Abstract
The effects of alcohol abuse on HIV disease progression have not been definitively established. A prospective, 30-month, longitudinal study of 231 HIV+ adults included history of alcohol and illicit drug use, adherence to antiretroviral therapy (ART), CD4+ cell count, and HIV viral load every 6 months. Frequent alcohol users (two or more drinks daily) were 2.91 times (95% CI: 1.23–6.85, p = 0.015) more likely to present a decline of CD4 to ≤200 cells/μl, independent of baseline CD4+ cell count and HIV viral load, antiretroviral use over time, time since HIV diagnosis, age, and gender. Frequent alcohol users who were not on ART also increased their risk for CD4 cell decline to ≤200 cells/mm3 (HR = 7.76: 95% CI: 1.2–49.2, p = 0.03). Combined frequent alcohol use with crack-cocaine showed a significant risk of CD4+ cell decline (HR = 3.57: 95% CI: 1.24–10.31, p = 0.018). Frequent alcohol intake was associated with higher viral load over time (β = 0.259, p = 0.038). This significance was maintained in those receiving ART (β = 0.384, p = 0.0457), but not in those without ART. Frequent alcohol intake and the combination of frequent alcohol and crack-cocaine accelerate HIV disease progression. The effect of alcohol on CD4+ cell decline appears to be independent of ART, through a direct action on CD4 cells, although alcohol and substance abuse may lead to unmeasured behaviors that promote HIV disease progression. The effect of alcohol abuse on viral load, however, appears to be through reduced adherence to ART.
Introduction
Alcohol consumption increased worldwide through-out the 1980s and has been stable since that time, with a global mean adult per capita intake of 5.1 liters of pure alcohol per year.1 In 2007, alcohol abuse was the 3rd leading lifestyle-related cause of death with approximately 5% of the U.S. population drinking heavily, and 15% of the population indulging in binge drinking.2 Similarly, alcohol consumption is common in populations with HIV disease, with some studies showing a prevalence of alcohol abuse up to 50% in this population.3–5 Alcohol is an immunosuppressant acting directly through T cell apoptosis,6,7 mitochondrial damage, 8–14 inhibition of T cell responses, natural killer (NK) cell activity, and macrophage phagocytic activity.15 Indirectly, alcohol also suppresses the immune system through alcohol-induced malnutrition and liver disease. Because of its effects on immunity, there has been concern that alcohol consumption among HIV+ individuals may increase susceptibility to opportunistic infections and accelerate disease progression.
Early observational studies did not find an association between alcohol consumption and HIV disease progression.16–19 Animal and in vitro studies during this period, however, suggested a significant effect. Alcohol caused an altered cytokine response and reduced macrophage-reactive oxygen species production in response to HIV infection in studies with transgenic mice, which could lead to accelerated development of AIDS.20,21 Changes in the relative proportions of T cell subsets in the thymus, increased losses of CD4+ and CD8+ cells, and susceptibility to AIDS-associated pathogens were also observed.22–24 Alcohol caused in vitro suppression of human lymphocyte proliferative response to HIV antigens and decreased the production of cytokines in a dose-related manner. In addition, in vivo exposure to alcohol caused an increase in HIV-1 replication in peripheral blood mononuclear cells (PBMCs) that was associated with a decrease in T-helper and suppressor cell function.25,26 More recent simian studies have provided evidence that chronic alcohol intake prior to and during HIV infection results in a higher viral set point, more rapid progression to end-stage disease, and exacerbation of the AIDS wasting syndrome through increased expression of tumor necrosis factor-α and atrogin-1.27–29
Several studies on alcohol and HIV disease progression after the introduction of antiretroviral therapy (ART) have established that alcohol results in reduced viral load response and CD4+ cell reconstitution, and poorer adherence to ART.30–32 A cross-sectional analysis of HIV-1-infected drug users found that heavy alcohol users (defined as alcohol intake three to four or more times per week), who were receiving highly active antiretroviral therapy (HAART), were four times less likely to achieve undetectable viral load and two times more likely to have CD4+ cell counts below 500 cells/μl than moderate drinkers or abstainers.32 A small prospective study of HIV+ patients receiving HAART found no difference in the proportion of those who attained undetectable viral loads or the mean CD4+ cell count between abstainers, moderate alcohol drinkers (<60 g/day), and heavy alcohol drinkers (>60 g/day).33 However, a larger longitudinal study of HIV+ persons with a history of alcohol problems found that alcohol use was a significant predictor of poorer adherence to ART31 and was negatively associated with HIV viral load suppression.34 A prospective study of 161 HIV+ women on ART also found that poorer adherence to ART was significantly associated with virologic failure, and that alcohol use was a significant predictor of lower adherence.30
These findings suggest that alcohol may accelerate disease progression through poorer adherence to ART treatment; however, the recent report by Samet et al.35 of a 7-year prospective study on the association between heavy alcohol intake and lower CD4+ cell counts in an HIV+ cohort not on ART indicates that alcohol may directly influence disease progression through an effect on CD4+ cell count. Evidence of the promotion of T cell apoptosis by ethanol offers a plausible mechanism by which this may happen.36,37 In addition, alcohol consumption has been found to decrease mitochondrial DNA and promote T cell apoptosis through increased systemic oxidative stress.36 In the current investigation, we examined the associations of alcohol use with HIV disease progression, measured by CD4+ cell count decline and HIV viral load, in a cohort of HIV+ drug users.
Materials and Methods
Study design
A prospective, longitudinal study was conducted on a cohort of 231 HIV-infected men and women recruited between March 2002 and December 2005. Participants were eligible for the study if they had documented HIV seropositive status, were age 18 years or older, and were active alcohol or illicit drug users (determined by urine drug toxicology). The study protocol was approved by the Florida International University Institutional Review Board, and all participants gave written informed consent prior to enrollment in the study.
Participants were followed monthly for 30 months. Demographics were collected at the initial screening visit. Biannually, blood was drawn for assessment of CD4+ cell count and HIV viral load. Physical examination, history of alcohol and drug use in the preceding 6 months, report of health care utilization/hospitalizations and intercurrent events, and current HIV medication use and adherence were documented.
Participant examination protocol
Physical examination and medical history were performed by a nurse practitioner after determination of eligibility, and every 6 months thereafter. The medical history included a medication history of all prescribed medications used in the previous 6 months, including ART. Participants were asked to bring the medications they were taking at each assessment visit. In addition, adherence to antiretrovirals was measured by two questionnaires, one on type of antiretrovirals and their prescribed schedule of intake and changes in the last 6 months (initiation, switches, and discontinuations) and a validated modified ACTG questionnaire recalling their adherence in the past week. The participants were also asked if they had been taking ART as prescribed in the previous 6 months. Charts were reviewed for information on prescription and historical adherence, including whether the participants were ART naive. Adherence with ART was also determined by attainment of undetectable HIV-1 RNA viral load (<400 copies/ml). Venous blood was drawn for CD4+ cell count and viral load.
Alcohol and drug abuse questionnaire
The alcohol and drug abuse questionnaire was administered by trained interviewers, and includes pattern and changes of substance abuse in the past 6 months, frequency of use [monthly, two to three times per month, weekly, two to four times per week, daily, and more than daily (specific daily use)], and route of consumption. In the case of alcohol, the questionnaire asks whether beer, wine, or liquor was consumed and the usual amounts. In addition, the questionnaire at baseline also asks for age of initiation of substance abuse and past patterns of consumption. In this specific population, medical record reviews were used to confirm the history of alcohol consumption and alcoholism, and the drug abuse instrument was validated against urine toxicology analysis.
Cytometric and HIV viral load assays
Lymphocyte phenotype was determined with a four-color immunophenotyping panel of monoclonal antibodies. Differential counts were determined using a Coulter MaxM hematology instrument and corroborated with cytocentrifuge smears. Viral load was obtained by the reverse transcriptase polymerase chain reaction using the Roche Amplicor reagents and protocol.
Statistical analysis
Descriptive statistics were used to characterize the population (N = 231). Data were assessed for normality of distribution, and transformations as performed on variables when appropriate. HIV viral load was log transformed, and the square root taken of the CD4+ cell count to normalize the distribution. Differences in baseline categorical characteristics by alcohol use were assessed using χ2, continuous variables with nonnormal distribution using Mann–Whitney, and continuous variables with normal distribution using Student's t-test.
The association between alcohol use and time to decline of CD4+ cell count to ≤200 cells/μl over a 30-month time period was evaluated in participants who had a CD4+ cell count >200 cells/μl at baseline (N = 130), using Cox proportional hazards model. In the Cox analysis, CD4+ cell count ≤200 cells/μl was defined as a nonrecurrent endpoint and only participants who did not have missing CD4+ cell count data were entered into the analyses. The models were restricted to complete case analyses and the differences in drop-out rate of the alcohol categories were estimated to assess the magnitude of the bias introduced.
These analyses were conducted separately for abstainers (those who do not use alcohol), moderate alcohol use (defined as one or less than one drink daily), and frequent alcohol use (defined as two or more drinks daily). The same model was used including only participants who were not receiving antiretrovirals. Baseline CD4+ cell count and HIV viral load, years since diagnosis of HIV, age and gender as time fixed variables, and ART as a time-dependent variable were controlled for in the analyses. Similar analyses were run using the major alcohol and drug use combinations.
A general linear mixed model approach was used to assess the relationship of alcohol intake across all time points and HIV viral load from repeated observations on the same subjects over time. These models included gender, age, years since HIV diagnosis as fixed-time covariates, and ART and CD4+ cell count as time-dependent covariates. These models were used for the total population and repeated for subpopulation analyses (those not receiving antiretrovirals and those using alcohol and drug use combinations). The use of alcohol alone and alcohol combined with other illicit drugs was also assessed in those participants who adhered to ART and whose HIV RNA viral load was <400 copies/ml using Pearson's χ2. Only those participants who had been on ART for >6 months were included in this analysis. All analyses were conducted using two-sided tests and a significance level of 0.05. Analyses were performed with the SPSS-15 for Windows statistical software package (Pearson Prentice Hall, Inc., Upper Saddle River, NJ) and SAS 9.1 software (SAS Institute, Cary, NC).
Results
Population characteristics
We recruited 231 HIV-seropositive participants and followed them for 30 months with an 8% annual attrition rate. The population was 77% black, 13% white Hispanic, and 7% white non-Hispanic. Seventy-three percent were male with a mean age of 42 ± 7 years. Median (min, max) CD4+ cell count was 310 (2, 1254) cells/μl, and HIV viral load was 11,911(399, 750,001) copies/ml, with 63% reporting ART at the baseline visit. The majority of the population had incomes under the poverty level, and almost half were homeless (Table 1). The proportion of participants on antiretrovirals was consistent throughout the study ranging from 63% to 67% with 29–33% having an undetectable viral load (<400 copies/ml). Median CD4+ cell count ranged from 266 to 315 cells/μl, and HIV viral load from 2561 to 27,000 copies/ml through the 30 months of the study, with no directional trend of increase or decline.
Table 1.
Population Characteristics
| Characteristic | |
|---|---|
| Male | 73% |
| Ethnicity | |
| Black | 77% |
| White, non-Hispanic | 13% |
| White, Hispanic | 7% |
| Other | 3% |
| Mean age, years (SD) | 42.72 (6.92) |
| Average monthly income (SD) | $324.26 ($638.56) |
| Percent of homelessness | 49% |
| Years since HIV diagnosis (SD) | 8.2 (5.95) |
| Median CD4+ cell count, cells/μl (range) at baseline | 310 (2, 1254) |
| Median HIV viral load, copies/ml (range) at baseline | 11,911 (399, 750,001) |
| Range % of frequent alcohol use (≥2 drinks daily) over time | 20–23% |
| Median intake of drinks for frequent drinkers | 4 drinks |
| Rate of consistent use of frequent alcohol over time | 39% |
| Range of % ART use over time | 63–67% |
| Range of % viral load <400 copies/ml for ART users over time | 29–33% |
Alcohol was consumed by 54.5% (126 out of a total of 231 participants) of the population with beer the most commonly consumed alcoholic beverage; only 6% (14 out of total of 231 participants) of participants consumed wine or liquor. Frequent alcohol users, defined as two or more alcoholic drinks daily, made up 23% (29 frequent alcohol users out of 126 participants who used alcohol), with a median intake of four alcoholic drinks daily for frequent drinkers. The majority of participants (75%, 22 out of 29 frequent alcohol users) in our cohort who were frequent alcohol drinkers at baseline reported alcohol intake in more than half of their follow-up visits. Of these, 54% reported daily or more than daily drinking and 39% reported continued frequent alcohol intake. Of those who drank alcohol, 38.5% (49 out of 126 alcohol users), 59% (74 out of 126 alcohol users), and 45% (57 out of 126 alcohol users) also used powder cocaine, crack-cocaine, or marijuana, respectively. The analyses were conducted for the effect of alcohol alone as well as for alcohol in combination with drugs of abuse.
Characteristics of participants using antiretrovirals compared to those not on antiretrovirals
There were 87 participants who were not on antiretrovirals at the beginning of the study. These participants were significantly younger (mean age 41.1 ± 6.8 vs. 43.6 ± 6.9 years, p = 0.009), with higher percentage of women (36% vs. 22%, p = 0.02), and had significantly higher CD4+ cell counts (mean CD4+ cell counts 438 ± 296 vs. 334 ± 263, p = 0.006) than those who were on ART. The mean viral load of those not on ART was 98,758.5 ± 178,576. There were no significant differences in drug (cocaine and marijuana—the two main drugs of abuse in this cohort) and alcohol use between the two groups. Three hundred and twenty-five person-years of follow-up were completed for individuals on ART and 175 person-years for participants not on ART.
Characteristics of frequent alcohol users
Frequent alcohol users were significantly older (45.1 ± 6.85 years, p < 0.05) than the rest of the cohort (42.38 ± 6.9 years). There was no significant difference in gender, baseline HIV viral load or CD4+ cell count, antiretroviral use, income, years of education, prevalence of homelessness, hospitalizations, or emergency room visits between those who used alcohol frequently and those who did not. Attendance at scheduled doctor's visits for monitoring of HIV disease was also not significantly different between frequent alcohol users and abstainers. No significant differences in the above mentioned characteristics were found between those who used alcohol frequently combined with cocaine or marijuana and the rest of the cohort. There was no proportional difference in drop-out rate by drinking category at baseline, or for drinking categories at any point of the study (6.65% for frequent drinkers not on ART vs. 8% for those on ART, p = 0.6).
Relative risk for decline of CD4 ≤200 cells/μl by alcohol use
The effect of frequent alcohol use on the rate of decline of CD4+ cell count to <200 cells/μl over 30 months was assessed in 130 participants who had a baseline CD4+ cell count >200 cells/μl. Moderate alcohol use, classified as any reported use of one alcoholic drink daily or less in the past 6 months, did not significantly increase the rate of decline of CD4+ cells compared to abstainers. Frequent alcohol use over time, classified as two or more alcoholic drinks daily however, nearly tripled the risk of a decline of CD4+ cell count to ≤200 cells/μl (HR = 2.91: 95% CI: 1.23–6.85, p = 0.015) compared to moderate alcohol use and alcohol abstention after controlling for baseline CD4+ cell count and HIV viral load, ART as a time-dependent variable, years since self-reported HIV diagnosis, age, and gender (Table 2 and Fig. 1). Both baseline CD4+ cell count (HR = 0.993: 95% CI: 0.990–0.996) and HIV viral load (HR = 1.882: 95% CI: 1.215–2.917) were significantly related to CD4+ cell count decline in this model. A comparison of frequent alcohol users to abstainers also showed a significant risk of decline of CD4+ cell count, although to a lesser extent (HR = 2.39: 95% CI: 1.017–5.598, p = 0.046).
Table 2.
Alcohol Use and Risk of Decline of CD4+ to ≤ 200 Cells/μl Compared to Alcohol Abstention
| Variablea | HRb | 95% CI HR | p Value |
|---|---|---|---|
| Moderate alcohol use (<1 drink/day) | 0.795 | 0.332–1.904 | 0.607 |
| Frequent alcohol use (≥2 drinks/day) | 2.907 | 1.233–6.855 | 0.015 |
| Alcohol and crack cocaine use | 3.575 | 1.240–10.307 | 0.018 |
All Cox regression models were controlled for ART over time, baseline HIV viral load and CD4 cell count, years since diagnosis of HIV, age, and gender.
Hazard ratio.
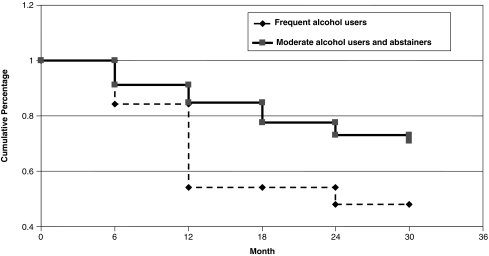
FIG. 1.
The effect of frequent alcohol use on the rate of decline of CD4+ cell count to ≤200 cells/μl over 30 months was assessed in 130 participants who had a baseline CD4+ cell count >200 cells/μl. Frequent alcohol use over time (two or more alcoholic drinks/day) significantly increased the decline of CD4+ cell count to ≤200 cells/μl. From Cox analysis we calculated an HR = 2.91: 95% CI: 1.23–6.85, p = 0.015.
Additionally, when the same model was used including only those participants who were not receiving antiretrovirals, frequent alcohol use over time significantly increased the risk of decline of CD4 ≤200 cells/mm3 (HR = 7.76:95% CI: 1.2–49.2, p = 0.03) compared to moderate alcohol use and alcohol abstention.
Relative risk for decline of CD4 ≤200 cells/μl by the combination of alcohol and drug use
To evaluate the synergistic effects of combined alcohol and drug use, the major drug use combinations, which included alcohol with cocaine and alcohol with marijuana, were also tested for relative risk of decline of CD4+ cell count to ≤200 cells/μl. Combining use of cocaine in any form with frequent alcohol use increased the risk of CD4+ cell decline (HR = 2.37: 95% CI: 1.016–5.509, p = 0.046), albeit not greater than that posed by frequent alcohol use alone. When evaluated by type of cocaine used, the combination of frequent alcohol use with cocaine in the form of crack showed the greatest risk for decline of CD4+ cell count to ≤200 cells/μl (HR = 3.57: 95% CI: 1.24–10.31. p = 0.018), independent of baseline CD4+ cell count and HIV viral load, ART as a time-dependent variable, years since self-reported HIV diagnosis, age, and gender (Table 2). Combined alcohol and marijuana use did not significantly increase risk, although the small sample size of combined alcohol and marijuana users may have limited the power to detect differences.
Alcohol use, ART, and HIV viral load
To evaluate the effect of frequent alcohol use across all time points of the study on HIV viral load, linear mixed effects models were constructed. HIV viral load averaged 0.259 log10 units higher in frequent alcohol drinkers compared to those who drank in moderation or abstained (β = 0.259, p = 0.038) after controlling for ART use, CD4+ cell count, gender, age, and years since HIV diagnosis. These results represent the mean difference in viral load between subjects who used alcohol frequently compared to those who used alcohol in moderation or abstained from alcohol, considering within subject variability in alcohol consumption over time.
Additional subset analyses of the effect of frequent alcohol use on HIV viral load were conducted utilizing the linear mixed effects model. When only those who were receiving antiretrovirals (N = 144) were included in the model, frequent alcohol use was independently associated with higher viral load after controlling for gender, age, and CD4+ cell count (β = 0.384, p = 0.0457). When only those who were not on ART (N = 87) were included in a similar mixed effects model, no statistical significance was found (β = 0.307, p = 0.4). Comparisons of HIV viral load in participants who used combined frequent alcohol and crack-cocaine with the rest of the population did not show statistically significant differences. Moderate alcohol drinkers also did not differ in their HIV viral load from alcohol abstainers.
Discussion
Our main findings show that frequent alcohol consumption is a predictor of CD4+ cell decline, as evidenced by a significantly greater decline of CD4 cell counts in participants who used ART, as well as those who were ART naive. Moreover, the combination of frequent alcohol and crack-cocaine use also significantly decreased CD4+ cell count over time, and the decrease appears to be independent of ART. In addition, frequent alcohol use increased plasma HIV viral load, although this relationship was statistically significant only in participants who were on ART. Thus, frequent alcohol consumption appears to affect HIV disease progression by accelerating the decline of CD4+ cell count and increasing viral load only in those receiving ART.
In our cohort, frequent alcohol users, whose median alcohol intake was four drinks daily, were not significantly different from moderate users or abstainers in factors such as stage of HIV disease, demographics, hospitalizations, or utilization of healthcare services. Similar to other studies, 31,32 we found in this alcohol and drug using cohort as a whole, a poor HIV viral load response in those on ART, with only 29–33% having viral load <400 copies/ml.
The effect of alcohol intake on HIV disease progression has not been clearly elucidated in epidemiological and clinical studies despite evidence from animal and in vitro research that ethanol accelerates disease progression through various mechanisms, including a direct action on CD4+ cell apoptosis. Alcohol and drug use, as well as other behaviors that might not have been measured by our study, are associated with a decrease in adherence to ART, which may affect disease progression in those receiving treatment.30,31,33 Consistent with our results of a more rapid decline in CD4+ cell count in frequent alcohol users independent of ART, and even greater risk of CD4+ cell decline in those not on ART, a recent study found lower CD4+ cell counts in heavy alcohol users who were not on ART, supporting evidence for an effect of alcohol on acceleration of HIV disease progression independent of its effects on medication adherence.35
Effect of alcohol use on CD4+ cell count
Our study finds that frequent alcohol intake was predictive of a faster decline of CD4+ cells longitudinally in a cohort of active alcohol and drug users, of whom 63–67% reported ART over time. The progression of CD4+ cell decline was independent of ART over time, and was greater in those who combined frequent alcohol and crack-cocaine use. These results provide evidence that frequent alcohol use alone, or in combination with crack-cocaine, is a risk factor for accelerated HIV disease progression, specifically a faster decline of CD4+ cell count and increased HIV viral load. This is consistent with findings from a recent report of data from the HIV Alcohol Longitudinal Cohort (ALC) and the HIV Longitudinal Interrelationships of Viruses and Ethanol study (LIVE). This report compared the average difference in HIV disease parameters using generalized linear mixed effects models35 between alcohol users and those who abstained from alcohol, and showed that participants not on ART who were heavy drinkers had CD4+ cell counts that averaged 48.6 cells/μl lower than those who were abstinent. Similar to our findings, the above study found no impact of heavy alcohol use on HIV viral load in those not on ART, leading the authors to suggest that the decrement in CD4+ cell count was not mediated by increased viral load, rather it was related to a direct effect of alcohol on CD4+ cells or lymphocytes in general. They suggested that the large beneficial effect of ART on CD4+ cell count may make it difficult to see a moderate effect of heavy alcohol intake on CD4+ cell count.
Although these authors found no association in those on ART, a previous cross-sectional study by these investigators found lower CD4+ cell counts and significantly higher HIV viral load in those who were on ART and drank heavily after controlling for ART adherence.31 Our longitudinal findings of an effect of frequent alcohol intake on CD4+ cell decline in a combined cohort of participants with or without ART after controlling for ART use confirm these cross-sectional results. The significance of our results in this cohort, contrary to the findings of the 2007 Samet report,35 may be due to population differences. In contrast to our population, the majority of whom were active alcohol and drug users, less than half of the population in the report of Samet et al.35 was currently alcohol or drug dependent. Additionally, our study differs from that of Samet et al.35 in that we compared frequent alcohol intake to those who drank moderately or abstained, whereas, they compared heavy alcohol drinkers only to those who were abstinent.
Moderate alcohol intake, defined as one drink daily or less, did not show an association with CD4+ cell count decline or increased viral load in our study. This is in agreement with an earlier study32 that did not find a significantly lower CD4+ cell count or higher viral load in “light” drinkers, defined as once weekly or less, and with Samet et al.,35 who did not find an association of moderate alcohol intake with CD4+ cell count. An earlier cross-sectional study on an HIV-positive cohort on ART, however, showed significantly lower CD4+ cell count and higher HIV viral load in both moderate and at-risk drinkers, compared to abstainers, even after controlling for ART adherence.31 The discrepancies in the research findings with moderate alcohol use require further investigation.
In our study, frequent alcohol use among participants who were not receiving ART posed a significantly higher risk of declining CD4+ cell counts, but did not increase plasma HIV viral load. Frequent alcohol users who were on ART, however, had a significantly higher viral load than moderate alcohol drinkers and alcohol abstainers. These results suggest that the effect of alcohol on CD4 cells is through metabolic pathways and is independent of the deleterious effect that alcohol might have on adherence to antiretrovirals. The effect on viral load, however, appears to be mediated by lack of adherence to ART, since it was not significantly increased in those who were not receiving antiretrovirals.
The increased effect of combining frequent alcohol intake with crack-cocaine use on CD4+ cell count decline may be due to a combined action of both of these substances on CD4+ cells. Cocaine, and particularly crack-cocaine, has been found to increase the risk of progression to AIDS,38,39 with one study showing a faster decline of CD4+ cell count over a short period of 3–4 months in those who used cocaine compared to those who did not.40
Similar to the findings for alcohol, numerous studies have shown poor antiretroviral medication adherence in cocaine users, particularly during periods of persistent use.41–44 Recent research, however, that evaluated the effect of drug use on disease progression, after adjusting for the confounding effects of adherence to ART, suggests that drug use, including cocaine, may also accelerate disease progression independent of antiretroviral noncompliance.45 Our results confirm these findings, because despite significant effects of the combined substances on CD4+ cell counts, the combined use of alcohol and crack on viral load, which is more related to ART adherence, was not significant. In a previous study, we reported that crack-cocaine, independent of ART, accelerated the decline of CD4+ cell count and was a significant predictor of higher HIV viral load over more than 2 years of the study in a cohort with poor viral load control.38 The greater effect of combined frequent alcohol and crack-cocaine use on the CD4+ cell count in this study suggests that the effects of each substance may be additive.
Effect of alcohol use on HIV viral load
Our findings indicated that HIV viral load in participants reporting frequent alcohol use at any time point of the study had a higher mean HIV viral load independent of ART, compared to those who abstained or did not use alcohol frequently. However, when participants were divided into those receiving ART and those not on ART for subset analyses, those without ART who used alcohol frequently did not have significantly increased viral load as compared to those who were not frequent alcohol users. On the other hand, frequent alcohol users who were on ART had a significantly higher viral load across all time points than those who were either moderate alcohol drinkers or abstainers. Only a relatively low percentage (29–33%) of those on ART had attained viral load control (<400 copies/ml) over time, which is consistent with previous literature with similar populations. Samet et al.31 found that less than half of at-risk drinkers adhered to ART regimens, and others32 found that only 24% of those on ART in a population of drug users and only 14% of heavy alcohol users within this population were able to achieve controlled viral load. These results suggest that the mechanism for diminished HIV viral control in frequent alcohol drinkers is mediated by reduced adherence to ART, and not a direct result of alcohol abuse on viral load.
Limitations
This is a secondary subset analysis of a longitudinal nutrition study of a cohort of HIV-seropositive alcohol and drug users; therefore, this cohort was not originally powered for analyses on alcohol use. The statistical significance of these analyses, however, suggests that the effect size of alcohol on CD4 cell count is large as evidenced by the statistical significance obtained with the relatively small number (N = 29) of participants using alcohol frequently.
In addition, we recognize that multiple substance use, such as combined alcohol and cocaine, might be associated with behaviors and metabolic consequences not measured or considered in these analyses that may promote disease progression and CD4 cell loss, for example, poor adherence with prescribed medication and/or inadequate micronutrient and macronutrient intake. Because the patterns of substance abuse of this HIV-positive cohort might not be common or typical of other HIV+ populations, these findings can be generalized only to other infected populations with similar patterns of substance abuse. Further studies targeting HIV+ heavy alcohol users that control for other confounding behavioral and metabolic variables need to be conducted to confirm and extend these findings.
Conclusions
Our findings support the hypothesis that frequent alcohol intake, as well as the combination of frequent alcohol and crack-cocaine, accelerates HIV disease progression. The effect of alcohol on CD4+ cell decline appears to be independent of ART, through a direct action on CD4 cells, although alcohol and substance abuse may lead to unmeasured behaviors that promote HIV disease progression. The action of alcohol abuse on viral load, however, seems to be through reduced adherence to ART.
Acknowledgments
This work was supported by the National Institute on Drug Abuse (Grant R01-DA-14966). We would like to thank all of the participants in the study without whom advancement in the management of HIV would not be possible. We also thank the Camillus House of Miami, Florida for providing space and resources without which this study would have not been feasible. Appropriate informed consent was obtained for this study and clinical research was conducted in accordance with guidelines for human experimentation as specified by the U.S. Department of Health and Human Services and/or authors' institutions.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.WHO Global Status Report on Alcohol. 2004. [Jul 30;2009 ]. http://www.who.int/substance_abuse/publications/globalstatusreportalcohol2004_alcconsumpt.pdf http://www.who.int/substance_abuse/publications/globalstatusreportalcohol2004_alcconsumpt.pdf
- 2.http://www.cdc.gov/alcohol/index.htm. Reviewed April. 2008. [Apr 30;2009 ]. http://www.cdc.gov/alcohol/index.htm
- 3.Phillips SJ. Freedberg KA. Traphagen ET. Horton NJ. Samet JH. Screening for alcohol problems in HIV-infected primary care patients (abstract) J Gen Intern Med. 2001;16:165. [Google Scholar]
- 4.Samet JH. Phillips SJ. Horton NJ. Traphagen ET. Freedberg KA. Detecting alcohol problems in HIV-infected patients: Use of the CAGE questionnaire. AIDS Res Hum Retroviruses. 2004;20:151–155. doi: 10.1089/088922204773004860. [DOI] [PubMed] [Google Scholar]
- 5.Congliaro J. Gordon AJ. McGinnis KA. Tabeneck L. Justice AC. How harmful is hazardous alcohol use and abuse in HIV infection: Do healthcare providers know who is at risk? J Acquir Immune Defic Syndr. 2003;33:521–525. doi: 10.1097/00126334-200308010-00014. [DOI] [PubMed] [Google Scholar]
- 6.Kapasi AA. Geeta P. Goenka A, et al. Ethanol promotes T cell apoptosis through the mitochondrial pathway. Immunology. 2003;108:313–320. doi: 10.1046/j.1365-2567.2003.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barve SS. Kelkar SV. Gobejishvilli L. Joshi-Barve S. McClain CJ. Mechanisms of alcohol-mediated CD4+ T lymphocyte death: Relevance to HIV and HCV pathogenesis. Front Biosci. 2002;7:1689–1696. doi: 10.2741/A872. [DOI] [PubMed] [Google Scholar]
- 8.Balkan J. Vural P. Oztezcan S, et al. Increased LDL+VLDL oxidizability and plasma homocysteine levels in chronic alcoholic patients. J Nutr Sci Vitaminol (Tokyo) 2005;51(2):99–103. doi: 10.3177/jnsv.51.99. [DOI] [PubMed] [Google Scholar]
- 9.Yuksel N. Uzbay IT. Karakilic H. Aki OE. Etik C. Erbas D. Increased serum nitrite/nitrate (NOx) and malondialdehyde (MDA) levels during alcohol withdrawal in alcoholic patients. Pharmacopsychiatry. 2005;38(2):95–96. doi: 10.1055/s-2005-837809. [DOI] [PubMed] [Google Scholar]
- 10.Kopczynska E. Lampka M. Torlinski L. Ziolkowski M. The level of 8-iso-prostaglandin F2 alpha, 4-hydroxynonenal and malondialdehyde in alcohol dependent men during combined therapy. Psychiatr Pol. 2002;36(2):293–302. [PubMed] [Google Scholar]
- 11.Fromenty B. Grimbert S. Mansouri A, et al. Hepatic mitochondrial DNA deletion in alcoholics. Association with microvesicular steatatosis. Gastroenterology. 1995;108:193–200. doi: 10.1016/0016-5085(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 12.Mansouri A. Fromenty B. Berson A, et al. Multiple hepatic mitochondrial DNA deletions suggest premature oxidative aging in alcoholic patients. J Hepatol. 1997;27:96–102. doi: 10.1016/s0168-8278(97)80286-3. [DOI] [PubMed] [Google Scholar]
- 13.Mansouri A. Gaou I. De Kerguenec C, et al. An alcoholic binge causes massive degradation of hepatic mitochondrial DNA in mice. Gastroenterology. 1999;117:181–190. doi: 10.1016/s0016-5085(99)70566-4. [DOI] [PubMed] [Google Scholar]
- 14.Cahill A. Cunningham CC. Adachi M, et al. Effects of alcohol and oxidative stress on liver pathology: The role of the mitochondrion. Alcohol Clin Exp Res. 2002;26(6):907–915. [PMC free article] [PubMed] [Google Scholar]
- 15.Watson RR. Borgs P. Witte M, et al. Alcohol, immunomodulation, and disease. Alcohol Alcohol. 1994;29(2):131–139. [PubMed] [Google Scholar]
- 16.Kaslow RA. Blackwelder WC. Ostrow DG, et al. No evidence for a role of alcohol or other psychoactive drugs in accelerating immunodeficiency in HIV-1 positive individuals. JAMA. 1989;261:3424–3429. [PubMed] [Google Scholar]
- 17.Coates RA. Farewell VT. Raboud J, et al. Cofactors of progression to acquired immunodeficiency syndrome in a cohort of male sexual contacts of men with human immunodeficiency virus disease. Am J Epidemiol. 1990;132(4):717–722. doi: 10.1093/oxfordjournals.aje.a115713. [DOI] [PubMed] [Google Scholar]
- 18.Veugelers PJ. Page KA. Tindall B, et al. Determinants of HIV progression among homosexual men registered in the Tricontinental Seroconverter Study. Am J Epidemiol. 1994;140(8):747–758. doi: 10.1093/oxfordjournals.aje.a117322. [DOI] [PubMed] [Google Scholar]
- 19.Penkower L. Dew MA. Kingsley L, et al. Alcohol consumption as a cofactor in the progression of HIV infection and AIDS. Alcohol Alcohol. 1995;12(6):547–552. doi: 10.1016/0741-8329(95)00042-9. [DOI] [PubMed] [Google Scholar]
- 20.Wang JY. Liang B. Watson RR. Alcohol consumption alters cytokine release during murine AIDS. Alcohol Alcohol. 1997;14(2):155–159. doi: 10.1016/s0741-8329(97)83138-2. [DOI] [PubMed] [Google Scholar]
- 21.Bautista A. Chronic alcohol intoxication attenuates human immunodeficiency virus-1 glycoprotein 120-induced superoxide anion release by isolated Kupffer cells. Alcohol Clin Exp Res. 1998;22(2):474–480. [PubMed] [Google Scholar]
- 22.Watson RR. Odeley OE. Darban HR. Lopez MC. Modification of lymphoid subsets by chronic ethanol consumption in C57BL/6 mice infected with LP-BM4 murine leukemia virus. Alcohol Alcohol. 1992;27(4):417–424. [PubMed] [Google Scholar]
- 23.Bermudez LE. Petrofsky M. Kolonoski P. Young LS. An animal model of Mycobacterium avium complex disseminated infection after colonization of intestinal tract. J Infect Dis. 1992;165:75–79. doi: 10.1093/infdis/165.1.75. [DOI] [PubMed] [Google Scholar]
- 24.Darban H. Watson RR. Darban JR. Shahbazian LM. Modification of resistance to Streptococcus pneumonia by dietary ethanol, immunization, and murine retroviral infection. Alcohol Clin Exp Res. 1992;16(5):846–851. doi: 10.1111/j.1530-0277.1992.tb01880.x. [DOI] [PubMed] [Google Scholar]
- 25.Bagasra O. Kajdacsy-Balla A. Lischner HW. Effects of alcohol ingestion on in vitro susceptibility of peripheral blood mononuclear cells to infection with HIV and of selected T-cell functions. Alcohol Clin Exp Res. 1989;13(5):636–643. doi: 10.1111/j.1530-0277.1989.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 26.Bagasra O. Kajdacsy-Balla A. Lischner HW. Pomerantz RJ. Alcohol intake increases human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells. J Infect Dis. 1993;167:789–797. doi: 10.1093/infdis/167.4.789. [DOI] [PubMed] [Google Scholar]
- 27.Molina PE. McNurlan M. Tathmacher J, et al. Chronic alcohol accentuates nutritional, metabolic, and immune alterations during asymptomatic simian immunodeficiency virus infection. Alcohol Clin Exp Res. 2006;30(12):2065–2078. doi: 10.1111/j.1530-0277.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- 28.Bagby GJ. Zhang P. Purcell JE. Didier PJ. Nelson S. Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcohol Clin Exp Res. 2006;30(10):1781–1790. doi: 10.1111/j.1530-0277.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- 29.Molina PE. Lang CH. McNurian M. Bagby GJ. Nelson S. Chronic alcohol accentuates simian acquired immunodeficiency syndrome-associated wasting. Alcohol Clin Exp Res. 2008;32(1):138–147. doi: 10.1111/j.1530-0277.2007.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howard AA. Arnsten JH. Lo Y, et al. A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS. 2002;16(16):2175–2182. doi: 10.1097/00002030-200211080-00010. [DOI] [PubMed] [Google Scholar]
- 31.Samet JH. Horton NJ. Meli S. Freedberg KA. Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28(4):572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- 32.Miguez MJ. Shor-Posner G. Morales G. Rodriguez A. Burbano X. HIV treatment in drug abusers: Impact of alcohol use. Addict Biol. 2003;8:33–37. doi: 10.1080/1355621031000069855. [DOI] [PubMed] [Google Scholar]
- 33.Fabris P. Tostitti G. Manfrin V, et al. Does alcohol intake affect highly active antiretroviral therapy (HAART) response in HIV-positive patients? J Acquir Immune Defic Syndr. 2000;25(1):92–93. doi: 10.1097/00042560-200009010-00013. [DOI] [PubMed] [Google Scholar]
- 34.Palepu A. Horton NJ. Tibbetts N. Meli S. Samet JH. Uptake and adherence to highly active antiretroviral therapy among HIV-infected people with alcohol and other substance use problems: The impact of substance abuse treatment. Addiction. 2004;99:361–368. doi: 10.1111/j.1360-0443.2003.00670.x. [DOI] [PubMed] [Google Scholar]
- 35.Samet JH. Cheng DM. Libman H. Nunes DP. Alperen JK. Saitz R. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr. 2007;46(2):194–199. doi: 10.1097/QAI.0b013e318142aabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kapasi AA. Geeta P. Goenka A, et al. Ethanol promotes T cell apoptosis through the mitochondrial pathway. Immunology. 2003;108:313–320. doi: 10.1046/j.1365-2567.2003.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barve SS. Kelkar SV. Gobejishvilli L. Joshi-Barve S. McClain CJ. Mechanisms of alcohol-mediated CD4+ T lymphocyte death: relevance to HIV and HCV pathogenesis. Front Biosci. 2002;7:1689–1696. doi: 10.2741/A872. [DOI] [PubMed] [Google Scholar]
- 38.Baum MK. Rafie C. Lai S. Sales S. Page B. Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2008;50:93–99. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- 39.Webber MP. Schonenbaum EE. Gourevitch MN. Buono D. Klein RS. A prospective study of HIV disease progression in female and male drug users. AIDS. 1999;13(2):257–262. doi: 10.1097/00002030-199902040-00014. [DOI] [PubMed] [Google Scholar]
- 40.Siddiqui NS. Brown LS Jr. Makuch RW. Short-term declines in CD4 levels associated with cocaine use in HIV-1 seropositive, minority injecting drug users. J Natl Med Assoc. 1993;85:293–296. [PMC free article] [PubMed] [Google Scholar]
- 41.Lucas GM. Gebo KA. Chaisson RE. Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16:767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 42.Arnsten JH. Demas PA. Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinkin CH. Barclay TR. Castellon SA, et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behave. 2007;11:185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campa A. Jayaweera DT. Rafie C. Sales S. Page JB. Baum MK. When access to antiretroviral for all is not enough. J Public Admin Manage. 2007;12(3):147–149. [Google Scholar]
- 45.Lucas GM. Griswold M. Gebo KA. Keruly J. Chaisson RE. Moore RD. Illicit drug use and HIV-1 disease progression: A longitudinal study in the era of highly active antiretroviral therapy. Am J Epidemiol. 2006;163:412–420. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]