Abstract
Catalase, glutathione peroxidase1 (GPx1), and peroxiredoxin (Prx) II are the principal enzymes responsible for peroxide elimination in RBC. We have now evaluated the relative roles of these enzymes by studying inactivation of GPx1 and Prx II in human RBCs. Mass spectrometry revealed that treatment of GPx1 with H2O2 converts the selenocysteine residue at its active site to dehydroalanine (DHA). We developed a blot method for detection of DHA-containing proteins, with which we observed that the amount of DHA-containing GPx1 increases with increasing RBC density, which is correlated with increasing RBC age. Given that the conversion of selenocysteine to DHA is irreversible, the content of DHA-GPx1 in each RBC likely reflects total oxidative stress experienced by the cell during its lifetime. Prx II is inactivated by occasional hyperoxidation of its catalytic cysteine to cysteine sulfinic acid during catalysis. We believe that the activity of sulfiredoxin in RBCs is sufficient to counteract the hyperoxidation of Prx II that occurs in the presence of the basal level of H2O2 flux resulting from hemoglobin autoxidation. If the H2O2 flux is increased above the basal level, however, the sulfinic Prx II begins to accumulate. In the presence of an increased H2O2 flux, inhibition of catalase accelerated the accumulation of sulfinic Prx II, indicative of the protective role of catalase. Antioxid. Redox Signal. 12, 1235–1246.
Introduction
Heme iron in deoxyhemoglobin (deoxyHb) is in the ferrous state in red blood cells (RBCs). The binding of O2 to heme iron results in electron delocalization, with the Fe(II)–O2 bond being in equilibrium with the Fe(III)–superoxide anion (O2·–) bond (34, 43). Occasionally, the superoxide anion is released instead of oxygen, resulting in the autoxidation of Hb to metHb with iron in the ferric state, which cannot bind O2. The superoxide anion is dismutated to H2O2, which can be further converted to the hydroxyl radical, and other hydroperoxides. In RBCs, the autoxidation of 3% of total Hb to metHb is estimated to occur each day (20, 42). Oxygen transport by RBCs is thus a substantial contributor to oxidative stress. RBCs are equipped with various antioxidant enzymes to cope with reactive oxygen species (ROS) produced as the result of the autoxidation of hemoglobin (Hb). Enzymes responsible for the elimination of H2O2 in RBCs include catalase, glutathione peroxidase (GPx) 1, and peroxiredoxins (Prxs) (17, 20, 21, 23, 24, 35).
GPx, which contains a selenocysteine (Sec) at its active site, catalyzes the reduction of hydroperoxides by glutathione (GSH) (13). There are at least four types of GPx in mammalian cells, but GPx1 is the only type present in RBCs (24). Mammalian cells express six different Prx enzymes (11, 33), with Prx II being especially abundant in RBCs (22–24). Proteomic analysis has revealed that RBCs also contain small amounts of Prx I and Prx VI (27). All Prx enzymes contain a conserved cysteine residue (designated the peroxidatic cysteine, CP) that corresponds to Cys-51 of Prx II (33). Four types of Prx (Prx I to Prx IV) contain an additional conserved Cys residue (the resolving cysteine, CR) that corresponds to Cys-172 in Prx II. The Prx enzymes that contain two conserved cysteine residues are thus designated 2-Cys Prxs, whereas Prx VI is referred to as 1-Cys Prx because it contains only the CP. In 2-Cys Prx enzymes, which are homodimers, CP–SH of one subunit is selectively oxidized by peroxides to CP–SOH, which then reacts with CR–SH of the other subunit to produce an intermolecular disulfide bond (33). Reduction of the disulfide intermediate is mediated by thioredoxin (Trx) (10). Despite the fact that cysteine is much less sensitive to oxidation by peroxides than is selenocysteine, the bimolecular rate constant for CP of Prx II was estimated to be 1.3 × 107 M–1 s–1 (31), which is approaching that for GPx.
GPx is susceptible to inactivation by its own substrates. Exposure of purified GPx1 to various hydroperoxides gradually results in its irreversible inactivation (7, 32). The mechanism of inactivation of GPx by peroxide has remained unknown. Prx enzymes are also inactivated during catalysis. The CP–SOH intermediate generated during catalysis occasionally undergoes further oxidation to sulfinic acid (CP–SO2H), leading to inactivation of peroxidase function (48). This hyperoxidation occurs only when Prx is engaged in the catalytic cycle. Reactivation of 2-Cys Prx enzymes is achieved by reduction of the CP–SO2H moiety in a reaction that requires ATP hydrolysis and is catalyzed by sulfiredoxin (Srx), with reducing equivalents such as GSH and Trx (5, 19, 46). Hyperoxidation to sulfinic acid is not restricted to Prx enzymes. Critical cysteine residues of many other proteins including glyceraldehyde-3-phosphate dehydrogenase (GAPDH) also undergo this modification. In contrast, reduction by Srx is highly selective. Among the Prx isoforms, only the sulfinic forms of the 2-Cys Prx subgroup (Prx I to Prx IV), not those of Prx V or Prx VI, are reduced by Srx (47). Moreover, Srx does not act on the sulfinic form of GAPDH (47). This specificity is due to the fact that Srx physically associates with the 2-Cys Prxs but not with other sulfinic proteins.
Among the antioxidant enzymes in RBCs, the activity of glutathione peroxidase 1 (GPx1) was shown to be strongly influenced by lifestyle and environmental factors such as use of dietary supplements and smoking habit and proposed as a strong predictor of cardiovascular risk, which is associated with oxidative stress (1, 6, 38). However, the mechanism underlying the influence of oxidative stress on GPx 1 activity in RBCs has remained obscure. The average lifespan of human RBCs is 120 days. Given the limited capacity of RBCs to replace damaged proteins by de novo synthesis, inactivation of antioxidant enzymes would be expected to perturb the balance between oxidant production and elimination and thereby to accelerate the accumulation of ROS. We now show that oxidative stress induces an inactivation of GPx1 in RBCs and the inactivation is associated with the irreversible conversion of the Sec residue to dehydroalanine (DHA). We developed a convenient blot method for the detection of DHA-containing proteins, with the use of which we found that the amount of inactivated GPx1 is greater in RBCs of higher density. In contrast, immunoblot analysis revealed that the sulfinic form of Prxs was detected only when the H2O2 flux was increased above the basal level attributable to Hb autoxidation. These observations offer insight into the relative roles of catalase, GPx1, and Prxs in the elimination of H2O2 in RBCs.
Materials and Methods
Materials
N-(biotinoyl)-N-9′(iodoacetyl)ethylenediamine (BIAM) was obtained from Molecular Probes (Carlsbad, CA). Horseradish peroxidase (HRP)-conjugated secondary antibodies, EZ-linked N-hydroxysuccinimide biotin, protein G–Sepharose beads, and HRP-conjugated streptavidin were from Pierce (Rockford, IL). Enhanced chemiluminescence (ECL) reagents, Prx I, Prx II, Prx VI, catalase, yeast glutathione reductase, a mouse monoclonal antibody to GPx1, as well as rabbit polyclonal antibodies to Prx I, to Prx II, to the sulfinic forms of 2-Cys Prxs, to Prx VI, to the sulfinic forms of Prx VI, to Srx, to GAPDH, to the sulfinic forms of GAPDH, or to catalase were obtained from Young-In Frontier (Seoul, Korea). Cystamine-2HCl was from Sigma-Aldrich (St. Louis, MO).
Measurement of the selenol content of GPx1 after labeling with BIAM
Human RBCs were lysed by ultrasonic treatment in a solution containing 50 mM potassium phosphate (pH 6.5), 1 mM EDTA, 0.5% Triton X-100, 1% SDS, aprotinin (1 mg/ml), leupeptin (1 mg/ml), 1 μM phenylmethylsulfonyl fluoride, and 10 mM BIAM; the solution was rendered free of O2 by bubbling with N2 gas. The lysates (or GPx1 purified human RBCs) were then incubated for 30 min at room temperature, after which the labeling reaction was terminated by the addition of β-mercaptoethanol to a final concentration of 20 mM and the reaction mixture was centrifuged at 10,000 g for 10 min at 4°C. The resulting supernatant was diluted two-fold with PBS, subjected to immunoprecipitated as described below.
Immunoprecipitation and immunoblot analysis of GPx1
RBC lysates or reaction mixtures were incubated at 4°C first overnight with antibodies to GPx1 and then for 2 h in the additional presence of protein G–Sepharose beads. The beads were then separated by centrifugation and washed three times with ice-cold PBS, and the bead-bound proteins were fractionated by SDS-PAGE on a 14% gel and transferred to a nitrocellulose membrane for immunoblot analysis with antibodies to GPx1. Immune complexes were detected with HRP-conjugated secondary antibodies and ECL reagents.
Identification of DHA in GPx1 by MS/MS sequencing
GPx1 (30 μg) purified from human RBCs was incubated with or without 1 mM H2O2 at 37°C for 1 h and then subjected to digestion with 0.6 μg of bovine trypsin at 37°C for 15 h. The resulting peptides were fractionated by HPLC on a C18 column with a linear gradient of 0 to 100% acetonitrile in 0.1% trifluoroacetic acid and at a flow rate of 1 ml/min. All fractions were collected and analyzed by MALDI-TOF mass spectrometry (MS) with a Voyager ion mirror reflector mass spectrometer (ABI, Foster City, CA). Mass spectra were interpreted with the use of the MS-Fit program (http://prospector.ucsf.edu/prospector/mshome.html). For peptide sequencing, the peptides were subjected to LC-ESI-Q-TOF tandem MS (Micomass, UK). The acquired spectra were processed and used to identify the peptide sequence with the use of MassLynx 4.0 software (Waters Co., Milford, MA).
Preparation of biotin-conjugated cysteamine
Cystamine · 2HCl (150 μl of a 50 mM solution in 0.1 M NaHCO3) was incubated for 1 h at 25°C with 50 μl of 50 mM EZ-linked N-hydroxysuccinimide biotin in DMF with vigorous shaking. The reaction was stopped by the addition of 50 μl of 90 mM dithiothreitol, and the reaction mixture was then incubated for an additional 1 h with shaking before fractionation by HPLC on a C18 column (4.6 by 25 cm; Vydac, W.R. Grace & Co., Deerfield, IL) with a linear gradient of 0 to 100% acetonitrile and at a flow rate of 1 ml/min. Fractions corresponding to the peak of biotin-conjugated cysteamine at 20.5 min were pooled and dried.
Detection of DHA-containing GPx1 after reaction with biotin-conjugated cysteamine
GPx1 was immunoprecipitated from RBC lysates with antibodies to GPx1, and the precipitates (or GPx1 purified from human RBCs) were subjected to alkylation with 10 mM iodoacetamide in the presence 1% SDS for 30 min at room temperature. Proteins were precipitated with trichloroacetic acid, dissolved in 0.1 M NaHCO3 (pH 10.0) containing 1% SDS and 5 mM biotin-conjugated cysteamine, and incubated at 37°C for 18 h. Biotinylated proteins were then detected by SDS-PAGE and blot analysis with HRP-conjugated streptavidin as described above.
Assay of GPx1 activity
Human RBC lysate (40 μg of protein) was incubated for 15 h at 4°C in the wells of a 96-well plate coated with antibodies to GPx1. After washing the plate twice with a solution containing 20 mM Tris-HCl (pH 7.5), 500 mM NaCl, and 0.3% Tween-20, the reaction was performed in 200 μl of a solution containing 0.5 mM EDTA, 200 μM NADPH, 0.2 U of yeast glutathione reductase, 1 mM GSH, 1 mM t-butyl hydroperoxide, and 100 mM Tris-HCl (pH 7.0). The reaction was initiated by the addition of t-butyl hydroperoxide, and NADPH oxidation was monitored for 30 min at 30°C by measurement of the decrease in absorbance at 340 nm. The initial rate of the reaction was determined from the linear portion of the time course.
Purification of GPx1 from RBCs
GPx1 was purified from 4 l of human RBCs by a modified version of a previously described method (2). RBCs lysates were prepared, proteins were precipitated from the lysates by the addition of solid (NH4)2SO4, and the precipitated proteins were dialyzed as described (2). The dialysate was subjected to sequential chromatographies on a column of CM-Sepharose (120 by 45 cm), a column of DEAE-Sepharose column (50 by 60 cm), a phenyl-5PW HPLC column (21.5 by 15 cm; tosoHass), and a Superdex-200 HPLC column (10 by 30 cm). Column fractions were assayed by immunoblot analysis with antibodies to GPx1.
Purification of Sec49 → Cys GPx1 mutant from Escherichia coli
Human GPx1 mutant in which Sec49 was replaced by cysteine residue were generated by standard PCR-mediated site-directed mutagenesis using GPx1 cDNA obtained from HeLa cells as the template and complementary primers containing a TGC that converts the codon (TGA) for Sec to one for Cys. The final mutated PCR products were ligated into the the NdeI and EcoRI sites of pET17b vector to generate pET 17b- GPx1-U49C. Escherichia coli BL21(DE3) competent cells (Novagen, Darmstadt, Germany) were transformed with pET17b-GPx1-U49C; cultured at 37°C for 5 h in 20ml of LB medium with ampicillin (100 μg/ml); and then transferred to 2 liters of fresh LB medium, incubated at 37°C. When the absorbance of the culture at 600 nm reached 0.5, expression was induced by incubation of the cells for 3 h with 0.5 mM isopropyl-β-d-thiogalactopyranoside. The cells were harvested by centrifugation, and stored at −70°C until used. The Sec49 → Cys mutant was purified according to a method similar to that described for the purification of GPx1 from RBC.
Results
Effects of RBC aging on the catalytic activity and selenol content of GPx1 in RBCs
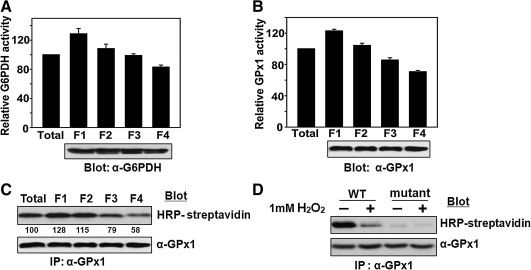
It is generally believed that the density of RBCs increases with RBC aging. We therefore fractionated human RBCs from healthy adult donors by centrifugation on a discontinuous density gradient of Percoll to obtain cells of four different mean densities (37). The activity of G6PDH, a marker of RBC aging (3, 29), decreased gradually with increasing RBC density (Fig. 1A). The activity of GPx1 also decreased with increasing RBC density, with the amount of GPx1 as determined by immunoblot analysis remaining constant (Fig. 1B). To determine whether the reduced GPx1 activity in the RBCs of higher density is accompanied by loss of selenol, we subjected RBC lysates to alkylation at pH 6.5 with BIAM. GPx1 was then immunoprecipitated and subjected to blot analysis with HRP-conjugated streptavidin (Fig. 1C). Although human GPx1 contains five Cys residues in addition to the active site Sec, selenol is selectively alkylated at pH 6.5 because it exists in the ionized form (–Se–), whereas thiols are in the protonated form (–SH) at this pH. The band intensity for BIAM-labeled GPx1 decreased with increasing RBC density, suggesting that a substantial proportion of GPx1 molecules in aged RBCs do not contain selenol. To provide concrete evidence that the selenol is the site of alkylation by BIAM, we prepared a mutant GPx1, in which Sec 49 was changed to Cys, and carried out the BIAM labeling experiment. Wild-type GPx1 was intensively labeled with BIAM, whereas no labeling was apparent with the mutant (Fig. 1D). In addition, H2O2 treatment decreased the labeling intensity of wild-type GPx1. These results indicate that Sec 49 is the only site of modification by BIAM and that oxidation of the selenol prevents BIAM labeling.
FIG. 1.
Effect of aging on the catalytic activity and selenol content of GPx1 in RBCs. (A–C) Fresh RBCs obtained from a healthy human adult were separated on the basis of their density by centrifugation at 4,000 g for 15 min at 4°C on a discontinuous density gradient consisting of 85, 80, 76, 72, 69, and 66% Percoll (from the bottom up), as described previously (8). Among the six discrete bands obtained, the two minor bands at the top and bottom were discarded and the four middle bands (F1 to F4 for the least dense to the most dense, respectively) were used. The activity of G6PDH (A) in each fraction were measured according to the procedure described previously (29), and the activity of GPx1 (B) were measured, and their activities were normalized by the corresponding value for RBCs before fractionation. The fractions were also assayed for the selenol content of GPx1 (C); RBC lysates were subjected to alkylation with BIAM, GPx1 was immunoprecipitated (IP) from the lysates with antibodies to GPx1, and BIAM-labeled GPx1 in the precipitates was detected by blot analysis with HRP-conjugated streptavidin to measure selenol content (SeH). The band intensities are the average of three independent experiments. The presence of equal amounts of protein among assay mixtures was confirmed by immunoblot analysis with antibodies to G6PDH (A) or to GPx1 (B, C). Activity data in A and B are means ± SD of triplicates from a representative experiment. (D) Wild-type (WT) and the Sec49 → Cys mutant (mutant) GPx1(10 μg) were incubated in the absence or presence of 1 mM H2O2 for 1 h at 37°C and precipitated with trichloroacetic acid. The precipitated proteins were subjected to BIAM labeling, followed by HRP-conjugated streptavidin blot analysis as described in C.
Conversion of Sec to DHA in GPx1 treated with H2O2
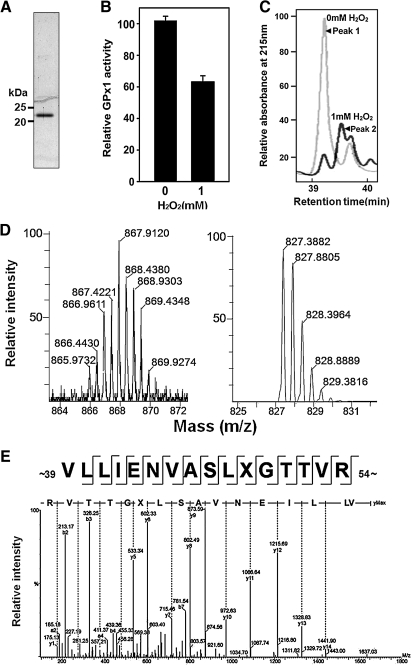
Tryptic peptides prepared from selenoproteins have previously been observed to have lost Sec residues as a result of their conversion to DHA during protein purification or digestion (25, 30). To determine whether GPx1 might lose selenium under oxidative stress, we incubated human GPx1 with 1 mM H2O2 for 1 h at 37°C. Such treatment resulted in ∼40% loss of peroxidase activity (Fig. 2B). Samples of GPx1 that had been incubated in the absence or presence of H2O2 were digested with trypsin, and the resulting peptides were fractionated by HPLC on a C18 column. Peptides were eluted between 20 and 60 min under the conditions described in Materials and Methods (not shown). Although the elution profiles of the two samples were similar, the pattern of peaks eluting between 39 and 40 min differed (Fig. 2C); treatment of GPx1 with H2O2 resulted in a decrease in the size of peak 1 (39.2 min) and the appearance of a new peak (peak 2) at 39.6 min. Peaks 1 and 2 were analyzed by LC-ESI-Q-TOF MS/MS. A selenium isotope distribution of 0.89% 74Se, 9.37% 76Se, 7.63% 77Se, 23.77% 78Se, 49.61% 80Se, and 8.73% 82Se rendered the Sec-containing peptide readily distinguishable from other peptides. Expanded spectra of peak 1 revealed an isotopic distribution typical of Se-containing peptides with a principal m/z peak at [M + 2H+]2+ = 867.9 (Fig. 2D). The monoisotopic mass of 1733.8 Da calculated from this value corresponds to residues 39 TO 54 (VLLIENVASLUGTTVR, where U denotes Sec) of GPx1 (theoretical mass = 1733.8 Da). Expanded ion signals of peak 2 yielded the usual isotopic distribution attributable to 13C (Fig. 2D), in contrast to the unusual distribution for the ions of peak 1. The principal m/z peak at [M + 2H+]2+ = 827.4 corresponds to a molecular mass of 1652.8 Da (theoretical mass of 1652.9 Da). The observed difference of 81.0 Da between the molecular masses derived from the principal ions of peaks 1 and 2 is consistent with the loss of H2Se (81.0 Da) and the concomitant generation of DHA in H2O2 -treated GPx1. The sequence of the peptide corresponding to the principal ion of peak 2 and resulting from the conversion of Sec to DHA was confirmed by MS/MS analysis (Fig. 2E). The amino acid sequence determined from the y ion series was VLLIENVASLXGTTVR, which is identical to that inferred for the peptide corresponding to the principal ion of peak 1 with the exception that Sec at position 49 is replaced by an unknown residue X. The mass difference of 69.0 Da between y5 and y6 ions suggests that this unknown residue is DHA. Together, these results thus indicated that Sec is converted to DHA during exposure of GPx1 to H2O2.
FIG. 2.
Identification of DHA in H2O2-treated GPx1 by MS. (A) Purified human GPx1 was subjected to SDS-PAGE analysis on a 14% gel. Size markers are indicated. (B) Purified Gpx1 (30 μg) was incubated in the absence or presence of 1 mM H2O2 for 1 h at 37°C and then assayed for GPx1 activity. Data are means ± SD of triplicates from a representative experiment. (C) GPx1 incubated with (dark gray line) or without (light gray line) H2O2 as in B was subjected to tryptic digestion, and the resulting peptides were fractionated by HPLC on a C18 column. Peaks eluting between 39 and 40 min are shown. (D) Expanded LC-ESI-Q-TOF tandem MS spectra for peptides corresponding to peak 1 (left panel) or peak 2 (right panel) from C. The isotopic distribution was normalized relative to the largest peak. (E) Tandem MS spectrum obtained from fragmentation of the doubly charged ion with an m/z of 827.4 from peak 2 in D. The y ion series defined the indicated amino acid sequence. The mass difference of 69.0 Da between the y5 and y6 ions defined residue X as DHA.
Detection of DHA-containing GPx1 in aged RBCs with the use of biotin-conjugated cysteamine
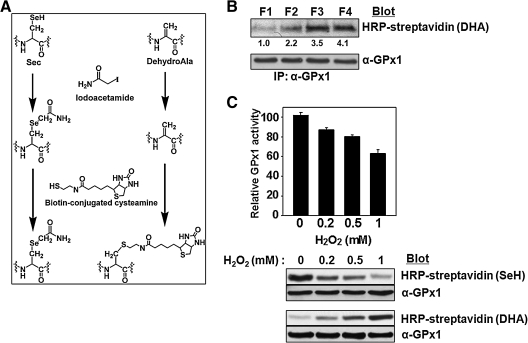
In order to detect DHA-containing GPx1 in cell lysates, we developed a method for specific labeling of DHA-containing proteins with biotin-conjugated cysteamine. This method, which relies on the well-established Michael addition of cysteamine to the DHA moiety (Fig. 3A), involves the immunoprecipitation of GPx1 from cell extracts and the alkylation of Cys–SH and intact Sec–SeH in the precipitated proteins with iodoacetamide. The precipitated proteins are then incubated with biotin-conjugated cysteamine to biotinylate DHA-containing GPx1, which is detected by SDS-PAGE followed by blot analysis with HRP-conjugated streptavidin.
FIG. 3.
Effect of aging and H2O2 treatment on the DHA content of GPx1 as revealed by blot analysis after reaction with biotin-conjugated cysteamine. (A) Chemical reactions underlying the biotinylation of GPx1 containing DHA. After alkylation of free SH and SeH groups by iodoacetamide, DHA residues are biotinylated with biotin-conjugated cysteamine. (B) Fresh RBCs were separated into four fractions (F1, F2, F3, and F4) on the basis of their density (age) as in Fig. 1, and GPx1 was immunoprecipitated from the lysate of each fraction and analyzed for DHA content by sequential reaction with iodoacetamide and biotin-conjugated cysteamine as outlined in A. The band intensities are average of three independent experiments. (C) After incubation of purified GPx1 (10 μg) at 37°C for 1 h with various amounts (0, 0.2, 0.5, or 1 mM ) of H2O2, GPx activity, selenol content(SeH), and DHA-content were measured as described above.
We applied this method to determine whether the conversion of Sec to DHA in GPx1 occurs with aging of RBCs. Indeed, the blot intensity of the band recognized by HRP-conjugated streptavidin increased gradually with increasing RBC density, which is correlated with increasing RBC age (Fig. 3B), indicating that the Sec residue of GPx1 is converted to DHA in a time-dependent manner during exposure to the mild oxidative stress resulting from heme autoxidation. The DHA detection method was validated using purified GPx1 (Fig. 3C). When purified enzyme was exposed for 1 h to various amounts (0, 0.2, 0.5, or 1 mM) of H2O2, the enzyme activity and the selenol content measured by BIAM labeling decreased in parallel in association with the increased H2O2 concentration. On the other hand, the DHA content measured by biotin-conjugated cysteamine increased with increased H2O2 concentration.
Effects of aging on production of the sulfinic forms of Prxs and GAPDH
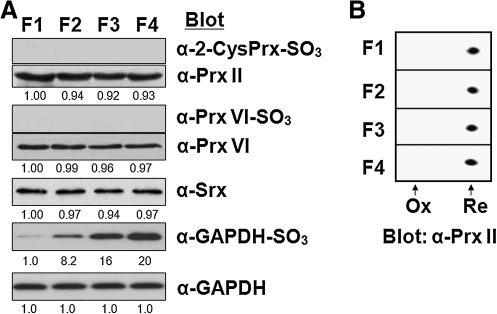
We also investigated whether the inactivated sulfinic forms of Prx enzymes accumulate in aged RBCs. Sulfinic Prxs can be detected by immunoblot analysis with antibodies that recognize a specific sequence surrounding the CP–SO2H (47). Because the active site sequence (DFTFVCPTEI) is the same for 2-Cys Prxs (Prx I to IV) and because the sizes of Prx I and Prx II are identical, the sulfinic forms of Prx I and Prx II cannot be differentiated by immunoblot analysis. Sulfinic Prx VI, however, can be distinguished because its active site sequence (DFTPVCTTEL) differs from that for 2-Cys Prxs and because specific antibodies that recognize the sulfinic form are available. Immunoblot analysis with the antibodies to sulfinic Prxs revealed that neither the sulfinic form of Prx I or Prx II nor that of Prx VI is detected in RBCs of higher density, indicating sulfinic Prxs do not accumulate during the aging process (Fig. 4A). In contrast, the amount of the sulfinic form of GAPDH increased markedly during aging (Fig. 4A). Sulfinylation of proteins induces an acidic shift in their position on two-dimensional gels. Analysis of the age-related RBC fractions by 2D-PAGE did not reveal an acidic shift of Prx II (Fig. 4B), consistent with the results of immunoblot analysis with the antibodies to sulfinic 2-Cys Prxs. Given that Prx II is abundant in RBCs, the conversion of even a small proportion of Prx II molecules to the sulfinic form would be expected to be readily detected by both types of analysis. Srx is expressed in RBCs and its abundance remained unchanged during aging (Fig. 4A).
FIG. 4.
Effects of aging on generation of the sulfinic forms of Prx enzymes or GAPDH. (A) Fresh RBCs were separated into four fractions (F1, F2, F3, and F4) on the basis of their density (age), and lysates of each fraction were subjected to immunoblot analysis with antibodies specific for the sulfinic form of 2-Cys Prxs, Prx VI, or GAPDH. Equal loading of proteins was confirmed by immunoblot analysis with antibodies to Prx II, to Prx VI, and to GAPDH, respectively. Immunoblot analysis was also performed with antibodies to Srx. The band intensities are average of two independent experiments. (B) Lysates of the age-related fractions of RBCs were also subjected to two-dimensional PAGE on a 13-cm Immobiline DryStrips (Amersham; pH 4 to 7, linear) and probed by immunoblot analysis with antibodies to Prx II(20). The positions of hyperoxidized (Ox) and reduced (Re) forms of Prx II are indicated.
Effects on antioxidant enzymes in RBCs of extracellular H2O2 produced by GO
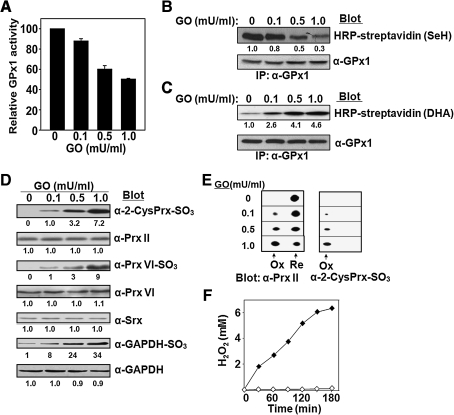
H2O2 passes through the plasma membrane of RBCs, and antioxidant enzymes in RBCs eliminate ROS that originate from the external environment and thereby protect other cells from oxidative injury induced by phagocytic cells or toxins (44). To examine the effects of extracellular H2O2 on RBCs, we added various amounts of glucose oxidase (GO) to these cells (50% hematocrit) suspended in DMEM containing a high concentration of glucose. GO catalyzes the oxidation glucose with concomitant production of H2O2. Incubation of RBCs with GO at 37°C for 3 h resulted in concentration-dependent decreases in the activity (Fig. 5A) and selenol content (Fig. 5B) of GPx1 as well as an increase in the DHA content of GPx1 (Fig. 5C). Although the sulfinic forms of Prx II and Prx VI were not detected in aged RBCs (Fig. 4), their accumulation was apparent in cells incubated in the presence of GO at 0.1 mU/ml and increased further at higher concentrations of GO (Fig. 5D). This accumulation of sulfinic Prx II was confirmed by 2D-PAGE, with 0, 10, 25, and 70% of Prx II being estimated to be oxidized to the sulfinic form in the presence of GO at 0, 0.1, 0.5, and 1.0 mU/ml, respectively (Fig. 5E). The amount of Srx in RBCs was not affected by the presence of GO (Fig. 5D). The sulfinic form of GAPDH was detected in RBCs even in the absence of GO (Fig. 4A), but its abundance increased in the presence of GO (Fig. 5D). The amount of H2O2 produced by GO under our experimental conditions was estimated. In the absence of RBCs, GO at 1 mU/ml generated H2O2 at a rate of ∼45 μM/min (Fig. 5F). However, accumulation of H2O2 was not detected when the same amount of GO was added to the suspension of RBCs (Fig. 5F); indeed, no accumulation of H2O2 was detected even at a GO concentration of 50 mU/ml, which could produce H2O2 at a rate of ∼2.25 mM/min in the absence of RBCs (not shown). These observations indicate that human blood is able to metabolize H2O2 efficiently by catalase in RBCs. Nevertheless, the entry of H2O2 into RBCs induces oxidative damage to many proteins including G6PDH and GAPDH. In addition, loss of the Sec residue of GPx1 is accelerated even at a low rate of H2O2 entry (4.5 μM/min, as generated by GO at 0.1 mU/ml). Furthermore, the hyperoxidation of Prx II and Prx VI, which was not observed during normal aging of RBCs, becomes apparent at the rate of H2O2 entry.
FIG. 5.
Effects on antioxidant enzymes in RBCs of extracellular H2O2 produced by GO. (A–E) Various amounts (0, 0.1, 0.5, or 1 mU) of glucose oxidase (GO) were added to 1 ml of RBCs at a 50% hematocrit in DMEM containing a high concentration (4,500 mg/l) of glucose. After incubation for 3 h with gentle shaking at 37°C, the RBCs were lysed and subjected to the following analyses: (A) Measurement of GPx1 activity and its normalization by that of RBCs incubated in the absence of GO. Data are means ± SD of triplicates from a representative experiment. (B, C) Determination of the selenol (SeH) and DHA contents, respectively, of GPx1. Equal loading of proteins was confirmed by immunoblot analysis with antibodies to GPx1. (D) Immunoblot analysis with antibodies specific for the sulfinic forms of 2-Cys Prxs, Prx VI, or GAPDH. Equal loading of proteins was confirmed by immunoblot analysis with antibodies to Prx II, to Prx VI, or to GAPDH, respectively. Immunoblot analysis was also performed with antibodies to Srx. The band intensities shown in B–D are average of two independent experiments. (E) Two-dimensional PAGE followed by immunoblot analysis with antibodies to Prx II and to the sulfinic form of 2-Cys Prxs. The positions of hyperoxidized (Ox) and reduced (Re) forms of Prx II are indicated. (F) The H2O2 concentration generated by incubation of GO at 1 mU/ml in the absence (solid diamonds) or presence (open diamonds) of RBCs at a 50% hematocrit was measured on the basis of ferrous oxidation of xylenol orange (45).
Effects of N-phenylhydroxylamine on antioxidant enzymes in RBCs
A variety of drugs including sulfonamides and industrial chemicals such as aniline induce hemolytic anemia (4, 15, 40). These arylamine compounds are metabolized in the liver, and the resulting N-hydroxyarylamines react with oxyHb to produce superoxide anion.
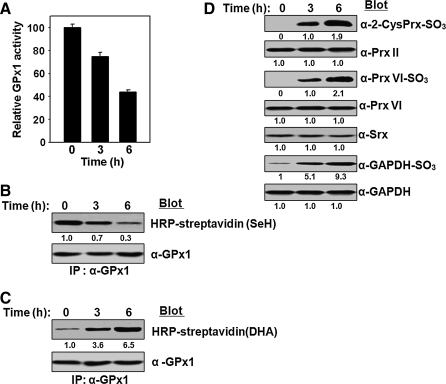
To examine the effects of such extra oxidative stress produced internally by an environmental chemical, we incubated a 50% hematocrit of RBCs with 200 μM N-phenylhydroxylamine for various times. The activity (Fig. 6A) and selenol content (Fig. 6B) of GPx1 decreased with time whereas the DHA content of GPx1 increased (Fig. 6C) on exposure of RBCs to N-phenylhydroxylamine. ROS produced by N-phenylhydroxylamine also induced hyperoxidation of Prx II, Prx VI, and GAPDH (Fig. 6D).
FIG. 6.
Effects of N-phenylhydroxylamine on antioxidant enzymes in RBCs. N-phenylhydroxy-lamine (200 μM) was added to a 50% hematocrit of RBCs in DMEM containing a high glucose concentration (4500 mg/l). After incubation for various times (0, 3, or 6 h) with gentle shaking at 37°C, the RBCs were lysed and subjected to the following analyses: (A) Measurement of GPx1 activity and its normalization relative to that of cells incubated in the absence of N-phenylhydroxylamine (time 0). Data are means ± SD of triplicates from a representative experiment. (B, C) Determination of the selenol (SeH) and DHA contents, respectively, of GPx1. Equal loading of proteins was confirmed by immunoblot analysis with antibodies to GPx1. (D) Immunoblot analysis with antibodies specific for the sulfinic forms of 2-Cys Prxs, Prx VI, or GAPDH. Equal loading of proteins was confirmed by immunoblot analysis with antibodies to Prx II, to Prx VI, and to GAPDH, respectively. Immunoblot analysis was also performed with antibodies to Srx. The band intensities shown in B–D are the average of two independent experiments.
Effect of catalase inhibition on Prx II hyperoxidation
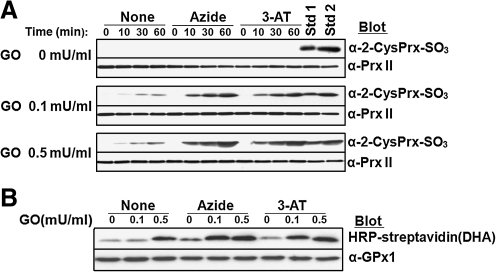
Catalase is inhibited specifically by 3-amino-1,2,4-triazole (3-AT) (26). This inhibitory action is only apparent in the presence of H2O2 because it is exerted on compound I. Catalase is inhibited nonspecifically by azide. Sodium azide (10 mM) or 3-AT (50 mM) was added to a 50% hematocrit of RBCs in DMEM containing high glucose before exposure of the cells to various concentrations of GO. After incubation of the RBC suspension for various times, cell lysates were subjected to immunoblot analysis with antibodies to the sulfinic form of 2-Cys Prxs (Fig. 7A). RBC lysates analyzed in Fig. 5E were included in the immunoblot analysis as hyperoxidation standards; in standards 1 and 2, ∼10, and ∼25%, respectively, of Prx II was hyperoxidized as the result of incubation with GO at 0.1 and 0.5 mU/ml, respectively, for 3 h. In the absence of GO, inhibition of catalase did not induce Prx II hyperoxidation (Fig. 7A). In the presence of GO at 0.1 or 0.5 mU/ml, Prx II was slowly hyperoxidized and this effect was accelerated by a factor of 5 to 10 when catalase was inhibited (Fig. 7A). We also evaluated the effect of catalase inhibition on GPx1-DHA formation (Fig. 7B). Although the effect was not as pronounced as that on Prx II hyperoxidation, the absence of catalase activity clearly increased GPx1-DHA formation. These results suggest that the absence of catalase activity is not enough of a burden to the Prx II and GPx1 systems to result in hyperoxidation of Prx II or irreversible oxidation of GPx1 unless H2O2 enters RBCs from the external environment.
FIG. 7.
Effect of catalase inhibition on Prx II hyperoxidation and GPx1-DHA formation. RBCs at a 50% hematocrit in DMEM containing high glucose (4500 mg/l) were incubated at 37°C first for 10 min in the absence or presence of 10 mM sodium azide or 50 mM 3-AT and then for the indicated times (0, 10, 30, and 60 min) in the additional presence of various concentrations (0, 0.1, or 0.5 mU/ml) of GO. (A) The cells were then lysed and subjected to immunoblot analysis with antibodies specific for the sulfinic form of 2-Cys Prxs. Equal loading of proteins was confirmed by immunoblot analysis with antibodies to Prx II. Lysates of RBCs that had been treated with GO at 0.1 mU/ml (Std 1) or 0.5 mU/ml (Std 2) for 3 h (Fig. 5E) were included in the immunoblot analysis as standards; in these standard samples, ∼10 and ∼25% of Prx II was hyperoxidized as judged on the basis of 2D-PAGE analysis. (B) RBCs that had been incubated for 60 min with various concentrations (0, 0.1, or 0.5 mU/ml) of GO in the absence or presence of 10 mM sodium azide or 50 mM 3-AT as in A were lysed, and the lysates were then subjected to the determination of DHA contents. Equal loading of proteins was confirmed by immunoblot analysis with antibodies to GPx1.
Discussion
We have shown that the Sec residue of GPx1 is converted to DHA during aging of RBCs in vivo. Our observation that treatment of purified GPx1 with H2O2 induces the conversion of Sec to DHA suggests that an oxidative environment promotes the conversion of GPx–SeH to GPx–SeO2H and subsequent loss of H2SeO2 via β-elimination. Treatment of GPx with H2O2 has been shown to generate GPx–SeO2H (41). We developed a convenient method for estimation of the amount of DHA–GPx1 in cell homogenates. This blot-based method depends on specific addition of biotin-conjugated cysteamine to the DHA residue followed by detection of biotinylated protein based on its interaction with streptavidin. With the use of this method, we found that conversion of the Sec residue of GPx1 to DHA occurred during aging of RBCs in vivo as well as in RBCs exposed to H2O2 generated either externally by GO or internally as a result of N-phenylhydroxylamine-induced Hb autoxidation.
Selenocysteine is not the only source of DHA in cells. Cysteine residues of many proteins are present in the form of Cys–SO2H in normal tissues (14) and some of them are converted to DHA (39). However, DHA is not frequently found in positions corresponding to Cys residues. This difference is likely attributable to the greater strength of the C–S bond (272 kJ/mol) compared with that of the C–Se bond (234 kJ/mol) (25). These multiple sources of DHA are consistent with our observation that direct blot analysis of crude RBC extracts yielded many positive bands (not shown). It was thus necessary to immunoprecipitate GPx1 before alkylation and labeling with biotin-conjugated cysteamine in order to measure DHA specifically in GPx1.
The concentration of Prx II was reported to be ∼240 μM (28). In contrast, Prx I and Prx VI are present at concentrations that are only ∼1% of that of Prx II (data not shown). During the catalytic cycle of Prx II, the intermolecular Cp-CR disulfide is reduced by Trx1, which has been suggested to be the limiting step in the process of H2O2 elimination in RBCs because the concentration of thioredoxin reductase 1 (TrxR1), the enzyme required for the reduction of oxidized Trx1, is low in RBCs (23). Nevertheless, Prx II molecules were found as monomers with the CP residue in the thiol state in RBCs obtained from normal individuals (23), suggesting that the reducing capacity of the Trx system in RBCs is sufficient to maintain Prx II in its reduced form while confronting the basal level of H2O2 flux originating from Hb autoxidation. It appears, however, that the Trx system does not have much extra capacity to cope with a small increase in H2O2 flux, given that Prx II molecules were found in the disulfide-linked dimeric state in RBCs treated for 10 min with H2O2 at a concentration as low as 0.5 μM (23).
Although Prx II is mainly responsible for dealing with the basal level of H2O2 flux originating from Hb autoxidation (20, 23), we did not detect sulfinic Prx II in aged RBCs. These findings are likely attributable to the fact that RBCs contain Srx at a sufficient concentration to counteract the hyperoxidation. If we assume that autoxidation occurs at a rate of 3% of total Hb a day in the 50% hematocrit suspension and that all superoxide anions produced from the autoxidation are dismutated to H2O2, the rate of H2O2 production in the suspension would be ∼0.1 μM/min. When RBCs at a 50% hematocrit were incubated for 3 h with GO at 0.1 mU/ml, which generates H2O2 at a rate of ∼4.5 μM/min, ∼10% of Prx II was found to be hyperoxidized. The hyperoxidation rate is proportional to the rate at which Prx eliminates H2O2 (48). This result suggests that the additional flux of H2O2 at a rate of ∼4.5 μM/min increases the hyperoxidation of Prx II to a level that exceeds the capacity of Srx in RBCs. Inhibition of catalase by azide or 3-AT did not increase H2O2 flux enough to induce accumulation of hyperoxidized Prx II, suggesting that Srx might still be able to counteract the additional flux of H2O2 arising from the inactivation of catalase function. However, catalase inhibition accelerated Prx II hyperoxidation 5- to 10-fold in RBCs exposed to GO at 0.1 or 0.5 mU/ml, suggesting that catalase becomes important for removal of H2O2 if the flux of H2O2 is increased, such as a result of exposure of RBCs to external H2O2 or to a toxin that increases intracellular H2O2 production. GPx1-DHA formation was enhanced moderately when catalase was inhibited by by azide or 3-AT.
Although ping-pong kinetics make it difficult to describe the enzymatic characteristics of Prx, GPx, and catalase by conventional Vmax and Km terms, various kinetic data suggest that Prx II is responsible for eliminating low concentrations of peroxides, whereas catalase scavenges H2O2 efficiently at high concentrations (20, 23, 31). This conclusion is also supported by in vivo evidence: Mice that lack Prx II develop hemolytic anemia (22), whereas RBC-related defects are not apparent in catalase-deficient mice (17).
Prx II, with a concentration of ∼240 μM (∼100 μM in blood), is one of the most abundant proteins in RBCs. Prx II would thus be expected to be able to remove H2O2 rapidly if its level in blood increases up to ∼100 μM. However, as suggested before (23), this removal would be attributable to one-time noncatalytic scavenging, given that all Prx II molecules would accumulate as the disulfide-linked dimer of the catalytic cycle as a result of the limited capacity of the Trx system in RBCs. Reduction of the dimeric Prx II might take as long as 20 min (23). Under these circumstances, elimination of additional H2O2 would depend on catalase action. Indeed, RBCs that lack catalase are highly sensitive to exogenous H2O2 (18), suggesting that catalase is essential for protection against higher levels of H2O2. The antioxidant role of GPx in various types of cells including RBCs has been discussed for many years (9). Recently, GPx1 has been suggested to play a minor role in the elimination of H2O2 from RBCs, given that mice lacking GPx1 appear normal (16) and RBCs derived from these mice show a virtually normal defense against exogenous H2O2 so long as catalase function is intact (21). The primary physiological substrate of GPx1 has been proposed to be organic peroxides rather than H2O2 (21). Special care has to be taken, however, in extrapolating the results obtained with GPx 1 KO mice to physiology of human RBCs because the relative activities of antioxidant enzymes in RBCs are different in the two species (20).
Catalase, as a dismutase, does not require reducing equivalents to eliminate H2O2, whereas GPx1 and Prx II require the GSH and Trx systems, respectively, both of which derive reducing equivalents from NADPH. Glutathione reductase, TrxR, and G6PDH, which are critical for the production of GSH, reduced Trx, and NADPH, respectively, are all sensitive to inactivation by H2O2 (3, 12, 36). GPx1 and Prx II are thus expected to become less efficient when RBCs are exposed to high levels of oxidative stress for long periods because the recycling of GSH and Trx becomes rate limiting. Even under such stressful circumstances, catalase alone is able to remove H2O2 rapidly as a result of its high turnover rate. However, two problems arise. First, organic peroxides will accumulate because catalase cannot remove them. Second, the intracellular concentration of H2O2 will be much higher than that maintained in the presence of fully functional GPx1 and Prx II because catalase is not able to function effectively when the H2O2 concentration falls. The steady-state level of H2O2 in mouse RBCs is estimated to be 0.05 nM (20). This extremely low level of H2O2, which is mainly attributable to the activity of Prx II, ensures protection of cell components from oxidative damage. The energy-consuming function of Prx II is thus needed in addition to the energy-independent catalase for RBC homeostasis.
GPx1 and Prx II are inactivated as the result of oxidative modification of Sec and Cys residues, respectively, at the active site. Our results now show that GPx1 inactivation is irreversible and does not require continuous turnover, whereas Prx II inactivation is reversible and progresses only when the enzyme goes through the catalytic cycle continuously (48). We found that the inactive DHA–GPx1 accumulates in RBCs with age even under the basal condition of H2O2 flux originating from Hb autoxidation. The inactive, sulfinic form of Prx II, however, does not accumulate under this condition. Our results indicate that the amount of sulfinic Prx II increases transiently when the flux of H2O2 increases temporarily above the basal level, but it might be removed slowly by the action of Srx. When exposed to such an increased H2O2 flux for long periods, however, the inactivated forms of both Prx II and GPx1 accumulate, as seen in RBCs exposed to GO, to N-phenylhydroxylamine, or to a catalase inhibitor in the presence of a low concentration of GO. Under these circumstances, catalase becomes the main player, and the concentrations of intracellular H2O2 and organic peroxides are expected to rise.
RBCs protect other tissues against oxidative damage by taking up and metabolizing peroxides (44). Given that the rate of DHA–GPx1 accumulation in RBCs depends on peroxide flux, the content of DHA–GPx1 in each RBC likely reflects total oxidative stress experienced by the cell during its lifetime. In addition to genetic polymorphisms, exposure to chemicals such as N-phenylhydroxylamine and sulfonamides, pathological conditions such as diabetes and local inflammation, and an insufficient intake of antioxidants are all expected to affect the rate of GPx1 inactivation. Our present data suggest that DHA–GPx1 in RBCs might be a suitable surrogate marker for evaluation of oxidative stress in the body.
Abbreviations Used
- 3-AT
3-amino-1,2,4-triazole
- BIAM
N-(biotinoyl)-N-9′(iodoacetyl) ethylenediamine
- DHA
dehydroalanine
- DMEM
Dulbecco's modified Eagle's medium
- DMF
N,N-dimethylformamide
- DTT
dithiothreitol
- FOX
ferrous oxidation of xylenol orange
- G6PDH
glucose-6-phosphate dehydrogenase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GO
glucose oxidase
- GPx
glutathione peroxidase
- GSH
glutathione
- Hb
hemoglobin
- HEL92.9
human erythroblastic leukemia
- LC-ESI-Q-TOF
liquid chromatography-electrospray ionization-quadrupole time-of-flight
- MALDI-TOF
matrix-assisted laser desorption-ionization time-of-flight
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- Prxs
peroxiredoxins
- ROS
reactive oxygen species
- Sec
selenocysteine
- Srx
sulfiredoxin
- Trx
thioredoxin
- TrxR
thioredoxin reductase
Acknowledgments
This study was supported by Korean Science and Engineering Foundation grants (National Honor Scientist Program grant 2006-05106 and grant FPR0502-470 of the 21C Frontier Functional Proteomics Projects) to S.G.R.
Author Disclosure Statement
S.G.R. is a member of the advisory board of Young-In Frontier.
References
- 1.Andersen HR. Nielsen JB. Nielsen F. Grandjean P. Antioxidative enzyme activities in human erythrocytes. Clin Chem. 1997;43:562–568. [PubMed] [Google Scholar]
- 2.Awasthi YC. Beutler E. Srivastava SK. Purification and properties of human erythrocyte glutathione peroxidase. J Biol Chem. 1975;250:5144–5149. [PubMed] [Google Scholar]
- 3.Bartosz G. Aging of the erythrocyte. VII. On the possible causes of inactivation of red cell enzymes. Mech Ageing Dev. 1980;13:379–385. doi: 10.1016/0047-6374(80)90079-2. [DOI] [PubMed] [Google Scholar]
- 4.Beutler E. The hemolytic effect of primaquine and related compounds: A review. Blood. 1959;14:103–139. [PubMed] [Google Scholar]
- 5.Biteau B. Labarre J. Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 6.Blankenberg S. Rupprecht HJ. Bickel C. Torzewski M. Hafner G. Tiret L. Smieja M. Cambien F. Meyer J. Lackner KJ. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349:1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 7.Blum J. Fridovich I. Inactivation of glutathione peroxidase by superoxide radical. Arch Biochem Biophys. 1985;240:500–508. doi: 10.1016/0003-9861(85)90056-6. [DOI] [PubMed] [Google Scholar]
- 8.Boyer C. Kahn A. Cottreau D. Marie J. [Mechanism of decrease in erythrocyte enzyme activities during red cell aging in the newborn and the adult] Nouv Rev Fr Hematol Blood Cells. 1977;18:229–231. [PubMed] [Google Scholar]
- 9.Brigelius–Flohe R. Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med. 1999;27:951–965. doi: 10.1016/s0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- 10.Chae HZ. Chung SJ. Rhee SG. Thioredoxin-dependent peroxide reductase from yeast. J Biol Chem. 1994;269:27670–27678. [PubMed] [Google Scholar]
- 11.Chae HZ. Robison K. Poole LB. Church G. Storz G. Rhee SG. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc Natl Acad Sci USA. 1994;91:7017–7021. doi: 10.1073/pnas.91.15.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dincer Y. Akcay T. Alademir Z. Ilkova H. Effect of oxidative stress on glutathione pathway in red blood cells from patients with insulin-dependent diabetes mellitus. Metabolism. 2002;51:1360–1362. doi: 10.1053/meta.2002.35192. [DOI] [PubMed] [Google Scholar]
- 13.Flohe L. Brigelius–Flohe R. Selenoproteins of the glutathione system. In: Hatfield dl., editor. Selenium. Its Molecular Biology and Role in Human Health. Boston, Dordrecht, London: Kluwer Academic Publishers; 2001. pp. 161–172. [Google Scholar]
- 14.Hamann M. Zhang T. Hendrich S. Thomas JA. Quantitation of protein sulfinic and sulfonic acid, irreversibly oxidized protein cysteine sites in cellular proteins. Methods Enzymol. 2002;348:146–156. doi: 10.1016/s0076-6879(02)48634-x. [DOI] [PubMed] [Google Scholar]
- 15.Harrison JH., Jr. Jollow DJ. Role of aniline metabolites in aniline-induced hemolytic anemia. J Pharmacol Exp Ther. 1986;238:1045–1054. [PubMed] [Google Scholar]
- 16.Ho YS. Magnenat JL. RBronson RT. Cao J. Gargano M. Sugawara M. Funk CD. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem. 1997;272:16644–16651. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- 17.Ho YS. Xiong Y. Ma W. Spector A. Ho DS. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem. 2004;279:32804–32812. doi: 10.1074/jbc.M404800200. [DOI] [PubMed] [Google Scholar]
- 18.Jacob HS. Ingbar SH. Jandl JH. Oxidative hemolysis and erythrocyte metabolism in hereditary acatalasia. J Clin Invest. 1965;44:1187–1199. doi: 10.1172/JCI105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong W. Park SJ. Chang TS. Lee DY. Rhee SG. Molecular mechanism of the reduction of cysteine sulfinic acid of peroxiredoxin to cysteine by mammalian sulfiredoxin. J Biol Chem. 2006;281:14400–14407. doi: 10.1074/jbc.M511082200. [DOI] [PubMed] [Google Scholar]
- 20.Johnson RM. Goyette G., Jr Ravindranath Y. Ho YS. Hemoglobin autoxidation and regulation of endogenous H2O2 levels in erythrocytes. Free Radic Biol Med. 2005;39:1407–1417. doi: 10.1016/j.freeradbiomed.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Johnson RM. Goyette G G., Jr Ravindranath Y. Ho YS. Red cells from glutathione peroxidase-1-deficient mice have nearly normal defenses against exogenous peroxides. Blood. 2000;96:1985–1988. [PubMed] [Google Scholar]
- 22.Lee TH. Kim SU. Yu SL. Kim SH. Park DS. Moon HB. Dho SH. Kwon KS. Kwon HJ. Han YH. Jeong S. Kang SW. Shin HS. Lee KK. Rhee SG. Yu DY. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood. 2003;101:5033–5038. doi: 10.1182/blood-2002-08-2548. [DOI] [PubMed] [Google Scholar]
- 23.Low FM. Hampton MB. Peskin AV. Winterbourn CC. Peroxiredoxin 2 functions as a noncatalytic scavenger of low-level hydrogen peroxide in the erythrocyte. Blood. 2007;109:2611–2617. doi: 10.1182/blood-2006-09-048728. [DOI] [PubMed] [Google Scholar]
- 24.Low FM. Hampton MB. Winterbourn CC. Peroxiredoxin 2 and peroxide metabolism in the erythrocyte. Antioxid Redox Signal. 2008;10:1621–1630. doi: 10.1089/ars.2008.2081. [DOI] [PubMed] [Google Scholar]
- 25.Ma S. Caprioli RM. Hill KE. Burk RF. Loss of selenium from selenoproteins: conversion of selenocysteine to dehydroalanine in vitro. J Am Soc Mass Spectrom. 2003;14:593–600. doi: 10.1016/S1044-0305(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 26.Margoliash E. Novogrodsky A. Schejter A. Irreversible reaction of 3-amino-1:2:4-triazole and related inhibitors with the protein of catalase. Biochem J. 1960;74:339–348. doi: 10.1042/bj0740339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marzocchi B. Ciccoli L. Tani C. Leoncini S. Rossi V. Bini L. Perrone S. Buonocore G. Hypoxia-induced post-translational changes in red blood cell protein map of newborns. Pediatr Res. 2005;58:660–665. doi: 10.1203/01.PDR.0000180545.24457.AC. [DOI] [PubMed] [Google Scholar]
- 28.Moore RB. Mankad MV. Shriver SK. Mankad VN. Plishker GA. Reconstitution of Ca(2+)-dependent K +transport in erythrocyte membrane vesicles requires a cytoplasmic protein. J Biol Chem. 1991;266:18964–18968. [PubMed] [Google Scholar]
- 29.Ninfali P. Palma F. Baronciani L. Piacentini G. Glucose-6-phosphate dehydrogenase activity and protein turnover in erythroblasts separated by velocity sedimentation at unit gravity and Percoll gradient centrifugation. Mol Cell Biochem. 1991;106:151–160. doi: 10.1007/BF00230181. [DOI] [PubMed] [Google Scholar]
- 30.Palacios O. Lobinski R. Investigation of the stability of selenoproteins during storage of human serum by size-exclusion LC-ICP-MS. Talanta. 2007;71:1813–1816. doi: 10.1016/j.talanta.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Peskin AV. Low FM. Paton LN. Maghzal GJ. Hampton MB. Winterbourn CC. The high reactivity of peroxiredoxin 2 with H2O2 is not reflected in its reaction with other oxidants and thiol reagents. J Biol Chem. 2007;282:11885–11892. doi: 10.1074/jbc.M700339200. [DOI] [PubMed] [Google Scholar]
- 32.Pigeolet E. Corbisier P. Houbion A. Lambert D. Michiels C. Raes M. Zachary MD. Remacle J. Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev. 1990;51:283–297. doi: 10.1016/0047-6374(90)90078-t. [DOI] [PubMed] [Google Scholar]
- 33.Rhee SG. Kang SW. Chang TS. Jeong W. Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001;52:35–41. doi: 10.1080/15216540252774748. [DOI] [PubMed] [Google Scholar]
- 34.Rifkind JM. Ramasamy S. Manoharan PT. Nagababu E. Mohanty JG. Redox reactions of hemoglobin. Antioxid Redox Signal. 2004;6:657–666. doi: 10.1089/152308604773934422. [DOI] [PubMed] [Google Scholar]
- 35.Rotruck JT. Pope AL. Ganther HE. Swanson AB. Hafeman DG. Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 36.Rundlof AK. Arner ES. Regulation of the mammalian selenoprotein thioredoxin reductase 1 in relation to cellular phenotype, growth, and signaling events. Antioxid Redox Signal. 2004;6:41–52. doi: 10.1089/152308604771978336. [DOI] [PubMed] [Google Scholar]
- 37.Salvo GP. Caprari P. Samoggia P. Mariani G. Salvati AM. Human erythrocyte separation according to age on a discontinuous "Percoll" density gradient. Clin Chim Acta. 1982;122:293–300. doi: 10.1016/0009-8981(82)90290-x. [DOI] [PubMed] [Google Scholar]
- 38.Schnabel R. Lackner KJ. Rupprecht HJ. Espinola–Klein C. Torzewski M. Lubos E. Bickel C. Cambien F. Tiret L. Munzel T. Blankenberg S. Glutathione peroxidase-1 and homocysteine for cardiovascular risk prediction: Results from the AtheroGene study. J Am Coll Cardiol. 2005;45:1631–1637. doi: 10.1016/j.jacc.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 39.Seo J. Jeong J. Kim YM. Hwang N. Paek E. Lee KJ. Strategy for comprehensive identification of post-translational modifications in cellular proteins, including low abundant modifications: application to glyceraldehyde-3-phosphate dehydrogenase. J Proteome Res. 2008;7:587–602. doi: 10.1021/pr700657y. [DOI] [PubMed] [Google Scholar]
- 40.Singh H. Purnell E. Smith C. Mechanistic study on aniline-induced erythrocyte toxicity. Arh Hig Rada Toksikol. 2007;58:275–285. doi: 10.2478/v10004-007-0018-2. [DOI] [PubMed] [Google Scholar]
- 41.Wendel A. Pilz W. Ladenstein R. Sawatzki G. Weser U. Substrate-induced redox change of selenium in glutathione peroxidase studied by x-ray photoelectron spectroscopy. Biochim Biophys Acta. 1975;377:211–215. doi: 10.1016/0005-2744(75)90303-4. [DOI] [PubMed] [Google Scholar]
- 42.Winterbourn CC. Free-radical production and oxidative reactions of hemoglobin. Environ Health Perspect. 1985;64:321–330. doi: 10.1289/ehp.8564321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winterbourn CC. McGrath BM. Carrell RW. Reactions involving superoxide and normal and unstable haemoglobins. Biochem J. 1976;155:493–502. doi: 10.1042/bj1550493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winterbourn CC. Stern A. Human red cells scavenge extracellular hydrogen peroxide and inhibit formation of hypochlorous acid and hydroxyl radical. J Clin Invest. 1987;80:1486–1491. doi: 10.1172/JCI113230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolff SS. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Meth Enzymol. 1994;233:182–189. [Google Scholar]
- 46.Woo HA. Chae HZ. Hwang SC. Yang KS. Kang SW. Kim K. Rhee SG. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science. 2003;300:653–656. doi: 10.1126/science.1080273. [DOI] [PubMed] [Google Scholar]
- 47.Woo HA. Jeong W. Chang TS. Park KJ. Park SJ. Yang JS. Rhee SG. Reduction of cysteine sulfinic acid by sulfiredoxin is specific to 2-cys peroxiredoxins. J Biol Chem. 2005;280:3125–3128. doi: 10.1074/jbc.C400496200. [DOI] [PubMed] [Google Scholar]
- 48.Yang KS. Kang SW. Woo HA. Hwang SC. Chae HZ. Kim K. Rhee SG. Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site cysteine to cysteine-sulfinic acid. J Biol Chem. 2002;277:38029–38036. doi: 10.1074/jbc.M206626200. [DOI] [PubMed] [Google Scholar]