Abstract
Objective
To examine the longitudinal relation between perceived stress in the previous month and perimenstrual symptom severity across two cycles among regularly menstruating, healthy women (n = 259).
Methods
At baseline (11 days before the first cycle), participants completed the 4-item Perceived Stress Scale (PSS) for the previous month (first cycle exposure) and questionnaires on lifestyle factors. On cycle day 22 of a standardized 28-day cycle, participants again completed the PSS for the previous week (second cycle exposure) and each week rated the severity (none, mild, moderate, severe) of 17 psychological and physical symptoms (e.g., crying, cramping, pain). Mixed models estimated the association between perceived stress scores and number of moderate/severe symptoms and symptom severity scores, allowing both stress and perimenstrual symptoms to vary by cycle.
Results
Adjusting for age, education, passive and active smoking, and waist/height ratio (WHtR), high stress (fourth quartile PSS) was associated with an increased risk of reporting ≥8 or more (OR 7.2, 3.3-15.8) and ≥5 (OR 2.5, 1.6-4.1) symptoms as moderate/severe during the perimenstrual period compared with lower stress (quartiles one, two, and three). Stress scores were positively (p < 0.0001) associated with increased symptom severity scores for total, psychological, and physical symptoms.
Conclusions
These analyses show that higher perceived stress precedes an increased severity of perimenstrual symptoms. Stress reduction programs may be an effective, nonpharmaceutical treatment for physical and psychological symptom relief.
Introduction
Premenstrual syndrome (PMS) is a common disorder among reproductive-aged women, associated with disruption of familial and social relationships, work interference and absenteeism, and increased healthcare costs.1–3 Approximately 40%–60% of reproductive-aged women experience PMS.4–6 Although pharmaceutical treatment has proved to be successful for many women who suffer from moderate to severe PMS, medications are not always effective or appropriate.7,8 It is, therefore, of public health importance to identify factors, such as psychosocial stress, that contribute to increased symptom severity and may be potentially modifiable.
PMS is a collection of physical, behavioral, and psychological symptoms that occur in the late luteal phase of the menstrual cycle, abate during menses, and are generally absent during the week after menses.9 Although >90 premenstrual symptoms have been identified, the most commonly reported include anger, anxiety, mood swings, depression, fatigue, decreased concentration, breast swelling and tenderness, general aches, and abdominal bloating.10,11
Despite the high prevalence of PMS, few risk factors have been consistently identified,12–14 little is known about the etiology of symptoms, and no universal treatment exists. A suspected etiological pathway is linked to slight irregularities in the normal variation of ovarian hormones throughout the menstrual cycle, as ovulation suppression is known to avert premenstrual symptoms;15 however, differences in reproductive hormone levels have not been consistently identified.16–19 Although not entirely clear, psychosocial stress may impact the severity of perimenstrual symptoms (premenstrual/menstrual weeks) through activation of the hypothalamic-pituitary-ovarian (HPO) axis, thus altering ovarian hormone levels, or stimulation of the sympathetic nervous system, leading to altered levels of neurotransmitters and other brain processes.9
Longitudinal studies are necessary to differentiate between stress as a cause or contributor and stress as a consequence of PMS because of the higher potential for reverse causality in retrospective or cross-sectional studies. Our aim, therefore, was to assess the association between psychosocial stress in the previous month and risk of perimenstrual symptoms, using a longitudinal design among otherwise healthy women.
Materials and Methods
Study population
The BioCycle Study was designed to explore ovarian function across the menstrual cycle in healthy women. A detailed description of the unique design, participants, and methods has been published.20 In brief, premenopausal women were recruited from western New York. Inclusion criteria were age 18–44, no chronic health conditions or psychiatric conditions (including premenstrual dysphoric disorder [PMDD]), regular self-reported cycle length between 21 and 35 days, a body mass index (BMI) between 18 and 35 kg/m2, and no current use of oral contraceptives or exogenous hormones.20 At baseline, participants had physical and anthropometric measures taken and completed questionnaires on lifestyle, physical activity (International Physical Activity Questionnaire, IPAQ),21 and family medical and health history. Participants received both verbal and written instructions in the use of a fertility monitor (Clearblue® Easy Fertility Monitor, Inverness Medical, Inc., Waltham, MA) to assist in scheduling visits to correspond with specific phases of the menstrual cycle.22,23 All participants signed an informed consent, and this study was approved by Institutional Review Boards at the University at Buffalo and the National Institutes of Health.
The study included one baseline clinic visit and 16 cycle visits (8 per cycle) over two menstrual cycles, scheduled to occur on approximately days 2, 7, 12, 13, 14, 18, 22, and 27 of a 28-day cycle, adjusted for cycle length (day 1 = start of menses). These visits were timed to capture the periods representative of hormonal variation throughout phases of the cycles: menstrual (day 2), follicular (day 7), ovulation (days 12, 13, 14), and luteal (days 18, 22, 27). Women were followed longitudinally for either one (n = 9) or two (n = 250) menstrual cycles. Compliance was high, with 94% completing 7 or 8 visits per cycle.20
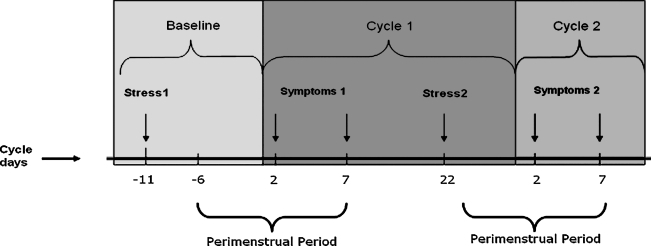
At baseline (approximately 11 days before the first cycle), participants completed the 14-item Perceived Stress Scale (PSS)24 for the previous month (Fig. 1). Additionally, at four clinic visits per cycle (days 2, 7, 14, 22), participants completed the 4-item PSS24 for the past week and a questionnaire designed to assess the presence and severity of 17 recognizable menstrual symptoms in the previous week.25,26 Participants rated perceived symptom severity as none, mild, moderate, or severe. Because perimenstrual symptoms peak in severity 2–3 days before the onset of menses, subside during the menstrual week, and are generally absent during the postmenstrual week,9,27 we focused our analysis on the assessments from the premenstrual week (day 2) and the menstrual week (day 7) for a total of four symptom assessments across two menstrual cycles.
FIG. 1.
Timeline of perceived stress and perimenstrual symptom assessments in the BioCycle Study. Day 1, onset of menses and start of menstrual cycle. Symptoms, perimenstrual symptom assessment for previous week. Symptoms 1 pertains to cycle 1 (premenstrual and menstrual week), and Symptoms 2 pertains to cycle 2 (premenstrual and menstrual week). Stress, stress assessment for previous week (perceived stress scale). Stress 1 is the exposure for Symptoms 1, and Stress 2 is the exposure for Symptoms 2.
At the screening and baseline clinic visits, information was collected about demographics and lifestyle using validated questions selected largely from major national surveys (e.g., National Health and Nutrition Examination Survey [NHANES] and National Health Interview Survey [NHIS]). On days 2, 7, 14, and 22, participants also completed a standardized 24-hour dietary recall from which mean caloric intake and caffeine intake were calculated. At the baseline visit, a trained research assistant measured height, weight, and waist circumference at the level of the natural waist28 using standardized techniques, from which BMI and waist/height ratio (WHtR) were calculated and categorized (< 0.5, ≥ 0.5).29 Age, education, income, alcohol intake, active and passive smoking, caloric intake, caffeine intake, BMI, WHtR (<0.5, ≥0.5), physical activity, gravidity, age at first menstrual period, and recent colds, flu, or other illness as reported at the clinic visits were considered as potential confounders because of their known or possible associations with stress and PMS.
Because physical activity may be a protective factor for both PMS severity30–32 and high stress,33–35 we assessed several components of baseline physical activity (past 7 days) as potential confounders, including IPAQ categories (low, moderate, and high activity level) and weekly total metabolic equivalents (METS), a measure of energy expenditure. Total weekly METS were calculated using the formula:
 |
Statistical analysis
The 4-item PSS scores were analyzed as continuous and as a dichotomous variable (high stress = fourth quartile PSS, low/moderate stress = quartiles one, two, and three). For brevity, the low/moderate stress category is referred here after as low stress. In terms of temporal relations, the 4-item PSS score (questions 2, 6, 7, and 14), as a subset of the baseline 14-item PSS score, served as the stress exposure in cycle 1, whereas the 4-item PSS scale administered at midcycle 1 (PSS score from cycle day 22 with reference to the previous week) was the stress exposure for symptoms in cycle 2 (Fig. 1). The 4-item version of the PSS scale was carefully selected as a subset of the 14-item PSS by the authors of the scale and validated by the scale's authors and others.24,37 It has excellent reliability and validity as a short-form stress scale (coefficient alpha reliability = 0.72; correlation with smoking rates at 3 months = 0.37).24 In addition, we further classified the women by stress pattern preceding each cycle into four groups: low stress both cycles (reference), high stress 1 and low stress 2, low stress 1 and high stress 2, and high stress both cycles.
The 17 perimenstrual symptoms were classified as moderate/severe or mild/none, and the number of moderate/severe symptoms were summed. Moderate and severe symptoms were combined to ensure sufficient statistical power to detect associations, given that few women (1%–5%) reported symptoms classified as severe because of the healthy nature of the population and that women with PMDD were not eligible. We examined risk of reporting ≥5 moderate/severe symptoms because this is one of the criteria for diagnosing PMDD6 and risk of ≥8 as a more severe classification. We created a symptom severity score26,38 at each time point by summing the severity ratings (0 = none, 1 = mild, 2 = moderate, 3 =severe) for each symptom. The total severity score was the sum of all 17 symptom ratings. The psychological severity score was the sum of the 5 psychological symptom ratings (depression/sadness, irritability, anxiety, anger, crying spells), and the physical severity score was the sum of the 12 physical symptoms. Symptom severity scores were treated as continuous and were transformed (square root) to approximate a normal distribution (transformed mean = 2.90, median = 3.0, interquartile range = 2.0, 3.9).
We also classified each cycle as probable moderate/severe PMS using two criteria. Criterion 1, adapted from Borenstein et al.,2,39 was ≥3 moderate/severe symptoms during the premenstrual week (1 of which must be psychological) and a total symptom severity score during the premenstrual week at least 30% greater than the total score during the postmenstrual week. However, we did not have information on the extent to which menstrual symptoms affect daily life/relationships; therefore, we also considered a second criterion (criterion 2), which classified cycles as moderate/severe PMS, using the same method except with ≥5 moderate/severe symptoms during the premenstrual week.
Time-varying multivariable mixed models were used to account for the correlation of measures within each woman across two menstrual cycles, allowing both stress and symptoms to vary by cycle and week (premenstrual and menstrual). Using generalized estimating equations, we estimated unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CI) for risk of experiencing specific moderate/severe symptoms, multiple moderate/severe symptoms, and moderate/severe PMS. We also estimated the risk of ≥8 moderate/severe symptoms across both cycles by whether the stress level changed from cycle 1 to cycle 2, using women who reported low stress preceding both cycles as the referent. We used a linear mixed model to estimate the effect of stress level on symptom severity scores. Factors were considered confounders in the analysis and retained in the final adjusted model if they changed the coefficient for stress by >10%.
To determine if the timing of baseline PSS administration impacted results, women who completed the baseline PSS after the first cycle clinic visit (n = 7) or >30 days before the first clinic visit (n =14) were excluded in sensitivity analyses. Finally, we examined recent physical activity level, age, and race/ethnicity as effect modifiers of the association between stress and perimenstrual symptoms by including an interaction term in the mixed models. We used Statistical Analysis System software (SAS 9.1, Cary, NC) for data analysis.
Results
Participants, aged 18–44 years, were 60% non-Hispanic white, and nearly 90% had some college education (Table 1).20 Most were single (68%), and < 4% reported being current smokers. A comparison of participant sociodemographic and lifestyle characteristics by level of baseline perceived stress showed no differences, with the exception of decreased age, caffeine intake, and age at first menstrual period (p < 0.05). Although not statistically significant, women with high perceived stress tended to have shorter menstrual cycles and higher BMI and WHtR.
Table 1.
Distribution of Participants According to Stress at Baseline and Characteristics
| Characteristic | Low stressa n (%) | High stressa n (%) | p valueb |
|---|---|---|---|
| Sociodemographics | |||
| Age, years | |||
| 18–24 | 96 (48.5) | 36 (60.0) | 0.03 |
| 25–29 | 33 (16.7) | 9 (15.0) | |
| 30–34 | 11 (5.6) | 8 (13.3) | |
| 35–39 | 28 (14.1) | 4 (6.7) | |
| 40–44 | 30 (15.1) | 3 (5.0) | |
| Income (thousands of dollars) | |||
| <20 | 39 (19.9) | 16 (26.7) | 0.47 |
| 20–<40 | 47 (24.0) | 14 (23.3) | |
| 40–<75 | 53 (27.0) | 18 (30.0) | |
| ≥75 | 57 (29.1) | 12 (20.0) | |
| Highest educational level | |||
| High school or less | 25 (12.6) | 8 (13.3) | 0.85 |
| Some college | 94 (47.5) | 26 (43.3) | |
| Bachelor's degree or higher | 79 (39.9) | 26 (43.3) | |
| Race | |||
| Non-Hispanic white | 124 (62.6) | 30 (50.0) | 0.21 |
| Non-Hispanic black | 36 (18.2) | 14 (23.3) | |
| Other | 38 (19.2) | 16 (26.7) | |
| Marital status | |||
| Single, never married | 129 (65.1) | 47 (78.3) | 0.10 |
| Married | 54 (27.3) | 12 (20.0) | |
| Divorced/widowed/separated | 15 (7.6) | 1 (1.7) | |
| Lifestyle | |||
| Cigarette smoking | |||
| Nonsmoker/unexposed to passive smoke | 80 (40.4) | 19 (31.7) | 0.45 |
| Nonsmoker/exposed to passive smoke | 111 (56.1) | 38 (63.3) | |
| Current smoker | 7 (3.5) | 3 (5.0) | |
| Alcohol use (past 12 months) | |||
| None | 61 (31.0) | 24 (40.7) | 0.38 |
| Light use (<1 drink per week) | 41 (20.8) | 14 (23.7) | |
| Moderate (1–3 drinks per week) | 59 (30.0) | 13 (22.0) | |
| Heavy (4+ drinks per week) | 36 (18.3) | 8 (13.6) | |
| Caffeine consumption (daily mg)c,d | 65.7 [17.2-146.8] | 28.4 [3.5-97.1] | 0.04 |
| Physical activity | |||
| IPAQ category | |||
| Low | 21 (10.6) | 4 (6.7) | 0.62 |
| Moderate | 71 (35.9) | 21 (35.0) | |
| High | 106 (53.5) | 35 (58.3) | |
| Total METSc,e (weekly total) | 3303 [1470-6333] | 3545.3 [1396-6912] | 0.57 |
| Health | |||
| Waist/height ratio | |||
| <0.5 | 163 (82.7) | 43 (72.9) | 0.09 |
| ≥0.5 | 34 (17.3) | 16 (27.1) | |
| Waist circumference,c (cm) | 73.4 [68.5-79.7] | 74.5 [68.5-80.3] | 0.71 |
| Body mass indexf (kg/m2) | 24.1 (3.7) | 24.5 (4.4) | 0.36 |
| Age at first menstrual periodc | 12.8 [12.0-13.7] | 12.3 [11.1-13.0] | 0.01 |
| Menstrual cycle lengthf (days) | |||
| Cycle 1 | 29.2 (4.6) | 28.0 (4.4) | 0.10 |
| Cycle 2 | 28.9 (3.6) | 28.1 (2.7) | 0.41 |
High stress = fourth quartile PSS score; Low stress = quartiles one, two, and three.
p values for categorical variables derived from chi-square test.
Median [interquartile range]; p values derived from Kolmogorov-Smirnov test.
Average intake from four 24-hour dietary recalls.
Total METs = duration (hours) × activity intensity (METS).
Mean (standard deviation); p values derived from t test.
IPAQ, International Physical Activity Questionnaire-long form; METS, metabolic equivalents.
High perceived stress in the previous month was associated with a significantly increased risk of moderate/severe symptoms for all 5 psychological and the majority of physical symptoms, with odds ranging from 2.0 to 3.0 for most of the symptoms (Table 2). Adjustment for age, education, and passive and active smoking did not have an appreciable impact on results.
Table 2.
Perceived Stress During Previous Cycle and Risk of Specific Moderate/Severe Perimenstrual Symptoms, Odds Ratios and 95% Confidence Intervals
| |
|
High stress vs. low Unadjusted estimates |
High stress vs. low Adjustedaestimates |
||
|---|---|---|---|---|---|
| Moderate severe perimenstrual symptoms | n (%)b | OR | 95% CI | OR | 95% CI |
| Psychological | |||||
| Depression or sadness | 70 (14.3) | 2.96 | [1.58-5.47] | 2.94 | [1.58-5.47] |
| Crying spells | 40 (8.2) | 3.55 | [1.67-7.61] | 3.78 | [1.75-8.08] |
| Anger, agression, short temper | 115 (23.5) | 1.95 | [1.13-3.38] | 2.16 | [1.25-3.74] |
| Tension or irritability | 159 (32.5) | 2.14 | [1.32-3.46] | 2.32 | [1.42-3.74] |
| Anxiety or nervousness | 102 (20.9) | 2.03 | [1.13-3.67] | 2.03 | [1.13-3.63] |
| Physical | |||||
| Generalized body aches | 86 (17.6) | 2.07 | [1.13-3.78] | 1.99 | [1.09-3.63] |
| Abdominal bloating | 182 (37.2) | 2.44 | [1.51-3.94] | 2.56 | [1.57-4.14] |
| Lower back pain | 114 (23.3) | 2.05 | [1.11-3.82] | 1.99 | [1.07-3.71] |
| Fatigue | 118 (24.1) | 2.51 | [1.44-4.39] | 2.48 | [1.42-4.35] |
| Breast tenderness | 139 (27.8) | 2.05 | [1.19-3.56] | 2.10 | [1.20-3.67] |
| Swelling of hands or feet | 30 (6.1) | 1.93 | [0.66-5.64] | 2.51 | [0.84-7.39] |
| Abdominal cramping | 200 (40.9) | 1.52 | [0.98-2.34] | 1.48 | [0.95-2.27] |
| Headache | 94 (19.2) | 1.71 | [1.01-2.89] | 1.90 | [1.13-3.19] |
| Insomnia | 31 (6.3) | 2.45 | [0.96-6.23] | 2.66 | [1.04-6.82] |
| Acne | 80 (16.4) | 1.29 | [0.66-2.53] | 1.21 | [0.61-2.39] |
| Appetite change | 93 (19.0) | 2.91 | [1.51-5.58] | 3.29 | [1.70-6.42] |
| Any food cravings | 171 (34.1) | 1.86 | [1.10-3.13] | 1.97 | [1.17-3.32] |
| Craving for chocolate | 113 (23.1) | 1.92 | [1.06-3.49] | 2.12 | [1.17-3.82] |
| Craving for sweets | 107 (21.9) | 2.01 | [1.14-3.56] | 2.14 | [1.21-3.78] |
| Craving for salt | 72 (14.3) | 2.28 | [1.02-5.05] | 2.80 | [1.25-6.30] |
| Other food cravings | 47 (9.6) | 3.06 | [1.35-6.89] | 3.13 | [1.39-7.10] |
Adjusted for age, education, passive and active smoking.
Number and percentage of cycles in which these symptoms were reported as moderate/severe during the premenstrual week.
High perceived stress was strongly associated with risk of experiencing multiple moderate/severe symptoms and moderate/severe PMS in the subsequent cycle. During the premenstrual week, ≥8 moderate/severe symptoms were reported in 17% of cycles, ≥5 moderate/severe symptoms in 36% of cycles, and 32% were classified as moderate/severe PMS by criterion 1 and 24% based on criterion 2 (Table 3). In unadjusted analyses, high perceived stress was significantly associated with a 2.66-fold increased risk of reporting ≥5 and an almost 8-fold increased risk of reporting ≥8 moderate/severe perimenstrual symptoms. High stress was significantly associated with a >2-fold increased risk of moderate/severe PMS using either criterion 1 or 2. Adjustment for age, education, passive and active smoking, and WHtR did not appreciably change these estimates, and results were similar when assessing stress scores on a continuous scale.
Table 3.
Perceived Stress during Previous Cycle and Risk of Multiple Moderate/Severe Perimenstrual Symptoms and PMS, Odds Ratios and 95% Confidence Intervals
| |
|
High stress vs. low stress |
Total stress score (continuous) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| |
|
Unadjusted |
Adjusteda |
Unadjusted |
Adjusteda |
||||
| n (%)b | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Number of moderate/severe symptoms | |||||||||
| Risk of 8+ moderate or severe symptoms vs. < 8 | 81 (16.6) | 7.77 | [3.5-17.12] | 7.17 | [3.29-15.80] | 1.46 | [1.26-1.69] | 1.45 | [1.25-1.68] |
| Risk of 5+ moderate or severe symptoms vs. < 5 | 177 (36.2) | 2.66 | [1.64-3.13] | 2.53 | [1.55-4.14] | 1.26 | [1.16-1.37] | 1.25 | [1.15-1.36] |
| Moderate/severe PMS | |||||||||
| Criterion 1c | 149 (31.6) | 2.05 | [1.13-3.74] | 2.08 | [1.14-3.78] | 1.19 | [1.08-1.31] | 1.20 | [1.08-1.32] |
| Criterion 2d | 114 (24.2) | 2.51 | [1.40-4.40] | 2.49 | [1.34-4.66] | 1.22 | [1.11-1.34] | 1.22 | [1.11-1.36] |
Adjusted for age, education, passive and active smoking, waist/height ratio.
Number and percent of participants reporting these symptoms as moderate/severe during the premenstrual week.
Criterion 1 = 3+ moderate/severe PMS symptoms, 1 of which is psychological, and total symptom severity score during premenstrual week is at least 30% greater than the score during the week after menses.
Criterion 2 = same as criterion 1, except requiring 5 + moderate/severe PMS symptoms to be present.
PMS, premenstrual syndrome.
Most women (63%, n = 148) had low stress preceding both cycles, 16% (n = 38) had high stress preceding cycle 1 but low stress preceding cycle 2, 11% (n = 27) had low stress preceding cycle 1 but high stress preceding cycle 2, and 9% (n = 22) had high stress preceding both cycles (Table 4). For women whose stress levels increased or decreased from cycle 1 to cycle 2, their symptom severity patterns appeared to follow their stress levels. Among women whose stress levels decreased from high (cycle 1) to low (cycle 2), the percentage who reported ≥8 moderate/severe symptoms dropped from 29% in cycle 1 to only 5% in cycle 2 (p = 0.006). To a similar effect, for women whose stress level increased from low (cycle 1) to high (cycle 2), more women reported ≥8 symptoms during the cycle that was preceded by high stress (22% vs. 18%). Compared with those with low stress preceding both cycles, women with high stress preceding both cycles were 25 times more likely to report ≥8 moderate/severe symptoms (p < 0.0001).
Table 4.
Changing Stress Patterns and Risk of ≥ 8 Moderate/Severe Symptoms, Odds Ratios and 95% Confidence Intervals
| |
|
Risk of ≥8 moderate/severe symptoms vs. <8a |
|||||
|---|---|---|---|---|---|---|---|
| |
|
8+ symptoms: n (%) |
Unadjusted |
Adjustedb |
|||
| Stress pattern group | n (%)c | Cycle 1d | Cycle 2d | OR | 95% CI | OR | 95% CI |
| Low stress both cycles | 148 (63) | 7% | 10% | 1.0 | Referent | 1.0 | Referent |
| High stress cycle 1, low stress cycle 2 | 38 (16) | 29% | 5% | 3.3 | [0.9-11.4] | 2.7 | [0.8-9.5] |
| Low stress cycle 1, high stress cycle 2 | 27 (11) | 18% | 22% | 5.2 | [1.4-20.1] | 5.1 | [1.4-18.2] |
| High stress both cycles | 22 (9) | 27% | 50% | 25.0 | [6.1-102.5] | 21.8 | [5.3-90.0] |
Odds ratios (ORs) for ≥8 moderate/severe symptoms were calculated across both cycles using mixed models.
Adjusted for age, education, passive and active smoking, and waist/height ratio.
Number and percentage of participants in each stress group (i.e., low stress both cycles).
Percentage who reported ≥8 moderate/severe symptoms in each cycle within each stress group (i.e., low stress both cycles).
Finally, high stress and total stress scores were positively associated (p < 0.0001) with increased symptom severity scores for total, psychological, and physical symptoms in both adjusted and unadjusted models (Table 5).
Table 5.
Mixed Model Linear Regression of Stress Scores During Previous Cycle on Total, Psychological, and Physical Symptom Severity Scores
| |
Unadjusted estimates |
Adjustedaestimates |
||||
|---|---|---|---|---|---|---|
| Outcome/stress measure | Betab | SE | pc | Betab | SE | pc |
| Total symptom score | ||||||
| Total PSS score | 0.052 | 0.008 | < 0.0001 | 0.057 | 0.008 | <0.0001 |
| High stress vs. low stress | 0.73 | 0.13 | < 0.0001 | 0.78 | 0.12 | <0.0001 |
| Psychological symptom score | ||||||
| Total PSS score | 0.039 | 0.005 | < 0.0001 | 0.043 | 0.005 | <0.0001 |
| High stress vs. low stress | 0.53 | 0.08 | < 0.0001 | 0.56 | 0.08 | <0.0001 |
| Physical symptom score | ||||||
| Total PSS score | 0.039 | 0.007 | < 0.0001 | 0.043 | 0.007 | <0.0001 |
| High stress vs. low stress | 0.55 | 0.06 | < 0.0001 | 0.60 | 0.11 | <0.0001 |
Adjusted estimates are adjusted for age, education, passive and active smoking.
Symptom severity scores were transformed with the square root. Beta estimates are on the transformed scale.
p value calculated from mixed model linear regression.
SE, standard error beta.
Results were unchanged in sensitivity analyses, where participants (n = 21) were excluded who took the baseline PSS >30 days before the first cycle clinic visit or after the first cycle clinic visit, and there was no significant effect modification by physical activity, age, or race/ethnicity (p > 0.20).
Discussion
In this longitudinal study, women with high stress in the previous month were significantly more likely to report an increased number and severity of perimenstrual symptoms in the subsequent cycle. Changing stress levels across the two cycles were associated with a changing pattern of symptom severity. Among those whose stress levels changed from one cycle to the next, more moderate/severe symptoms were reported during the cycle that was preceded by higher stress levels. Although the sample size of the subgroups in this analysis was small, this certainly suggests that stress patterns may be associated with differing perimenstrual symptom patterns. For those who experienced high stress levels preceding both cycles, it is possible there is a cumulative effect of chronic stress on symptom severity, as 50% of these women reported ≥8 moderate/severe symptoms in the second cycle, up from 27% in the first cycle, although a longer longitudinal study would be necessary to fully explore this relationship. Furthermore, we are unable to differentiate the stress measured in this study as either chronic or acute, and these types of stressors may exert different effects on the menstrual cycle.
Our study is subject to several limitations. First, our assessment of perimenstrual symptoms was based on a weekly collection of symptoms rated for severity. We expect that recall of symptoms from the prior week is not subject to substantial recall error because of this limited time period. Although gold standard diagnosis generally requires prospective collection of daily symptoms for two cycles,40 most previous studies of this association used monthly or yearly recall of symptoms.4,41–47 Weekly symptom collection is an alternative to daily collection that is thought to reduce participant burden and increase compliance without involving long-term retrospective recall.11 Furthermore, whereas retrospective assessments of PMS have been criticized for the potential for participants to inflate the severity of symptoms, these criticisms apply mainly to retrospective assessments that reflect an entire menstrual cycle or several cycles and rely solely on memory to differentiate between phases of the cycle.48 The current study required women to recall symptoms over the past week and did not require women to recall over multiple phases of the cycle. However, we acknowledge the limitations associated with weekly reporting of perceived symptom severity. To this end, it was not our intent to diagnose participants with overt PMS but to analyze and describe the longitudinal association between stress and perimenstrual symptom patterns.
In addition, it is possible there is a circularity to the association between high stress and high symptom severity, in that stress may contribute to increased PMS symptom severity, which may in turn cause higher stress levels during the premenstrual period. We attempted to minimize this potential bias, however, by using the stress measure from the symptom-free interval (i.e., late follicular phase/early luteal phase) that preceded the premenstrual time period as the exposure. It is less likely that stress experienced during the symptom-free interval could be fully attributed to PMS symptoms. Finally, although our analysis is longitudinal by design and the stress assessments preceded that of the perimenstrual symptoms, we cannot delineate causality nor fully rule out reverse causality.
This study also has many strengths. Longitudinal analysis allows for the determination of a temporal association between perceived stress and perimenstrual symptoms in the next cycle, which previous cross-sectional studies were not equipped to analyze. We were also able to examine the effects of changing stress levels on premenstrual symptom severity. Our study was conducted exclusively among healthy, reproductive-aged women with no psychological or other chronic diseases, many of which conditions served as confounders in previous studies, as they mimic PMS symptoms.11 Other studies included women on oral contraceptives or antidepressant medications, often prescribed as a treatment for PMS/PMDD and associated with decreased severity of symptoms.49–51 Finally, our study was able to time clinic visits to specific phases of the cycle with the aid of fertility monitors tracking hormone levels across the cycles.
Results from this longitudinal study add to results of a small number of cross-sectional studies that have reported a positive association between psychosocial stress and premenstrual symptoms.4,41–47,52,53 There has been only one similar longitudinal study, and that study found that women with high stress levels during the previous month, particularly during the follicular phase, had increased risk of dysmenorrhea (painful menstruation).54 In contrast, no predictive value of daily stress was found in a small cohort of 25 women with severe PMS, although the full range of severity was not considered; there was no comparison group of women without PMS; and estimates were not adjusted for confounders.55 Other studies have found that past traumatic experiences were associated with greater severity of PMS symptoms.56 Moreover, the percentage of cycles where at least one moderate symptom was reported and the percentage classified as moderate/severe PMS in our study were similar to those reported in other studies.5,46,57
It is not clear how stress may contribute to increased perimenstrual symptom severity, although stress-induced changes in ovarian hormone levels and neurotransmitters may be involved. Stress has been shown to cause hormonal changes through the HPO axis, causing alterations in ovarian hormones that may render a woman more susceptible to menstrual disorders.58 Alternatively, Rabin et al.59 and others60,61 have suggested that PMS was related to an activation of the hypothalamic-pituitary-adrenal (HPA) axis or a heightened sensitivity to its function, in that those who are more sensitive to increased cortisol are those who develop PMS.59 A third potential mechanism relates to the impact of stress levels on the neurotransmitters epinephrine, norepinephrine, and serotonin, which have also been shown to be altered in women with PMS.16,19,62,63 Woods et al.53 found that increased cortisol levels were associated with increased fluid retention and related symptoms (i.e., body aches, bloating, swelling, breast tenderness) and that altered levels of norepinephrine and epinephrine were associated with anxiety and mood-related symptoms. Finally, it has also been suggested that the stressed physiological state leads to a heightened sensitivity to an increased severity of menstrual symptoms.64
Overall, these results extend those of previous cross-sectional studies suggesting that the severity of perimenstrual symptoms may be stress-related. Results are likely generalizable to populations similar to our healthy, reproductive-aged women without diagnosed PMDD. Given the increased direct and indirect healthcare and occupational costs associated with PMS,1,2 we agree with Wang et al.54 that stress reduction programs for reducing psychosocial stress may be a potentially noninvasive and cost-effective method for PMS relief compared with pharmaceutical treatments.
Acknowledgments
We are indebted to all the investigators and staff at the Epidemiology Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the University at Buffalo for their respective roles in the study, their dedication and effort, and assistance in study implementation. In addition, we recognize the BioCycle participants for their extraordinary commitment to the study.
This work was funded by intramural funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Results were presented at the Annual Meeting of the Society of Pediatric and Perinatal Epidemiologic Research, Anaheim, California, June 22–23, 2009.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Borenstein J. Chiou CF. Dean B. Wong J. Wade S. Estimating direct and indirect costs of premenstrual syndrome. J Occup Environ Med. 2005;47:26–33. doi: 10.1097/01.jom.0000150209.44312.d1. [DOI] [PubMed] [Google Scholar]
- 2.Borenstein JE. Dean BB. Endicott J, et al. Health and economic impact of the premenstrual syndrome. J Reprod Med. 2003;48:515–524. [PubMed] [Google Scholar]
- 3.Dean BB. Borenstein JE. A prospective assessment investigating the relationship between work productivity and impairment with premenstrual syndrome. J Occup Environ Med. 2004;46:649–656. doi: 10.1097/01.jom.0000131796.62115.84. [DOI] [PubMed] [Google Scholar]
- 4.Hourani LL. Yuan H. Bray RM. Psychosocial and lifestyle correlates of premenstrual symptoms among military women. J Womens Health. 2004;13:812–821. doi: 10.1089/jwh.2004.13.812. [DOI] [PubMed] [Google Scholar]
- 5.Tabassum S. Afridi B. Aman Z. Tabassum W. Durrani R. Premenstrual syndrome: Frequency and severity in young college girls. J Pak Med Assoc. 2005;55:546–549. [PubMed] [Google Scholar]
- 6.Yonkers KA. O'Brien PM. Eriksson E. Premenstrual syndrome. Lancet. 2008;371:1200–1210. doi: 10.1016/S0140-6736(08)60527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rapkin A. A review of treatment of premenstrual syndrome and premenstrual dysphoric disorder. Psychoneuroendocrinology. 2003;28(Suppl 3):39–53. doi: 10.1016/s0306-4530(03)00096-9. [DOI] [PubMed] [Google Scholar]
- 8.Rapkin AJ. New treatment approaches for premenstrual disorders. Am J Manag Care. 2005;11:S480–S491. [PubMed] [Google Scholar]
- 9.Freeman EW. Halbreich U. Premenstrual syndromes. Psychopharmacol Bull. 1998;34:291–295. [PubMed] [Google Scholar]
- 10.Johnson SR. Premenstrual syndrome, premenstrual dysphoric disorder, and beyond: A clinical primer for practitioners. Obstet Gynecol. 2004;104:845–859. doi: 10.1097/01.AOG.0000140686.66212.1e. [DOI] [PubMed] [Google Scholar]
- 11.Freeman EW. Premenstrual syndrome and premenstrual dysphoric disorder: Definitions and diagnosis. Psychoneuroendocrinology. 2003;28(Suppl 3):25–37. doi: 10.1016/s0306-4530(03)00099-4. [DOI] [PubMed] [Google Scholar]
- 12.Bertone-Johnson ER. Hankinson SE. Johnson SR. Manson JE. Cigarette smoking and the development of premenstrual syndrome. Am J Epidemiol. 2008;168:938–945. doi: 10.1093/aje/kwn194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caan B. Duncan D. Hiatt R. Lewis J. Chapman J. Armstrong MA. Association between alcoholic and caffeinated beverages and premenstrual syndrome. J Reprod Med. 1993;38:630–636. [PubMed] [Google Scholar]
- 14.Gold EB. Bair Y. Block G, et al. Diet and lifestyle factors associated with premenstrual symptoms in a racially diverse community sample: Study of Women's Health Across the Nation (SWAN) J Womens Health. 2007;16:641–656. doi: 10.1089/jwh.2006.0202. [DOI] [PubMed] [Google Scholar]
- 15.Jarvis CI. Lynch AM. Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967–978. doi: 10.1345/aph.1K673. [DOI] [PubMed] [Google Scholar]
- 16.Blum I. Lerman M. Misrachi I, et al. Lack of plasma norepinephrine cyclicity, increased estradiol during the follicular phase, and of progesterone and gonadotrophins at ovulation in women with premenstrual syndrome. Neuropsychobiology. 2004;50:10–15. doi: 10.1159/000077935. [DOI] [PubMed] [Google Scholar]
- 17.Lentz MJ. Woods N. Heitkemper M. Mitchell E. Henker R. Shaver J. Ovarian steroids and premenstrual symptoms: A comparison of group differences and intraindividual patterns. Res Nurs Health. 2007;30:238–249. doi: 10.1002/nur.20188. [DOI] [PubMed] [Google Scholar]
- 18.Redei E. Freeman EW. Daily plasma estradiol and progesterone levels over the menstrual cycle and their relation to premenstrual symptoms. Psychoneuroendocrinology. 1995;20:259–267. doi: 10.1016/0306-4530(94)00057-h. [DOI] [PubMed] [Google Scholar]
- 19.Woods NF. Lentz MJ. Mitchell ES. Shaver J. Heitkemper M. Luteal phase ovarian steroids, stress arousal, premenses perceived stress, and premenstrual symptoms. Res Nurs Health. 1998;21:129–142. doi: 10.1002/(sici)1098-240x(199804)21:2<129::aid-nur4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Wactawski-Wende J. Schisterman EF. Hovey K, et al. BioCycle Study: Design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol. 2009;23:171–184. doi: 10.1111/j.1365-3016.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig CL. Marshall AL. Sjostrom M, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 22.Howards PP. Schisterman EF. Wactawski-Wende J. Reschke JE. Frazer AA. Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: The BioCycle Study. Am J Epidemiol. 2009;169:105–112. doi: 10.1093/aje/kwn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behre HM. Kuhlage J. Gassner C, et al. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: Comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod. 2000;15:2478–2482. doi: 10.1093/humrep/15.12.2478. [DOI] [PubMed] [Google Scholar]
- 24.Cohen S. Kamarck T. Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 25.Endicott J. Nee J. Harrison W. Daily Record of Severity of Problems (DRSP): Reliability and validity. Arch Womens Ment Health. 2006;9:41–49. doi: 10.1007/s00737-005-0103-y. [DOI] [PubMed] [Google Scholar]
- 26.Taylor JW. The timing of menstruation-related symptoms assessed by a daily symptom rating scale. Acta Psychiatr Scand. 1979;60:87–105. doi: 10.1111/j.1600-0447.1979.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 27.Halbreich U. Backstrom T. Eriksson E, et al. Clinical diagnostic criteria for premenstrual syndrome and guidelines for their quantification for research studies. Gynecol Endocrinol. 2007;23:123–130. doi: 10.1080/09513590601167969. [DOI] [PubMed] [Google Scholar]
- 28.Lohman TG, editor; Roche AF, editor; Martorell R, editor. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 29.Ashwell M. Hsieh SD. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr. 2005;56:303–307. doi: 10.1080/09637480500195066. [DOI] [PubMed] [Google Scholar]
- 30.Bianchi-Demicheli F. Ludicke F. Lucas H. Chardonnens D. Premenstrual dysphoric disorder: Current status of treatment. Swiss Med Weekly. 2002;132:574–578. doi: 10.4414/smw.2002.10055. [DOI] [PubMed] [Google Scholar]
- 31.Rasheed P. Al-Sowielem LS. Prevalence and predictors of premenstrual syndrome among college-aged women in Saudi Arabia. Ann Saudi Med. 2003;23:381–387. doi: 10.5144/0256-4947.2003.381. [DOI] [PubMed] [Google Scholar]
- 32.Stoddard JL. Dent CW. Shames L. Bernstein L. Exercise training effects on premenstrual distress and ovarian steroid hormones. Eur J Appl Physiol. 2007;99:27–37. doi: 10.1007/s00421-006-0313-7. [DOI] [PubMed] [Google Scholar]
- 33.Aldana SG. Sutton LD. Jacobson BH. Quirk MG. Relationships between leisure time physical activity and perceived stress. Percept Mot Skills. 1996;82:315–321. doi: 10.2466/pms.1996.82.1.315. [DOI] [PubMed] [Google Scholar]
- 34.Rimmele U. Seiler R. Marti B. Wirtz PH. Ehlert U. Heinrichs M. The level of physical activity affects adrenal and cardiovascular reactivity to psychosocial stress. Psychoneuroendocrinology. 2009;34:190–198. doi: 10.1016/j.psyneuen.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 35.Schnohr P. Kristensen TS. Prescott E. Scharling H. Stress and life dissatisfaction are inversely associated with jogging and other types of physical activity in leisure time—The Copenhagen City Heart Study. Scand J Med Sci Sports. 2005;15:107–112. doi: 10.1111/j.1600-0838.2004.00394.x. [DOI] [PubMed] [Google Scholar]
- 36.Ainsworth BE. Haskell WL. Whitt MC, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell AM. Crane PA. Kim Y. Perceived stress in survivors of suicide: Psychometric properties of the Perceived Stress Scale. Res Nurs Health. 2008;31:576–585. doi: 10.1002/nur.20284. [DOI] [PubMed] [Google Scholar]
- 38.Borenstein JE. Dean BB. Leifke E. Korner P. Yonkers KA. Differences in symptom scores and health outcomes in premenstrual syndrome. J Womens Health. 2007;16:1139–1144. doi: 10.1089/jwh.2006.0230. [DOI] [PubMed] [Google Scholar]
- 39.Borenstein JE. Dean BB. Yonkers KA. Endicott J. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068–1075. doi: 10.1097/01.AOG.0000259920.73000.3b. [DOI] [PubMed] [Google Scholar]
- 40.Futterman LA. Rapkin AJ. Diagnosis of premenstrual disorders. J Reprod Med. 2006;51:349–358. [PubMed] [Google Scholar]
- 41.Deuster PA. Adera T. South-Paul J. Biological, social, and behavioral factors associated with premenstrual syndrome. Arch Fam Med. 1999;8:122–128. doi: 10.1001/archfami.8.2.122. [DOI] [PubMed] [Google Scholar]
- 42.Heilbrun AB., Jr. Frank ME. Self-preoccupation and general stress level as sensitizing factors in premenstrual and menstrual distress. J Psychosom Res. 1989;33:571–577. doi: 10.1016/0022-3999(89)90064-0. [DOI] [PubMed] [Google Scholar]
- 43.Lane T. Francis A. Premenstrual symptomatology, locus of control, anxiety and depression in women with normal menstrual cycles. Arch Womens Ment Health. 2003;6:127–138. doi: 10.1007/s00737-003-0165-7. [DOI] [PubMed] [Google Scholar]
- 44.Lustyk MK. Widman L. Paschane A. Ecker E. Stress, quality of life and physical activity in women with varying degrees of premenstrual symptomatology. Women Health. 2004;39:35–44. doi: 10.1300/J013v39n03_03. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell ES. Woods NF. Lentz MJ. Differentiation of women with three perimenstrual symptom patterns. Nurs Res. 1994;43:25–30. [PubMed] [Google Scholar]
- 46.Strine TW. Chapman DP. Ahluwalia IB. Menstrual-related problems and psychological distress among women in the United States. J Womens Health. 2005;14:316–323. doi: 10.1089/jwh.2005.14.316. [DOI] [PubMed] [Google Scholar]
- 47.Zhao G. Wang L. Qu C. [Prevalence of premenstrual syndrome in reproductive women and its influential factors.] Zhonghua Fu Chan Ke.Za Zhi. 1998;33:222–224. [PubMed] [Google Scholar]
- 48.Richardson JT. Questionnaire studies of paramenstrual symptoms. Psychol Women Q. 1990;14:15–42. [Google Scholar]
- 49.Freeman EW. Kroll R. Rapkin A, et al. Evaluation of a unique oral contraceptive in the treatment of premenstrual dysphoric disorder. J Womens Health Gend Based Med. 2001;10:561–569. doi: 10.1089/15246090152543148. [DOI] [PubMed] [Google Scholar]
- 50.Freeman EW. Rickels K. Yonkers KA. Kunz NR. McPherson M. Upton GV. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737–744. doi: 10.1016/s0029-7844(01)01530-7. [DOI] [PubMed] [Google Scholar]
- 51.Freeman EW. Rickels K. Sondheimer SJ. Polansky M. Xiao S. Continuous or intermittent dosing with sertraline for patients with severe premenstrual syndrome or premenstrual dysphoric disorder. Am J Psychiatry. 2004;161:343–351. doi: 10.1176/appi.ajp.161.2.343. [DOI] [PubMed] [Google Scholar]
- 52.Brown MA. Lewis LL. Cycle-phase changes in perceived stress in women with varying levels of premenstrual symptomatology. Res Nurs Health. 1993;16:423–429. doi: 10.1002/nur.4770160606. [DOI] [PubMed] [Google Scholar]
- 53.Woods NF. Lentz MJ. Mitchell ES. Heitkemper M. Shaver J. Henker R. Perceived stress, physiologic stress arousal, and premenstrual symptoms: Group differences and intra-individual patterns. Res Nurs Health. 1998;21:511–523. doi: 10.1002/(sici)1098-240x(199812)21:6<511::aid-nur5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 54.Wang L. Wang X. Wang W, et al. Stress and dysmenorrhoea: A population-based prospective study. Occup Environ Med. 2004;61:1021–1026. doi: 10.1136/oem.2003.012302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beck LE. Gevirtz R. Mortola JF. The predictive role of psychosocial stress on symptom severity in premenstrual syndrome. Psychosom Med. 1990;52:536–543. doi: 10.1097/00006842-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Koci A. Strickland O. Relationship of adolescent physical and sexual abuse to perimenstrual symptoms (PMS) in adulthood. Issues Ment Health Nurs. 2007;28:75–87. doi: 10.1080/01612840600996281. [DOI] [PubMed] [Google Scholar]
- 57.Hylan TR. Sundell K. Judge R. The impact of premenstrual symptomatology on functioning and treatment-seeking behavior: Experience from the United States, United Kingdom, and France. J Womens Health Gend Based Med. 1999;8:1043–1052. doi: 10.1089/jwh.1.1999.8.1043. [DOI] [PubMed] [Google Scholar]
- 58.Nepomnaschy PA. Welch K. McConnell D. Strassmann BI. England BG. Stress and female reproductive function: A study of daily variations in cortisol, gonadotrophins, and gonadal steroids in a rural Mayan population. Am J Hum Biol. 2004;16:523–532. doi: 10.1002/ajhb.20057. [DOI] [PubMed] [Google Scholar]
- 59.Rabin D. Gold PW. Margioris AN. Chrousos GP. Stress and reproduction: Physiologic and pathophysiologic interactions between the stress and reproductive axes. Adv Exp Med Biol. 1988;245:377–387. doi: 10.1007/978-1-4899-2064-5_29. [DOI] [PubMed] [Google Scholar]
- 60.Roca CA. Schmidt PJ. Altemus M, et al. Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. J Clin Endocrinol Metab. 2003;88:3057–3063. doi: 10.1210/jc.2002-021570. [DOI] [PubMed] [Google Scholar]
- 61.Rabin DS. Schmidt PJ. Campbell G, et al. Hypothalamic-pituitary-adrenal function in patients with the premenstrual syndrome. J Clin Endocrinol Metab. 1990;71:1158–1162. doi: 10.1210/jcem-71-5-1158. [DOI] [PubMed] [Google Scholar]
- 62.Inoue Y. Terao T. Iwata N, et al. Fluctuating serotonergic function in premenstrual dysphoric disorder and premenstrual syndrome: Findings from neuroendocrine challenge tests. Psychopharmacology (Berl) 2007;190:213–219. doi: 10.1007/s00213-006-0607-9. [DOI] [PubMed] [Google Scholar]
- 63.Odink J. Van der Ploeg HM. Van den BH. Van Kempen GM. Bruinse HW. Louwerse ES. Circadian and circatrigintan rhythms of biogenic amines in premenstrual syndrome (PMS) Psychosom Med. 1990;52:346–356. doi: 10.1097/00006842-199005000-00008. [DOI] [PubMed] [Google Scholar]
- 64.Hammarback S. Damber JE. Backstrom T. Relationship between symptom severity and hormone changes in women with premenstrual syndrome. J Clin Endocrinol Metab. 1989;68:125–130. doi: 10.1210/jcem-68-1-125. [DOI] [PubMed] [Google Scholar]