Abstract
Background
Some think chronic fatigue syndrome (CFS) and fibromyalgia (FM) are variants of the same illness process. This would imply that CFS patients with and without comorbid FM have similar biological underpinnings. To test this, we compared serotonergic-based responses, plasma prolactin (PRL), and self-reported measures of fatigue to intravenous infusion of tryptophan among patients with CFS alone, CFS + FM, and healthy controls.
Methods
Men and women with CFS alone or CFS + FM and healthy subjects, none with current major depressive disorder (MDD), were given 120 mg of l-tryptophan per kg lean body mass intravenously (i.v.). Before and after tryptophan infusion, blood samples were collected, and plasma PRL, tryptophan, and kynurenine concentrations were determined.
Results
Women with CFS alone, but not CFS + FM, showed upregulated plasma PRL responses compared with controls. There were no differences among groups of men. Plasma tryptophan and kynurenine concentrations did not differ among groups.
Conclusions
These results indicate that women with CFS alone have upregulated serotonergic tone that is not seen in those with comorbid FM. The lack of effect in men suggests a mechanism that might explain, in part, the increased prevalence of CFS in women. The data support the interpretation that CFS in women is a different illness from FM.
Introduction
Chronic fatigue syndrome (CFS) and fibromyalgia (FM) are medically unexplained illnesses predominantly affecting women.1 The hallmark symptom of CFS is debilitating fatigue, which is accompanied by such symptoms as impaired concentration, headaches, unrefreshing sleep, and muscle/joint pain.2 When muscle and joint pain are widespread and tender points are frequent, patients can fulfill the case definition for FM as well3; 37% of women with CFS in our Pain & Fatigue Center also fulfilled criteria for FM.4 There is a good deal of overlap between the symptoms of these two syndromes, and controversy exists about whether these are two distinct disorders or simply variants along a spectrum of a single illness.5
The symptoms of fatigue and widespread pain suggest a central nervous system (CNS) origin for FM and CFS. The neurotransmitter serotonin (5-HT) plays a role in both fatigue and pain sensitivity; therefore, 5-HT has been implicated in the symptomatology of both FM and CFS.6 For example, when brain 5-HT is increased either by exercise or administration of the 5-HT precursor tryptophan, fatigue follows.7–10 Importantly however, under these conditions, pain sensitivity decreases, and in contrast, pain sensitivity increases when levels of brain 5-HT are low.10–12 That fatigue follows an upregulated system whereas pain follows a downregulated system suggests pathophysiological differences between syndromes of severe fatigue and widespread pain with tenderness—CFS and FM, respectively.
Researchers have used a variety of pharmacological probes to test serotonergic tone in CFS patients. Some studies suggest increased serotonergic tone via increased release of 5-HT and upregulated postsynaptic receptor levels,13–16 whereas others have reported no evidence for altered 5-HT in CFS.17–19 Only one study has focused on FM, and it too reported increases.20 This unexpected result may have stemmed from the use of buspirone as probe, which also targets dopaminergic receptors. This lack of specificity may also explain, in part, the variable result in CFS.
Another important possible reason for these inconsistencies stems from the fact that previous studies of central 5-HT in CFS and in FM have not specified whether their patient populations comprised patients with CFS or FM alone or patients having both diagnoses. Therefore, the studies done to date have likely involved heterogeneous populations with unknown proportions of those with CFS + FM vs. CFS or FM alone. Despite its syndromic overlap with CFS, FM is proposed to have the opposite serotonergic regulation from CFS, namely, deficient central 5-HT signaling21–23; therefore, the presence of comorbid FM may have confounded the results of previous studies of central 5-HT in CFS patients.
5-HT is synthesized from the amino acid tryptophan via the enzyme tryptophan hydroxylase, which is not normally saturated with tryptophan. As a result, tryptophan loading increases tryptophan concentrations and 5-HT synthesis in the brain in humans24,25 as well as animals.26,27 In fact, tryptophan can be considered a highly specific 5-HT probe, and its effects are not limited to the increased activation of a few select 5-HT receptor subtypes. To date, tryptophan has not been used to probe the serotonergic system in CFS patients.
The major objective of this study was to assess brain serotonergic activity indirectly via plasma prolactin (PRL) response to intravenous tryptophan in patients with CFS alone or CFS + FM compared with control subjects. The difference in serotonergic regulation between CFS and FM led us to hypothesize that we would see a significant increase in PRL in the CFS alone patients but not in those in those with CFS + FM, compared with controls. Finally, we evaluated sex differences in serotonergic-based responses.
Materials and Methods
Subjects
This study was approved by the Institutional Review Board of the New Jersey Medical School. All procedures were carried out with the adequate understanding and written consent of the subjects. Subjects were recruited through the NIAID-funded New Jersey CFS Cooperative Research Center. Women with CFS were referred by their physician or were self-referred in response to media reports, advertisement, or information provided on the Center website. Control subjects were solicited by advertisement or referred by patients. Patients were eligible for study if they fulfilled the 1994 case definition for CFS.2 Therefore, these patients had no known cause for their symptoms based on a physical examination or the results of full blood chemistry examinations, which included tests of thyroid and liver function, electrolytes, urea, full blood count, and erythrocyte sedimentation rate. CFS subjects with comorbid FM were identified using the diagnostic criteria of the American College of Rheumatology.3 Controls were eligible if they reported being in good health, showed no abnormalities on the physical examination, and were not exercising regularly. Subjects were excluded if a structured psychiatric diagnostic interview, the computerized version of the Diagnostic Interview Schedule (DIS),28 showed them positive for psychotic disorders, substance abuse, or eating disorders. In addition, because of the well-known effects of tryptophan in reducing plasma PRL in patients with major depressive disorder (MDD),14 we also excluded 10 subjects (all with CFS and 2 with coexisting FM) with current depression (within the preceding month). Severity of CFS was determined using a method previously described.29 All subjects were medication free for at least 2 weeks prior to testing.
Procedure and measures
Nonmenopausal female subjects were tested during the late follicular phase of their menstrual cycle (7–14 days after the first day of the last menses). Testing took place in the afternoon after insertion of an intravenous (i.v.) catheter in the antecubital vein. Baseline blood samples were taken 30 and 45 minutes after catheter insertion (t = 0 and t = 15, respectively). l-Tryptophan (Ajinomoto U.S.A. Inc., Raleigh, NC) was infused over the following 30 minutes (120 mg/kg lean body mass). After completion of the infusion, blood samples were obtained at 15-minute intervals over the next hour. An additional two samples were taken 30 minutes apart. Plasma was collected and stored at −70°C.
Plasma PRL was determined using a commercially available radioimmunoassay kit (ICN Pharmaceuticals Inc., Costa Mesa, CA). Interassay and intra-assay coefficients of variation (CV) were 8.2 and 4.1%, respectively. The minimum detectable dose was 0.5 ng/mL. Plasma kynurenine and total tryptophan were measured using reverse-phase HPLC with 3-nitro-l-tyrosine as the internal standard, as previously described,30 with some modifications.31 Briefly, 100 μL of plasma was diluted with 100 μL of 50 μM 3-nitro-l-tyrosine, and proteins were precipitated by the addition of 25 μL of 2M trichloroacetic acid. A reverse-phase 55-mm LiChroCART 55-4 cartridge packed with Purosphere STAR RP18 (3 μm grain size) (Merk, Darmstadt, Germany) and a C18 precolumn (Merck) were used with a Waters Breeze HPLC system (Milford, MA). The elution buffer contained 15 mM acetic acid-sodium acetate (pH 4.0) with 27 mM acetonitrile, and the flow rate was 0.9 mL/min. The ratios of the integrated areas under the tryptophan and kynurenine peaks to the area under the 3-nitro-l-tyrosine peak were calculated and used to determine the concentrations in the tryptophan and kynurenine peaks. The CV of the internal standard was <5%.
Self-reported measures of energy before and in response to tryptophan infusion were derived from the activation dimension A of the short form of the activation-deactivation checklist (AD ACL).32 The questionnaire was administered before tryptophan infusion (t = 0) and at 30-minute intervals after completion of the infusion.
Data analysis
Plasma prolactin, tryptophan, and kynurenine concentrations and AD ACL Energy and Tired scores were each evaluated as a function of time, diagnostic group, and sex. Our primary analyses evaluated differences between the control group and each patient group, CFS only or CFS+FM. These analyses employed mixed model regression (mixed models procedure; SPSS, Inc., Chicago, IL) with random subject and intercept terms. Age and duration of illness were analyzed using a 2-way ANOVA as a function of sex and patient group. Group differences in CFS severity scores were evaluated with the chi-square statistic.
Results
Table 1 shows clinical and baseline physiological characteristics of the sample. The men were generally younger than the women, and whereas there was no difference in the age between groups of women, control men were significantly younger than CFS men (p < 0.05). The duration of illness did not differ as a function of sex or CFS group, and there was no difference in CFS severity between the two patient groups or sexes.
Table 1.
Clinical Characteristics of Sample
| |
CFS only |
CFS+FM |
Controls |
|||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
| n | 15 | 7 | 8 | 3 | 10 | 6 |
| Age, years | 41.5 ± 7.3 | 38.7 ± 8.8 | 46.0 ± 6.6 | 32.5 ± 10.6 | 42.3 ± 11.4 | 25.2 ± 2.9 |
| Illness duration, months | 82.2 ± 54.4 | 84.2 ± 60.1 | 57.6 ± 19.5 | 86.0 ± 65.1 | — | — |
| % severe CFS | 42.9 | 50.0 | 60.0 | 50.0 | — | — |
CFS, chronic fatigue syndrome; FM, fibromyalgia.
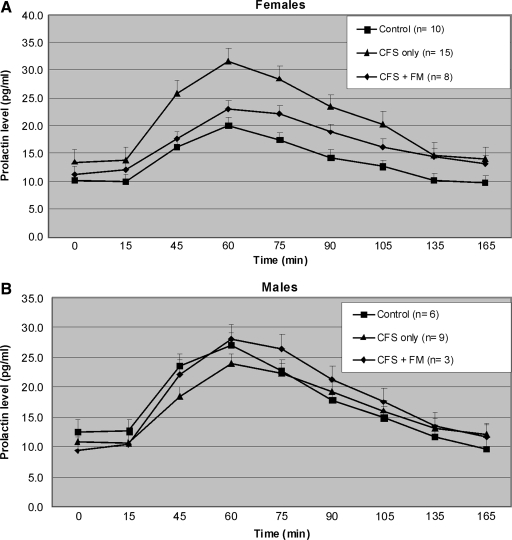
Figure 1 shows the PRL response over time for women (Fig. 1A) and men (Fig. 1B). For both men and women, there were increasing plasma PRL levels after infusion with a peak response at about 60 minutes (or 30 minutes postinfusion). Baseline plasma PRL concentrations were similar in the three diagnostic groups. Although the greatest response in the women was seen in the CFS only group, there was no apparent difference in PRL response among diagnostic groups in the men. For women, the mean (±SD) maximum PRL increases from baseline were 9.9 ± 11.2, 18.6 ± 14.2, and 11.9 ± 4.8 pg/mL for controls, CFS only, and CFS+FM, respectively, whereas comparable values for men were 14.5 ± 10.9, 13.4 ± 9.4, and 18.9 ± 11.8, respectively. Mixed model ANOVA, separated by sex, showed an interaction between diagnosis and time for women [F(16,240) = 1.84, p = 0.03], but not for men [F(16,104) = 0.8, p = 0.62]. These data indicate that the magnitude of the PRL response over time varied as a function of diagnostic group, but only among the women.
FIG. 1.
Plasma prolactin response to exogenous tryptophan (120 mg/kg lean body weight) administered i.v. between 15 and 45 minutes. Among women (A), tryptophan appears to induce a greater plasma prolactin response in the CFS only group, and perhaps in CFS+FM group, than in the control group. There is no apparent difference among the men (B). All subjects exhibited a return to baseline concentration by 90-minutes postinfusion. Values are given as means ± SEM. CFS, chronic fatigue syndrome; FM, fibromyalgia.
To evaluate differences between diagnostic groups within the group of women, we compared PRL changes over time between the control group and each patient group, CFS only and CFS+FM, in separate mixed model analyses. Results showed differences over time between control and CFS only groups [F(8,184) = 2.2, p = 0.03] but not between control and CFS+FM groups [F(8,128) = 0.6, p = 0.76]. Point by point comparisons indicated significantly higher PRL levels in CFS only than control women at all the time points between 75 and 105 minutes postinfusion, inclusive. There were no differences in the baseline levels, and time points after 105 minutes suggested a return to similar baseline levels. Thus, CFS only women, but not men, showed a significantly greater PRL response to tryptophan challenge than did control subjects.
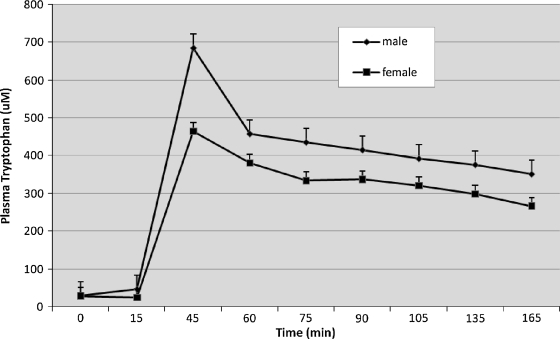
Mean (±SD) levels of plasma tryptophan did not differ among study groups at baseline (29.7 ± 7.4, 30.9 ± 8.4, and 27.0 ± 7.7 pg/mL for controls, CFS only, and CFS + FM, respectively). Figure 2 shows that after the infusion, plasma concentrations of tryptophan immediately increased approximately 200–300 times, followed by a slow decrease over time. Final tryptophan levels were higher than baseline. Although a mixed model ANOVA showed a greater increase in tryptophan over time in men than women [F(8, 330) = 4.0, p < 0.001], concentrations did not vary as a main effect or interaction with diagnosis. These data confirm that men, who were generally larger, received higher doses of tryptophan.
FIG. 2.
Plasma tryptophan concentrations increased significantly over time following tryptophan infusion, moreso in men than in women. However, there were no differences as a function of diagnostic group or interactions of diagnosis with time. Values are given as means ± SEM.
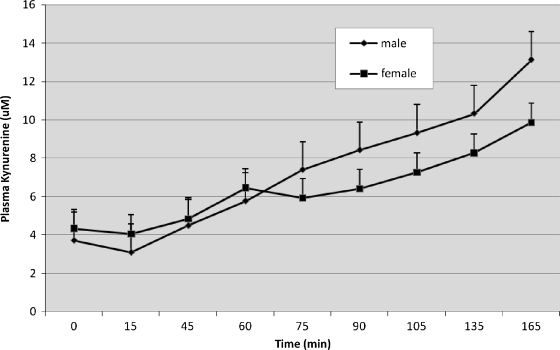
Figure 3 shows increasing plasma kynurenine concentrations over time after infusion. The response of men and women was generally similar over time, except between the 60-minute and 75-minute points, where there was a pause in the women's increase. Mixed model ANOVA showed a greater slope over time in men than women [F(8, 330) = 4.0, p < 0.001]. Plasma kynurenine concentrations did not vary as a function of diagnosis or any interaction. These data confirm the expectation that men, who received higher doses of tryptophan, also produced higher levels of this metabolite.
FIG. 3.
Plasma kynurenine concentrations increased significantly in response to tryptophan infusion, moreso in men than in women. However, there were no differences as a function of diagnostic group or interactions of diagnosis with time. Values are given as means ± SEM.
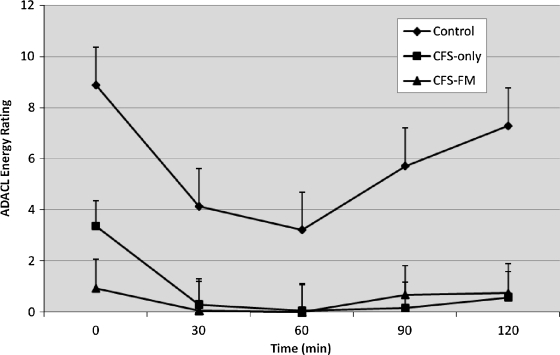
There was no significant effect of sex on AD ACL Energy scores are shown in Figure 4 as a function of time and group. Repeated measures ANOVA showed an effect of time in both the control (F(4, 56) = 11.2, p < 0.001) and the CFS only group (F(4, 76) = 12.9, p < 0.001) but not in the CFS+FM group, which remained at floor levels over time. Post hoc comparisons showed that, relative to baseline, the control group reported less Energy at the 30, 60, and 90 minute assessments and that Energy had recovered to baseline levels at 120 minutes. On the other hand, the CFS only group reported less Energy at all the postinfusion assessments; that is, Energy did not recover to baseline levels by 120 minutes. Sex showed no main or interacting effects with time in these analyses. AD ACL Tired scores showed a similar pattern of response, albeit in the appropriate direction (data not shown). Thus, these analyses suggest that CFS patients may have a different response to tryptophan challenge than do controls. However, the AD ACL did not perform well for the fatigued patients because of marked floor effects prior to tryptophan infusion.
FIG. 4.
ADACL energy scores decreased in CFS patients and healthy subjects after tryptophan infusion. Both patient groups had significantly lower Energy scores than controls prior to infusion. Energy scores decreased in response to tryptophan infusion in CFS patients (p = 0.002) and healthy subjects (p = 0.003), but a decrease was not evident in CFS + FM patients (p = 0.30). Minimum and maximum possible scores are 5 and 20, respectively. ADACL, activation-deactivation checklist.
Discussion
Women with CFS alone, but not those with CFS + FM, had a greater PRL response to exogenous tryptophan than did control women. These differences were not found in men with CFS in either group. There were no significant differences in the plasma concentrations of tryptophan or its principal metabolite, kynurenine, between any group either at baseline or in response to tryptophan infusion after groups were stratified by sex. These results suggest differences in central effects of tryptophan among the study groups in producing the enhanced PRL responses to tryptophan seen in CFS only patients.
CFS is predominantly a problem in women's health, occurring more than twice as often in women as in men.33 The reason for this sex predominance in favor of women is unknown, but the data reported here suggest one mechanism may be via differences in serotonergic function and responsiveness to biological probes. Against this interpretation is the result of an earlier study in men with CFS using fenfluramine as a probe of serotonergic function, which showed an upregulated response compared with controls.15 No differences were found, however, in a mixed gender study in CFS using the same probe.17 Nonetheless, the data reported here using the same experimental protocol to study women and men with CFS do support a biological difference between the sexes that requires further study.
Gender differences as well as the use of pharmacological agents that were not specific to the serotonergic system may explain some of the inconsistent results of prior studies concerning serotonergic tone in CFS. Only one group reduced patient pool heterogeneity by studying only one sex,15,16 and they chose men, substantially less at risk for CFS than women. The probes used in their studies were the 5-HT1A receptor agonist buspirone and the 5-HT-releasing agent d-fenfluramine. Another mixed gender study also found an upregulated PRL response to probe with buspirone,13,16 although buspirone may be having this effect because it also interacts with the dopaminergic system.34 Studies of mixed gender patients with CFS probed with fenfluramine yielded varying results, with one finding significant increases of PRL to the probe14 and two others finding no difference from controls.17,19 Another study in a mixed gender group used the 5-HT2C receptor agonist M-chlorophenylpiperazine (mcpp) and found no evidence for upregulated serotonergic responding.18 However, this probe also has antagonistic properties at 5-HT2A receptors as well as some affinity for α2 adrenoreceptors34 and, thus, is unclear as to its 5-HT-specific effects.
Although gender and nature of the probe may explain these discrepancies in part, another critical variable that has not been studied in any of these reports is the presence of absence of comorbid FM. In fact, the proposed increase in central 5-HT in CFS is virtually opposite that proposed for FM patients. The 5-HT metabolite, 5-HIAA, was at a lower level in the cerebrospinal fluid of patients with FM compared with healthy controls, thus suggestive of decreased central serotonergic tone.35,36 Because plasma PRL levels were higher than in controls in women with CFS alone and similar to levels in controls in women with CFS + FM, we expect that levels might be below those of controls in a future study comparing female patients with FM alone with healthy controls. This would be additional evidence for decreased serotonergic tone in this patient group.
In a more recent study looking at 5-HT1A receptors using positron emission tomography (PET) studies in CFS patients, patients with comorbid FM were excluded. The binding potential of [11C]WAY-100635 was reduced in CFS patients in a number of brain regions, and the authors suggested this may reflect a downregulation of receptors in response to “overall increased serotonergic synaptic transmission.”37 Thus, this is additional evidence supporting increased central serotonergic tone specific to CFS alone.
Differences in PRL responses were not due to differences in the metabolism of infused tryptophan to kynurenine or plasma concentrations of total tryptophan achieved in this study. We, therefore, conclude that these effects of tryptophan on PRL release result from differential central effects of tryptophan across patient groups. In previous studies, baseline plasma free tryptophan levels were reported to be significantly greater38 or lower18 among CFS patients than healthy subjects, but total tryptophan concentrations did not differ,18,39 akin to results found in the current study.
We have previously reported significantly lower baseline AD ACL Energy scores and higher Tired scores in CFS patients.40 Exogenous tryptophan (30 mg/kg) is known to increase both subjective and objective measures of fatigue,41–43 which was also evident in our study with respect to the decrease and increase in AD ACL Energy and Tired scores, respectively, in response to tryptophan infusion. The peak reduction in Energy scores was greater in healthy subjects than in either patient group, and the response in controls, but not patients, waned over time. This difference between patients and controls is most likely driven by a floor effect in the patient groups, each of whom began the study with Energy scores already close to the minimum possible score (minimum and maximum possible scores of 5 and 20, respectively). Developing more sensitive ways to capture subjective and objective fatigue is an important research goal.
As the findings in this study turn on the differences between CFS occurring alone or with comorbid FM, an obvious question concerns the specificity of each diagnosis using clinical criteria dependent on self-reported symptoms. Although CFS patients often have widespread pain, they do not often show the multiple tender points that characterize FM. Obviously, finding biomarkers that differentiate CFS from FM would be an invaluable step forward in adding to the specificity of diagnosis. The data reported here suggest that probing central serotonergic pathways may provide such a biomarker.
In summary, the results of this study are important in that we found only women with CFS alone showed an upregulated PRL response to i.v. tryptophan. This biological difference across the sexes may explain, in part, the skew toward women in illness prevalence. Importantly, the different results based on the presence or absence of comorbid FM strongly suggest that CFS and FM have different underlying pathophysiological underpinnings. A critical next step will be to extend these studies to include all three patient groups: those with CFS alone, those with CFS + FM, and those with FM alone.
Limitations
We cannot be sure that the differences reported here are specific to the tryptophan infusion or might represent some nonspecific response to a brain-active probe; answering this question would require doing another experiment with a different probe of PRL, such as thyrotropin-releasing hormone (TRH). It remains possible that our results may have occurred from a tryptophan effect in decreasing dopaminergic tone, independent of changes in 5-HT,44 or that differences between the two patient groups in PRL response may have to do with differences in biosynthetic or metabolic products of tryptophan, other than 5-HT, that impact PRL release.45,46 Those questions remain to be answered. The near to the floor baseline measures of Energy and Tired in the CFS groups prevent a clear interpretation of negative findings; it remains possible that more appropriate measures might produce behavioral effects that are consistent with the PRL response. Finally, because CFS is primarily a disease of women, our ability to identify and recruit male patients, especially those with CFS + FM, was restricted; thus, our conclusions were limited by the smaller number of men studied.
Acknowledgments
This work was supported by NIH grant AI-34427 and AI-54478. We acknowledge Beth Israel Medical Center (B.H.N.) and NYU College of Dentistry (M.N.J.) for supporting the preparation of this article.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Prins JB. van der Meer JW. Bleijenberg G. Chronic fatigue syndrome. Lancet. 2006;367:346–355. doi: 10.1016/S0140-6736(06)68073-2. [DOI] [PubMed] [Google Scholar]
- 2.Fukuda K. Straus SE. Hickie I, et al. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe F. Smythe HA. Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 4.Ciccone DS. Natelson BH. Comorbid illness in the chronic fatigue syndrome: A test of the single syndrome hypothesis. Psychosom Med. 2003;62:268–275. doi: 10.1097/01.psy.0000033125.08272.a9. [DOI] [PubMed] [Google Scholar]
- 5.Barsky AJ. Borus JF. Functional somatic syndromes. Ann Intern Med. 1999;130:910–921. doi: 10.7326/0003-4819-130-11-199906010-00016. [DOI] [PubMed] [Google Scholar]
- 6.Parker AJR. Wessely S. Cleare AJ. The neuroendocrinology of chronic fatigue syndrome and fibromyalgia. Psychol Med. 2001;31:1331–1345. doi: 10.1017/s0033291701004664. [DOI] [PubMed] [Google Scholar]
- 7.Fernstrom JD. Fernstrom MH. Exercise, serum free tryptophan, and central fatigue. J Nutr. 2006;136:553S–559S. doi: 10.1093/jn/136.2.553S. [DOI] [PubMed] [Google Scholar]
- 8.Davis JM. Alderson NL. Welsh RS. Serotonin and central nervous system fatigue: Nutritional considerations. Am J Clin Nutr. 2000;72(Suppl 2):573S–578S. doi: 10.1093/ajcn/72.2.573S. [DOI] [PubMed] [Google Scholar]
- 9.Blomstrand E. Amino acids and central fatigue. Amino Acids. 2001;20:25–34. doi: 10.1007/s007260170063. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura M. Furue H. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J Pharmacol Sci. 2006;101:107–117. doi: 10.1254/jphs.crj06008x. [DOI] [PubMed] [Google Scholar]
- 11.Messing RB. Fisher LA. Phebus L. Lytle LD. Interaction of diet and drugs in the regulation of brain 5-hydroxyindoles and the response to painful electric shock. Life Sci. 1976;18:707–714. doi: 10.1016/0024-3205(76)90182-x. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Garcia JA. Serotonergic modulation of spinal sensory circuits. Curr Top Med Chem. 2006;6:1987–1996. doi: 10.2174/156802606778522159. [DOI] [PubMed] [Google Scholar]
- 13.Bakheit AMO. Behan PO. Dinan TG. Gray CE. O'Keane V. Possible upregulation of hypothalamic 5-hydroxytryptamine receptors in patients with postviral fatigue syndrome. BMJ. 1992;304:1010–1012. doi: 10.1136/bmj.304.6833.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleare AJ. Bearn J. Allain T, et al. Contrasting neuroendocrine responses in depression and chronic fatigue syndrome. J Affective Disord. 1995;34:283–289. doi: 10.1016/0165-0327(95)00026-j. [DOI] [PubMed] [Google Scholar]
- 15.Sharpe M. Hawton K. Clements A. Cowen PJ. Increased brain serotonin function in men with chronic fatigue syndrome. BMJ. 1997;315:164–165. doi: 10.1136/bmj.315.7101.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharpe M. Clements A. Hawton K, et al. Increased prolactin response to buspirone in chronic fatigue syndrome. J Affective Disord. 1996;41:71–76. doi: 10.1016/0165-0327(96)00075-4. [DOI] [PubMed] [Google Scholar]
- 17.Yatham LN. Morehouse RL. Chisholm BT, et al. Neuroendocrine assessment of serotonin (5-HT) function in chronic fatigue syndrome. Can J Psychiatry. 1995;40:93–96. [PubMed] [Google Scholar]
- 18.Vassallo CM. Feldman E. Peto T, et al. Decreased tryptophan availability but normal postsynaptic 5-HT2c receptor sensitivity in chronic fatigue syndrome. Psychol Med. 2001;31:585–591. doi: 10.1017/s0033291701003580. [DOI] [PubMed] [Google Scholar]
- 19.Bearn J. Allain T. Coskeran P, et al. Neuroendocrine responses to d-fenfluramine and insulin-induced hypoglycemia in chronic fatigue syndrome. Biol Psychiatry. 1995;37:245–252. doi: 10.1016/0006-3223(94)00121-I. [DOI] [PubMed] [Google Scholar]
- 20.Malt EA. Olafsson S. Aakvaag A. Lund A. Ursin H. Altered dopamine D2 receptor function in fibromyalgia patients: A neuroendocrine study with buspirone in women with fibromyalgia compared to female population based controls. J Affective Disord. 2003;75:77–82. doi: 10.1016/s0165-0327(02)00025-3. [DOI] [PubMed] [Google Scholar]
- 21.Yunus MB. Dailey JW. Aldag JC. Masi AT. Jobe PC. Plasma tryptophan and other amino acids in primary fibromyalgia: A controlled study. J Rheumatol. 1992;19:90–94. [PubMed] [Google Scholar]
- 22.Russell IJ. Michalek JE. Vipraio GA. Fletcher EM. Wall K. Serum amino acids in fibrositis/fibromyalgia syndrome. J Rheumatol (Suppl) 1989;19:158–163. [PubMed] [Google Scholar]
- 23.Moldofsky H. Warsh JJ. Plasma tryptophan and musculoskeletal pain in non-articular rheumatism (“fibrositis syndrome”) Pain. 1978;5:65–71. doi: 10.1016/0304-3959(78)90025-8. [DOI] [PubMed] [Google Scholar]
- 24.Moir AT. Eccleston D. The effects of precursor loading in the cerebral metabolism of 5-hydroxyindoles. J Neurochem. 1968;15:1093–1108. doi: 10.1111/j.1471-4159.1968.tb06827.x. [DOI] [PubMed] [Google Scholar]
- 25.Gillman PK. Bartlett JR. Bridges PK, et al. Indolic substances in plasma, cerebrospinal fluid, and frontal cortex of human subjects infused with saline or tryptophan. J Neurochem. 1981;37:410–417. doi: 10.1111/j.1471-4159.1981.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 26.van der Stelt HM. Broersen LM. Olivier B. Westenberg HG. Effects of dietary tryptophan variations on extracellular serotonin in the dorsal hippocampus of rats. Psychopharmacology (Berl) 2004;172:137–144. doi: 10.1007/s00213-003-1632-6. [DOI] [PubMed] [Google Scholar]
- 27.Fernstrom JD. Effects of the diet and other metabolic phenomena on brain tryptophan uptake and serotonin synthesis. Adv Exp Med Biol. 1991;294:369–376. doi: 10.1007/978-1-4684-5952-4_34. [DOI] [PubMed] [Google Scholar]
- 28.Marcus S. Robins LN. Bucholz K. Quick Diagnostic Interview Schedule 3R, version 1. St. Louis, MO: Washington University School of Medicine; 1990. [Google Scholar]
- 29.Natelson BH. Johnson SK. DeLuca J, et al. Reducing heterogeneity in chronic fatigue syndrome: A comparison with depression and multiple sclerosis. Clin Infect Dis. 1995;21:1204–1210. doi: 10.1093/clinids/21.5.1204. [DOI] [PubMed] [Google Scholar]
- 30.Widner B. Werner ER. Schennach H. Wachter H. Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 1997;43:2424–2426. [PubMed] [Google Scholar]
- 31.Laich A. Neurauter G. Widner B. Fuchs D. More rapid method for simultaneous measurement of tryptophan and kynurenine by HPLC. Clin Chem. 2002;48:579–581. [PubMed] [Google Scholar]
- 32.Thayer RE. The activation-deactivation adjective check list (AD ACL). The biopsychology of mood and arousal. New York: Oxford University Press; 1989. pp. 178–180. [Google Scholar]
- 33.Jason LA. Richman JA. Rademaker AW, et al. A community-based study of chronic fatigue syndrome. Arch Intern Med. 1999;159:2129–2137. doi: 10.1001/archinte.159.18.2129. [DOI] [PubMed] [Google Scholar]
- 34.Riblet LA. Taylor DP. Eison MS. Stanton HC. Pharmacology and neurochemistry of buspirone. J Clin Psychiatry. 1982;43:11–18. [PubMed] [Google Scholar]
- 35.Russell IJ. Vaeroy H. Javors M. Nyberg F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550–556. doi: 10.1002/art.1780350509. [DOI] [PubMed] [Google Scholar]
- 36.Legangneux E. Mora JJ. Spreux-Varoquaux O, et al. Cerebrospinal fluid biogenic amine metabolites, plasma-rich platelet serotonin and [3H]-imipramine reuptake in the primary fibromyalgia syndrome. Rheumatology (Oxf ) 2001;40:290–296. doi: 10.1093/rheumatology/40.3.290. [DOI] [PubMed] [Google Scholar]
- 37.Cleare AJ. Messa C. Rabiner EA. Grasby PM. Brain 5-HT1A receptor binding in chronic fatigue syndrome measured using positron emission tomography and [11C]WAY-100635. Biol Psychiatry. 2005;57:239–246. doi: 10.1016/j.biopsych.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 38.Castell LM. Yamamoto T. Phoenix J. Newsholme EA. The role of tryptophan in fatigue in different conditions of stress. Adv Exp Med Biol. 1999;467:697–704. doi: 10.1007/978-1-4615-4709-9_90. [DOI] [PubMed] [Google Scholar]
- 39.Georgiades E. Behan WM. Kilduff LP, et al. Chronic fatigue syndrome: New evidence for a central fatigue disorder. Clin Sci (Lond) 2003;105:213–218. doi: 10.1042/CS20020354. [DOI] [PubMed] [Google Scholar]
- 40.Peckerman A. LaManca JJ. Qureishi B, et al. Baroreceptor reflex and integrative stress responses in chronic fatigue syndrome. Psychosom Med. 2003;65:889–895. doi: 10.1097/01.psy.0000079408.62277.3d. [DOI] [PubMed] [Google Scholar]
- 41.Lieberman HR. Corkin S. Spring BJ. Wurtman RJ. Growdon JH. The effects of dietary neurotransmitter precursors on human behavior. Am J Clin Nutr. 1985;42:366–370. doi: 10.1093/ajcn/42.2.366. [DOI] [PubMed] [Google Scholar]
- 42.Lieberman HR. Corkin S. Spring BJ, et al. The effects of tryptophan and tyrosine on human mood and performance. Psychopharmacol Bull. 1984;20:595–598. [PubMed] [Google Scholar]
- 43.Cunliffe A. Obeid OA. Powell-Tuck J. A placebo-controlled investigation of the effects of tryptophan or placebo on subjective and objective measures of fatigue. Eur J Clin Nutr. 1998;52:425–430. doi: 10.1038/sj.ejcn.1600581. [DOI] [PubMed] [Google Scholar]
- 44.Emiliano AB. Fudge JL. From galactorrhea to osteopenia: Rethinking serotonin-prolactin interactions. Neuropsychopharmacology. 2004;29:833–846. doi: 10.1038/sj.npp.1300412. [DOI] [PubMed] [Google Scholar]
- 45.Moroni F. Tryptophan metabolism and brain function: Focus on kynurenine and other indole metabolites. Eur J Pharmacol. 1999;375:87–100. doi: 10.1016/s0014-2999(99)00196-x. [DOI] [PubMed] [Google Scholar]
- 46.Kostoglou-Athanassiou I. Treacher DF. Wheeler MJ. Forsling ML. Melatonin administration and pituitary hormone secretion. Clin Endocrinol (Oxf ) 1998;48:31–37. doi: 10.1046/j.1365-2265.1998.00341.x. [DOI] [PubMed] [Google Scholar]