not collected immediately surrounding each illness, a specific etiology could not be assigned to an individual illness, but analysis of the clinical data showed a significant association between serological diagnosis of a viral infection and the occurrence of bronchitis (P=.007), pneumonia (P=.02), any LRI (P=.002), and any respiratory infection (P=.02) but not URI (P=.17) according to Mantel‐Haenszel chi‐square.
DISCUSSION
This report demonstrates the wide range of respiratory viruses that circulate in LTCFs. Previous reports of viral disease in these populations have focused primarily on outbreaks of specific viruses, most often influenza. 7 , 13 , 14 , 15 , 16 , 17 There have also been a number of prospective studies that have examined the role of other respiratory viruses such as RSV and parainfluenza in nursing homes, but most have been limited to a single season or institution. 2 , 11 , 18 , 19 , 20 This study is unique in that 33 nursing homes over a 3‐year period were under surveillance, and evaluation of eight different viral infections was undertaken. Although this trial was not designed to examine the specific microbiological etiology of respiratory illnesses, the collection of blood at baseline and 1 year provided an opportunity for serological diagnosis of multiple viral infections over time. In addition to the well‐recognized pathogens influenza and RSV, infections due to hMPV, coronaviruses OC43 and 229E, and parainfluenza viruses were identified throughout the study period. Rather than large outbreaks of single pathogens, a veritable cornucopia of viruses circulated in individual nursing homes each year.
A rise in viral‐specific antibody according to EIA remains sensitive and specific for detection of infection for most respiratory viruses in elderly people, even when compared with new molecular methods such as reverse transcription polymerase chain reaction (RT‐PCR). 9 , 21 Several exceptions are worth noting, one being influenza, in which response to vaccination may complicate the serological diagnosis of infection. In the current study, 24 and 19 residents were diagnosed with influenza A and B, respectively. None of the subjects infected with influenza A showed antibody rises to influenza B, and vice versa, suggesting that antibody rises were not vaccine induced. In addition, vaccine effect was less likely, because serum samples were obtained 7 to 9 months after immunization, when vaccine induced antibody has typically decreased to near baseline. 22 Another limitation of serology for viral diagnosis is the inability to identify rhinovirus. Rhinovirus is a ubiquitous pathogen that has been shown to cause outbreaks of respiratory disease in nursing homes, but serological diagnosis is not possible, because multiple serotypes exist. 23 Because rhinoviruses are a frequent cause of the common cold, it is not surprising that no association was found between URI and serological viral diagnosis.
Presently, there are limited data in elderly people on the newly described virus hMPV and its role as a cause of illness in LTCFs. Outbreaks of hMPV infection with significant morbidity and mortality in LTCFs have been reported in the United States, Canada, and Japan. 16 , 24 In each report, diagnosis was made using RT‐PCR, and the number of documented cases was small. In this study, hMPV was the most common infection diagnosed, accounting for 24% of the documented viral infections. Asymptomatic serological infection has been described in 9.5% of young and 1.5% of elderly adults. 9 The clinical syndromes were not assessed in the current study, and therefore the true effect of hMPV could not be assessed. However, the frequency of infection indicates that prospective studies of hMPV in LTCFs are needed.
Similar to hMPV, coronaviruses are difficult to detect using standard viral cultures. Thus, few data are available on the effect of these viruses in long‐term care. In a prospective study of 11 nursing homes in the United Kingdom using serology for diagnosis, 11% of acute respiratory infections were due to coronavirus OC43 or 229E. 18 Outbreaks of respiratory illness due to coronavirus OC43 mimicking influenza have also been described in nursing homes. 25 Coronaviruses OC43 and 229E were common in the current study, accounting for 6% and 11% of infections, respectively. Two new strains of human coronaviruses have recently been identified, NL63 and HKU1, and will require further study to determine whether these viruses are also important pathogens in this population. 26 , 27
The current study demonstrates the complex nature of respiratory tract infections in LTCFs. Influenza infection was identified in 11% of residents, which confirms the need for improved influenza vaccines in this population, yet the broad range of other viruses circulating each year was impressive, underscoring the importance of viral‐specific diagnosis during outbreak investigations or clinical trials of vaccines or antivirals. These data should provide useful information for those wishing to pursue clinical trials related to respiratory viral infections in nursing home populations.
ACKNOWLEDGMENTS
The authors wish to thank Edward E. Walsh, MD, for his thoughtful review of the manuscript and the staff of the Metabolic Research Unit and Nutritional Evaluation Laboratory of Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University and the participating nursing homes for help in recruitment of subjects and analysis of blood micronutrients.
Conflict of Interest: Dr. Falsey has received research grants from Sanofi‐pasteur. She serves on the advisory board for Quidel Inc. and has consulted with Sanofi‐pasteur, Glaxo‐Smith Kline, Alnylam, Medimmune, and Merck and has received received honoraria. Dr. Hamer is on the speaker's bureau for Glaxo Smith Kline and owns shares of Inverness Medical Innovations, Inc., a company that makes respiratory diagnostics products. Dr. Meydani has funding in this area of study from the U.S. Department of Agriculture, National Institute on Aging (NIA), and Roche (for provision of the supplements for the study). This work was supported by NIA, National Institutes of Health Grant 1R01‐AG13975 and U.S. Department of Agriculture (USDA) agreement 58‐1950‐9‐001. The study was also supported by HRCA/Harvard Research Nursing Home PO1 AG004390 to Dr. Lipsitz, which facilitated recruitment of subjects and conduct of the study at Hebrew Rehabilitation Center.
Author Contributions: Dr. Falsey analyzed the viral data and wrote the manuscript. Dr. Dallal assisted with the analysis of data and reviewed and had a final say about the manuscript. Maria Formica and Gloria Andolina performed the viral assays and assisted with analysis of the viral data. Dr. Hamer was involved in the conceptualization of the study design, was responsible for data acquisition, and assisted with data interpretation and editing of the final manuscript. Lynette Leka was responsible for data acquisition. Dr. Meydani had full access to all of the data in the study and was responsible for the integrity of the data and the accuracy of the data analysis, as well as its acquisition. Dr. Meydani was responsible for the study concept and design and interpretation of study findings and assisted in the drafting and final editing of the manuscript.
Sponsor's Role: The sponsor provided the funding for the original vitamin E study but did not provide funds for virologic testing or analysis.
REFERENCES
- 1. Mylotte JM. Nursing home‐acquired pneumonia. Clin Infect Dis 2002;35:1205–1211. [DOI] [PubMed] [Google Scholar]
- 2. Orr PH, Peeling RW, Fast M et al Serological study of responses to selected pathogens causing respiratory tract infection in the institutionalized elderly. Clin Infect Dis 1996;23:1240–1245. [DOI] [PubMed] [Google Scholar]
- 3. Muder RR. Pneumonia in residents of long‐term care facilities: Epidemiology, etiology, management, and prevention. Am J Med 1998;105:319–330. [DOI] [PubMed] [Google Scholar]
- 4. Ellis SE, Coffey CS, Mitchel EF et al Influenza‐ and respiratory syncytial virus‐associated morbidity and mortality in the nursing home population. J Am Geriatr Soc 2003;51:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drinka PJ, Krause P, Nest L et al Risk of acquiring influenza A in a nursing home from a culture‐positive roommate. Infect Control Hosp Epidemiol 2003;24:872–874. [DOI] [PubMed] [Google Scholar]
- 6. Drinka PJ, Gravenstein S, Langer E et al Mortality following isolation of various respiratory viruses in nursing home residents. Infect Control Hosp Epidemiol 1999;20:812–815. [DOI] [PubMed] [Google Scholar]
- 7. Sorvillo FJ, Huie SF, Strassburg MA et al An outbreak of respiratory syncytial virus pneumonia in a nursing home for the elderly. J Infect 1984;9:252–256. [DOI] [PubMed] [Google Scholar]
- 8. Meydani SN, Leka LS, Fine BC et al Vitamin E and respiratory tract infections in elderly nursing home residents: A randomized controlled trial. JAMA 2004;292:828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Falsey AR, Erdman D, Anderson LJ et al Human metapneumovirus infections in young and elderly adults. J Infect Dis 2003;187:785–790. [DOI] [PubMed] [Google Scholar]
- 10. Falsey AR, McCann RM, Hall WJ et al The “common cold” in frail older persons: Impact of rhinovirus and coronavirus in a senior daycare center. J Am Geriatr Soc 1997;45:706–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Falsey AR, Treanor JJ, Betts RF et al Viral respiratory infections in the institutionalized elderly: Clinical and epidemiologic findings. J Am Geriatr Soc 1992;40:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Falsey AR, McCann RM, Hall WJ et al Acute respiratory tract infection in daycare centers for older persons. J Am Geriatr Soc 1995;43:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Faulks JT, Drinka PJ, Shult P. A serious outbreak of parainfluenza type 3 on a nursing unit. J Am Geriatr Soc 2000;48:1216–1218. [DOI] [PubMed] [Google Scholar]
- 14. Hall WN, Goodman RA, Noble GR et al An outbreak of influenza B in an elderly population. J Infect Dis 1981;144:297–302. [DOI] [PubMed] [Google Scholar]
- 15. Centers for Disease Control. Outbreaks of influenza among nursing home residents—Connecticut, United States. MMWR Morb Mortal Wkly Rep 1985;34:478–482. [PubMed] [Google Scholar]
- 16. Honda H, Iwahashi J, Kashiwagi T et al Outbreak of human metapneumovirus infection in elderly inpatients in Japan. J Am Geriatr Soc 2006;54:177–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drinka PJ, Gravenstein S, Krause P et al Outbreaks of influenza A and B in a highly immunized nursing home population. J Fam Pract 1997;45:509–514. [PubMed] [Google Scholar]
- 18. Nicholson KG, Baker DJ, Farquhar A et al Acute upper respiratory tract viral illness and influenza immunization in homes for the elderly. Epidemiol Infect 1990;105:609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drinka PJ, Gravenstein S, Krause P et al Non‐influenza respiratory viruses may overlap and obscure influenza activity. J Am Geriatr Soc 1999;47:1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hornsleth A, Siggaard‐Andersen J, Hjort L. Epidemiology of herpesvirus and respiratory virus infections. Part 1. Serologic findings. Geriatrics 1975;30:61–68. [PubMed] [Google Scholar]
- 21. Falsey AR, Formica MA, Walsh EE. Diagnosis of respiratory syncytial virus infection: Comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J Clin Microbiol 2002;40:817–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Couch RB, Keitel WA, Cate TR. Improvement of inactivated influenza virus vaccines. J Infect Dis 1997;176:S38–S44. [DOI] [PubMed] [Google Scholar]
- 23. Louie JK, Yagi S, Nelson FA et al Rhinovirus outbreak in a long term care facility for elderly persons associated with unusually high mortality. Clin Infect Dis 2005;41:262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boivin G, De Serres G, Hamelin ME et al An outbreak of severe respiratory tract infection due to human metapneumovirus in a long‐term care facility. Clin Infect Dis 2007;44:1152–1158. [DOI] [PubMed] [Google Scholar]
- 25. Birch CJ, Clothier HJ, Seccull A et al Human coronavirus OC43 causes influenza‐like illness in residents and staff of aged‐care facilities in Melbourne, Australia. Epidemiol Infect 2005;133:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lau SK, Woo PC, Yip CC et al Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol 2006;44:2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bastien N, Anderson K, Hart L et al Human coronavirus NL63 infection in Canada. J Infect Dis 2005;191:503–506. [DOI] [PMC free article] [PubMed] [Google Scholar]


